Abstract
Glial cells surround neuronal endings and isolate them within specialized compartments. This architecture is found at synapses in the central nervous system, as well as at receptive endings of sensory neurons. Recent studies are beginning to uncover the contributions of glial compartments to the functions of the ensheathed neurons. However, the cellular and molecular processes that guide compartment morphogenesis remain unknown. The main sensory organ of Caenorhabditis elegans, the amphid, provides an experimentally tractable setting in which to address the mechanisms underlying glial compartment formation. Amphid development is stereotyped and amphid structure is easily assayed. We recently uncovered a molecular tug of war that regulates the size of the amphid sensory compartment. The Nemo-like kinase LIT-1 interacts with the glial cytoskeleton to promote compartment growth, a process that also involves components of the retromer complex, while the Patched-related transmembrane protein DAF-6 keeps this expansion in check. Here we discuss how regulation of secretion by the cytoskeleton could guide the sculpting of glial compartments.
Keywords: amphid, che-14, compartment, daf-6, lit-1, receptive ending, retromer
The Glial Compartment of the Amphid
Fundamental anatomical features of neurons are specialized processes, dendrites and axons, that detect stimuli and propagate them through the nervous system. The termini of these neurites often lie within compartments formed by glia. We have used the main sense organ of C. elegans, the amphid, to understand how these glial compartments are formed. Each amphid consists of 12 sensory neurons and two glial cells, the sheath (AMsh) and socket (AMso) cells (Fig. 1A and B). The cell bodies of amphid neurons reside between the first and second bulb of the pharynx and extend two processes: an axon that enters the nerve ring (the main neuropil of the worm) and a dendrite that reaches to the tip of the nose. The two glial cells extend processes that run parallel to the dendrites. At the nose tip, a stereotypical structure is formed (Fig. 1C). The AMsh and AMso glia connect through adherens junctions to form a continuous channel. The dendrites penetrate the AMsh glial cell to enter this channel, and the entry sites are also sealed by adherens junctions. We refer to the pocket formed by the AMsh glia as the amphid compartment. Single or double sensory cilia extend from each dendrite. Some cilia traverse the length of the compartment and are exposed to the environment through an opening formed by the AMso glia, while others are embedded within the AMsh glia. The AMso channel is lined with cuticle and seems to function to allow the sensory cilia to sample the environment. The AMsh glia is an active secretory cell that fills the compartment with extracellular matrix material (ECM).1

Figure 1. The amphid sensory organ. (A) Cartoon of an adult C. elegans hermaphrodite. Two bilaterally symmetric amphids are located in the head (only one is depicted). (B) Each amphid consists of 12 neurons (red; only one is depicted) and two glial cells, the sheath (AMsh; green) and the socket (AMso; blue). (C) Processes from the neurons and glia come together at the tip of the nose. The glial cells align to form the amphid sensory compartment. The amphid sensory neurons extend single-ciliated, double-ciliated, wing-like, or finger-like receptive endings. Apical junctions between the dendrites and the sheath glia, as well as between the sheath and socket glia, establish an isolated compartment for the sensory cilia. This niche is filled with matrix material secreted by the sheath glia. Adapted from Perkins et al., 1986. For a more detailed discussion of C. elegans glia, see reference 27.
DAF-6, a Regulator of Glial Compartment Size
The first insights into the molecular mechanisms that guide amphid compartment morphogenesis came from studies of the gene daf-6. Mutations in daf-6 were originally isolated in a screen for animals unable to become dauer larvae.2 The underlying cause of this dauer entry defect appears to be a bloated sensory compartment that fails to open to the environment.3,4 The apparently normal sensory cilia in these animals are trapped within this deformed channel and lack access to external stimuli (compare Figure 2A and D with Figure 2B and E). daf-6 encodes a Patched-related transmembrane protein that localizes to the sensory compartment membrane, as well as to surfaces of other tubular structures.5 We recently demonstrated that in daf-6 mutants, the sensory compartment forms normally during embryogenesis, but abnormally expands shortly after its formation, suggesting a role for daf-6 in restricting glial compartment expansion.6
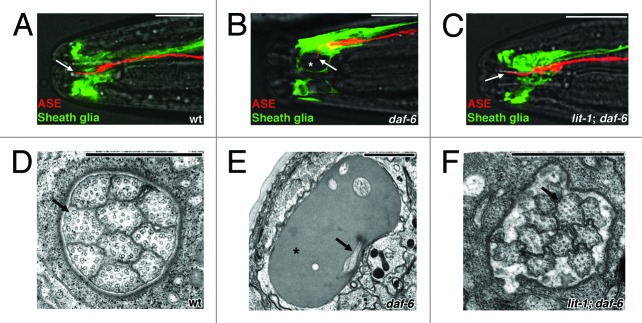
Figure 2. Suppression of daf-6 defects by lit-1. (A-C) Fluorescence microscopy images of the amphid sensory organs from wild-type animals, daf-6 mutants, and lit-1; daf-6 double mutants. The ASE neuron is shown in red (mCherry) and the amphid sheath in green (GFP). The ASE neuron extends a single cilium (arrow) through the length of the amphid sensory compartment in the wild type. In daf-6 animals, the cilium is bent and not exposed to the environment, and the amphid pocket is bloated (asterisk). Both defects are suppressed by mutation of lit-1. Anterior is to the left. White scale bars, 10 μm. (D-F) Electron micrographs of cross-sections through the amphid sensory compartment of the same genotypes. Arrows point to sensory cilia, asterisk marks bloated sensory compartment. Black scale bars, 1 μm. Adapted from reference 6.
