Abstract
The JAK-STAT signaling pathway plays a central role in transducing stress and growth signals in the hypertrophic heart. Unlike most signal transducers, JAKs and STATs signal in a number of different ways, both within the JAK-STAT pathway and in collaboration with other signaling pathways. In this review, we discuss how IL-6 activates cells lacking IL-6 receptors through trans-signaling and examine JAK-STAT pathway interaction with GPCR-linked pathways both within and between cells. Finally, we discuss recent studies showing how the JAK-STAT pathway can intersect with a general transcriptional regulatory mechanism to effect transcription of STAT-dependent stress response genes.
Keywords: Il-6 trans-signaling, JAK-STAT signaling pathway, angiotensin II, autocrine signaling, cardiac hypertrophy, cardiac lineage protein-1, endothelin-1, paracrine signaling, transcription elongation factor b, αB-crystallin
Introduction
The heart responds to increased demand by mounting an adaptive or compensatory response to increase cardiac function and normalize cardiac output. To achieve this, cardiomyocytes increase synthesis of sarcomeres, their primary contractile unit, and assemble them into an expanded arrangement of myofibrils.1 To accommodate these additional myofibrils, the cardiomyocyte enlarges or hypertrophies. While this allows the heart to meet increased demand in the short term, prolonged load imposition causes this adaptive response to turn maladaptive or decompensatory. Cardiomyocytes become metabolically depleted and die causing severe erosion in systolic and diastolic function that eventually leads to heart failure.
Underlying the adaptive and maladaptive hypertrophic responses are distinct genetic programs controlling cardiomyocyte contractility, stress response and metabolic energy production.2-10 Each program is a response to hypertrophic signals, cytokines and stress hormones. A variety of experimental approaches have identified distinct signal transduction pathways linking these signals to the genomic stress response mounted by cardiomyocytes.3,11,12 Among the most prominent signal transducers involved in cardiac hypertrophy are the MAPKinase, calmodulin-dependent phosphatase and JAK-STAT signaling pathways.13-15 Ongoing research on the role of JAK-STAT signaling in cardiac hypertrophy has provided new insights into how this signaling pathway can “repurpose” its signal transducers to play a much wider and more influential role in controlling how cardiomyocytes sense and respond to hypertrophic stress. In this review, we discuss three examples of how JAKs and STATs can interact with other signal transducers and transcriptional regulators within the same cell and between different cells to orchestrate the hypertrophic response.
The JAK-STAT Pathway
The JAK-STAT pathway was originally identified as a receptor-activated pathway responsive primarily to interferon-gamma and members of the interleukin-6 family, such as IL-6, cardiotrophin 1 (CT-1) and leukemia inhibitory factor (LIF).16-22 The signaling pathway formed by these latter ligands and their IL6-α/gp130 receptor plays an important role in biology and has long been exemplary of the JAK-STAT pathway itself. But further study has shown that this simple JAK-STAT signaling paradigm is representative of only a portion of the signaling pathways that use JAK and STAT proteins to transmit extracellular signals. JAK kinases have been shown to associate with a wider spectrum of receptor types such as tyrosine kinase or G-protein linked receptors and activated JAKs are able to phosphorylate other receptors and adaptor proteins suggesting that their substrate specificity need not be confined to IL6-α/gp130 type receptors or STATs alone.23-25 This allows the JAK kinase to transduce a wider spectrum of signals via STATs or other signaling molecules thereby widening the number of possible intercellular interactions that can be mediated by JAK-STAT signaling.
The JAK-STAT pathway differs from most signaling pathways in that one of its cytoplasmic signal transducers, the STAT protein, is itself the transcription factor activated by the JAK kinases. While much is known about how these two signal transducers form the JAK-STAT signaling pathway or act in conjunction with other receptor systems to transmit diverse signals, much less is known about how STATs interact with the transcriptional apparatus to bring about transcription of STAT-dependent genes. Here again, the prominence of the JAK-STAT pathway in transmitting hypertrophic signals to cardiomyocyte nuclei has afforded us the opportunity to study such interactions. Our laboratory’s long-standing interest in hypertrophic signaling through the JAK-STAT pathway has recently intersected with our more recent studies of the transcriptional regulator CLP-1 (cardiac lineage protein-1) in controlling RNA polymerase II-dependent transcription. These studies have led to new insights into how the JAK-STAT signaling pathway can act to potentiate transcription of STAT-dependent genes by interacting with the more general components of the basal transcriptional apparatus.
In this review, we will examine how the JAK-STAT pathway broadens its signaling “bandwidth” allowing it to transmit extracellular signals for a number of important processes, particularly those related to the cellular response to hypertrophic stress-inducing stimuli. We will focus on the cardiovascular system in which the JAK-STAT pathway has been shown to play a prominent role in transducing as well as responding to stress signals in the hypertrophic mammalian heart.
