Abstract
The C. elegans neuroectodermal seam cells provide a tractable and well-established model for studying the stem cell mode of division, due to the reiterative asymmetric divisions occurring during larval development. They are, however, not generally considered to be ‘true’ stem cells, owing to their eventual terminal differentiation and the lack of a defined stem cell niche—a microenvironment that promotes the proliferation and prevents the differentiation of the stem cells that reside within. Here, we discuss the concept of the niche in relation to the seam, with reference to our recent findings suggesting that the stem-like properties of the seam cells are maintained at least in part through protection from differentiation signals emanating from the surrounding hypodermal syncytium. Determining the applicability of the niche concept will require definition of these signals and will have important implications for the status of seam cells in the context of stem cell biology.
Keywords: seam cells, stem cells, niche, proliferation, differentiation
Introduction
Of the many uses of C. elegans as a model for studying developmental biology, the application of the worm to enhancing our understanding of stem cells ranks among the most topical; advantages as a model organism, combined with the possession of cell lineages which can serve as models of stem cell biology, make this creature an extremely powerful tool.
Stem cells are of fundamental importance to development; they proliferate, providing the material for growth and renewal, but also have the potential to produce differentiated cell types and thus specialized tissues. Medically too, stem cells are of great interest; the effects of aberrations in stem cell development can be profound, as demonstrated by the implication of stem cells in an ever widening range of human cancers (for a review see ref. 1). Furthermore, though the science of artificial generation of tissues and organs from stem cells in vitro is still in its infancy, progress will depend on a deeper understanding of stem cell biology.
The developmental potential and significance of stem cells means that the regulation of their division and fate is of paramount importance; the balance between proliferation and differentiation must be tightly coordinated with the requirements of development. We have used the seam cells as models of stem cell divisions. The seam lineage comprises a specialized epithelial tissue, consisting of multipotent lateral hypodermal cells which lie along each side of the worm and which undergo reiterative divisions during larval development. Specifically, we have been examining the roles of the C. elegans RUNX and CBFβ homologs, rnt-1 and bro-1, respectively, in promoting seam cell proliferation and self-renewal. Members of the RUNX family of transcription factors, present throughout the animal kingdom, form heterodimeric complexes with CBFβ, which increases the DNA-binding affinity and specificity of its RUNX partner. Working together, RUNX/CBFβ are well-established as key players in stem cell developmental pathways.2 In the worm, both families are represented by a single gene, meaning that the potentially confounding effects of redundancy experienced in other systems are avoided.
Our finding that the GATA factor ELT-1 directly regulates bro-13 led to further functional dissection of the elt-1 gene and the discovery that it has a dual role in maintaining the stem-like fate of seam cells. As well as working through the BRO-1/RNT-1 complex to promote proliferation, ELT-1 performs an additional, bro-1/rnt-1-independent role. Through the repression of the fusogen eff-1, which is required for heterotypic fusion of seam daughters with the hypodermis,4 ELT-1 prevents seam cells fusing with the surrounding hypodermis; in elt-1(RNAi) animals, ectopic fusion is observed, coupled with loss of the stem-like seam fate and acquisition of the hypodermal, differentiated fate.
The apparent relationship between the departure of cells from the seam, fusion with the hpy7 syncytium and loss of the stem-like seam fate raised the question of whether seam cells reside in a niche. Here, in the light of these findings, the ‘stem-like’ status of the seam cells, together with the applicability (or not) of the niche concept to this system, is examined further.
Seam Cells and the Stem Cell Concept
A unique feature of stem cells is their ability to self-renew—giving rise to more stem cells as well as producing differentiated cell types. This ability places stem cells in a powerful position, with immense potential to influence development. Consequently, tight and robust regulation is required, both of the number as well as the pattern of their divisions.
