Abstract
While life requires water, many organisms, known as anhydrobiotes, can survive in the absence of water for extended periods of time. Although discovered 300 years ago, we know very little about the fascinating phenomenon of anhydrobiosis. In this paper, we summarize our previous findings on the desiccation tolerance of the Caenorhabditis elegans dauer larva. A special emphasis is given to the role of trehalose in protecting membranes against desiccation. We also propose a simple mechanism for this process.
Keywords: Caenorhabditis elegans, anhydrobiosis, dauer, desiccation, membrane packing, membrane phase transition, membrane protection, metabolic depression, trehalose
Approximately 70% of the Earth’s surface is covered by water, a substance that has extraordinary physical properties. It is therefore not surprising that most of life on our planet depends on water.1 Clearly, life without water does not exist. However, many organisms, known as anhydrobiotes, can temporarily survive in the absence of water. Antony van Leeuwenhoek first discovered anhydrobiotes 300 years ago when he observed dry material from his roof gutter. After adding water to it and examining it under the microscope, he was surprised that some previously dry “animalcules” (probably tardigrades or rotifers) started moving following rehydration. Although his findings did not immediately interest his contemporaries, they became a hot topic of debate 20 years after his death, and discussions about anhydrobiosis have continued, albeit interruptedly, for another 200 years.2,3
As David Wharton elegantly describes, anhydrobiosis is “the ability to survive the cessation of metabolism due to water loss.”3 It is also a state in which the metabolism of the organism either stops completely or decreases to a level that cannot be measured and is distinguished from death by its reversibility.2 Once the organism reabsorbs water, metabolism increases and the organism exhibits all vital signs. The most extreme examples of this are bacterial spores that can revive after 25–40 million years4 or plant seeds that can germinate after 1,300 years.5 Today, we know that anhydrobiotic species exist among bacteria, yeast, plants, rotifers, crustaceans, tardigrades and insects as well as nematodes.6
The first observation of an anhydrobiotic nematode (Anguina tritici) was made in 1743 and since then, many other studies have been conducted to assess behavioral responses and survival capabilities.7 Encouraged by the discovery of James Clegg8 that dehydrated Artemia cysts contain high amounts of glycerol, John Crowe began investigating the metabolic changes in Aphelencus avenae,9,10 including the synthesis of disaccharide trehalose. In vitro models suggested that trehalose is involved in protecting phospholipid bilayers against damage inflicted during desiccation.11 However, whether this mechanism exists in vivo remained unclear until recently.
Despite many studies on anhydrobiosis in nematodes, the molecular mechanisms underlying this phenomenon are poorly understood. One of the major breakthroughs came in 2002 when a plant gene was discovered in a nematode. Browne et al.12 identified that a gene, which is upregulated in A. avenae upon desiccation, is highly homologous to a late embryogenesis abundant (LEA) gene induced by water deprivation in many plants. Following this discovery, homologs of this protein were identified in many other taxa,13 suggesting that anhydrobiotes may use similar mechanisms for coping up with desiccation stress.
Anhydrobiosis is highly common among nematodes, and studying these organisms teaches us about the behavior and biochemistry of anhydrobiotic animals. However, such research is limited because genetic manipulations are almost impossible in non-model organisms. We thought that using Caenorhabditis elegans for the investigation of anhydrobiosis would bring advancement into the field because it is one of the best model organisms. Recently, we discovered that the dauer larva of C. elegans is an anhydrobiote.14 This finding allowed us to use powerful genetic methods, such as mutagenesis, RNA interference and transgenomics, on C. elegans to elucidate the molecular mechanisms underlying anhydrobiosis.
