Abstract
Octopamine (OA) appears to function as the invertebrate counterpart of norepinephrine (NE) in the modulation of a number of key behaviors. In C. elegans, OA signaling is complex, mediated by at least three distinct α-adrenergic-like receptors and appears to activate more global peptidergic signaling cascades that have the potential to dramatically amplify the octopaminergic signal. These OA-dependent peptidergic signaling cascades involve an array of neuropeptides that activate receptors throughout the nervous system and have the potential to both directly and indirectly modulate locomotory decision-making. In this commentary we highlight the use of C. elegans as a model to expand our understanding of noradrenergic signaling in mammals, specifically as it relates to the role of NE in anti-nociception.
Keywords: octopamine, neuropeptide, norepinephrine, aversive behavior, pain
Introduction
Catecholamines have long been implicated in the modulation of pain. The emergence of noradrenergic signaling in our understanding of anti-nociception fueled the discovery of selective noradrenergic ligands for use as analgesic agents.1 Although C. elegans contains only 302 neurons, it exhibits many complex behaviors, including chemotaxis, thermotaxis and mechanosensation and its rapid reversal in the presence of noxious stimuli can be at least operationally defined as the perception of pain. More importantly, these complex behaviors can be modulated by an array of monoamines and neuropeptides and understanding how these ligands interact to modulate fast neurotransmission mediated at the level of individual neurons is one of the principle goals of C.elegans systems neuroscience.2-6 In Mills et al.,7 we defined the role of OA in the modulation of aversive behavior, as a prelude to more in depth cell-based studies. In this commentary, we further highlight the similarities between noradrenergic signaling in mammals and octopaminergic signaling in C. elegans and the potential utility of the C. elegans model to better understand monoaminergic/peptidergic interactions and, more specifically, the role of noradrenergic signaling in mammalian anti-nociception.
Octopaminergic Signaling in C. elegans
C. elegans does not synthesize NE, but contains a structurally similar molecule OA that lacks the 3′ hydroxyl of NE. Given the structural similarity of NE and OA and the conservation of their respective G-protein coupled receptors, OA has been considered the invertebrate counterpart of NE. In C. elegans, OA is synthesized from tyramine (TA) in the two RIC interneurons and the gonadal sheath cells, based on the expression of tyramine β-hydroxylase (TBH-1), the rate-limiting enzyme for OA biosynthesis.8 OA signals nutritional status in C. elegans and appears to increase with starvation, although this has never been measured experimentally. OA acts antagonistically to serotonin (5-HT) to modulate a host of key behaviors, including pharyngeal pumping, egg-laying and locomotion.9,10 In addition, OA inhibits aversive behavior mediated by the two ASH sensory neurons.6 The RICs receive limited sensory input from the dopaminergic CEPs, required for mechanical food sensation and the nociceptive ADLs, form gap junctions with the ASHs and AWBs, and synapse on a limited number of synaptic partners, suggesting the RICs not only signal synaptically but also humorally.11 Indeed, OA receptors appear to be expressed on many neurons not directly innervated by the RICs. Three OA receptors have been identified, SER-3, SER-6 and OCTR-1, that appear to couple to Gαq, Gαq and Gαi respectively, based on heterologous expression and genetic analyses.6,12,13 All three C. elegans OA receptors appear to be most similar to mammalian α-adrenergic receptors, with SER-6 most identical to mammalian α1-receptors and OCTR-1 most identical to mammalian α2-receptors. In contrast to the situation in insects, no β-adrenergic receptor ortholog has been identified in the C. elegans genome. In addition, although C. elegans contains unique monoamine-gated Cl-channels specific for other monoamines, including 5-HT, TA and dopamine (DA), no OA-gated channel has yet been identified and no OA-specific reuptake protein has been identified in C. elegans, in contrast to 5-HT and DA, so that the mechanism of OA inactivation is unclear.14-16 Given the role of OA in modulating ASH-mediated aversive behavior and NE in anti-nociception in mammals discussed below, we questioned whether the pathways of OA and NE “inhibition” might be at least partially conserved in these two distantly-related organisms.