In electron microscopy serial reconstructions, Ward and colleagues1 noted the presence of ECM-containing vesicles, likely produced by the Golgi apparatus of the AMsh glia, that appear to fuse with the sensory compartment membrane. We have suggested that these matrix-laden vesicles may be the driving force behind sensory compartment expansion, and that DAF-6 restricts compartment expansion either by antagonizing vesicle secretion, or by promoting vesicle reuptake. A role for DAF-6 in regulating vesicle dynamics is consistent with its sequence similarity to Patched, the receptor for the Hedgehog signaling molecule. In the Hedgehog pathway, Patched has been suggested to influence trafficking of vesicles loaded with Smoothened, the downstream effector of the pathway.7 Another Patched family member in C. elegans, ptc-1, has been proposed to regulate vesicle dynamics during germ-cell cytokinesis.8 Moreover, DAF-6 is required for the formation of other tubular structures in C. elegans5 and considerable evidence implicates vesicle trafficking in the control of tubulogenesis.9 Finally, in addition to its membrane localization, DAF-6 is also found in subcortical puncta that could represent vesicles.5
LIT-1 Kinase Drives Amphid Channel Expansion
Since DAF-6 restricts the expansion of the amphid sensory compartment, we reasoned that mutations in genes that drive compartment expansion might suppress the defects of daf-6 mutants. Indeed, from a screen for suppressors of daf-6, we recovered several mutants in which glial compartment shape was restored. One of these mutants affects the gene encoding the Nemo-like kinase LIT-1 (Fig. 2C and F), which seems to play a key role in compartment expansion, as lit-1 single mutants have compartments that are too small to accommodate all sensory cilia.6 Mutations in lit-1 also enhance the amphid morphogenesis defects of che-14 mutants. The CHE-14 protein is similar to Drosophila and vertebrate Dispatched, and work from the Labouesse lab has shown that this protein is an important regulator of apical secretion.10 The genetic interaction between lit-1 and che-14 further supports the notion that secretion is likely to play a key role in AMsh compartment morphogenesis.
LIT-1 is an important regulator of Wnt signaling in many developmental contexts in C. elegans, where it translocates into the nucleus to regulate transcription.11 However, the Wnt signaling pathway does not appear to be involved in sensory compartment morphogenesis.6 Mutants in core Wnt components do not affect compartment formation and are not able to suppress the expanded compartments of daf-6 mutants. Instead, we found that for glial compartment expansion, LIT-1 functions at the glial compartment surface, together with its activating kinase MOM-4. LIT-1 localizes to this surface through its highly conserved C-terminal domain. Truncation of this domain does not affect developmental processes mediated by Wnt signaling, but is sufficient to suppress daf-6 mutant defects. Consistent with this observation, LIT-1 nuclear localization is not disrupted by a C-terminal truncation, but localization to the glial compartment surface is abolished.
To understand how the C-terminus of LIT-1 anchors the protein to the glial compartment surface, we performed a yeast two-hybrid screen to identify proteins that interact specifically with this domain. We found that one binding partner is actin. Strikingly, using fluorescence electron microscopy (fEM),12 we found that although actin is generally distributed at the cortical surface throughout the glial cell, it is greatly enriched around the sensory compartment. Furthermore, while cortical actin could be disrupted by application of actin depolymerizing agents, these failed to remove actin and LIT-1 from the compartment surface6 These observations suggest that actin surrounding the compartment may be the anchor for LIT-1, although technical challenges have not allowed direct testing of this idea to date.