IL-6 Cytokine Signaling: Pathway Diversity and Alternative Mechanisms
The IL-6 cytokine family mediates various aspects of cardiac hypertrophy including decreased diastolic function, increased cell size and protein content, and altered cellular metabolism.26-28 To mediate these diverse responses to hypertrophic stress, IL-6 cytokines signal through an equally diverse array of receptors and signaling pathways. For example, to induce homodimerization of the gp130 signal transducer and activation of JAK kinases, IL-6 binds to the IL-6R receptor while CT-1 and LIF bind to the LIFRβ receptor.29,30 Along with receptor diversification, the signaling pathway associated with these receptors can diversify by employing any one of five different STAT isoforms or even diverge completely from the canonical JAK-STAT pathway to include other pathways such as the ERK1/2 and PI3Kinase/Akt pathways.31 Diversification of receptor and signaling pathways has provided a way for JAK-STAT signaling to detect different stresses associated with a variety of cardiomyopathies such as hypertrophy, myocardial infarction, and ischemia/reperfusion injury, and respond in a variety of ways by fostering a state of cytoprotection, anti-apoptosis, cell survival or hypertrophic growth.32
Many of these diverse effects can be delineated by cytokine and the receptor-signaling pathways these cytokines activate. For example, in addition to mediating the hypertrophic as well as cytoprotective response in cardiomyocytes through activation of STATs 1 and 3,21,22,33-35 LIF and its receptor can also activate two non-STAT pathways, the MEK/ERK/p90 cascade leading to activation of ERK5 and the PI3Kinase/Akt pathway leading to activation of the S6 kinase.36,37 The same is true for CT-1 and its role in ventricular remodeling in the hypertrophic and failing heart.38-41 As with LIF, CT-1 uses different signaling pathways downstream of the LIFRβ/gp130 complex to stimulate different responses to different stimuli. For anti-apoptotic and/or survival effects, CT-1 activates the p42/p44 (MEK1/2-ERK1/2) and the PI3Kinase/Akt pathways.42,43 In cardiac hypertrophy, recent findings show that while the JAK-STAT and MEK1-ERK1/2 pathways are activated in response to CT-1, establishment of hypertrophy was dependent only upon activation of the MEK5-ERK5 pathway.44
In addition to LIF and CT-1, IL-6 signaling is activated in a number of cardiomyopathies in response to inducers such as inflammatory cytokines and neurohormones.45,46 As with LIF and CT-1, studies of IL-6 have shown activation of the ERK1/2 and Akt/S6 kinase signaling pathways.47 But unlike LIF and CT-1, various studies have shown that this is achieved by a unique kind of signaling mechanism. Rather than bind to IL-6R receptors in the plasma membrane of responding cells, IL-6 binds to a soluble, non-membranous form of IL-6R, called sIL-6R, that is extracellular in nature and not physically associated or tethered to any one cell. Upon binding the IL-6 cytokine, the IL-6/sIL-6R complex associates with gp130 transducers on the surface of cells, activating them to transduce the IL-6 signal to downstream signal transduction pathways.48-51 The use of soluble IL-6 receptors to transmit the IL-6 signal is intriguing for two reasons: it provides an alternative IL-6 signaling pathway that can act either independently or in conjunction with IL-6 signaling through the membrane-bound IL-6 receptor, and second, it provides a way for gp130-positive cells completely lacking a membrane IL-6 receptor a means of responding to IL-6.
The ability to enhance signaling in IL-6-responsive cells or confer this ability to cells lacking the IL-6 receptor appears to be a critical feature in both hypertension and cardiac hypertrophy.52 Three studies of sIL-6R signaling have demonstrated the importance of either enhancing IL-6 responsiveness or conferring it on a wider number of cells to evoke a greater physiological response, e.g., hypertension or hypertrophy. Hirota et al. showed that in mice doubly transgenic for DNA constructs constitutively expressing IL-6 and IL-6R, increased expression and distribution of this ligand-receptor pair initiated cardiac hypertrophy whereas single transgenics did not.53 Similar results were obtained with cultured cardiomyocytes stimulated with the hypertrophic agent phenylephrine (PE) but in these studies increased IL-6 responsiveness and establishment of hypertrophy were achieved by treating cells with increased levels of the soluble IL-6 receptor along with the IL-6 ligand.47 These results suggested that activation of both membrane-bound and soluble IL-6 receptors were needed to effectively express the hypertrophic phenotype. It remained to be determined if the soluble receptor signaling mechanism was essential for establishing hypertrophy. Coles et al. addressed this question by using an in vivo model in which cardiac hypertrophy is induced by chronic administration of Ang II.52 They first showed the critical need for IL-6 signaling in hypertrophy by showing that hearts of IL-6 knockout mice did not undergo hypertrophy with Ang II treatment. To assess the need for the soluble IL-6 receptor in establishing hypertrophy, these same researchers inhibited IL-6R trans-signaling in Ang II-treated mice using a soluble form of the gp130 receptor (sgp130FC). Surprisingly, hearts in these mice became hypertrophic suggesting that sIL-6R signaling was not required. Together, these experiments showed that while IL-6 signaling is critical for establishing hypertrophy, certain hypertrophic stimuli, e.g., PE, may be unable to activate the membrane-bound IL-6 signaling pathway to the extent needed for mounting a response. In this case, activation of the soluble receptor pathway may be needed to maximize IL-6 signaling, STAT3 activation and transcription of STAT3-dependent hypertrophic stress response genes. In the case of Ang II, the soluble receptor pathway is apparently not needed for mounting a hypertrophic response suggesting that this stimulus can maximally activate IL-6 signaling through membrane-bound receptors. An alternative possibility is that Ang II can directly induce expression of IL-6 receptors in IL-6 receptor-negative cells and in this way maximize IL-6 signaling independently of the soluble IL-6 receptor mechanism. Perhaps comparative analysis of Ang II-IL-6 signaling in hypertensive vascular smooth muscle cells, which requires soluble IL-6 receptor signaling, vs. that in hypertrophic cardiomyocytes may provide some insights into the molecular rationale for using the soluble IL-6 receptor mechanism.
