Abstract
Background.
Resveratrol, a plant-derived polyphenol, has shown promising effects on insulin sensitivity and glucose tolerance in animal models and is also reported to have cardioprotective properties, but human studies are limited. In a pilot study, we tested the hypothesis that resveratrol improves glucose metabolism and vascular function in older adults with impaired glucose tolerance (IGT).
Methods.
Ten subjects aged 72 ± 3 years (M ± SD) with IGT were enrolled in a 4-week open-label study of resveratrol (daily dose 1, 1.5, or 2 g). Following a standard mixed meal (110 g carbohydrate, 20 g protein, 20 g fat), we measured 3-hour glucose and insulin area under the curve (AUC), insulin sensitivity (Matsuda index), and secretion (corrected insulin response at 30 minutes). Endothelial function was assessed by reactive hyperemia peripheral arterial tonometry (reactive hyperemia index) before and 90 minutes postmeal. Results did not differ by dose, so data were combined for analysis.
Results.
At baseline, body mass index was 29 ± 5 kg/m2, fasting plasma glucose 110 ± 13 mg/dL, and 2-hour glucose 183 ± 33 mg/dL. After 4 weeks of resveratrol, fasting plasma glucose was unchanged, but peak postmeal (185 ± 10 vs 166 ± 9 mg/dL, p = .003) and 3-hour glucose AUC (469 ± 23 vs 428 ± 19, p = .001) declined. Matsuda index improved (3.1 ± 0.5 vs 3.8 ± 0.5, p = .03), and corrected insulin response at 30 minutes was unchanged (0.6 ± 0.1 vs 0.5 ± 0.5, p = .49). There was a trend toward improved postmeal reactive hyperemia index (baseline vs resveratrol postmeal delta −0.4 ± 0.2 vs 0.2 ± 0.3, p = .06). Weight, blood pressure, and lipids were unchanged.
Conclusions.
At doses between 1 and 2 g/day, resveratrol improves insulin sensitivity and postmeal plasma glucose in subjects with IGT. These preliminary findings support the conduct of larger studies to further investigate the effects of resveratrol on metabolism and vascular function.
Resveratrol (3,5,4′-trihydroxystilbene) is a plant-derived polyphenolic compound mainly known for its antioxidant and phytoestrogenic properties. Interest in this compound has increased in recent years, first from its identification as a chemopreventive agent for skin cancer and subsequently from reports that it activates sirtuins and extends the life span of lower organisms, including rodents (1). Resveratrol has demonstrated promising effects on insulin secretion, insulin sensitivity, and glucose tolerance in a variety of animal models (2,3). Notably, resveratrol prevented the negative metabolic effects of excess calorie intake, improving glucose tolerance, lowering insulin levels, and significantly increasing survival of middle-aged mice (4). Resveratrol has also been shown to increase mitochondrial biogenesis and appears to mimic the beneficial effects of caloric restriction on glucose metabolism (5–7).
Resveratrol has also been proposed to have cardioprotective effects. Resveratrol possesses weak activity as a phytoestrogen (8), antioxidant properties (9), and has been shown to both enhance synthesis and decrease inactivation of the vasorelaxant nitric oxide (10). Resveratrol may also promote vascular relaxation by inhibiting synthesis of the potent vasoconstrictor thromboxane A2 and by other nitric oxide-independent mechanisms (11).
However, despite the many health claims made on its behalf and its widespread use as a nutritional supplement, formal studies of resveratrol in humans are very limited and no studies of its metabolic effects have been reported. Further, questions about resveratrol bioavailability, dosing range, and safety also need to be addressed (12–14). We therefore conducted a pilot study of resveratrol treatment as an initial step in assessing its potential to improve glucose tolerance, insulin sensitivity, and vascular function. For this initial investigation, we studied the effects of resveratrol in subjects with impaired glucose tolerance (IGT) who have definite but not-yet-severe metabolic dysregulation, which may be most amenable to intervention. We chose to focus on older adults for two important reasons. First, IGT is in large part an age-related phenomenon, affecting up to 30% of older adults (15) and constitutes a major risk factor for the development of both diabetes and cardiovascular disease (16). In addition, although lifestyle modification was exceptionally effective in preventing progression from IGT to diabetes in older participants (age 60–85) in the Diabetes Prevention Program, metformin was not (17), highlighting the need for alternate pharmacologic approaches for older adults with IGT.