Our two-hybrid studies also revealed that the LIT-1 C-terminus can bind to the Wiskott-Aldrich syndrome protein (WASP), a regulator of actin polymerization.13 A mutation in the WASP-encoding locus, wsp-1, suppresses the compartment defects of daf-6 mutants, and double mutants between lit-1 and wsp-1 suppress daf-6 defects to the same extent as lit-1 mutations alone.6 Thus, like LIT-1, WASP seems to be required to promote amphid sensory compartment expansion, and likely functions in the same pathway as LIT-1. Together, these results raise the possibility that LIT-1 promotes channel growth by regulating actin polymerization. Although such a role for lit-1 has not been previously described, at least one other study lends credence to this hypothesis: LIT-1 was recently shown to promote anchor cell invasion through the basement membrane during vulval morphogenesis in C. elegans and carcinoma cell invasion in an ex vivo metastasis model.14 Both processes require extensive remodeling of the actin cytoskeleton, consistent with our scheme for LIT-1 function.
LIT-1, Actin and Vesicles
We hypothesize that DAF-6 restricts sensory compartment expansion by modulating vesicle trafficking. Might LIT-1 also affect this process? Although LIT-1 had not been previously implicated in the control of vesicle trafficking, many studies demonstrate key roles for WASP-dependent actin polymerization in endocytosis.15 A handful of studies also support a role for actin polymerization in exocytosis. For example, secretory granules become coated with actin before their secretion by cultured pancreatic acinar cells,16 and WASP-mediated actin polymerization drives secretion by neuroendocrine cells.17 Antibodies directed against yeast WASP inhibit fusion of purified vacuoles,18 while in Drosophila, WASP and the WASP interacting protein (WIP) are required for the targeted exocytosis of pre-fusion vesicles during myoblast fusion.19 LIT-1, a serine/threonine kinase, could effect changes in WASP activity by direct phosphorylation. Indeed, in other contexts, WASP can be activated through the phosphorylation of serines 483 and 484.20 Taken together, these observations suggest plausible roles for LIT-1, WASP and actin in exocytosis.
Further strengthening this hypothesis is our recent identification of a role for some components of the retromer complex in sensory compartment morphogenesis.21 The retromer complex recognizes the cytoplasmic tails of endocytosed transmembrane proteins through a cargo-selection module composed of the proteins VPS26, VPS29 and VPS35. A membrane coating/bending subunit composed of the sorting nexins SNX1/2 and SNX5/6 then promotes the budding of cargo-enriched vesicles from the endosome. These vesicles are targeted to the Golgi apparatus, and from there, cargo and membrane can be recycled back to the plasma membrane.22 Like lit-1 mutations, mutations in vps-29, snx-1 and another sorting nexin, snx-3, are able to suppress daf-6 defects. These observations suggest a role for vesicular trafficking in controlling compartment size. Surprisingly, the remaining parts of the classic retromer complex, VPS26, VPS29 and SNX5/6, do not appear to be important for compartment formation,21 suggesting either that VPS-29, SNX-1 and SNX-3 recycle a new class of cargoes, or that their role is cargo-independent and restricted to trafficking of membrane material to promote compartment growth.
Enter the Neurons
Based on our studies, a plausible but highly speculative model for the control of AMsh glial compartment size is depicted in Figure 3. We posit that an important point of regulation for compartment size control is the addition and removal of membrane from the glial compartment surface. The retromer components SNX-1, SNX-3 and VPS-29 could be important in generating vesicles that fuse with the compartment lumen to expand its diameter. Vesicle fusion could be facilitated by the coating of vesicles with actin, and this process might be regulated by phosphorylation of WASP by the MOM-4/LIT-1 kinase module. CHE-14 might also promote aspects of vesicle fusion with the compartment membrane. DAF-6 could act either in vesicle reuptake, or to block vesicle fusion. Although speculative, the model offers several testable predictions. For example, LIT-1 should be able to phosphorylate WASP on specific residues, and mutating these residues should mimic a WASP null mutant. Expression of protein inhibitors of vesicle trafficking could also shed light on how important this process is in compartment growth.

Figure 3. A speculative model for the regulation of sensory compartment size. Components of the retromer complex are important for the generation of vesicles. These vesicles become coated with actin after activation of WASP by the MOM-4/LIT-1 kinase module. Fusion of the coated vesicles with the plasma membrane drives expansion of the compartment. CHE-14 promotes fusion and thus expansion, while DAF-6 keeps this expansion in check by inhibiting vesicle exocytosis. See text for details.