Signaling through Non-gp130 Receptors: Variations in JAK-Receptor Coupling
For the IL-6 family of cytokines, mounting the appropriate response to a wide array of physiological conditions often relies on using different receptors to respond to different cytokines. By varying the type of cytokine (IL-6, CT-1 and LIF), cytokine receptor (IL-6R, sIL-6R and LIFRβ), and receptor signaling pathway (MEK, PI3K, Akt and JAK-STAT), cells can react to a wider variety of stress or other stimuli by activating the appropriate sets of genes and mounting the appropriate physiological response. Another way in which the JAK-STAT pathway can respond to a wider array of physiological conditions is by transducing signals received by receptors other than the IL6-R/gp130 receptor complex. In this signaling paradigm, it is the JAK kinase that determines variation in signaling, gene expression, and physiological effects through its association with other non-gp130 receptors and activation when these receptors bind non-IL-6 cytokines. One example of this type of signaling can be found in the hypertensive heart.
While cardiac hypertrophy can occur in response to a number of pathological conditions, the most widespread is elevated arterial blood pressure or hypertension. Hypertension results from over-activation of the renin-angiotensin system or RAS in the kidneys leading to elevated levels of the circulating hormonal peptide Angiotensin II or Ang II.54,55 This RAS is called systemic RAS and the Ang II it produces is responsible for controlling blood pressure by regulating vasoconstriction. In hypertension, increased Ang II and vasoconstriction raises peripheral arterial resistance to a point where the heart must work harder to pump blood. In response, cardiomyocytes increase their contractility by increasing the extent to which they mechanically stretch. Under these conditions, the Ang II AT1 receptor can act as a stretch-sensing receptor.56 One of the genes induced by stretch-activated AT1 receptors is angiotensinogen whose active product, Ang II, can auto-activate Ang II receptors on cardiomyocytes. This activation of RAS in cardiomyocytes is called local RAS and its continued activation by stretch or Ang II signaling can maintain the hypertrophic state.57,58
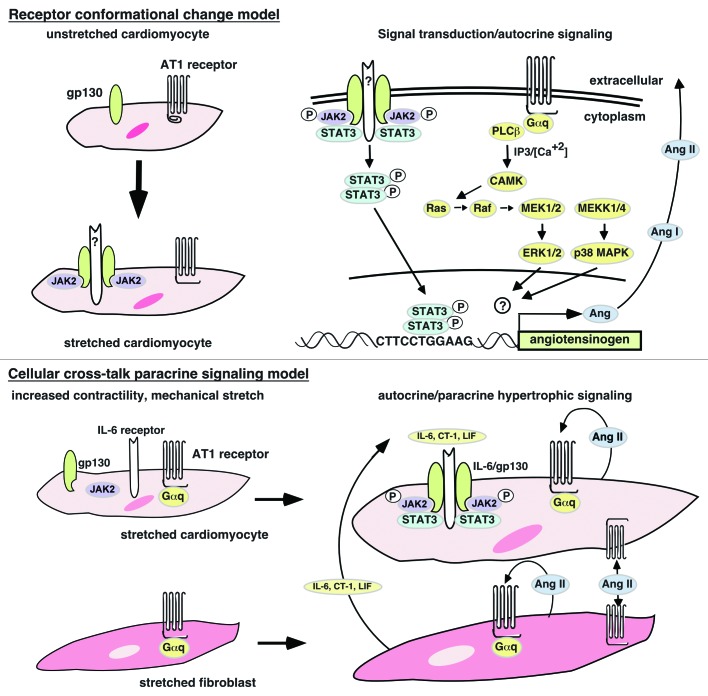
As with IL-6, deciphering how Ang II or mechanical stretch signals via the AT1 receptor to evoke the hypertrophic response is best done in cultured cardiomyocytes under controlled conditions. When cardiomyocytes are treated with Ang II, the AT-1 receptor is activated leading to phosphorylation/activation of JAK2 and STATs 1, 3, 5A and 5B.59,60 Similarly, when cardiomyocytes are mechanically stretched in the absence of Ang II, the AT-1 receptor is activated and JAKs 1 and 2, STATs 1 and 3 and gp130 are phosphorylated and activated as is the G-protein linked ERK signaling pathway.56,61 The phosphorylation of gp130 by JAK kinases activated by the canonical IL-6R/gp130-JAK-STAT pathway is unlikely since the time course of IL-6 cytokine production (60–120 min) is incompatible with JAK-STAT activation (2–5 min).61 This suggests that the gp130 complex and the AT1 receptor can be activated directly by stretch, perhaps via stretch-induced conformational changes that expose binding sites for JAK kinases or G proteins.56 An alternative explanation more in keeping with the heart’s cellular composition is to have Ang II or IL-6 cytokines produced in other cells signal to cardiomyocytes in paracrine fashion. Sano et al. have shown that Ang II can induce IL-6, LIF and CT-1 in cardiac fibroblasts and that these cytokines can activate gp130-linked receptors to induce cardiomyocyte hypertrophy.62 Stretch experiments have shown that both cardiomyocytes as well as cardiac fibroblasts can produce Ang II suggesting that autocrine as well as paracrine signaling can be a motive force for sustained hypertrophy;63-66 however, see reference 67. These possibilities are illustrated in Figure 1.