In addition, stem cells are also associated with—perhaps even defined by—the stem cell niche. The niche is the microenvironment of the stem cell population that is responsible for exerting control over the cells lying within, maintaining the stem, proliferative fate and repressing terminal differentiation. The importance of the niche has been recognized for decades, following the finding that the proliferative potential of stem cells was context-dependent and seemed to depend on association of the stem cells with their natural cellular environment.5 Subsequent work in Drosophila and C. elegans led to the definition of the stem cell niche at a cellular level6,9 and a clearer understanding of the relationship between stem cells, their environment and the signals underlying their divisions and fate. Within the niche, pro-mitotic, anti-differentiation signals maintain the proliferative state of the stem cell population. As cells leave the niche, the signaling environment to which they are exposed changes and differentiation results.
In the worm, the germ line has been widely recognized as containing the only bona fide stem cells in the worm.7,8 Germ cell nuclei lying at the distal ends of the gonad arms proliferate continuously for the entire lifetime of the worm, retaining the potential to give rise to all the differentiated cell types found in the next generation of worms. In addition, the germline represents a paradigm of the stem cell niche, the first to be defined at the cellular level. The germ nuclei reside in a microenvironment that maintains them in the proliferative state and prevents differentiation. Fine cytoplasmic processes extending out from the cell body of the distal tip cell ensure that the stem cells maintain contact with the niche9,10 and more recently it has been suggested that additional, long-range signals are also involved in maintenance of the stem cell pool.11 Clearly, the microenvironment of stem cells represents a complex matrix of signaling interactions, absolutely required for maintenance of the stem fate. Dissecting these interactions will be key to unravelling stem cell regulation.
Since the work on the C. elegans germline, which first defined the stem cell niche at a cellular level, the principle of the niche signaling to stem cells to promote proliferation, or prevent differentiation, has been found to apply broadly and has been defined in numerous different systems.12-16 Indeed, the concept of the niche has become so intimately linked with stem cells that it is seen as a defining feature; stem cells would not be termed stem cells if they did not reside in a defined niche.
How then does the C. elegans seam lineage compare with the germline? As indicated above, unlike the germline the seam has not been considered to be a “true” stem cell lineage, hence the use of “stem cell-like”. However, seam cells do share important properties with stem cells and the differences are perhaps less significant than previously thought.
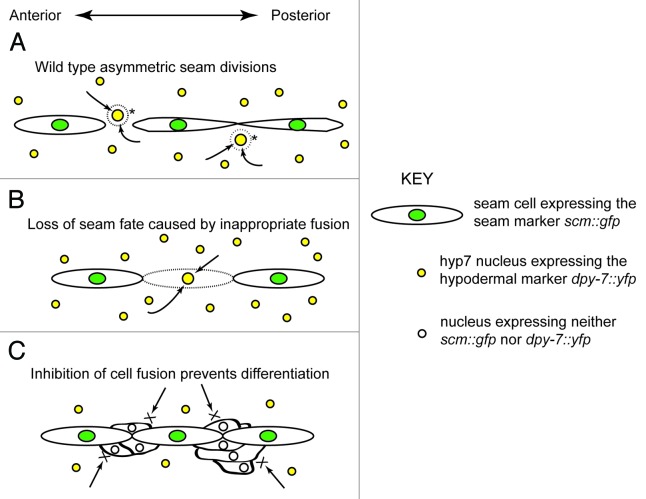
Crucially, the seam cells have the ability to both self-renew, and to give rise to differentiated cell types; hypodermal, glial and neuronal cells are all contributed by the seam lineage.17 Throughout larval development, the seam cells undergo repeated divisions, separated by a period of quiesence. At each division, individual cells either divide asymmetrically, giving rise to one stem-like daughter and one daughter which ceases proliferating and instead goes on to differentiate (Fig. 1), or symmetrically, producing two stem-like daughters, both of which will divide again, thus expanding the number of progenitors.
Figure 1.