C. elegans has a short life cycle consisting of four larval stages prior to adulthood. In unfavorable environmental conditions such as overconfluency, limited food or elevated temperature, growth is arrested in an alternative and distinct developmental stage known as the dauer. Dauers can survive several months in the absence of food and are resistant to various environmental stresses. When the conditions are favorable again, they resume the reproductive cycle.15 Regardless of the environmental conditions, large, pure dauer populations can easily be achieved by growing the temperature sensitive dauer-constitutive mutant strain daf-2(e1370) in liquid culture. At 15°C, these worms remain in the reproductive cycle, while at 25°C, their growth is arrested in the dauer stage.16
To test the anhydrobiotic ability of C. elegans, we first developed a desiccation assay. In this assay, worms were desiccated in sealed chambers under defined relative humidity (RH) at constant temperature. We then rehydrated them with distilled water and counted the surviving organisms. Using this assay, we tested all developmental stages of C. elegans and observed that only dauers could survive desiccation, provided that they were first “preconditioned.” During preconditioning, dauers were kept in 98% RH for 4 days. Remarkably, even under this condition, an outside osmotic pressure of 27 atm was exerted on the worms, which extracted more than 80% of their body water. When desiccated at lower RH, worms survived a loss of up to 98% of body water. Even in 0% RH, a condition under which they lost almost all of body water content, 10% of worms survived. Upon rehydration, all surviving worms became active, and within a few days, developed into fertile adults (Vid. S1).
It is intriguing that only the dauers survive desiccation. We can explain this from a metabolic and an evolutionary perspective. As they do not feed, dauers depend on glycogen and fat as energy sources. Energy is produced slowly via anaerobic pathways, such as glycolysis and malate dismutation.17 It could be that the ametabolic state is easy to achieve when the metabolism is already depressed. In addition to that, dauers are probably more frequently exposed to desiccation stress than other stages in the nature. Adults die soon after the reproductive cycle is completed, whereas dauers can survive for many months. It is possible that during that time, dauers experience desiccation several times. From an evolutionary point of view, it is more likely that populations of C. elegans grow again from surviving dauers following environmental stress. This could be the natural selection mechanism that favored gaining desiccation tolerance to the C. elegans dauer.
Knowing that the C. elegans dauer is an anhydrobiote, we next sought to elucidate the role of trehalose in anhydrobiosis. First, we observed that the level of trehalose rapidly increases in dauers upon preconditioning and decreases to basal levels after rehydration. This suggests that these organisms have a mechanism that senses the decrease in environmental humidity and that responds by inducing trehalose synthesis.
Next, we wanted to determine how trehalose-deficient worms respond to desiccation. Worms synthesize trehalose from glucose 6-phosphate and UDP-glucose in two steps. The first step is catalyzed by trehalose 6-phosphate synthase (TPS) and yields trehalose 6-phosphate, which is then dephosphorylated into trehalose.18 C. elegans has two genes coding for TPS: tps-1 (ZK54.2) and tps-2 (F19H8.1). Recently, we generated a mutant strain lacking both genes (daf-2;ΔΔtps) and showed that it was unable to synthesize trehalose or trehalose-derived lipids (maradolipids).19 Even after preconditioning, daf-2;ΔΔtps dauers were unable to survive harsh desiccation. Strikingly, in the dead worms, giant fat droplets accumulated in the vicinity of the gut, which was probably caused by the merging of smaller droplets. This was seen only after rehydration, which implies that the effect of desiccation is manifested upon water influx. In such worms, we also observed significant damage in cell membranes and membranous organelles. Altogether, these findings clearly indicate that desiccation changes the physical state of the membrane, making it more susceptible to merging with neighboring membranes or being ruptured upon rehydration. Trehalose plays an essential role in this process by protecting the membranes against those changes, since even minor damage to the cell membrane can result in cell and organism death.