Figure 1. Models of mechanical stretch activation of JAK kinases and STAT proteins. Conformational model. Mechanical stretch is postulated to induce conformational changes in the gp130 and AT1 receptors that expose binding sites for JAK2 kinases and Gαq proteins, respectively. It is unclear how or if gp130 dimerizes in order to allow JAK2 kinase cross-phosphorylation. The AT1 receptor acting as a stretch receptor will activate the RAS/MEK/ERK/p38 pathway leading to upregulation of the angiotensinogen gene. The transcription factor activated by p38 or ERK1/2 has not been identified. Paracrine model. Mechanical stretch initiates signal transducer association with receptors as in conformational model. Continued signaling after mechanical stretch exhaustion is performed by Ang II produced in response to stretch-activated AT1 receptors to give autocrine signaling as well as paracrine signaling. Ang II-activated cardiac fibroblasts produce IL-6 family cytokines that activate the canonical JAK-STAT pathway in cardiomyocytes via paracrine signaling thereby maintaining the post-mechanical stretch hypertrophic state.
In hypertrophy, autostimulatory production of Ang II following exhaustion of the mechanical stretch signal may be one way the hypertrophic state is sustained. Since auto-stimulation of the AT-1 receptor by Ang II activates STATs to enter the nucleus,68,69 this loop likely involves STAT-dependent activation of RAS-related genes, the most likely candidate being the angiotensinogen gene whose gene product is proteolytically processed to give Ang II. To examine this possibility, Mascareno and colleagues treated cardiomyocytes with Ang II and found that this led to upregulation of the angiotensinogen gene.70 To demonstrate a direct linkage with an activated AT-1 receptor-JAK-STAT pathway, they showed that Ang II could stimulate STATs 3 and 6 to bind as a heterodimer to a STAT-binding element within the promoter of the angiotensinogen gene to activate its transcription. To determine if these in vitro results held in vivo, Mascareno and colleagues examined the genetically hypertensive SHR rat strain and showed that hypertensive but not normal hearts expressed nuclear STATs that were bound to the STAT-binding site within the angiotensinogen gene promoter.70 These findings, together with those of Sano et al.,62 suggest that both Ang II autocrine and paracrine signaling can act to maintain hypertension leading to hypertrophy: autocrine stimulation of cardiomyocyte AT-1 receptors to produce more angiotensinogen and Ang II, and paracrine stimulation of cardiac fibroblast AT-1 receptors to produce IL-6 cytokines that feedback onto IL-6 receptors on cardiomyocytes to increase angiotensinogen gene expression (Fig. 1). Nyui et al.71 have shown that in the absence or presence of Ang II, MAPKinase is activated in stretched cardiomyocytes by LIF acting through the LIFRβ/gp130 receptor. More recently, Lal et al. have extended these observations to show that prolonged stretch of cardiac fibroblasts and cardiomyocytes activates the p38 kinase to increase transcription of the angiotensinogen gene.66 Together, these observations show how JAK-STAT signaling contributes to the interactions between cardiac fibroblasts and cardiomyocytes so critical to the development, function and response of the heart to stress stimuli.72 These studies suggest that cellular interactions of this type may rely, in part, on the cross-talk between JAK-STAT signaling pathways in each cell type. In the following section, we discuss how JAK-STAT pathways can cross-talk with non-STAT signaling pathways within cells to mount a genomic response to a potentially broader array of extracellular stimuli.