The role of seam cell contacts and boundaries in maintaining the stem-like fate. (A) During asymmetric seam cell divisions, anterior daughters (marked by asterisks) leave the line of the seam and lose expression of scm::gfp (green shading). Breakdown of the cell membrane likely allows differentiation signals from the surrounding hypodermis (arrows) to enter the cells, which consequently switch on expression of the hypodermal marker, dpy-7::yfp (yellow shading). In this diagram, the two posterior-most seam cells have already joined up after the division. This contact is essential for subsequent divisions of the seam; maintenance of the correct (i.e., end-to-end) cell contacts is required for propagation of the stem fate. (B) In animals subjected to knockdown of ajm-1, dlg-1, elt-1 or let-413 by RNAi, inappropriate loss of cell membranes is observed (dashed line). As in (A), dissolution of the membrane surrounding seam cells may facilitate the entry of differentiation signals from the hypodermis, resulting in affected seam cells making the transition from the stem to the differentiated fate. (C) In eff-1 mutants the anterior daughters of seam divisions fail to fuse with the hypodermal syncytium. These cells leave the line of the seam, accumulating alongside the lineage. They cease dividing and most lose scm::gfp expression. They are, however, unable to differentiate–DPY-7::YFP is never observed–and thus are in what we term a state of “developmental limbo.” According to our model, the differentiation signals from the hypodermis (arrows, as in A and B), which would normally enter these cells as the membrane breaks down, are prevented from doing so. As a result, differentiation does not occur; the membrane effectively acts as a protective niche, shielding the seam from the influence of the surrounding hypodermis.
With respect to proliferation and differentiation then, seam cells appear to meet the criteria for stem cells. However, this is not the whole story. At the end of larval development, throughout which the seam cells have proceeded to divide, the entire lineage is programmed to terminally differentiate; the seam cells, which up to that point have remained distinct, fuse together to produce a syncytium that runs the entire length of the body. No more divisions occur. This clearly represents a stark contrast with the germline, and places limits on the extent to which the seam cells can be seen as having the ability to self-renew. However, it is important to note that, while the seam cells do indeed terminally differentiate, up until this point they nevertheless serve as models of the stem cell mode of division; the invariant, reiterative divisions of the seam cells, some symmetric and some asymmetric, provide a superb model for dissecting how the balance between proliferation and differentiation is regulated. In this context, whether a developmental program that terminates division potential is subsequently activated is irrelevant; for the entirety of larval development seam cell divisions can be analyzed in real time and at single cell resolution. On this basis, seam cells may not be stem cells in the narrowest of definitions, but they are certainly excellent models for analyzing stem cell divisions.
It is also worth noting that, in some heterochronic mutants (for example, let-718) the seam cells continue to divide beyond the L4-adulthood transition. The number of divisions in wild type animals is thus normally limited in some way, likely reflecting the development and short lifespan of the worm. Subsequent to the larval stages the seam is no longer required to contribute to the growth of the animal, which ceases at adulthood. Similarly, the three-week lifespan of the worm does not require the seam to provide cells to counter the effects of aging and degeneration in a way analogous to, for example, the stem cells found in human epithelia. These reasons, together with the additional seam divisions observed in mutants with defects in terminal differentiation, suggest that the seam cells in fact have the potential to continue dividing but that this potential is normally actively limited once the worm reaches adulthood.
There is an additional and significant apparent difference between the seam and other recognized lineages of stem cells—the niche. Indeed, perhaps the strongest argument against the seam cells being true stem cells is that no niche has so far been identified. This is, of course, not to say that the seam cells do not reside in a niche. Indeed, our recent findings suggest that the niche concept may well apply to the seam.
Evidence for a Seam Cell Niche
As noted above, RNAi knockdown of elt-1 revealed a role for this gene in repressing the fusogen, EFF-1. elt-1 acts to prevent EFF-1-dependent fusion of seam cells with hyp7, a syncytium of hypodermal nuclei which surround the seam dorsally and ventrally (Fig. 1).
We therefore decided to further investigate the relationship between this fusion event and the stem-like fate.
