Abstract
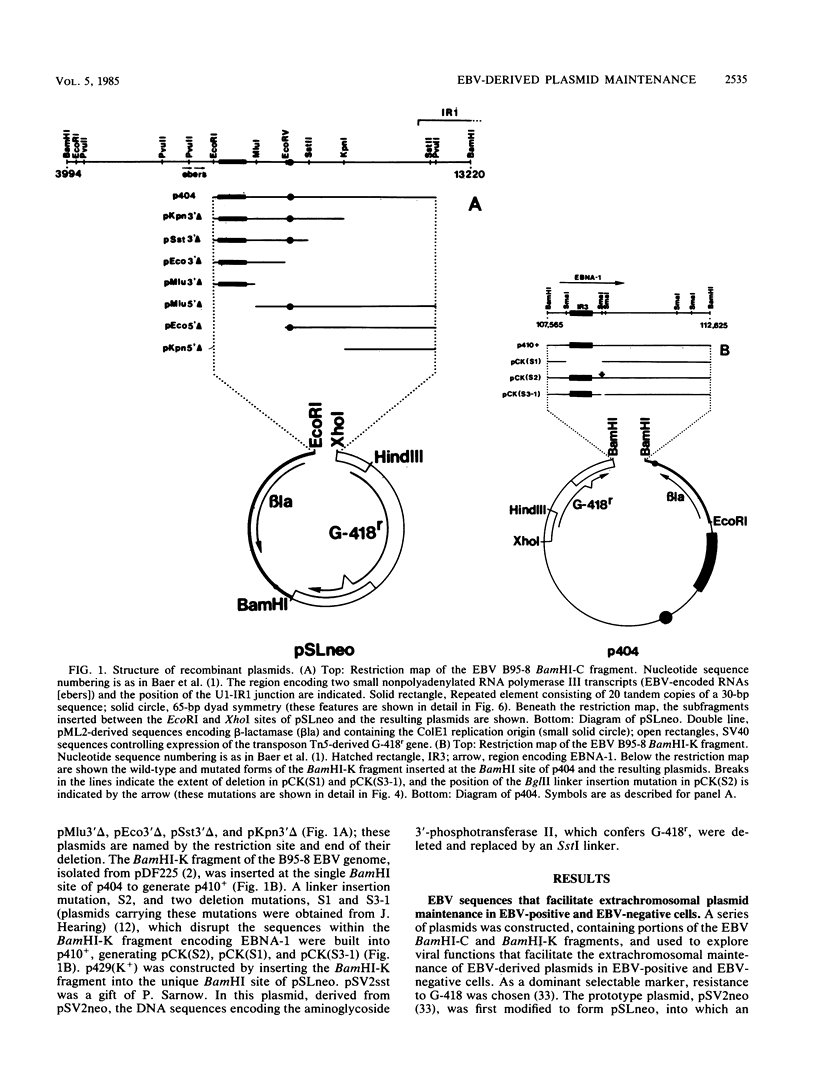
The Epstein-Barr virus (EBV) genome becomes established as a multicopy plasmid in the nucleus of infected B lymphocytes. A cis-acting DNA sequence previously described within the BamHI-C fragment of the EBV genome (J. Yates, N. Warren, D. Reisman, and B. Sugden, Proc. Natl. Acad. Sci. USA 81:3806-3810, 1984) allows stable extrachromosomal plasmid maintenance in latently infected cells, but not in EBV-negative cells. In agreement with the findings of Yates et al., deletion analysis permitted the assignment of this function to a 2,208-base-pair region (nucleotides 7315 to 9517 of the B95-8 strain of EBV) of the BamHI-C fragment that contained a striking repetitive sequence and an extended region of dyad symmetry. A recombinant vector, p410+, was constructed which carried the BamHI-K fragment (nucleotides 107565 to 112625 of the B95-8 strain, encoding the EBV-associated nuclear antigen EBNA-1), the cis-acting sequence from the BamHI-C fragment, and a dominant selectable marker gene encoding G-418 resistance in animal cells. After being transfected into HeLa cells, this plasmid persisted extrachromosomally at a low copy number, with no detectable rearrangements or deletions. Two mutations in the BamHI-K-derived portion of p410+, a large in-frame deletion and a linker insertion frameshift mutation, both of which alter the carboxy-terminal portion of EBNA-1, destroyed the ability of the plasmid to persist extrachromosomally in HeLa cells. A small in-frame deletion and linker insertion mutation in the region encoding the carboxy-terminal portion of EBNA-1, which replaced 19 amino acid codons with 2, had no effect on the maintenance of p410+ in HeLa cells. These observations indicate that EBNA-1, in combination with a cis-acting sequence in the BamHI-C fragment, is in part responsible for extrachromosomal EBV-derived plasmid maintenance in HeLa cells. Two additional activities have been localized to the BamHI-C DNA fragment: (i) a DNA sequence that could functionally substitute for the simian virus 40 enhancer and promoter elements controlling the expression of G-418 resistance and (ii) a DNA sequence which, although not sufficient to allow extrachromosomal plasmid maintenance, enhanced the frequency of transformation to G-418 resistance in EBV-positive (but not EBV-negative) cells. These findings suggest that the BamHI-C fragment contains a lymphoid-specific or EBV-inducible promoter or enhancer element or both.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl V., Henle G., Henle W., Kohn G. Demonstration of a herpes group virus in cultures of peripheral leukocytes from patients with infectious mononucleosis. J Virol. 1968 Jul;2(7):663–669. doi: 10.1128/jvi.2.7.663-669.