Signaling Pathway Crosstalk within Cells
The heart responds to the stress of increased workload by increasing cardiac output through increased synthesis and assembly of functional sarcomeres. Flawed synthesis and assembly could lead to functionally compromised sarcomeres, reduced contractility and cardiomyocyte elimination by apoptosis. To prevent this, stressed cardiomyocytes express αB-crystallin (CryAB), a heat shock protein, that binds to sarcomeric proteins such as titin to ensure the correct assembly of sarcomeres.73 Because of its protective functions, CryAB and other stress-induced heat shock proteins are often upregulated in response to hypertrophic stimuli.74,75 Within the CryAB gene is an intronic regulatory element (IRE) similar to one in the ventricular myosin light chain-2 gene that mediates myosin upregulation during hypertrophic stress.76 This sequence can bind the NFAT transcription factor indicating that CryAB is responsive to calcineurin-NFAT signaling activated by increases in intracellular Ca2+.77-80
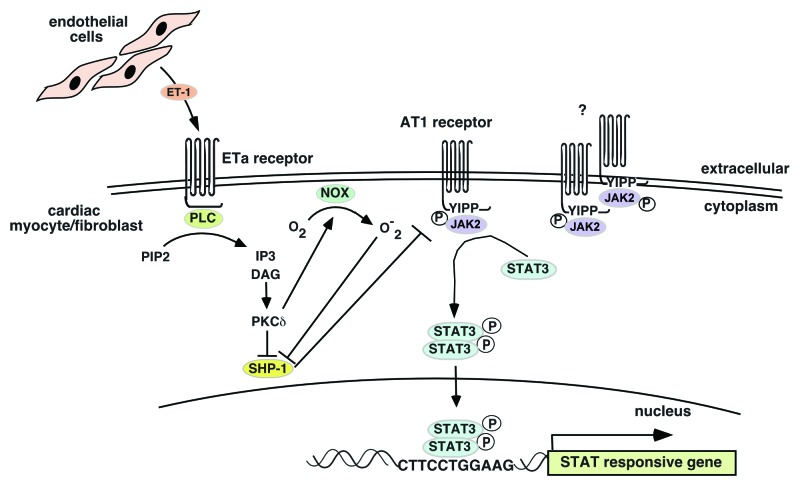
One signaling system known to activate Ca2+ channels under conditions of cardiac stress is the endothelin-1 (ET-1) signal transduction pathway.81,82 Our laboratory has investigated the response of cardiomyocytes to ET-1 and has shown that in addition to activation of the calcineurin-NFAT pathway, there is a distinct and essential involvement of STAT3 dimers for activating the CryAB gene.83 This suggests that ET-1 (ETa) receptors can signal via the JAK2 kinase to phosphorylate and activate STAT3 dimers. As a GPCR, ETa predominantly signals via IP3 kinase to activate the PKC/Raf/MEF/ERK signal transduction pathway. But also as a GPCR, ETa apparently shares with receptors as diverse as those for Ang II, stromal cell-derived factor-1α, cholecystokinin, monocyte chemotactic protein 1, angiotensin-(1–7), bradykinin B2 and opioid receptors, the ability to signal via JAK2 kinase.84 Unlike the AT1 receptor, ETa has no apparent sites for binding JAKs suggesting that JAK2 kinases must be activated “off-receptor” and then recruited to the receptor to phosphorylate bound STAT proteins (for which a binding site motif, YXXQ, exists85).86 While it is unclear as to how the ETa receptor could achieve this, Kurdi and Booz have put forth a model for non-canonical JAK2 activation by GPCRs that could provide some insight into how GPCRs can signal via JAK kinases under conditions of oxidative stress that are often seen in cardiomyopathies.84
According to the Kurdi and Booz model, GPCRs act to maintain JAK2 kinase phosphorylation and activity through inhibition of its phosphatase, SHP-1. GPCRs achieve this by activating PKCδ which either directly or indirectly inhibits SHP-1. One interpretation of this model is that GPCRs may be acting to keep levels of phospho-JAK2 kinase high enough to allow potential recruitment for phosphorylating either themselves or more likely, bound STATs. How JAK2 kinases are phosphorylated to begin with in the absence of a dimerizing “platform” (as in the canonical gp130 model) is unclear. One possibility is that JAK2 is activated by other receptors such as the AT1 or IL-6/gp130 family of receptors. In fact, there is evidence to suggest that ET-1 can potentiate Ang II signaling in establishing hypertrophy and activating the fibrotic program that causes fibrosis in hypertrophic hearts. Adiarto et al. have shown that endothelial cells in the heart can produce ET-1 which can bind to ETa receptors on cardiomyocytes and cardiac fibroblasts in AngII-infused hearts to induce cardiac hypertrophy and fibrosis.87 In support of the Kurdi and Booz model, these researchers also showed an increase in PKCδ indicating activation of the DAG/PKC pathway that inhibits SHP-1. These possibilities are illustrated in Figure 2.
Figure 2. Endothelin-1 receptor crosstalk with Ang II receptor to potentiate JAK2 kinase activity. Endothelial cell-derived ET-1 activates the ETa receptor on cardiomyocytes or cardiac fibroblasts. Activated ETa signals to PKCδ, which mediates inhibition of the SHP-1 JAK2 kinase phosphatase to allow phosphorylated JAK2 kinases to remain phosphorylated and active. These active JAK2 kinases can phosphorylate STAT3 associated with either the AT1 or ETa receptor (which lacks a JAK2 binding site but has one for STAT3). How JAK2 kinases become phosphorylated to begin with is not clear. There is some evidence that the AT1 receptor can dimerize in which case the associated JAK2 kinases can cross-phosphorylate themselves.