Noradrenergic Signaling in the Modulation of Mammalian Nociception
In mammals NE functions as both a neurotransmitter and neuromodulator, has wide-spread effects on a variety of physiological systems, and noradrenergic agents are used in the treatment of a number of disease states, including anxiety, depression, and schizophrenia.17 Adrenergic receptors are G-protein coupled and are grouped into two families, α and β, containing multiple subtypes. In general, α1- receptors (α1A,B,D) couple to Gαq, α2- receptors (α2A,B,C) to Gαi and β-receptors (β1–3) to Gαs.18
NE plays a complex role in the modulation of nociception. In healthy tissues, endogenous NE has little effect on the perception of pain, but NE can initiate either anti- or pro-nociceptive responses during chronic pain or in inflamed or injured tissues. For example, NE administered in the spinal cord can initiate a pronounced, dose-dependent anti-nociceptive response that is mediated by α2-receptors, based on extensive behavioral and pharmacological analyses.19 More importantly, the release of endogenous NE in the spinal cord from descending axons in the brainstem, such as those from the locus coeruleus and the A5/A7 nuclei, is also coupled to spinal anti-nociception and this NE-dependent anti-nociception is inhibited by α2-antagonists.20,21 NE appears to function pre-synaptically to inhibit nociceptive, substance P-containing, afferent fibers through α2A receptors, since neurotransmitter release from these fibers is inhibited both by NE or α2-agonists, such as clonidine.22,23 As noted above, the tonic activity of this intrinsic NE-mediated anti-nociceptive pathway appears to be quite low and may only be relevant with chronic injury or in other disease states. In addition, NE also appears to activate a number of additional inhibitory anti-nociceptive pathways by stimulating the α1-dependent release of an array of neuropeptides and other neuro-active ligands, such as γ-aminobutyric acid (GABA).24 For example, NE stimulates the secretion of vasopressin from the paraventricular nucleus of the hypothalamus and increases the pain threshold in a dose-dependent manner.25 To summarize, NE can suppress pain through the action of inhibitory α2-receptors (Gαi-coupled) directly on afferent nociceptors and by the activation of α1-receptors on inhibitory peptidergic, GABAergic and glycinergic interneurons (for review see refs. 26 and 27). In contrast, NE-mediated pro-nociceptive pathways have also been identified, highlighting the complexity of noradrenergic modulation, with NE increasing excitability of neurons in the dorsal root ganglion (DRG) of nerve injured animals through the α2-mediated-blockade of N-type Ca2+ channels and subsequent inhibition of Ca2+-activated K+ channels.28 NE appears to increase subthreshold membrane oscillation in the DRG suggesting a basis for at least some of the NE-mediated pronociceptive mechanisms operating in the periphery.29 To summarize, the effects of NE in the perception of pain in mammals is complex and mediated by multiple receptors.
Octopaminergic Inhibition of Aversive Behavior Mediated by the ASH Sensory Neurons
In C. elegans, OA delays aversive responses to the volatile repellant, 1-octanol mediated by the single pair of ASH sensory neurons.6 Upon the presentation of 1-octanol on a hair in front of a forward moving animal, reversal is usually initiated in about 4–5 sec at 100% 1-octanol and in about 10 sec at 30% 1-octanol, with the presence of food or exogenous 5-HT increasing responses to 30% 1-octanol to about 4–5 sec.30,31 OA delays aversive responses to both 100% 1-octanol and 30% 1-octanol in the presence of food or 5-HT to about 10 sec. Surprisingly, all three OA receptors are involved in this OA “inhibition.” OCTR-1 functions directly in the ASHs to inhibit Gαs signaling and inhibit food or 5-HT stimulation. For example, OA does not inhibit animals with OCTR-1 or Gαo knocked down in the ASHs.4 Conversely, the ASH RNAi knockdown of pde-4 that encodes a cAMP phosphodiesterase increases aversive responses in the absence of food and this increase is abolished by OA, presumably by decreasing the increased cAMP signaling after pde-4 RNAi knockdown.4 Interestingly, this OA inhibition is abolished by increasing the exogenous OA concentration from 4 mM to 10 mM. Apparently, increasing the OA concentration activates a second ASH-expressed OA receptor, SER-3 that antagonizes ASH OCTR-1 signaling, highlighting the complexity of octopaminergic modulation. A similar situation has been observed for NE modulation in the mammalian olfactory bulb, where the differential affinities of α1 and α2 noradrenergic receptor subtypes for NE have been postulated to account for the differential modulation of GABA release and olfactory processing.32
