Abstract
Autophagy is a cellular response activated by many pathogens, but the mechanism of activation is largely unknown. Recently we showed for the first time that rotavirus initiates the autophagy pathway through a calcium-mediated mechanism. Expression of the rotavirus-encoded NSP4, a pore-forming protein (viroporin), elicits the release of endoplasmic reticulum (ER) lumenal calcium into the cytoplasm of the infected cell. The increased cytoplasmic calcium activates a calcium signaling pathway involving calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) and 5′ adenosine monophosphate-activated protein kinase (AMPK) to trigger autophagy. Rotavirus further manipulates autophagy membrane trafficking to transport viral ER-associated proteins to viroplasms, sites of viral genome replication and immature particle assembly. Transport of viral proteins to viroplasms is required for assembly of infectious virus. Thus, NSP4, a multifunctional viral protein known to regulate infectious particle assembly, also modulates membrane trafficking by orchestrating the activation of autophagy to benefit viral replication.
Keywords: autophagy, calcium, kinase, stress, virus
Autophagy is a cellular catabolic process involving an intracellular membrane trafficking pathway important for the removal of damaged organelles and long-lived proteins. Apart from its role in maintaining cellular homeostasis, it is now recognized that autophagy also plays roles in innate immunity to remove intracellular microbial pathogens and to activate adaptive immunity. A number of pathogens exploit the autophagy process to enhance replication, but the mechanisms these pathogens use to initiate this process have not been elucidated.
We recently reported that rotavirus, which causes severe diarrheal disease in children and accounts for 450,000 deaths each year, manipulates autophagy to facilitate viral replication. Our study was motivated by our previous observation that the rotavirus nonstructural protein NSP4 colocalizes with the autophagy marker protein LC3 surrounding viroplasms, sites of viral genome replication and immature particle assembly. We have now demonstrated that rotavirus infection initiates autophagy and that autophagy provides a pro-viral, not an anti-viral, role in rotavirus replication. Inhibition of autophagy using a PtdIns3K inhibitor and infecting cells that lack the autophagy-initiation proteins ATG3 and ATG5 significantly reduces the yield of infectious rotavirus.
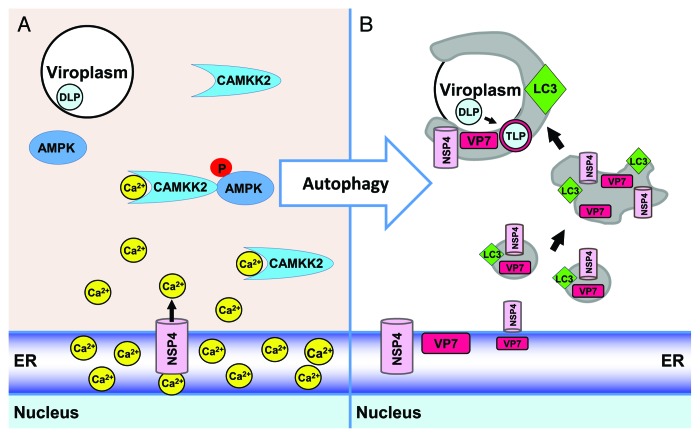
Seeking to define the mechanism of how rotavirus uses autophagy for enhanced replication, we discovered and characterized rotavirus as the first pathogen to initiate the autophagy process by triggering a calcium-mediated signaling pathway. Calcium ions are ubiquitous intracellular signaling molecules responsible for controlling a plethora of cellular processes. For this reason, cellular calcium homeostasis is tightly regulated. Rotavirus disrupts calcium homeostasis by expression of NSP4. NSP4, initially synthesized as an ER transmembrane glycoprotein, is a viroporin that releases ER lumenal calcium into the cytoplasm of infected cells. We found that elevation of cytoplasmic calcium ([Ca2+]cyto) alone is responsible for the initiation of autophagy because RNAi knockdown of NSP4, expression of a viroporin mutant, which does not increase [Ca2+]cyto, and chelation of [Ca2+]cyto following NSP4 expression all fail to induce autophagy. The increased [Ca2+]cyto activates a signaling pathway involving CAMKK2 and phosphorylation of AMPK to initiate autophagy. A specific inhibitor of CAMKK2, STO-609, not only inhibits AMPK phosphorylation and autophagy induction, but also significantly reduces the yield of infectious virus.
We examined the kinetics of NSP4-LC3 vesicle assembly and colocalization with viroplasms. At 4 h post infection (hpi), a time point when LC3-II and phosphorylated AMPK are concomitantly detected by western blot, NSP4 and LC3 colocalize in small puncta. The small puncta appear to fuse forming larger puncta at 5 hpi. At 6 hpi, NSP4, VP7 and LC3 are detected surrounding viroplasms, sites of viral genome replication and immature particle assembly. Interaction of NSP4 with immature particles at the interface between viroplasms and NSP4-LC3-containing membranes is an important and unique step in rotavirus infectious particle assembly. NSP4 binds to the immature particle in the viroplasm triggering particle budding through these membranes to facilitate assembly of the outer capsid proteins onto particles to form mature, infectious virions. One of the outer capsid proteins, VP7, is a lumenal ER membrane-associated protein. Treatment of rotavirus-infected cells with STO-609 inhibits NSP4 and VP7 trafficking to the viroplasms; neither NSP4 nor VP7 are detected surrounding viroplasms. Thus, this is a novel example of a virus that manipulates the autophagy membrane trafficking process to acquire and transport membranes containing viral proteins to the site of virus replication for infectious particle assembly.
Elucidating the calcium-mediated mechanism of rotavirus-initiated autophagy is significant as this may be a common mechanism for pathogen-induced autophagy. A number of other microbial pathogens (viruses, bacteria and parasites) disrupt calcium homeostasis increasing [Ca2+]cyto, as well as induce and require autophagy for their replication, but the mechanisms used by these pathogens to initiate the autophagy process are largely unknown. Determining whether increased [Ca2+]cyto is a common mechanistic link for induction of autophagy by pathogenic microbes is important because it raises the possibility of developing broad spectrum antimicrobial drugs that target this pathway (Fig. 1).
Figure 1. Overview of calcium-activated autophagy that transports viral proteins for infectious rotavirus assembly. (A) The rotavirus viroporin, NSP4, releases calcium (Ca2+) from the ER into the cytoplasm. Upon binding Ca2+, CAMKK2 is activated and phosphorylates AMPK leading to the initiation of autophagy. (B) Viroporin-mediated initiation of autophagy results in the transport of the ER-associated viral proteins NSP4 and VP7 to viroplasms, sites of viral genome replication and immature [double-layered particle (DLP)] formation. Upon autophagy initiation, small NSP4-LC3 puncta form. These fuse, forming larger puncta, which then engulf viroplasms. NSP4 mediates the assembly of the outer capsid proteins onto the DLP, forming the infectious virion [triple-layered particle (TLP)].
Glossary
Abbreviations:
- CAMKK2
calcium/calmodulin-dependent protein kinase kinase 2
- hpi
hours post infection
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/23959