JAK-STAT Interaction with the Basal Transcriptional Apparatus
Unlike most other signal transduction pathways that terminate in the activation of nuclear transcription factors, the STAT proteins are themselves transcription factors not only capable of transducing the hypertrophic signal from the cytoplasm but also acting to transcribe stress response genes in the nucleus. Our laboratory as well as others have shown that under hypertrophic conditions, the JAK2 kinase phosphorylates STATs, activating them to dimerize and translocate to the nucleus where they upregulate target genes.56,70,88,89 Exactly how the STAT proteins engage the basal transcriptional machinery to initiate transcription is not fully known and with the increasing complexity of the transcriptome, becoming much harder to fathom. Yet despite this, some progress is being made, particularly with respect to the interaction of the JAK-STAT pathway with the nuclear transcription factors and regulatory molecules that control RNA polymerase (pol) II activity and its accessibility to RNA pol II-dependent genes.
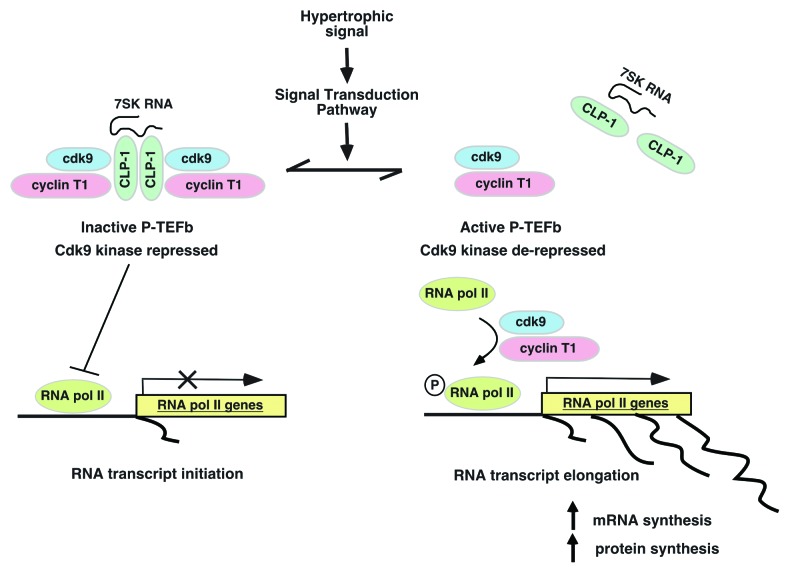
Our laboratory is studying one such nuclear regulatory molecule, CLP-1, and its involvement in the cellular response to hypertrophic stimuli.90-93 CLP-1, the mouse homolog of the human HEXIM1 gene,94-96 is a nuclear protein that regulates P-TEFb (transcription elongation factor b), a complex formed by cyclin dependent kinase (cdk) 9 with cyclin T1. CLP-1 reversibly inhibits P-TEFb kinase activity, repressing cdk9 when associated with P-TEFb and de-repressing cdk9 when dissociated. When de-repressed, cdk9 phosphorylates RNA pol II, switching it from an initiation state at the transcriptional start site to an elongation state that allows completion of nascent RNA chains (Fig. 3). This process, termed “promoter proximal pausing,” appears to regulate expression of genes such as developmental control and stress response genes that require rapid activation in response to changing cellular conditions.97,98 Emerging evidence suggests that the CLP-1-P-TEFb transcriptional regulatory machinery can act as the molecular “go-between” linking extracellular signals to genomic output. As a prominent transducer of hypertrophic signals in cardiomyocytes, the JAK-STAT pathway is a good candidate for interaction with the CLP-1-P-TEFb regulatory complex for controlling transcription of stress and STAT-dependent genes. Based on the CLP-1-P-TEFb model, for STATs to activate genes, they must in some way activate or de-repress P-TEFb. To examine this, we blocked the JAK-STAT pathway in hypertrophic cardiomyocytes using the JAK2 kinase inhibitor AG490 and found that more P-TEFb complexes retained CLP-1 keeping cdk9 activity repressed.99 Since inhibition of JAK2 kinase prevents STAT dimerization and mobilization to the nucleus, these results suggested that under normal conditions, STAT dimers might promote transcription by preventing binding of CLP-1 to P-TEFb complexes. Some evidence for direct STAT3 interaction with cdk9 in regulating gene transcription in this way has come from studies of two STAT3-inducible genes, the p21waf1 gene and the γ-fibrinogen gene.100,101
Figure 3. Schematic of CLP-1 control of P-TEFb and gene transcription. When CLP-1 is bound to P-TEFb, the cdk9 kinase is inhibited and RNA pol II remains in an initiation state in which “paused” RNA synthesis produces only nascent RNAs. Dissociating or preventing CLP-1 from binding to P-TEFb de-represses cdk9 to phosphorylate RNA pol II and activate it to an elongation state in which it completes nascent RNA transcripts. The association of CLP-1 with P-TEFb is reversible and potentially subject to control by other factors such as signal transducers or transcription factors in their effort to promote transcription of their target genes.