METHODS
Adults aged 65 and older were screened with a 75-g oral glucose tolerance test and those with fasting plasma glucose <126 mg/dL and 2-hour glucose ≥140 mg/dL were eligible to enroll. Subjects were excluded if they had a recent cardiovascular event, evidence of significant liver or renal disease; any active cancer; or prior history of estrogen-dependent neoplasm. Because of the possibility of CYP450-related drug interactions (18), treatment with the following drugs was exclusionary: antiepileptics, mexilitene, quinidine, cyclosporine, tacrolimus, HIV protease inhibitors, or high-dose statin therapy (>20 mg atorvastatin or rosuvastatin; >40 mg simvastatin, pravastatin, or lovastatin). Individuals taking resveratrol or antioxidant vitamins (other than a standard multivitamin preparation) within the prior 3 months were also excluded. The study protocol was approved by the Albert Einstein College of Medicine Institutional Review Board, and all participants provided written informed consent.
Resveratrol capsules were obtained from Biotiva, LLC, and independent verification of the resveratrol content of the capsules used in this study was performed in the laboratory of Rong-Fong Shen, PhD, Proteomics and Analytical Biochemistry Unit, National Institute on Aging at the National Institutes of Health. Subjects were randomly assigned to take open-label resveratrol for 4 weeks in one of the three doses: 1, 1.5, and 2 g/day, taken in divided doses. Subjects were instructed to maintain their usual dietary and physical activity patterns during their participation in the study.
Standard Meal Test
Subjects were studied following an overnight fast and after a test meal containing 110 g carbohydrate, 20 g protein, and 20 g fat. The meal consisted of standard breakfast foods: cereal, bread, juice, and milk. Blood sampling was performed fasting and 30, 60, 120, and 180 minutes following the meal through an indwelling intravenous catheter. Subjects were instructed to consume a standard meal and snack at home on the night prior to the standard meal test, in order to minimize metabolic variability between tests. The assigned resveratrol dose was administered with the standard meal during the test conducted at the conclusion of the 4-week treatment period. Insulin sensitivity was estimated using homeostasis model assessment (HOMA-IR): insulinfasting (mU/mL) × glucosefasting (mmol/L)/22.5 (19) and also from insulin and glucose levels obtained following the standard meal challenge using the Matsuda index: 10,000/√([fasting plasma glucose × fasting plasma insulin] [mean glucose × mean insulin]) (20,21). Insulin secretion was estimated using the corrected insulin response at 30 minutes: insulin30min (μU/mL)/glucose30min (mg/dL) × (glucose30min [mg/dL] − 70) (19). β-Cell function was assessed using the oral disposition index (DIO) calculated using the formula: (ΔI0– 30/ΔG0– 30) (1/I0) (22). Insulin and glucose area under the curve (AUC) were calculated using the trapezoidal method. Percent body fat was measured using bioimpedance analysis (RJL Systems, Clinton Township, MI).
Endothelial function testing was performed fasting and 90 minutes following the standard meal, using reactive hyperemia peripheral arterial tonometry (RH-PAT) (23,24). RH-PAT employs a finger plethysmographic device to detect pulsatile arterial volume changes, which are sensed by a pressure transducer and transferred to a computer for analysis (EndoPAT; Itamar Medical). Studies are performed with the patient at rest, in a comfortable thermoneutral environment, and any antihypertensive medications were held until after completion of the 90-minute postmeal test. Fingertip probes are placed on the index finger of both hands, and 5 minutes of baseline recording is obtained. Blood flow is then occluded in one arm for 5 minutes, using a standard blood pressure cuff. Recording continues in both fingers during occlusion and for 5 minutes after release of the cuff. The reactive hyperemia index is calculated as the ratio of the average pulse amplitude in the posthyperemic phase divided by the average baseline amplitude, with normalization to the signal in the control arm to compensate for any systemic changes. Test–retest repeatability testing in our laboratory among healthy controls resulted in a coefficient of variability of 15.2% for tests performed 2 hours apart and 16.4% for tests performed 1–4 weeks apart.