Identification of the signals that direct AMsh glial compartments to adopt their optimal size may provide an invaluable tool for manipulating the system to test mechanistic predictions. Our data suggest that neuronal cilia may be prime candidates for the source of these signals. Our EM studies of wild-type and daf-6 mutants suggest a possible correlation between the timing of compartment bloating and the appearance of cilia.6 Furthermore, in animals that fail to extend sensory cilia due to a mutation in the daf-19 locus (a gene that acts in neurons to coordinate cilia morphogenesis)23, both DAF-65 and LIT-16 fail to localize along the length of the compartment, suggesting that both arms of the compartment size control mechanism respond to neuronal input. It is interesting to note that although homologs of Hedgehog, the protein that engages Patched in Drosophila and vertebrates, do not exist in C. elegans,24 the C. elegans genome encodes many Hedgehog related molecules25 that might bind DAF-6. Experimental evidence also supports the hypothesis that DAF-6 may act as a receptor. The daf-6(m176) allele is predicted to substitute a lysine for an asparagine residue in the first extracellular loop of the protein. Importantly, daf-6(m176) mutants display an almost fully penetrant defect in amphid function,5 despite proper localization of DAF-6 to glial compartment membranes. Thus, the m176 mutation site may define an interaction domain with an extracellular signal, perhaps of neuronal origin.
General Principles?
Glia ensheath neurons in a number of settings. Oligodendrocyte and Schwann cell myelin surrounds CNS and peripheral axons, respectively; and non-myelinating Schwann cells ensheath peripheral sensory axons.26 Signals from axons control the diameter of the myelin sheath and the number of myelin layers. The concept, therefore, that neurons can dictate the size and structure of their glial sheath is well established. Our studies suggest that this notion may very likely extend to glial compartments that surround sensory receptive endings, and perhaps to synapses. The conservation of all the components we have identified raises the possibility that similar principles guide the morphogenesis of glial compartments in other settings. Beyond the glial compartment, the identification of interactions between LIT-1, actin and WASP suggests a general role for LIT-1 in actin regulation. Indeed, our preliminary studies suggest a role for LIT-1 in glial remodeling in C. elegans (G. Oikonomou and C. Procko, unpublished results). Further studies of Nemo-like kinases in cell invagination and metastasis may reveal whether this principle extends beyond the worm.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19343
References
- 1.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol. 1975;160:313–37. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 2.Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–71. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 3.Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–51. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- 4.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–87. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 5.Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8:893–906. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Oikonomou G, Perens EA, Lu Y, Watanabe S, Jorgensen EM, Shaham S. Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans. PLoS Biol. 2011;9:e1001121. doi: 10.1371/journal.pbio.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 8.Kuwabara PE, Lee MH, Schedl T, Jefferis GSA. A C. elegans patched gene, ptc-1, functions in germ-line cytokinesis. Genes Dev. 2000;14:1933–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/S0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 10.Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol. 2000;10:1098–107. doi: 10.1016/S0960-9822(00)00695-3. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BT, Kimble J. A new look at TCF and beta-catenin through the lens of a divergent C. elegans Wnt pathway. Dev Cell. 2009;17:27–34. doi: 10.1016/j.devcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, et al. Protein localization in electron micrographs using fluorescence nanoscopy. Nat Methods. 2011;8:80–4. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–34. doi: 10.1016/S0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 14.Matus DQ, Li X-Y, Durbin S, Agarwal D, Chi Q, Weiss SJ, et al. In vivo identification of regulators of cell invasion across basement membranes. Sci Signal. 2010;3:ra35. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galletta BJ, Mooren OL, Cooper JA. Actin dynamics and endocytosis in yeast and mammals. Curr Opin Biotechnol. 2010;21:604–10. doi: 10.1016/j.copbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A. 2000;97:1091–5. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader M-F. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15:520–31. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitzen G, Wang L, Thorngren N, Wickner W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J Cell Biol. 2002;158:669–79. doi: 10.1083/jcb.200204089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–86. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Cory GOC, Cramer R, Blanchoin L, Ridley AJ. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol Cell. 2003;11:1229–39. doi: 10.1016/S1097-2765(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomou G, Perens EA, Lu Y, Shaham S. Some, but not all, retromer components promote morphogenesis of C. elegans sensory compartments. Dev Biol. 2012;362:42–9. doi: 10.1016/j.ydbio.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–82. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 23.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;5:411–21. doi: 10.1016/S1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 24.Bürglin TR, Kuwabara PE. Homologs of the Hh signalling network in C. elegans. WormBook. 2006;28:1–14. doi: 10.1895/wormbook.1.76.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspöck G, Kagoshima H, Niklaus G, Bürglin TR. Caenorhabditis elegans has scores of hedgehog-related genes: sequence and expression analysis. Genome Res. 1999;9:909–23. doi: 10.1101/gr.9.10.909. [DOI] [PubMed] [Google Scholar]
- 26.Burkitt GH, Young B, Heath JW. Wheater's functional histology: a text and color atlas. New York: Churchill Livinstone; 1993. [Google Scholar]
- 27.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia. 2011;59:1253–63. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]


