Abstract
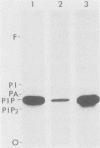
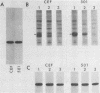
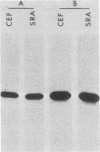
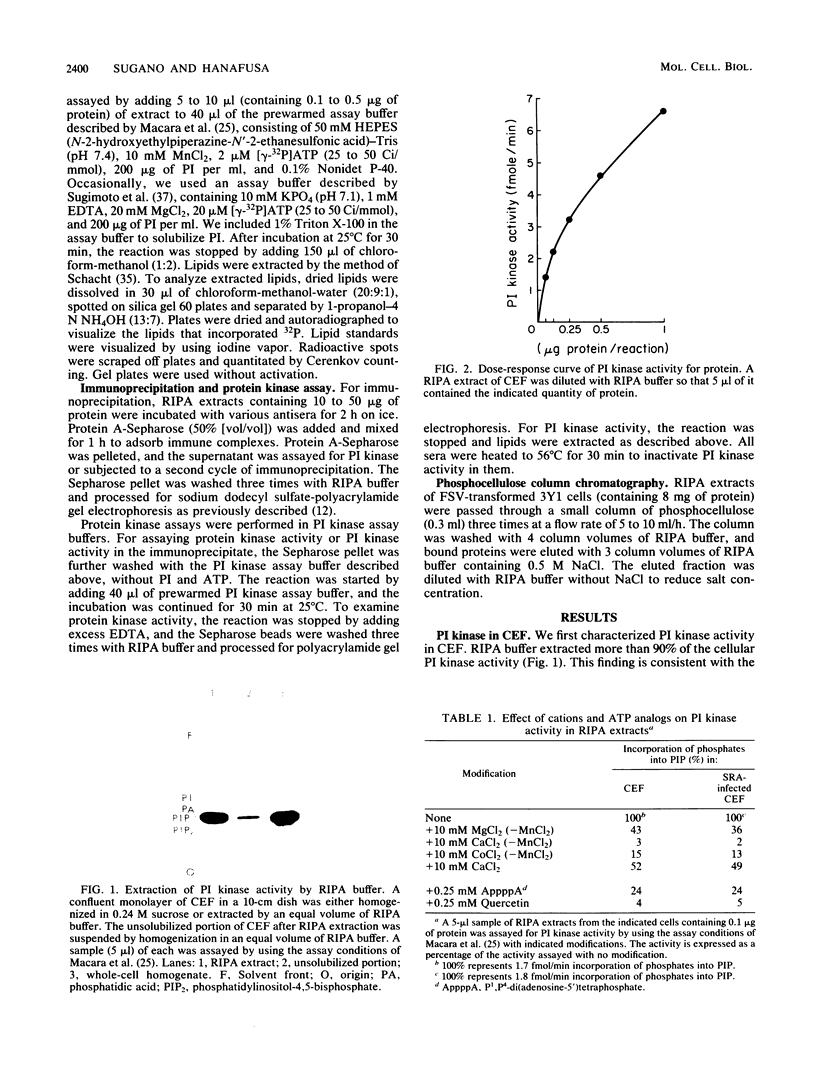
We assayed phosphatidylinositol (PI) kinase (EC 2.7.1.67) activity in detergent extracts of nontransformed or virus-transformed cells. Nontransformed chicken embryo fibroblasts (CEF) contain PI kinase activity with an apparent specific activity of 20 pmol/min per mg of protein. This activity sedimented as a single peak with a molecular weight of approximately 60,000 in a glycerol gradient, although immunoprecipitation with anti-p60src sera showed that the PI kinase activity is distinct from p60c-src. Extracts from CEF transformed by Rous sarcoma virus, Fujinami sarcoma virus, or avian sarcoma virus UR2 showed no elevation of PI kinase activity over nontransformed CEF. Removal of the oncogene products from extracts by immunoprecipitation did not change the level of PI kinase activity in extracts, suggesting that putative virus-coded PI kinases do not make a significant contribution to overall levels of PI kinase activity in transformed cells. Additionally, P140gag-fps was separated from cellular PI kinase by phosphocellulose chromatography. This partially purified fraction contained low PI kinase activity distinct from P140gag-fps, indicating that P140gag-fps has no detectable PI kinase activity.