Interestingly, at higher levels of ASH activation, OA inhibition also requires the expression of SER-6 in the AWB, ADL and ASI sensory neurons. SER-6 signaling requires an array of AWB, ADL and ASI-expressed neuropeptides. Surprisingly, the RNAi knockdown of individual peptide-encoding genes in one pair of sensory neurons can dramatically reduce OA inhibition, while the expression of the same peptide-encoding gene can rescue OA inhibition in a different pair of sensory neurons in null animals. How can a phenotype demonstrated by RNAi knockdown in one pair of neurons in wild-type animals be rescued by expression (overexpression?) in a different pair of neurons in null animals? We and others have observed a similar phenomenon with other neuropeptides, even after rigorously excluding the potential for RNAi spreading.33 Monoamines or neuropeptides are released both tonically and acutely in response to internal and environmental cues and activate a variety of extrasynaptic receptors on multiple neurons, suggesting that a changing “humoral” soup of monoamines/neuropeptides may, at least in part, define “behavioral state.” The composition of this “soup” is dependent on contributions from multiple neurons, suggesting that small increases or decreases in ligand release from any one neuron pair has the potential to alter signaling. Indeed, only 2-fold changes in the expression level of the TA receptor tyra-3 can have profound effects on locomotory behavior.34 These observations suggest that any overexpression in rescued animals has the potential to compensate for an absence of release from other neurons. This philosophy may also be potentially extrapolated to neural circuits that are modulated by multiple inputs, i.e., the loss of one modulator can be masked by the overexpression of another, i.e., neural circuits are not necessarily analogous to enzymatic pathways with one rate-limiting step operating at saturation. In the past, cell-specific rescue has been the “gold standard” for functional localization and for structural proteins this is certainly true. However, we have observed potential “off-target” phenotypes with the expression of many C. elegans G-protein coupled receptors, presumably because ligands for the receptors are either tonically released or the receptors themselves exhibit constitutive activity in the absence of ligand. The effects of G-protein signaling on neurotransmitter release in C. elegans are well documented and we have observed that the expression of many Gαs, Gαo and Gαq -coupled receptors in interneurons or motorneurons modulating locomotion often yield artifactual locomotory phenotypes, just as the expression of the gain-of-function G-proteins themselves. If neurotransmitter release from any neuron in a circuit has the potential to increase the output of the circuit, then neuron-specific RNAi knockdown may be more diagnostic than rescue, highlighting the need for both techniques in defining circuit modulation.
We hypothesize that SER-6 mediated G-protein signaling stimulates dense core vesicle and neuropeptide release from the ASI, ADL and AWB sensory neurons, although peptide release has not yet been measured directly. SER-6 appears to couple robustly to Gαq when heterologously expressed in Xenopus oocytes, based on the OA-dependent activation of an endogenous Ca2+- gated Cl- channel, but SER-6 coupling in vivo is unclear, especially given the previously described role for Gαs signaling in peptide release in the ASHs.4 It will important to identify the specific role of G-protein signaling on neuropeptide release from these neurons. Interestingly, in Drosophila motorneurons, OA-stimulated neuropeptide release occurs in the absence of extracellular calcium and requires synergistic Gαs signaling, the Ins(1,4,5)P3 receptor and Ca2+ mobilization from the endoplasmic reticulum mediated by the ryanodine receptor. This work suggests that neuropeptide release may occur in the absence of activity-dependent Ca2+ entry and that changes in Ca2+ dynamics examined by Ca2+ imaging may reflect more than simple neuronal excitation, i.e., the GPCR-dependent release of Ca2+ from internal stores may also be involved.35
Interestingly, some of the receptors for these neuropeptides appear to be expressed on additional sensory neurons mediating primarily attraction, not aversion, suggesting that reversal may be also facilitated by inhibiting tonic signaling from neurons favoring forward locomotion. Indeed, it appears that locomotory decision-making requires the integration of multiple tonic and acute sensory inputs and the modulation of tonic signaling from any of the C. elegans sensory neurons may have the potential to modulate locomotory behavior.36,37 For example, the C. elegans oxygen-sensing neurons function tonically to continuously monitor environment PO2 to modulate locomotory behavior, and the ablation of these oxygen-sensing neurons also modulates aversive responsive responses to 1-octanol (Hapiak and Komuniecki, unpublished).38 It will be important to define the subtle interactions among the 14 pairs of sensory neurons in the modulation of locomotory behavior, once we know more about how they signal and how they are modulated by the complex and changing mix of humorally-expressed monoamines and neuropeptides.