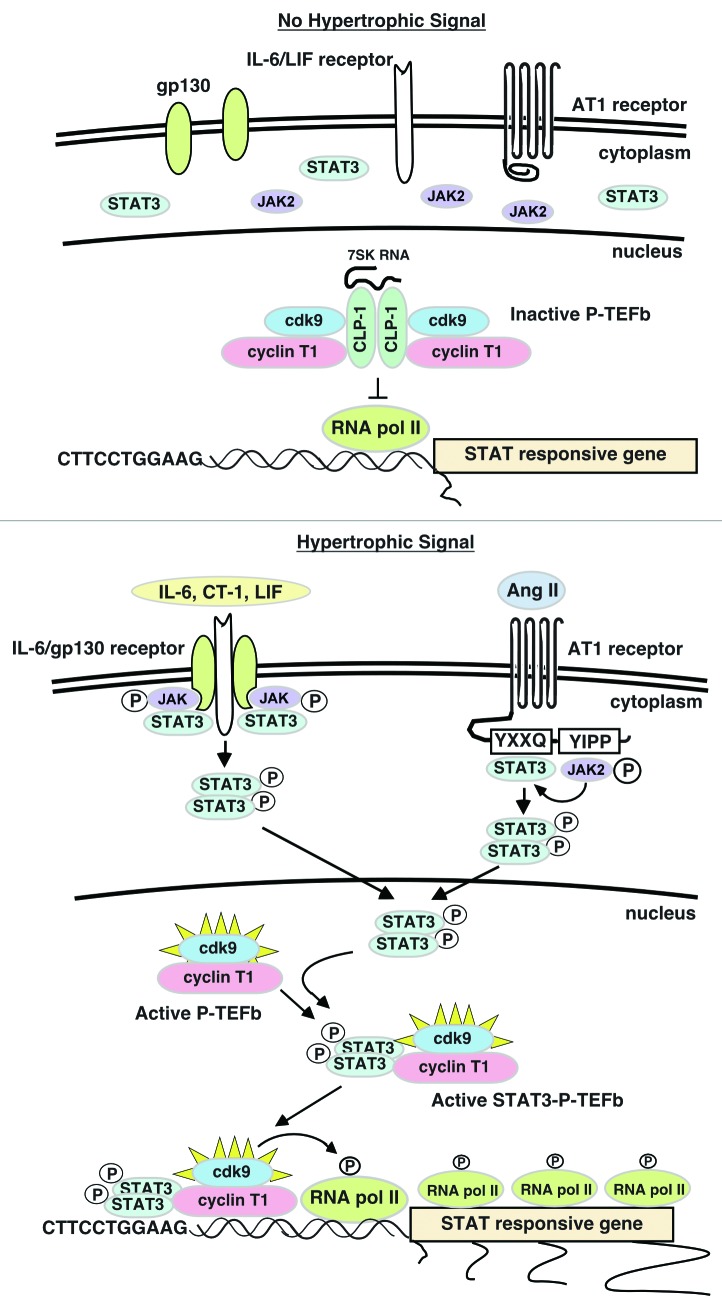
IL-6 treatment of HepG2 cells activates the IL-6Rα/gp130 receptor resulting in the phosphorylation and activation of STAT3 and transcription of STAT3-dependent genes, two of which, p21waf1 and γ-fibrinogen (FBG), were the subject of independent studies on how STAT3 interacts with transcriptional regulators to initiate gene transcription.100-103 In both cases, activated nuclear STAT3 dimers were shown to bind to cdk9 to form STAT3-cdk9 complexes that were then recruited to the STAT3-binding site within the promoter of the p21waf1 and γ-fibrinogen genes. With the STAT3-cdk9 complex localized to the proximal promoter, cdk9 can readily phosphorylate RNA pol II at the transcriptional start site, switching it from its initiation state to its elongation state and productive synthesis of full-length RNA transcripts. Giraud et al.100 went on to show that STAT3 can also recruit the chromatin-modifying proteins p300/CBP, a transcriptional co-activator and histone acetyltransferase, and BRG1, a chromatin remodeler, that act to make the proximal promoter region more accessible to RNA pol II.100,104-106 It appears from these studies that cdk9 and STAT3 are mutually dependent on each other for conferring full transcriptional competency to STAT-dependent genes: STAT3 brings cdk9 to the promoter region made accessible to RNA pol II through STAT3 recruitment of chromatin modifiers and remodelers while cdk9 phosphorylates the recruited RNA pol II to complete gene transcription. Conceivably, STAT3 dimers could be acting in the same way in hypertrophic cardiomyocytes to facilitate STAT-dependent gene transcription. STAT3 is of particular interest since it is known to upregulate predominantly cardioprotective genes in various hypertrophic models suggesting a role in compensatory hypertrophy.107,108 This potential regulatory mechanism is illustrated in Figure 4.