Assays were performed in the core laboratories of the Einstein Institute for Clinical and Translational Research: chemistry profiles, complete blood count, urinalysis, glucose, lipoproteins, insulin (radioimmunoassay), high sensitivity c-reactive protein (latex-enhanced turbidimetric assay), and adiponectin (radioimmunoassay; Linco).
Statistical Analysis
Data are presented as mean (±SD) for baseline values and mean (±SEM) for baseline versus resveratrol comparisons. Baseline and posttreatment variables (peak and AUC glucose, insulin, Matsuda index, etc.) were compared using a paired t-test. Since there were no apparent differences among the three resveratrol doses studied, the groups were combined for analysis. For RH-PAT index, the pre- and postmeal delta was also analyzed (baseline vs resveratrol) using paired t-test. Data analyses were performed using GraphPad Instat, v3.
RESULTS
Ten subjects (seven female) with a mean age of 72 ± 3 years were enrolled. The subjects were overweight to obese and moderately insulin resistant, with a mean body mass index of 29 ± 5 kg/m2 and HOMA-IR 5.1 ± 1.8. Mean fasting and 2-hour plasma glucose were 110 ± 13 mg/dL and 183 ± 33 mg/dL, respectively. Four subjects were being treated with antihypertensive medications and three with statins; doses of these medications remained unchanged during the study.
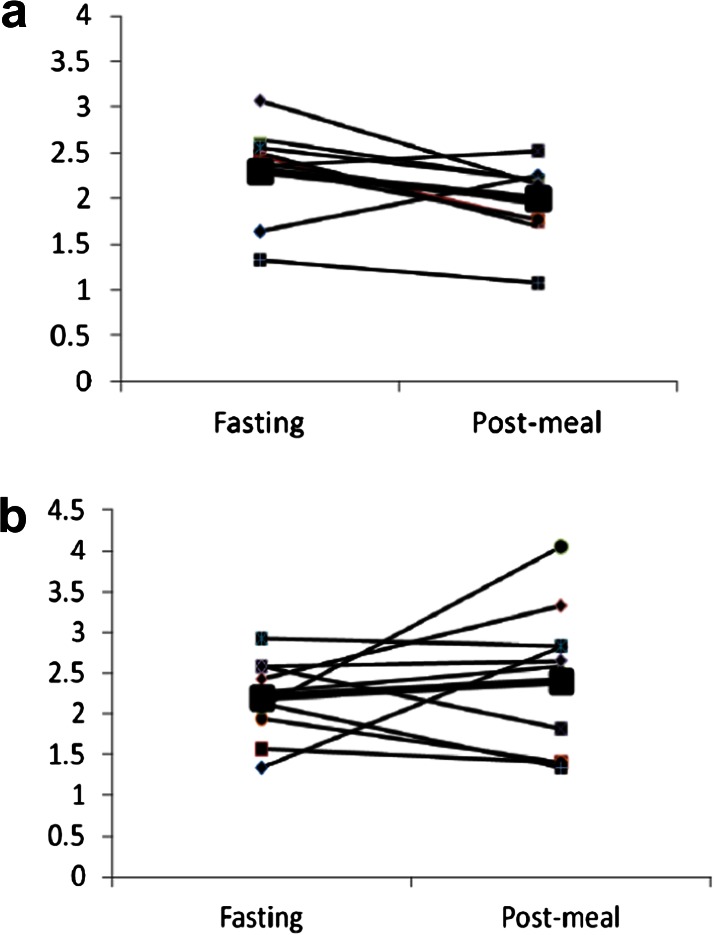
After 4 weeks of resveratrol, fasting glucose was unchanged, but peak postmeal glucose (185 ± 10 vs 166 ± 9 mg/dL, p = .003) and 3-hour glucose AUC (469 ± 23 vs 428 ± 19, p = .001) declined significantly (Figure 1a). Furthermore, postmeal insulin AUC fell by 18 ± 25% (p = .05; Figure 1b), and insulin sensitivity (using the Matsuda index) improved following treatment with resveratrol (3.1 ± 0.5 vs 3.8 ± 0.5, p = .03; Figure 2). Changes in HOMA-IR were not significant (3.6 ± 0.5 vs 3.3 ± 0.3, p = .13). Insulin secretion, as measured by corrected insulin response at 30 minutes, did not change significantly (0.60 ± 0.1 vs 0.50 ± 0.1, p = .49). Disposition index, a measure of β-cell function adjusted for insulin sensitivity, was 1.43 at baseline and 1.60 after resveratrol (p = .52). Weight, percent body fat, blood pressure, and fasting lipid profile were unchanged (Table 1). We also found no changes in the levels of high sensitivity c-reactive protein and adiponectin.