C. elegans: A Model for the NE-Mediated Modulation of Nociception in Mammals?
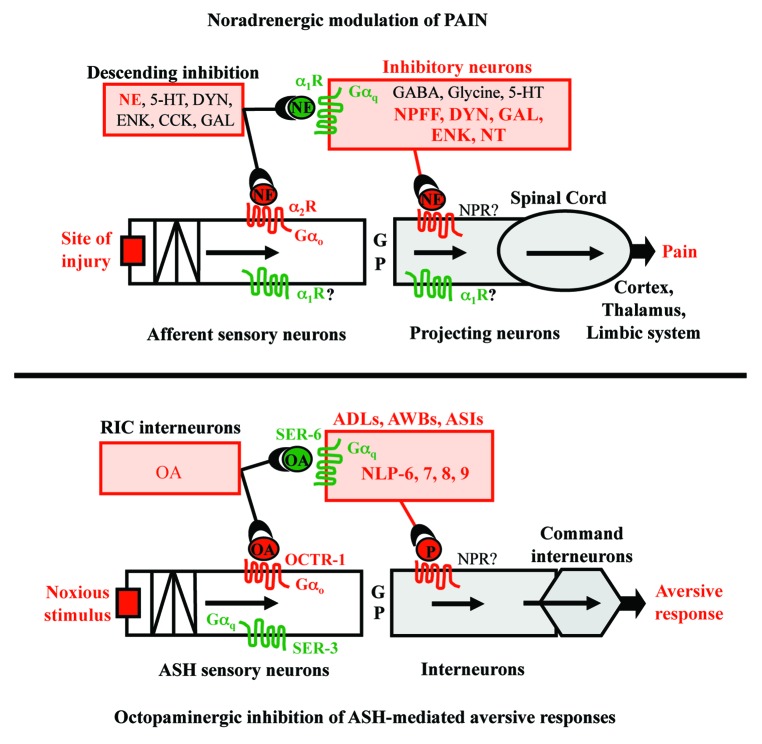
As outlined above, the octopaminergic modulation of ASH-mediated aversive behavior appears to mimic the noradrenergic modulation of chronic pain in mammals (Fig. 1).36 In mammals, Gαo-coupled α1-receptors presynaptically inhibit sensory input and Gαq-coupled α2 receptors activate more global inhibitory signaling cascades. Similarly, in C. elegans Gαo- coupled α1-like receptors (OCTR-1) presynaptically inhibit sensory input and Gαq-coupled α2-like receptors (SER-6) activate more global inhibitory peptidergic signaling cascades. Finally, both OA and NE are also capable of activating pronociceptive pathways. Together these observations suggest that understanding the OA-dependent modulation of aversive behavior in C. elegans may provide useful mechanistic insights into the modulation of chronic pain in mammals.
Figure 1. Octopamine inhibition of aversive responses in C.elegans mimics noradrenergic anti-nociception in mammals. 5-HT, serotonin; CCK, cholecystokinin; DYN, dynorphin; ENK, encephalin; G, glutamate; GABA, γ-aminobutyric acid; NE, norepinephrine; NPFF, neuropeptide FF; NT, neurotensin; OA, octopamine; P, peptide.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/20467
References
- 1.Smith H, Elliott J. Alpha(2) receptors and agonists in pain management. Curr Opin Anaesthesiol. 2001;14:513–8. doi: 10.1097/00001503-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–21. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glauser DA, Chen WC, Agin R, Macinnis BL, Hellman AB, Garrity PA, et al. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–99. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Kim K, Li C, Barr MM. FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci. 2007;27:7174–82. doi: 10.1523/JNEUROSCI.1405-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, et al. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–12. doi: 10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills H, Wragg R, Hapiak V, Castelletto M, Zahratka J, Harris G, et al. Monoamines and neuropeptides interact to inhibit aversive behaviour in Caenorhabditis elegans. EMBO J. 2011;31:667–78. doi: 10.1038/emboj.2011.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–60. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–4. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 10.Packham R, Walker RJ, Holden-Dye L. The effect of a selective octopamine antagonist, epinastine, on pharyngeal pumping in Caenorhabditis elegans. Invert Neurosci. 2010;10:47–52. doi: 10.1007/s10158-010-0107-9. [DOI] [PubMed] [Google Scholar]
- 11.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 12.Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450:553–6. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- 13.Suo S, Kimura Y, Van Tol HH. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci. 2006;26:10082–90. doi: 10.1523/JNEUROSCI.0819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, et al. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol. 1998;54:601–9. [PubMed] [Google Scholar]
- 15.Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–38. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–5. doi: 10.1038/35044083. [DOI] [PubMed] [Google Scholar]
- 17.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 18.Summers RJ, McMartin LR. Adrenoceptors and their second messenger systems. J Neurochem. 1993;60:10–23. doi: 10.1111/j.1471-4159.1993.tb05817.x. [DOI] [PubMed] [Google Scholar]
- 19.Danzebrink RM, Gebhart GF. Antinociceptive effects of intrathecal adrenoceptor agonists in a rat model of visceral nociception. J Pharmacol Exp Ther. 1990;253:698–705. [PubMed] [Google Scholar]