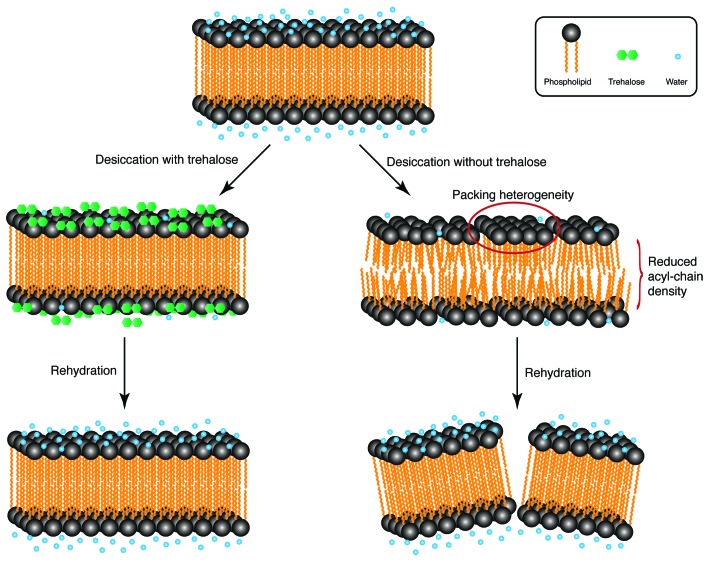
Next, we asked how trehalose keeps membranes intact. To address this question, we used attenuated total reflectance Fourier-transform infrared difference spectroscopy with hydration modulation20 on whole animals. This method provides extensive information about the physical state of membranes by assessing the vibrations of functional groups in phospholipids.21 To compare the differences between trehalose-producing (daf-2) and trehalose-deficient (daf-2;ΔΔtps) worms, infrared spectra of preconditioned dauers were measured during desiccation to 45% RH followed by rehydration to 97% RH. All spectral differences were then analyzed. In trehalose-producing dauers, all spectral changes occurring in the lipidic region of the spectrum during desiccation were reversed during rehydration. Therefore, desiccation and rehydration in these worms are reversible processes for lipids. However, we observed two striking irreversible changes in the desiccation spectra of trehalose-deficient dauers: broadening of antisymmetric methylene bands and an upshift of the symmetric methylene stretching frequency. The broadening of antisymmetric methylene bands indicates heterogeneous packing of acyl chains of lipids. In other words, lipids in some regions of the membrane are packed differently than in others. The upshift of the symmetric methylene stretching frequency indicates an increase in the volume of acyl chains, which means that the membranes expand during desiccation without trehalose. As these changes are not reversed during rehydration, it is possible that the membrane structure is not the same as it was prior to desiccation. We hypothesize that the altered lipidic order destabilizes membranes during dehydration and causes membrane damage upon water influx (Fig. 1).
Figure 1. A simple model of trehalose-mediated membrane protection during desiccation. In the hydrated state, phospholipid head groups interact with water. This interaction keeps the membrane in the liquid crystalline phase. During desiccation in the presence of trehalose, the hydration effect of water is compensated by the presence of sugar, thus preserving the lipid order; therefore, the membrane stays intact upon rehydration. However, without trehalose, desiccation leads to phospholipid packing heterogeneity and reduced acyl chain density, thereby destabilizing the membrane and resulting in damage to the membrane upon influx of water. Schematic representation only; items not drawn to scale.
How do our in vivo data connect to the data obtained from the in vitro experiments on the role of trehalose in membrane protection? Three hypotheses have previously been proposed for this effect: water replacement, water entrapment and vitrification.22 The water replacement hypothesis, first speculated by James Clegg23 in 1967 and since then extended significantly, suggests that the hydroxyl groups of sugars can substitute for the hydrogen bonding of water to polar residues. In this way, the hydration effect of water can be imitated by sugars during the dry state. The water entrapment hypothesis suggests that sugars entrap water molecules near the surfaces of macromolecules, therefore maintains their hydration in low water environments.24 Although this model was first proposed to explain protein stabilization, it can be generalized to membranes as well. Finally, the vitrification hypothesis suggests that sugars can form biological glasses upon dehydration, hence stabilizing subcellular structures by dramatically reducing the diffusion rate.25 Despite these different arguments, it appears to be that these three mechanisms are not mutually exclusive.