Figure 1.
Glucose and insulin profiles at baseline and after 4 weeks treatment with resveratrol. Glucose and insulin levels obtained fasting and 30, 60,120 and 180 minutes following meal challenge; area under the curve (AUC) calculated using trapezoidal method.
Figure 2.
Insulin sensitivity at baseline and after 4 weeks treatment with resveratrol. (a) Insulin sensitivity estimated by Matsuda index, using glucose and insulin levels at 30, 60, 120 and 180 minutes following meal challenge. Baseline vs. resveratrol, p = .03. (b) Insulin sensitivity estimated by HOMA-IR, using fasting glucose and insulin levels. Baseline vs. resveratrol, p = .13. Improved insulin sensitivity is indicated by an increase in Matsuda index and a decrease in HOMA-IR.
Table 1.
Metabolic and Safety Variables at Baseline and After 4 Weeks Treatment With Resveratrol
| Baseline | Resveratrol | p Value | |
| Weight (kg) | 74.0 (4.2) | 73.6 (4.1) | .95 |
| Body fat (%) | 34.7 (4.8) | 35.2 (4.8) | .94 |
| Cholesterol (mg/dL) | 176 (10) | 184 (12) | .62 |
| HDL cholesterol (mg/dL) | 51 (4) | 50 (4) | .86 |
| Triglycerides (mg/dL) | 103 (13) | 113 (13) | .59 |
| LDL cholesterol (mg/dL) | 104 (9) | 111 (11) | .63 |
| hs-CRP (mg/L) | 2.3 (0.6) | 2.8 (0.8) | .62 |
| Adiponectin (μg/mL) | 8.5 (1.0) | 8.3 (1.0) | .89 |
| Fasting glucose (mg/dL) | 103 (4) | 103 (4) | .99 |
| Fasting insulin (μU/mL) | 14.1 (1.8) | 12.4 (1.0) | .42 |
| CIR30 | 0.6 (0.1) | 0.5 (0.1) | .49 |
| Serum creatinine (mg/dL) | 0.8 (0.05) | 0.8 (0.04) | .99 |
| AST (U/L) | 18.3 (1.1) | 19.1 (1.0) | .60 |
| ALT (U/L) | 15.2 (1.1) | 16.9 (1.5) | .37 |
| BP systolic (mmHg) | 129 (5) | 126 (5) | .68 |
| BP diastolic (mmHg) | 72 (2) | 70 (3) | .59 |
Notes: Data obtained during standard meal challenge; data are mean (SEM); p values calculated from paired t-tests. ALT= alanine aminotransferase; AST= aspartate aminotransferase; CIR30 = corrected insulin response at 30 minutes; HDL= high density lipoprotein; hs-CRP = high sensitivity c-reactive protein; LDL= low density lipoprotein.
Endothelial function was assessed by RH-PAT (pre- and postmeal) during the standard meal test at baseline and after 4 weeks of resveratrol (Figure 3). Fasting RH-PAT index was essentially unchanged (2.3 ± 0.16 vs 2.2 ± 0.15, p = .8), but there was a trend toward improved postmeal endothelial function (higher RH-PAT score) following resveratrol (baseline postmeal delta −0.40 ± 0.15 vs treatment postmeal delta +0.21 ± 0.30, p = .06).
Figure 3.
