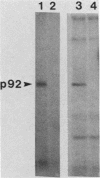
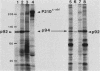
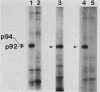
Abstract
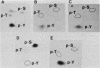
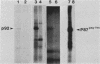
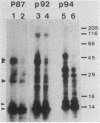
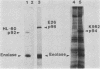
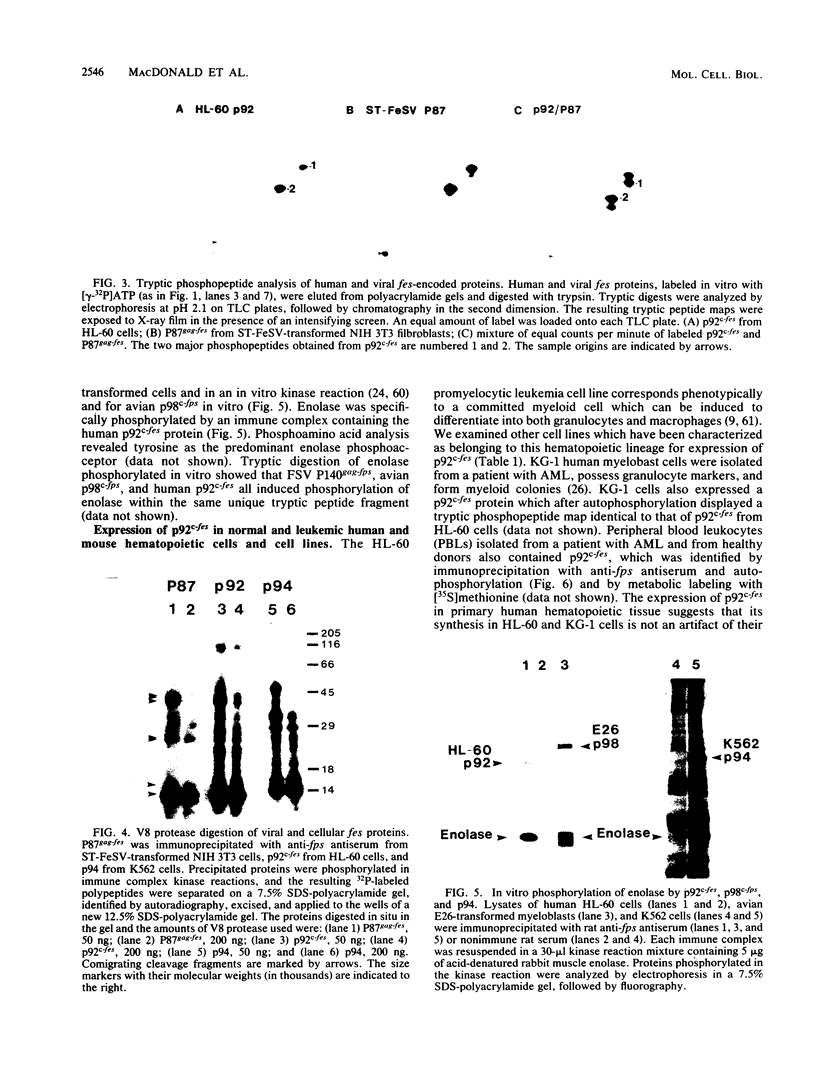
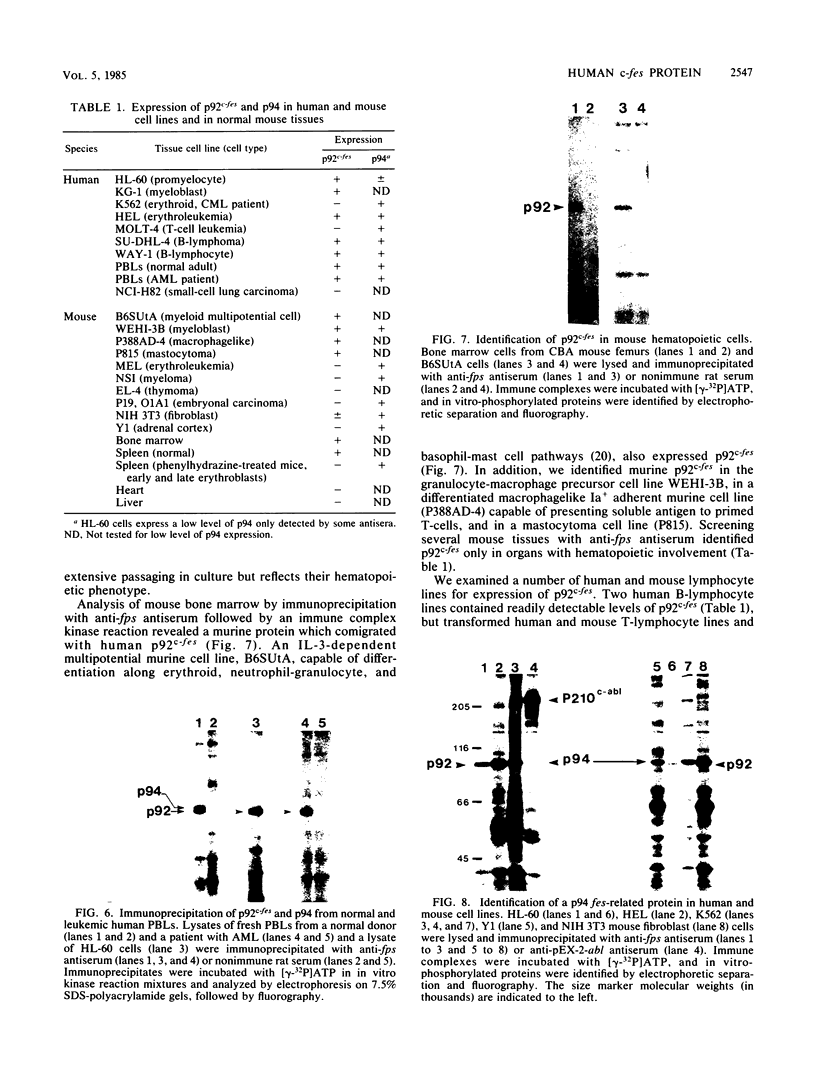
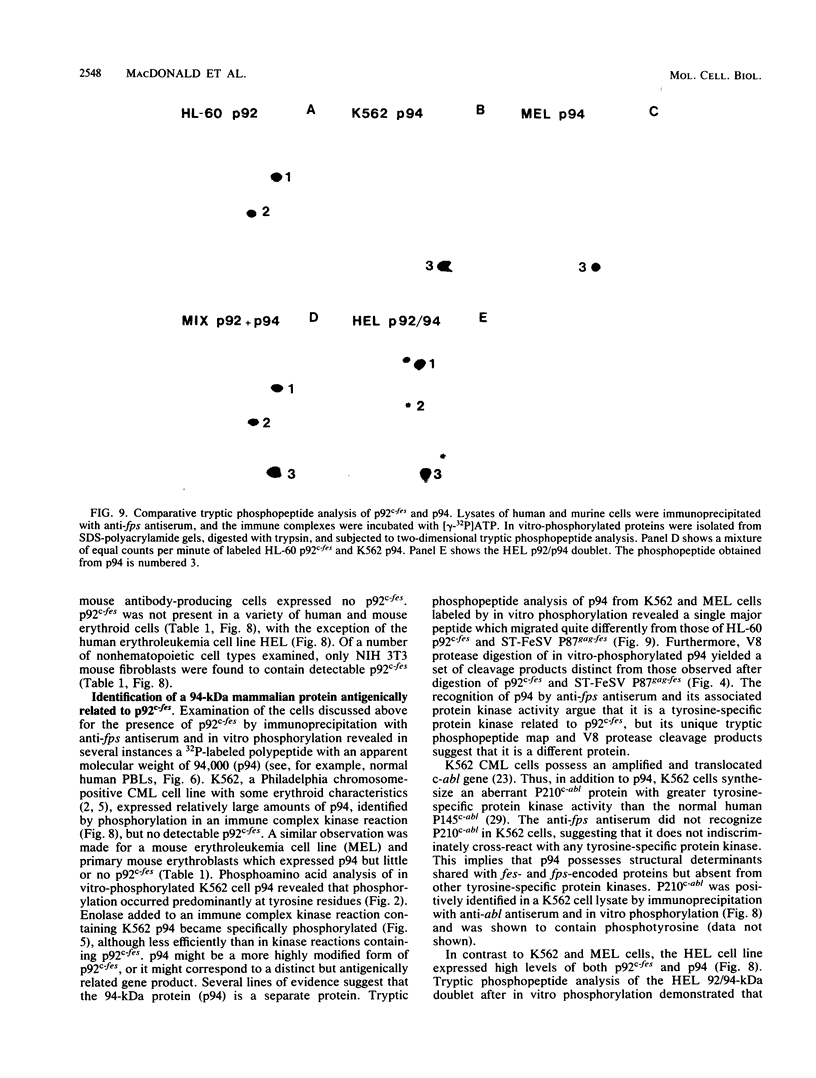
The avian c-fps and mammalian c-fes proto-oncogenes are cognate cellular sequences. Antiserum raised against the P140gag-fps transforming protein of Fujinami avian sarcoma virus specifically recognized a 92,000-Mr protein in human and mouse hematopoietic cells which was closely related in structure to Snyder-Theilen feline sarcoma virus P87gag-fes. This polypeptide was apparently the product of the human c-fes gene and was therefore designated p92c-fes. Human p92c-fes was associated with a tyrosine-specific protein kinase activity in vitro and was capable of both autophosphorylation and phosphorylation of enolase as an exogenous protein substrate. The synthesis of human and mouse p92c-fes was largely, though not entirely, confined to myeloid cells. p92c-fes was expressed to relatively high levels in a multipotential murine myeloid cell line, in more mature human and mouse granulocyte-macrophage progenitors, and in differentiated macrophage like cells as well as in the mononuclear fraction of normal and leukemic human peripheral blood. p92c-fes was not found in erythroid cells, with the exception of a human erythroleukemia line which retains the capacity to differentiate into macrophage like cells. These results suggest a normal role for the p92c-fes tyrosine kinase in hematopoiesis, particularly in granulocyte-macrophage differentiation. In addition, a distinct 94,000-Mr polypeptide, antigenically related to p92c-fes, was identified in a number of hematopoietic and nonhematopoietic human and mouse cells and was also found to be associated with a tyrosine-specific protein kinase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Donner L., Ruscetti S. K., Sherr C. J. Transformation-defective mutants of Snyder-Theilen feline sarcoma virus lack tyrosine-specific protein kinase activity. J Virol. 