As a result of elt-1 RNAi, seam cells were observed to inappropriately lose their boundaries, which were visualized using components of the apical junction complexes (e.g., AJM-1) as markers of the membrane. These junction complexes reside in the apical membranes of the cells and are critical for cell-cell attachment and potentially also for signaling. Following elt-1 RNAi, seam cells were observed to inappropriately lose their boundaries, as marked by AJM-1::mCherry, and were found to not just cease dividing but undergo a fate transformation; they leave the seam, moving into the hyp7 syncytium, and switch on expression of dpy-7, an established marker of the differentiated, hypodermal fate (Fig. 1). Interestingly, knockdown of the apical junction proteins AJM-1, DLG-1 and LET-413 leads to an apparently identical phenotype. While apical junction proteins may not be the direct targets of eff-1 (indeed, current hypotheses see apical junction breakdown as a relatively late event in the cell fusion process4,19), these components are clearly crucial for membrane integrity and thus, indirectly, the fate of the seam cells.
The relevance of this to the niche concept is not simply that the seam cells require a membrane boundary to proliferate. It goes without saying that all cells require integrity to divide and no-one would argue that, on the basis that the cell membrane represents a niche, any cell with the ability to divide is a stem cell. Rather, in the context of the seam lineage, the niche argument hinges on the fact that the boundary between the seam and hypodermal compartments does appear to protect the seam from differentiation; the membrane of the seam cells, which separates this lineage from hyp7, appears to represent the niche in this system.
In eff-1 mutants, proliferation and differentiation are uncoupled. Whereas in wild type animals anterior daughters of asymmetric seam divisions lose their proliferative potential, essentially at the same time as fusing with the hyp7 syncytium, in worms lacking functional EFF-1, these anterior daughters are unable to undergo the fusion process. As a result these cells, retaining their boundaries (which are clearly visible through the use of AJM-1::GFP), accumulate around the seam, unable to move out into the hypodermis; strikingly, while they lose their seam or “stem-like” fate (they switch off expression of the seam cell marker, scm::gfp, and were never observed to undergo subsequent divisions), they nevertheless fail to differentiate (they never express dpy-7::yfp). The result is that these cells are left in what we termed “developmental limbo”; they are neither capable of proliferation nor of differentiating.
The fact that proliferation can be decoupled from differentiation is significant; it could easily be assumed that differentiation simply results directly from the loss of the seam fate. Instead though, differentiation appears to be a distinct process and, importantly, a process that is blocked by the inability of the seam daughters to fuse with the hypodermis. This decoupling has implications for the concept of the niche and suggests that fusion is required for the differentiation process. Put another way, the prevention of fusion—and thus the maintenance of a boundary between the seam and the hypodermis—protects the seam from differentiation and allows cells to remain in their proliferative state.
The seam-hyp7 fate transition that accompanies fusion demonstrates that loss of the boundary around the seam cells facilitates the active process of differentiation. Looking back to the meaning of the niche, it is generally considered to apply to the microenvironment of stem cells, which plays an active role in maintaining their special characteristics by promoting proliferation and protecting the stem cell pool from differentiation. With respect to the latter property at least, the niche concept may be applicable; the seam-hypodermis boundary appears to protect the seam cells within from differentiation signals. This situation is analogous to that seen in the Drosophila male germline, where signals emanating from outside the niche promote differentiation of stem cell daughters.20 In the seam though, the exact nature of these signals remains to be determined. Are they diffusible cues, physically excluded from the seam merely by the membrane barrier between the seam and hypodermal compartments, or does the prevention of differentiation depend on a more complex signaling cascade across the membrane?
Furthermore, it remains unclear whether seam cells receive extrinsic signals to promote their proliferation, as might be expected under the niche hypothesis (based on, for example, the apparent role of proliferative signals emanating from the follicle stem cell niche in the Drosophila ovary21). On the one hand, it could be that the loss of proliferative ability—the ‘seam’ fate—is determined at division, and not by the subsequent movement of daughter cells away from the seam. This point of view could be argued on the basis that, in the eff-1 mutants described above, anterior daughters fail to divide and reliably express scm::gfp in spite of remaining adjacent to and in contact with the seam line (Fig. 1).