Reactive-hyperemia index (RHI) at baseline and after 4 weeks treatment with resveratrol. (a) RHI at study baseline. (b) RHI after 4 weeks treatment with resveratrol. RH-PAT performed fasting and 90 minutes post meal challenge; RHI is ratio of post-hyperemia pulse amplitude divided by baseline amplitude (see Methods for details). Baseline vs. resveratrol post-meal delta, p = .06.
All doses of resveratrol were well tolerated, and there were no serious adverse events or changes in laboratory safety parameters, including serum creatinine, liver enzymes, complete blood cell count, or urinalysis. Specifically, there were no instances of transaminase increases above the normal range, and all subjects completed the protocol. Among adverse events considered “possibly” related to study treatment, one subject reported mild diarrhea and two women reported increased “hot flashes.” Details of safety monitoring are shown in the Supplementary Table 1.
DISCUSSION
In this initial open-label pilot study of resveratrol treatment, we show evidence of improved meal tolerance and whole-body insulin sensitivity in adults with age-related glucose intolerance. Resveratrol at the doses studied was well tolerated and appeared to be safe. Further, we observed a trend toward improved postmeal endothelial function. Together, these results suggest that resveratrol shows promise as a new therapeutic strategy for an important and highly prevalent age-related metabolic disorder.
There is abundant evidence that defects in both insulin secretion and insulin action contribute to age-related IGT (25), suggesting that both could be appropriate targets for intervention in at-risk older adults. We chose to assess glucose tolerance and insulin sensitivity using a standard mixed meal challenge, which represents a more physiologic stimulus (including the contribution of incretin hormones to glucose homeostasis) than oral or intravenous glucose and may be better tolerated in older individuals (26). The use of meal challenge data to assess insulin sensitivity, rather than “gold standard” techniques, such as the euglycemic clamp, is an acknowledged limitation of our study. However, meal challenge protocols to measure insulin sensitivity have been widely used (25,26) and application of the Matsuda algorithm in this context has been validated (in nondiabetic and diabetic subjects), showing reasonable correlation (r = .55 to .70) with insulin sensitivity obtained during a frequently sampled intravenous glucose tolerance test (22,27). Future studies will be needed to confirm our finding of improved insulin sensitivity with resveratrol and to define the relative contributions of hepatic and peripheral insulin action. We were unable to detect significant changes in insulin secretion using calculated measures based on 30-minute glucose and insulin (corrected insulin response at 30 minutes) or using the oral disposition index, but tests with better sensitivity (c-peptide modeling or hyperglycemic clamp techniques) may be more informative.
Although in vivo evidence of resveratrol’s metabolic effects has been quite consistent, the relevant mechanisms are likely complex and remain incompletely understood. Resveratrol has been reported to be an activator of the mammalian sirtuin, Sirt1, which is involved in processes such as cell survival and glucose homeostasis (28,29). However, resveratrol’s activation of Sirt1 has been questioned and there is evidence that alternate pathways, including activation of AMP kinase, could be responsible for some of resveratrol’s metabolic effects (4,30).
Concerns have been raised about resveratrol’s bioavailability in vivo, and the appropriate doses for use in human studies are not known (31). Animal studies have employed doses from 5 to 400 mg/kg/day, and human clinical trials (mostly single-dose or short-term studies) have also included a wide range of doses, from 5 to 5,000 mg/day. We chose to explore the dose range of 1–2 g/day, balancing the strategy of testing higher doses for “proof of concept” with concern about possible drug toxicity since little human safety data are currently available for resveratrol (32). In our study, meal tolerance improved with these doses, but it did not return to levels seen in similar aged subjects with normal glucose tolerance studied using the same standard meal protocol (33). Further, we were unable to detect differences within the narrow dose range studied and expect that a wider range of resveratrol doses will be necessary to reveal evidence of a dose–response.