1981 Jul;39(1):246–254. doi: 10.1128/jvi.39.1.246-254.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Murnane M. J., Tonkonow B. L., Berman B. W., Mazur E. M., Cavallesco C., Jenko T., Snyder E. L., Forget B. G., Hoffman R. Embryonic-fetal erythroid characteristics of a human leukemic cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3509–3513. doi: 10.1073/pnas.77.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Cohen D. A., Kaplan A. M. Adherent Ia+ murine tumor lines with characteristics of dendritic cells. I. Morphology, surface phenotype, and induction of syngeneic mixed lymphocyte reactions. J Exp Med. 1981 Dec 1;154(6):1881–1898. doi: 10.1084/jem.154.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- DUNN T. B., POTTER M. A transplantable mast-cell neoplasm in the mouse. J Natl Cancer Inst. 1957 Apr;18(4):587–601. [PubMed] [Google Scholar]
- Dasgupta J. D., Garbers D. L. Tyrosine protein kinase activity during embryogenesis. J Biol Chem. 1983 May 25;258(10):6174–6178. [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Epstein A. L., Levy R., Kim H., Henle W., Henle G., Kaplan H. S. Biology of the human malignant lymphomas. IV. Functional characterization of ten diffuse histiocytic lymphoma cell lines. Cancer. 1978 Nov;42(5):2379–2391. doi: 10.1002/1097-0142(197811)42:5<2379::aid-cncr2820420539>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Shibuya M., Hanafusa H., Stephenson J. R. Transforming genes of avian (v-fps) and mammalian (v-fes) retroviruses correspond to a common cellular locus. Virology. 1983 Mar;125(2):480–486. doi: 10.1016/0042-6822(83)90219-2. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Ingman-Baker J., Hinze E., Levy J. G., Pawson T. Monoclonal antibodies to the transforming protein of Fujinami avian sarcoma virus discriminate between different fps-encoded proteins. J Virol. 1984 May;50(2):572–578. doi: 10.1128/jvi.50.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Adkins B., Beug H., Graf T. src- and fps-containing avian sarcoma viruses transform chicken erythroid cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7122–7126. doi: 10.1073/pnas.81.22.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol. 1983 Aug;11(7):649–660. [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. Evidence for two distinct c-src loci on human chromosomes 1 and 20. Nature. 