Abstract
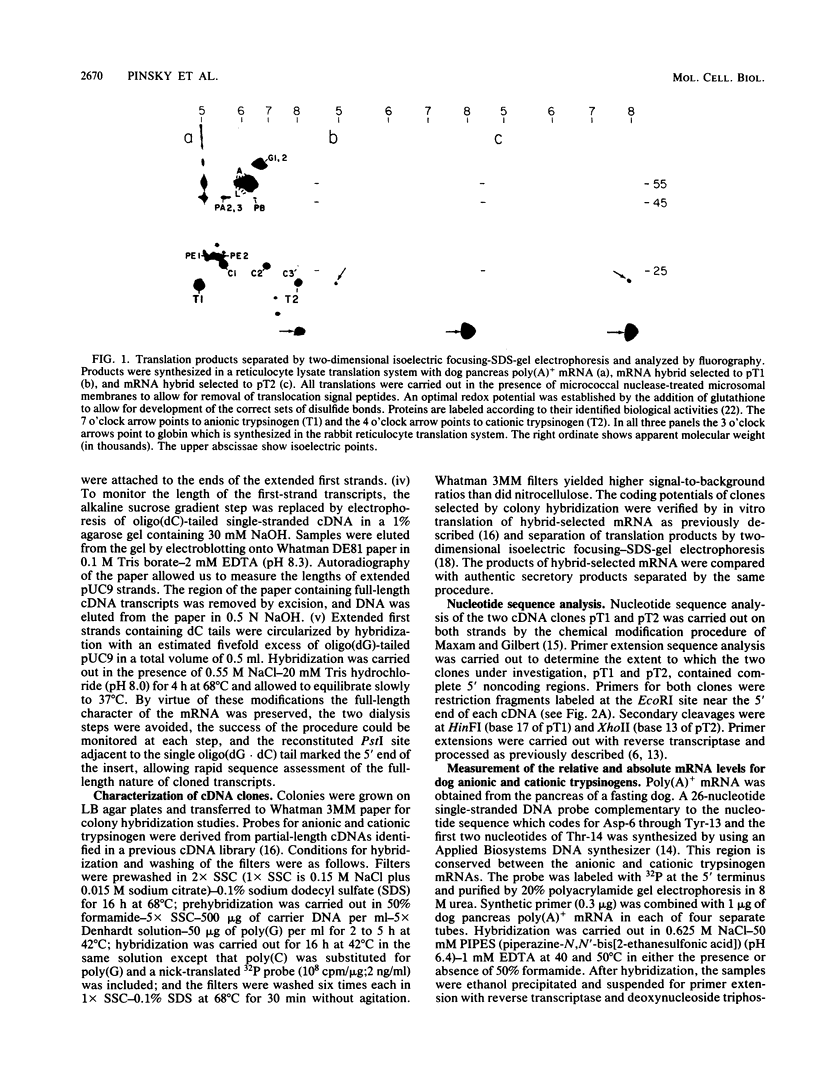
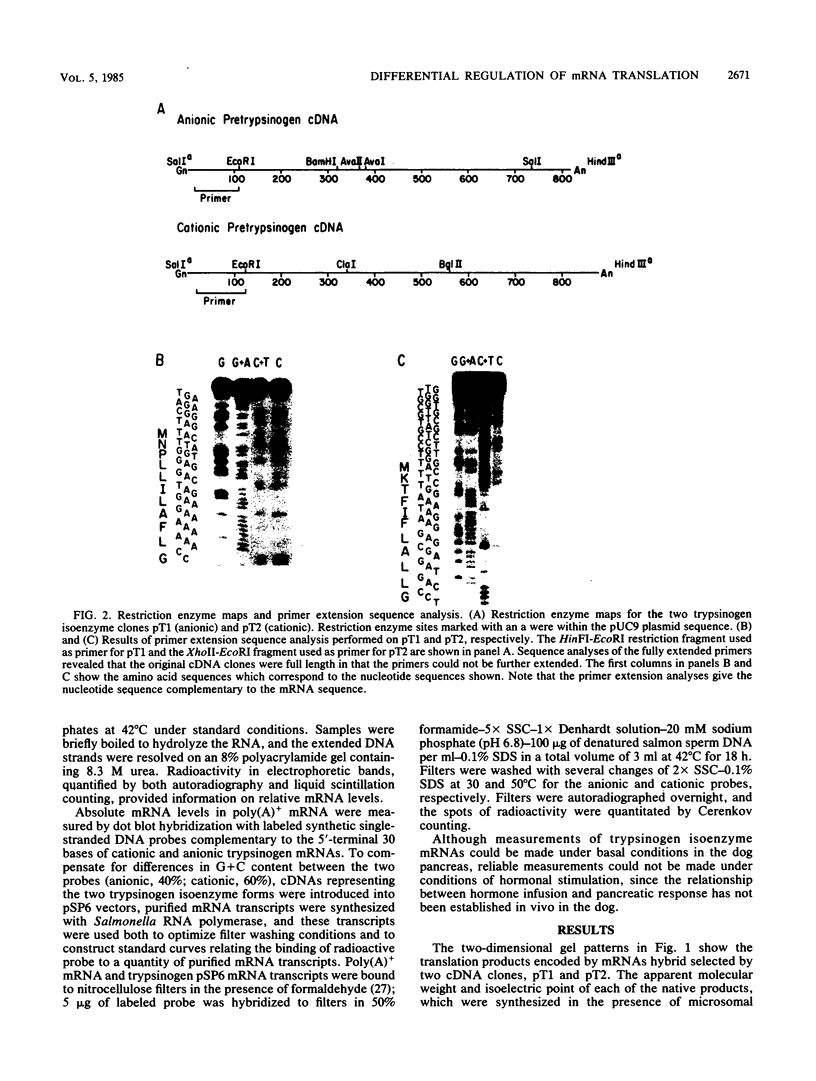
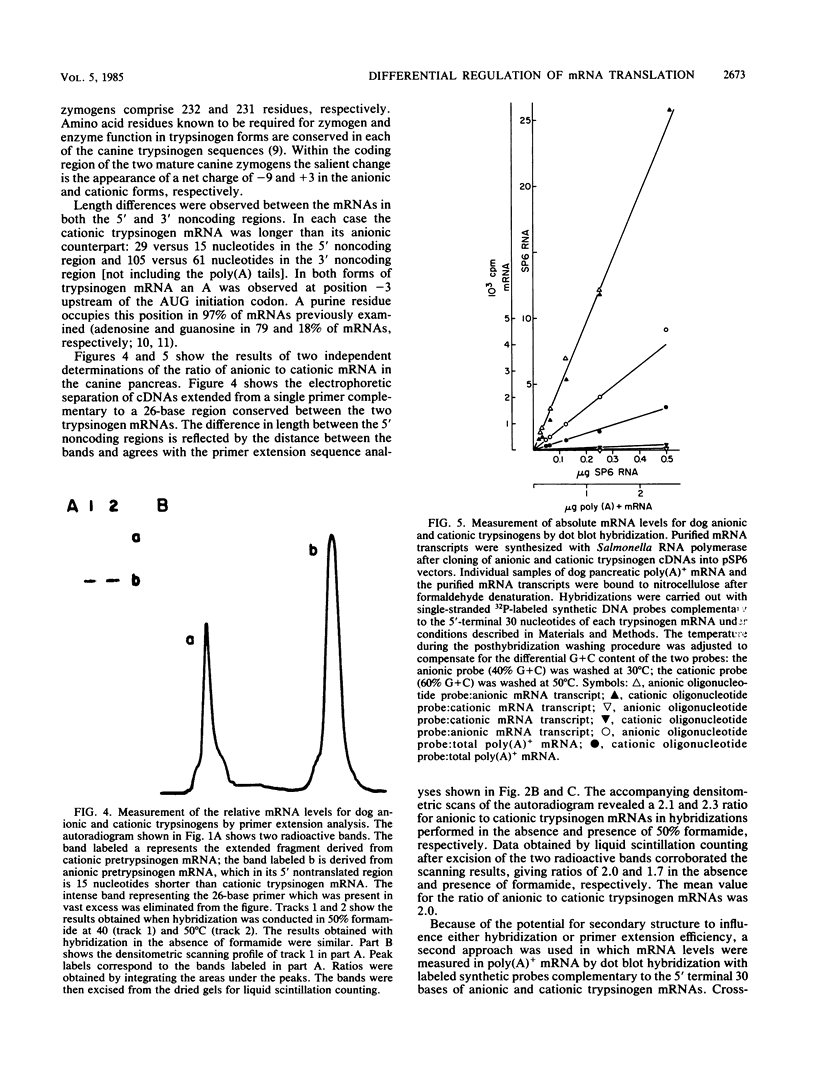
In the absence of changes in functional mRNA levels, stimulation of the pancreas with caerulein, a peptide analog of cholecystokinin, has been previously shown to increase the synthesis of anionic but not cationic trypsinogen. To look for structure-function correlations, a high-yield, full-length cDNA library has been constructed from canine pancreatic poly(A)+ mRNA. Full-length clones coding for the two major trypsinogen isoenzyme forms have been identified by colony hybridization and verified by in vitro translation of hybrid-selected mRNA in the presence of microsomal membranes and an optimal redox potential. Disulfide-bonded translation products were separated and identified by two-dimensional isoelectric focusing-sodium dodecyl sulfate-gel electrophoresis. Nucleotide sequence analysis allowed us to deduce the amino acid sequences for the anionic and cationic forms of canine trypsinogen, which contain 232 and 231 residues, respectively (77% amino acid identity), and the 15-residue amino terminal signal sequences (53% amino acid identity) associated with the two presecretory forms. Measurements of relative and absolute mRNA levels, when related to relative protein synthesis values, indicated that the translational efficiency of anionic trypsinogen mRNA exceeded that of cationic