- 20.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–94. doi: 10.1016/S0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 21.Proudfit HK. Pharmacologic evidence for the modulation of nociception by noradrenergic neurons. Prog Brain Res. 1988;77:357–70. doi: 10.1016/S0079-6123(08)62802-2. [DOI] [PubMed] [Google Scholar]
- 22.Takano M, Takano Y, Yaksh TL. Release of calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal polypeptide (VIP) from rat spinal cord: modulation by alpha 2 agonists. Peptides. 1993;14:371–8. doi: 10.1016/0196-9781(93)90055-L. [DOI] [PubMed] [Google Scholar]
- 23.Ueda M, Oyama T, Kuraishi Y, Akaike A, Satoh M. Alpha 2-adrenoceptor-mediated inhibition of capsaicin-evoked release of glutamate from rat spinal dorsal horn slices. Neurosci Lett. 1995;188:137–9. doi: 10.1016/0304-3940(95)11397-F. [DOI] [PubMed] [Google Scholar]
- 24.Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–84. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Yuan HF, Liu WY, Zhang XX, Feng JP, Ni N, et al. Norepinephrine regulates arginine vasopressin secretion in hypothalamic paraventricular nucleus relating with pain modulation. Neuropeptides. 2009;43:259–65. doi: 10.1016/j.npep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/S0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 27.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Honma Y, Yamakage M, Ninomiya T. Effects of adrenergic stimulus on the activities of Ca2+ and K+ channels of dorsal root ganglion neurons in a neuropathic pain model. Brain Res. 1999;832:195–206. doi: 10.1016/S0006-8993(99)01499-7. [DOI] [PubMed] [Google Scholar]
- 29.Xing JL, Hu SJ, Jian Z, Duan JH. Subthreshold membrane potential oscillation mediates the excitatory effect of norepinephrine in chronically compressed dorsal root ganglion neurons in the rat. Pain. 2003;105:177–83. doi: 10.1016/S0304-3959(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 30.Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci U S A. 2004;101:15512–7. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, et al. Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci. 2009;29:1446–56. doi: 10.1523/JNEUROSCI.4585-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nai Q, Dong HW, Linster C, Ennis M. Activation of alpha1 and alpha2 noradrenergic receptors exert opposing effects on excitability of main olfactory bulb granule cells. Neuroscience. 2010;169:882–92. doi: 10.1016/j.neuroscience.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida K, Hirotsu T, Tagawa T, Oda S, Wakabayashi T, Iino Y, et al. Odour concentration-dependent olfactory preference change in C. elegans. Nat Commun. 2012;3:739. doi: 10.1038/ncomms1750. [DOI] [PubMed] [Google Scholar]
- 34.Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature. 2011;472:313–8. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci U S A. 2011;108:4477–81. doi: 10.1073/pnas.1017837108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komuniecki R, Harris G, Hapiak V, Wragg R, Bamber B. Monoamines activate neuropeptide signaling cascades to modulate nociception in C. elegans: a useful model for the modulation of chronic pain? Invertebrate neuroscience: IN 2011. [DOI] [PubMed] [Google Scholar]
- 37.Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. Bioessays. 2012 doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 38.Busch KE, Laurent P, Soltesz Z, Murphy RJ, Faivre O, Hedwig B, et al. Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat Neurosci. 2012;15:581–91. doi: 10.1038/nn.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]