One of the most surprising results we obtained from desiccation studies of dauers deprived of trehalose was the appearance of vibrational frequency changes that are typically associated with a gain in the free volume of acyl chains. Membranes undergo phase transitions upon drying.22 Normally, the head groups of phospholipids are hydrated and hydrogen bonding to water keeps phospholipids at a certain distance from one another. When water is removed, phospholipids are packed more tightly, resulting in an increase in van der Waals’ forces between acyl chains. Consequently, the fluidity of the membrane decreases and the membrane changes from liquid crystalline state to a gel state. During this transition, membranes tend to merge with each other. The converse behavior of C. elegans lipids suggests that at least a fraction of the C. elegans membranes responds to desiccation differently than model membranes or purified membrane vesicles. Integral and peripheral membrane proteins, as well as other solutes, may be the cause of this surprising phenomenon. However, we did observe merging of small fat droplets. If this is due to the fusion of membrane layers surrounding fats, it suggests that despite their differential response, trehalose-free membranes are still prone to fusion during desiccation.
Crowe22 proposed that the main mechanism for the maintenance of lipids in a liquid crystalline state during desiccation involves lipid rafts, which are specialized membrane microdomains rich in cholesterol, sphingolipids and certain proteins.26 According to this model, trehalose keeps certain microdomains of the membrane in the liquid crystalline state, while other regions remain in the gel phase, creating a phase separation. We obtained evidence for a heterogeneous response of lipid packing to dessication of trehalose-deficient dauers. The deviation from the normal response to drying suggests that non-planar or even non-lamellar lipidic phases may form in trehalose-deficient strains upon desiccation. Whether these differently packed regions are the microdomains that are preserved by trehalose requires further investigation. A thorough analysis of the lyotropic phase transitions of C. elegans-specific lipids may reveal the physical role of trehalose in membrane integrity and its relation to anhydrobiosis. Preliminary studies in this direction indicate that in addition to trehalose synthesis, altered lipid headgroup diversity may be one of the physicochemical factors mediating desiccation tolerance during preconditioning.
Despite our better understanding of the phenomenon, anhydrobiosis still remains a mystery. Using C. elegans dauer as a model anhydrobiote provides an important opportunity to elucidate the molecular mechanisms underlying this phenomenon. Analyzing changes in the transcriptome and proteome of these organisms should help reveal the overall process of anhydrobiosis. Reverse genetics, when coupled with these methods, could provide even more information on the anhydrobiotic ability of C. elegans.
Supplementary Material
Acknowledgments
The authors would like to thank Jean-Marc Verbavatz, Daniela Vorkel and Hassan Khesbak for their collaborations to this project. We would also like to thank Diego de Mendoza for critically reading and providing input for the paper.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19040
References
- 1.Wiggins PM. Role of water in some biological processes. Microbiol Rev. 1990;54:432–49. doi: 10.1128/mr.54.4.432-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keilin D. The problem of anabiosis or latent life: history and current concept. Proc R Soc Lond B Biol Sci. 1959;150:149–91. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- 3.Wharton DA. Life at the limits: Organisms in extreme environments. Cambridge: Cambridge University Press, 2002. [Google Scholar]