On the other hand, it is important to note that such ‘limbo’ cells, though next to the seam line, are most definitely not ‘within’ it; although they do not fuse with the hypodermis, they nevertheless move out of the line of the seam immediately after division. The use of distinct fusogens for heterotypic and homotypic seam cell fusion—and the distinct developmental timings of these processes—clearly demonstrates that there are significant differences between the dorso-ventral and the lateral sides of the seam cells.4,22 Furthermore, the importance of contact between the seam cells for proliferation is well-established.23,24 Thus an intriguing possibility is that the interaction between the anterior and posterior ends of seam cells—as opposed to the dorsal and ventral surfaces—plays a key role in determining the fate of anterior daughters of asymmetric seam cell divisions. Perhaps it is by moving out of the line of the seam, and thus out of this “interaction zone,” that these cells lose their proliferative potential. Such a situation would certainly parallel that observed in the Drosophila germline, where physical attachment of stem cells to the niche, mediated by adherens junctions, are crucial for the transmission of self-renewal signals, and maintenance of the stem fate.25
Conclusions and Future Prospects
That the seam cells of the worm represent an invaluable model for the stem cell mode of division is beyond doubt. However, their relationship with true stem cells is more ambiguous. Central to this issue of identity is the concept of the niche, a term which has yet to be applied to the seam. Our findings suggest that the seam cells are indeed maintained in their proliferative state, in part, through protection from differentiation signals emanating from the surrounding hypodermis.
The next step will be to determine the precise nature of these putative differentiation cues and how they are transmitted to the seam. In addition, determining the origin and form of signals that cause the seam to proliferate throughout larval development remains a challenge, but is important. Defining the niche, if it is possible, should allow us to re-define the seam.
Acknowledgments
Work in A.W.’s laboratory is funded by BBSRC (BB/G018448/1), CRUK (C20933/A11782) and AICR (08-0458). C.B. was in receipt of an MRC Capacity Building Studentship.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19417
References
- 1.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–9. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 2.Appleford PJ, Woollard A. RUNX genes find a niche in stem cell biology. J Cell Biochem. 2009;108:14–21. doi: 10.1002/jcb.22249. [DOI] [PubMed] [Google Scholar]
- 3.Brabin C, Appleford PJ, Woollard A. The Caenorhabditis elegans GATA factor ELT-1 works through the cell proliferation regulator BRO-1 and the Fusogen EFF-1 to maintain the seam stem-like fate. PLoS Genet. 2011;7:e1002200. doi: 10.1371/journal.pgen.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, et al. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2:355–62. doi: 10.1016/S1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 5.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 6.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–30. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 7.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 8.Joshi PM, Riddle MR, Djabrayan NJV, Rothman JH. Caenorhabditis elegans as a model for stem cell biology. Dev Dyn. 2010;239:1539–54. doi: 10.1002/dvdy.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–99. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 10.Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–24. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- 11.McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJAA. A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci U S A. 2009;106:11617–22. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 13.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–60. doi: 10.1016/S0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 14.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 15.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–9. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 16.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/S1534-5807(02)00254-X. [DOI] [PubMed] [Google Scholar]
- 17.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 18.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 19.Gattegno T, Mittal A, Valansi C, Nguyen KCQ, Hall DH, Chernomordik LV, et al. Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol Biol Cell. 2007;18:1153–66. doi: 10.1091/mbc.E06-09-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005;118:665–72. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 21.O’Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008;182:801–15. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, et al. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. Dev Cell. 2007;12:683–98. doi: 10.1016/j.devcel.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin J, Kenyon C. Cell contact regulates neuroblast formation in the Caenorhabditis elegans lateral epidermis. Development. 1994;120:313–23. doi: 10.1242/dev.120.2.313. [DOI] [PubMed] [Google Scholar]
- 24.Silhánková M, Jindra M, Asahina M. Nuclear receptor NHR-25 is required for cell-shape dynamics during epidermal differentiation in Caenorhabditis elegans. J Cell Sci. 2005;118:223–32. doi: 10.1242/jcs.01609. [DOI] [PubMed] [Google Scholar]
- 25.Song X, Zhu C-H, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–7. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]