The vascular effects of resveratrol on isolated tissues or animal models are well described and include decreased platelet aggregation via inhibition of cyclooxygenase-1 and weak estrogen receptor agonist and antioxidant properties (34–36). Human data are limited, but a study of red grape polyphenols (containing trace amounts of resveratrol) showed improvement in flow-mediated vasodilation (37), and others have reported reduction in oxidative stress and increased expression of antioxidant genes following a single dose of resveratrol (38). We hypothesized that endothelial function would be enhanced in our subjects following treatment with resveratrol, both directly through effects on nitric oxide and also potentially as a consequence of improved glucose tolerance. In a previous study, we demonstrated reduced postmeal RH-PAT index in IGT, the timing of which coincided with peak postmeal glucose levels (32). These dual effects of resveratrol on endothelial function (directly and via improvement in glucose tolerance) may be synergistic. However, with the current study design, we are unable to determine whether any observed vascular effects may be direct or mediated by improvements in glucose metabolism, and further studies are needed to explore this. Further, our results are of borderline statistical significance and require confirmation.
Results of this pilot study should be interpreted with caution given the open-label uncontrolled study design. Although weight and reported physical activity levels did not change during the 4-week treatment period, it is possible that subtle changes in diet and/or exercise could have influenced these results. Furthermore, with the small number of subjects, we were likely underpowered to detect differences in some study outcomes, especially RH-PAT. Finally, although resveratrol was well tolerated and appeared to be safe in this cohort, much larger and longer-duration studies will be needed to appropriately assess safety concerns.
In conclusion, this pilot study provides the first evidence in humans that resveratrol may possess clinically relevant effects on glucose metabolism and vascular function. Future studies should include formal randomized placebo-controlled trials and efforts to explore the potential mechanisms for resveratrol’s cardiometabolic effects.
FUNDING
This study was supported in part by the Clinical and Translational Science Award (UL1 RR025750, KL2 RR025749, and TL1 RR025748) from the National Center for Research Resources, a component of the National Institutes of Health, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health. This study was also supported by the Einstein Diabetes Research and Training Center (5P60 DK20541) and the National Institute on Aging (P01 AG021654-01).
SUPPLEMENTARY MATERIAL
Supplementary Table 1 can be found at: http://biomedgerontology.oxfordjournals.
Acknowledgments
These results were presented as an abstract at the American Diabetes Association Annual Scientific Meeting in June, 2010. The authors wish to thank the staff of the Diabetes Clinical Trials Unit, the Clinical Research Center, the study participants, and Bronx CREED, funded by the National Institutes of Health's National Center for Minority Health & Health Disparities (P60 MD000514), which provided Spanish language translation for study documents.
References
- 1.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Labbe A, Garand C, Cogger V, et al. Resveratrol improves insulin resistance, hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;44:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labinskyy N, Csiszar A, Veress G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13:989–996. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;92:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 10.Cruz MN, Luksha L, Logman H, Poston L, Agewell S, Kublickiene K. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am J Physiol Heart Circ Physiol. 2006;290:H1969–H1975. doi: 10.1152/ajpheart.01065.2005. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu J. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- 12.Smoliga J, Vang O, Baur JA. Challenge of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr062. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 14.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 15.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 16.DECODE Study Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 17.Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–1081. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- 19.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Aloulou I, Brun JF, Mercier J. Evaluation of insulin sensitivity and glucose effectiveness during a standardized breakfast test: comparison with the minimal model analysis of an intravenous glucose tolerance test. Metabolism. 2006;55:676–690. doi: 10.1016/j.metabol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Utzschneider K, Prigeon R, Faulenbach M, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 24.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 26.Dalla Man C, Campioni M, Polonsky KS, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 27.Brun JF, Ghanassia E, Fedou C, Bordenave S, Raynaud de Mauverger E, Mercier J. Assessment of insulin sensitivity (SI) and glucose effectiveness (SG) from a standardized hyperglucidic breakfast test in type 2 diabetics exhibiting various levels of insulin resistance. Acta Diabetol. 2010 doi: 10.1007/s00592-010-0232-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 32.Brown V, Patel K, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crandall JP, Shamoon H, Cohen HW, et al. Post-challenge hyperglycemia in older adults is associated with increased cardiovascular risk profile. J Clin Endocrinol Metab. 2009;94:1595–1601. doi: 10.1210/jc.2008-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Sas DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005;42:281–289. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Miatello R, Vazquez M, Renna N, Cruzado M, Zumino AP, Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens. 2005;18:864–870. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Zern TL, Wood RJ, Greene C, et al. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;35:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 38.Ghanim H, Sia CL, Korzeniewski K, et al. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high fat, high carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]