1984 Nov 1;312(5989):70–71. doi: 10.1038/312070a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev Z., Leibovitz N., Segev O., Shilo B. Z. Expression of the src and abl cellular oncogenes during development of Drosophila melanogaster. Mol Cell Biol. 1984 May;4(5):982–984. doi: 10.1128/mcb.4.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Guilbert L. Differential expression of c-fos in hematopoietic cells: correlation with differentiation of monomyelocytic cells in vitro. EMBO J. 1984 Aug;3(8):1887–1890. doi: 10.1002/j.1460-2075.1984.tb02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Yokochi T., Chait A., Kannagi R. Human erythroleukemia cell line (HEL) undergoes a drastic macrophage-like shift with TPA. Blood. 1983 Oct;62(4):832–845. [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. In vitro transformation of murine pre-B lymphoid cells by Snyder-Theilen feline sarcoma virus. J Virol. 1983 Jun;46(3):993–1002. doi: 10.1128/jvi.46.3.993-1002.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Suard Y., Perucho M., Li Y., Kamata T., Feramisco J., Stavnezer E., Fogh J., Wigler M. H. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Cline M. J. Expression of cellular oncogenes during embryonic and fetal development of the mouse. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7141–7145. doi: 10.1073/pnas.81.22.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Boettiger D., Dexter T. M. Continuous in vitro generation of multipotential stem cell clones from src-infected cultures. Nature. 1984 Jul 19;310(5974):228–230. doi: 10.1038/310228a0. [DOI] [PubMed] [Google Scholar]
- Territo M. C., Cline M. J. Monocyte function in man. J Immunol. 1977 Jan;118(1):187–192. [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Hinze E., Pawson T. Mapping of multiple phosphorylation sites within the structural and catalytic domains of the Fujinami avian sarcoma virus transforming protein. J Virol. 1983 Apr;46(1):29–41. doi: 10.1128/jvi.46.1.29-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura Y., Buonassisi V., Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966 Mar;26(3):529–535. [PubMed] [Google Scholar]