Full text
PDF
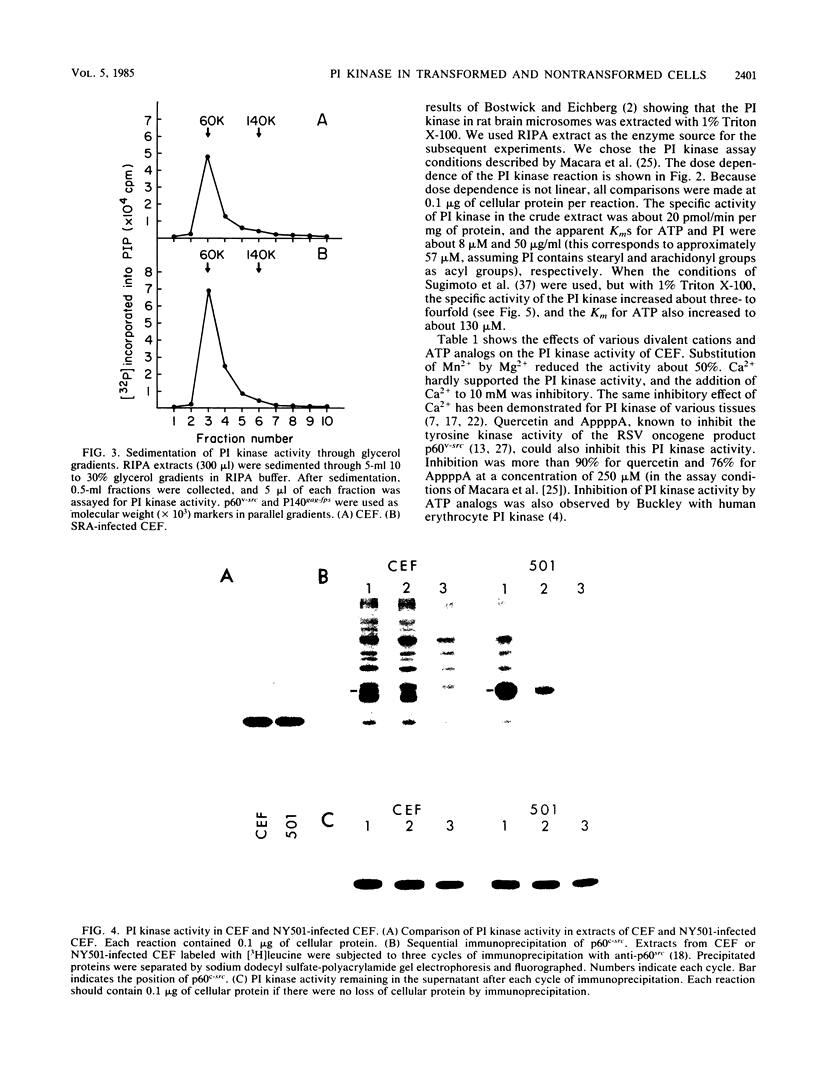



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bostwick J. R., Eichberg J. Detergent solubilization and hydrophobic chromatography of rat brain phosphatidylinositol kinase. Neurochem Res. 1981 Oct;6(10):1053–1065. doi: 10.1007/BF00964412. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Buckley J. T. Properties of human erythrocyte phosphatidylinositol kinase and inhibition by adenosine, ADP and related compounds. Biochim Biophys Acta. 1977 Jun 23;498(1):1–9. doi: 10.1016/0304-4165(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Clinton G. M., Roskoski R., Jr Tyrosyl and cyclic AMP-dependent protein kinase activities in BHK cells that express viral pp60src. Mol Cell Biol. 1984 May;4(5):973–977. doi: 10.1128/mcb.4.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodzin M., Kennedy E. P. Biosynthesis of diphosphoinositide in brain. J Biol Chem. 1965 Oct;240(10):3771–3780. [PubMed] [Google Scholar]
- Cooper P. H., Hawthorne J. N. Phosphatidylinositol kinase and diphosphoinositide kinase of rat kidney cortex: properties and subcellular localization. Biochem J. 1976 Oct 15;160(1):97–105. doi: 10.1042/bj1600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Insulin acutely increases phospholipids in the phosphatidate-inositide cycle in rat adipose tissue. J Biol Chem. 1982 Apr 25;257(8):4042–4045. [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Krueger J. G., Hanafusa H., Goldberg A. R. Only membrane-associated RSV src proteins have amino-terminally bound lipid. Nature. 1983 Mar 10;302(5904):161–163. doi: 10.1038/302161a0. [DOI] [PubMed] [Google Scholar]
- Graziani Y., Erikson E., Erikson R. L. The effect of quercetin on the phosphorylation activity of the Rous sarcoma virus transforming gene product in vitro and in vivo. Eur J Biochem. 1983 Oct 3;135(3):583–589. doi: 10.1111/j.1432-1033.1983.tb07692.x. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hajra A. K., Seguin E. B., Agranoff B. W. Rapid labeling of mitochondrial lipids by labeled orthophosphate and adenosine triphosphate. J Biol Chem. 1968 Apr 10;243(7):1609–1616. [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. The properties and subcellular distribution of phosphatidylinositol kinase in mammalian tissues. Biochim Biophys Acta. 1969 Jan 7;171(1):75–88. doi: 10.1016/0005-2744(69)90107-7. [DOI] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergil B., Sundler R. Phosphorylation of phosphatidylinositol in rat liver Golgi. J Biol Chem. 1983 Jul 10;258(13):7968–7973. [PubMed] [Google Scholar]