- 4.Cano RJ, Borucki MK. Revival and identification of bacterial-spores in 25-million-year-old to 40-million-year-old Dominican amber. Science. 1995;268:1060–4. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 5.Shen-Miller J, Mudgett MB, Schopf JW, Clarke S, Berger R. Exceptional seed longevity and robust growth - Ancient sacred lotus from china. Am J Bot. 1995;82:1367–80. doi: 10.2307/2445863. [DOI] [Google Scholar]
- 6.Watanabe M. Anhydrobiosis in invertebrates. Appl Entomol Zool (Jpn) 2006;41:15–31. doi: 10.1303/aez.2006.15. [DOI] [Google Scholar]
- 7.Perry RN. Desiccation survival of parasitic nematodes. Parasitology. 1999;119:S19–30. [PubMed] [Google Scholar]
- 8.Clegg JS. Free glycerol in dormant cysts of the brine shrimp Artemia salina, and its disappearance during development. Biol Bull. 1962;123:295–301. doi: 10.2307/1539275. [DOI] [Google Scholar]
- 9.Crowe JH, Madin KAC. Anhydrobiosis in tardigrades and nematodes. Trans Am Microsc Soc. 1974;93:513–24. doi: 10.2307/3225155. [DOI] [Google Scholar]
- 10.Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–99. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 11.Crowe JH, Crowe LM, Mouradian R. Stabilization of biological-membranes at low water activities. Cryobiology. 1983;20:346–56. doi: 10.1016/0011-2240(83)90023-8. [DOI] [PubMed] [Google Scholar]
- 12.Browne J, Tunnacliffe A, Burnell AM. Anhydrobiosis: plant desiccation gene found in a nematode. Nature. 2002;416:38. doi: 10.1038/416038a. [DOI] [PubMed] [Google Scholar]
- 13.Hand SC, Menze MA, Toner M, Boswell L, Moore D. LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol. 2011;73:115–34. doi: 10.1146/annurev-physiol-012110-142203. [DOI] [PubMed] [Google Scholar]
- 14.Erkut C, Penkov S, Khesbak H, Vorkel D, Verbavatz JM, Fahmy K, et al. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr Biol. 2011;21:1331–6. doi: 10.1016/j.cub.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Riddle DL. The dauer larva. In: Wood WB, ed. The nematode Caenorhabditis elegans. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, 1988:393-412. [Google Scholar]
- 16.Golden JW, Riddle DL. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci USA. 1984;81:819–23. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnell AM, Houthoofd K, O'Hanlon K, Vanfleteren JR. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp Gerontol. 2005;40:850–6. doi: 10.1016/j.exger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Behm CA. The role of trehalose in the physiology of nematodes. Int J Parasitol. 1997;27:215–29. doi: 10.1016/S0020-7519(96)00151-8. [DOI] [PubMed] [Google Scholar]
- 19.Penkov S, Mende F, Zagoriy V, Erkut C, Martin R, Pässler U, et al. Maradolipids: Diacyltrehalose glycolipids specific to dauer larva in Caenorhabditis elegans. Angew Chem Int Ed Engl. 2010;49:9430–5. doi: 10.1002/anie.201004466. [DOI] [PubMed] [Google Scholar]
- 20.Khesbak H, Savchuk O, Tsushima S, Fahmy K. The role of water H-bond imbalances in B-DNA substate transitions and peptide recognition revealed by time-resolved FTIR spectroscopy. J Am Chem Soc. 2011;133:5834–42. doi: 10.1021/ja108863v. [DOI] [PubMed] [Google Scholar]
- 21.Goormaghtigh E, Raussens V, Ruysschaert JM. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim Biophys Acta. 1999;1422:105–85. doi: 10.1016/s0304-4157(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 22.Crowe JH. Trehalose as a “chemical chaperone”: Fact and fantasy. . Adv Exp Med Biol. 2007;594:143–58. doi: 10.1007/978-0-387-39975-1_13. [DOI] [PubMed] [Google Scholar]
- 23.Clegg JS. Metabolic studies of cryptobiosis in encysted embryos of Artemia salina. Comp Biochem Physiol. 1967;20:801–9. doi: 10.1016/0010-406X(67)90054-0. [DOI] [Google Scholar]
- 24.Belton PS, Gil AM. IR and Raman-spectroscopic studies of the interaction of trehalose with hen egg-white lysozyme. Biopolymers. 1994;34:957–61. doi: 10.1002/bip.360340713. [DOI] [PubMed] [Google Scholar]
- 25.Green JL, Angell CA. Phase-relations and vitrification in saccharide-water solutions and the trehalose anomaly. J Phys Chem. 1989;93:2880–2. doi: 10.1021/j100345a006. [DOI] [Google Scholar]
- 26.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.