trypsinogen mRNA by 1.5- to 2.9-fold under basal conditions. Analysis of the 5' noncoding regions of trypsinogen mRNAs revealed a striking conservation of sequence (10 of 12 bases) between dog and rat anionic trypsinogen forms. This contrasted markedly with the divergence of the 5' noncoding regions observed between dog anionic and cationic trypsinogen mRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom K. S., Amaya E., Carbon J., Clarke L., Hill A., Yeh E. Chromatin conformation of yeast centromeres. J Cell Biol. 1984 Nov;99(5):1559–1568. doi: 10.1083/jcb.99.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne T., Scheele G. Amino acid sequences of transport peptides associated with canine exocrine pancreatic proteins. J Biol Chem. 1982 Apr 25;257(8):4133–4140. [PubMed] [Google Scholar]
- Cozzone A., Marchis-Mouren G. Messenger ribonucleic acid stability in rat pancreas and liver. Biochemistry. 1967 Dec;6(12):3911–3917. doi: 10.1021/bi00864a037. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E., Nakashima K., Shatkin A. J. Base-pairing in conserved 3' end of 18 S rRNA as determined by psoralen photoreaction and RNase sensitivity. J Biol Chem. 1980 Jun 10;255(11):4973–4975. [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J. Sequence analysis of zein cDNAs obtained by an efficient mRNA cloning method. Nucleic Acids Res. 1983 Jul 25;11(14):4891–4906. doi: 10.1093/nar/11.14.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman D. L. The disulphide bridges of trypsin. J Mol Biol. 1965 Jul;12(3):929–932. doi: 10.1016/s0022-2836(65)80340-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Two similar but nonallelic rat pancreatic trypsinogens. Nucleotide sequences of the cloned cDNAs. J Biol Chem. 1982 Aug 25;257(16):9724–9732. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pinsky S. D., LaForge K. S., Luc V., Scheele G. Identification of cDNA clones encoding secretory isoenzyme forms: sequence determination of canine pancreatic prechymotrypsinogen 2 mRNA. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7486–7490. doi: 10.1073/pnas.80.24.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Scheele G., Blackburn P. Role of mammalian RNase inhibitor in cell-free protein synthesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4898–4902. doi: 10.1073/pnas.76.10.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R., Carne T. Mechanism of compartmentation of secretory proteins: transport of exocrine pancreatic proteins across the microsomal membrane. J Cell Biol. 1980 Dec;87(3 Pt 1):611–628. doi: 10.1083/jcb.87.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Van Knippenberg P. H., Van Kimmenade J. M., Heus H. A. Phylogeny of the conserved 3' terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 1984 Mar 26;12(6):2595–2604. doi: 10.1093/nar/12.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]