- Kai M., Salway J. G., Hawthorne J. N. The diphosphoinositide kinase of rat brain. Biochem J. 1968 Feb;106(4):791–801. doi: 10.1042/bj1060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M., White G. L., Hawthorne J. N. The phosphatidylinositol kinase of rat brain. Biochem J. 1966 Nov;101(2):328–337. doi: 10.1042/bj1010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough K. M., Thompson W. Triphosphoinositide phosphodiesterase in developing brain of the rat and in subcellular fractions of brain. J Neurochem. 1970 Jan;17(1):1–11. doi: 10.1111/j.1471-4159.1970.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G., Marinetti G. V., Balduzzi P. C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci U S A. 1984 May;81(9):2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Perry M. E., Levy B. T. P1,P4-Di(adenosine-5')tetraphosphate inhibits phosphorylation of immunoglobulin G by Rous sarcoma virus pp60src. J Biol Chem. 1983 Apr 10;258(7):4055–4058. [PubMed] [Google Scholar]
- Mathey-Prevot B., Shibuya M., Samarut J., Hanafusa H. Revertants and partial transformants of rat fibroblasts infected with Fujinami sarcoma virus. J Virol. 1984 May;50(2):325–334. doi: 10.1128/jvi.50.2.325-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Harwood J. L., Coleman R., Hawthorne J. N. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta. 1967 Dec 5;144(3):649–658. doi: 10.1016/0005-2760(67)90053-7. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Salway J. G., Kai M., Hawthorne J. N. Triphosphoinositide phosphomonoesterase activity in nerve cellbodies, neuroglia and subcellular fractions from whole rat brain. J Neurochem. 1967 Oct;14(10):1013–1024. doi: 10.1111/j.1471-4159.1967.tb09512.x. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Sheltawy A., Brammer M., Borrill D. The subcellular distribution of triphosphoinositide phosphomonoesterase in guinea-pig brain. Biochem J. 1972 Jul;128(3):579–586. doi: 10.1042/bj1280579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]