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D. K., Robert M. F., Shedd D., Summers W. P., Robinson J. E., Wolak J., Stefano J. E., Miller G. Identification of Epstein-Barr nuclear antigen polypeptide in mouse and monkey cells after gene transfer with a cloned 2.9-kilobase-pair subfragment of the genome. Proc Natl Acad Sci U S A. 1984 Jan;81(1):43–47. doi: 10.1073/pnas.81.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Nonoyama M. Host cell regulation of induction of Epstein-Barr virus. J Virol. 1974 Jul;14(1):174–176. doi: 10.1128/jvi.14.1.174-176.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gussander E., Adams A. Electron microscopic evidence for replication of circular Epstein-Barr virus genomes in latently infected Raji cells. J Virol. 1984 Nov;52(2):549–556. doi: 10.1128/jvi.52.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing J. C., Nicolas J. C., Levine A. J. Identification of Epstein-Barr virus sequences that encode a nuclear antigen expressed in latently infected lymphocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4373–4377. doi: 10.1073/pnas.81.14.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A., Ripley S., Heller M., Kieff E. Chromosome site for Epstein-Barr virus DNA in a Burkitt tumor cell line and in lymphocytes growth-transformed in vitro. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1987–1991. doi: 10.1073/pnas.80.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G. W., Bjursell G., Klein G., Giovanella B. C., Singh S. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976 Mar 25;260(5549):302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Heller M., Petti L., O'Shiro E., Kieff E. Persistence of the entire Epstein-Barr virus genome integrated into human lymphocyte DNA. Science. 1984 Dec 14;226(4680):1322–1325. doi: 10.1126/science.6095452. [DOI] [PubMed] [Google Scholar]
- Nilsson K. High-frequency establishment of human immunoglobulin-producing lymphoblastoid lines from normal and malignant lymphoid tissue and peripheral blood. Int J Cancer. 1971 Nov 15;8(3):432–442. doi: 10.1002/ijc.2910080311. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robert M. F., Shedd D., Weigel R. J., Fischer D. K., Miller G. Expression in COS-1 cells of Epstein-Barr virus nuclear antigen from a complete gene and a deleted gene. J Virol. 1984 Jun;50(3):822–831. doi: 10.1128/jvi.50.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin S. F., Vaughan J. H., Carson D. A. Changes in the expression of two Epstein-Barr virus-associated antigens, EBNA and RANA, during the cell cycle of transformed human B lymphoblasts. Int J Cancer. 1980 Jul 15;26(1):9–12. doi: 10.1002/ijc.2910260103. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983 Oct 30;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Rösl F., Zentgraf H. Origin of replication in episomal bovine papilloma virus type 1 DNA isolated from transformed cells. EMBO J. 1984 Sep;3(9):2173–2178. doi: 10.1002/j.1460-2075.1984.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]