Figure 4. Interaction between the JAK-STAT pathway and the CLP-1-P-TEFb transcriptional regulatory mechanism. In the absence of hypertrophic signals, STAT3 remains cytoplasmic and CLP-1 inhibits P-TEFb. This keeps RNA pol II in the initiation state and STAT-responsive genes untranscribed. In the presence of hypertrophic signals, STAT3 is activated and STAT3 dimers are mobilized to the nucleus where they bind to cdk9-cyclin T1 complexes (active P-TEFb) and prevent binding by the CLP-1 repressor. The STAT3-P-TEFb complex is then recruited to STAT3 target genes via binding to specific STAT3 binding sites within the proximal promoter of these genes. Recruitment of chromatin modifiers by STAT3 (not shown) opens chromatin to access by RNA pol II, which upon phosphorylation by active cdk9 kinase resumes synthesis of paused, nascent RNA transcripts.
Together, these experiments show how two well-studied transcriptional processes can interact to form a novel mechanism for increasing the transcriptional readiness and output of target genes. It also suggests how potent gene activators such as the cdk9-cyclin T1 complex might “acquire” specificity for a given gene by in effect using the sequence recognition properties of binding partners such as STAT3 to be “guided” to that gene where it can activate RNA pol II and gene transcription. If true, such a model could explain how other non-sequence recognizing activators can be brought to specific genes to upregulate their transcription. These experiments also suggest that STAT-dependent genes that are stress response genes may be in a state of “paused” RNA synthesis, poised to respond to stress signals by rapidly completing transcript formation for translation into protein product. How this gene activation model might relate to STAT-dependent stress response genes in hypertrophic cardiomyocytes is presently under study in our laboratory.
Acknowledgments
Work from our laboratory cited in this review was supported by NIH grant 1RO1 HL 73399-01 to M.A.Q.S.
Glossary
Abbreviations:
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- MAPKinase
mitogen-activated protein kinase
- IL-6
interleukin-6
- CT-1
cardiotrophin-1
- LIF
leukemia inhibitory factor
- ERK
extracellular signal-regulated kinase
- IP3 kinase
inositol 1,4,5-trisphosphate
- MEK
MAPKinase kinase
- Ang II
angiotensin II
- RAS
renin-angiotensin system
- CryAB
αB-crystallin
- NFAT
nuclear factor of activated T cell
- ET-1
endothelin-1
- PKC
protein kinase C
- GPCR
G-protein coupled receptor
- CLP-1
cardiac lineage protein-1
- TEFb
transcription elongation factor b
- Cdk 9
cyclin-dependent kinase 9
- CBP
CREB-binding protein
- BRG1
Brahma-related gene-1
- HEXIM1
hexamethylene bis-acetamide inducible protein 1
- PI3K
phosphoinositide-3-kinase
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/20702
References
- 1.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–43. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265–72. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21(Suppl 5):121–31. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 5.Graham HK, Horn M, Trafford AW. Extracellular matrix profiles in the progression to heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiol (Oxf) 2008;194:3–21. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 6.Parker TG, Packer SE, Schneider MD. Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990;85:507–14. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–31. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 8.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–67. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 9.Sack MN, Kelly DP. The energy substrate switch during development of heart failure: gene regulatory mechanisms (Review) Int J Mol Med. 1998;1:17–24. doi: 10.3892/ijmm.1.1.17. [Review] [DOI] [PubMed] [Google Scholar]
- 10.Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–45. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 11.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Sugden PH. Signalling pathways in cardiac myocyte hypertrophy. Ann Med. 2001;33:611–22. [PubMed] [Google Scholar]
- 13.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 14.Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, et al. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol. 2007;212:311–22. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- 15.Mascareno E, Siddiqui MA. The role of Jak/STAT signaling in heart tissue renin-angiotensin system. Mol Cell Biochem. 2000;212:171–5. doi: 10.1023/A:1007157126806. [DOI] [PubMed] [Google Scholar]
- 16.Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–13. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 17.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuzawa J, Booz GW, Hunt RA, Shimizu N, Karoor V, Baker KM, et al. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3 : an autocrine loop for hypertrophy. Hypertension. 2000;35:1191–6. doi: 10.1161/01.hyp.35.6.1191. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara K, Saito Y, Harada M, Ishikawa M, Ogawa E, Miyamoto Y, et al. Involvement of cardiotrophin-1 in cardiac myocyte-nonmyocyte interactions during hypertrophy of rat cardiac myocytes in vitro. Circulation. 1999;100:1116–24. doi: 10.1161/01.cir.100.10.1116. [DOI] [PubMed] [Google Scholar]
- 20.Pan J, Fukuda K, Kodama H, Sano M, Takahashi T, Makino S, et al. Involvement of gp130-mediated signaling in pressure overload-induced activation of the JAK/STAT pathway in rodent heart. Heart Vessels. 1998;13:199–208. doi: 10.1007/BF01745045. [DOI] [PubMed] [Google Scholar]
- 21.Kodama H, Fukuda K, Pan J, Makino S, Baba A, Hori S, et al. Leukemia inhibitory factor, a potent cardiac hypertrophic cytokine, activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1997;81:656–63. doi: 10.1161/01.res.81.5.656. [DOI] [PubMed] [Google Scholar]
- 22.Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation. 1998;98:346–52. doi: 10.1161/01.cir.98.4.346. [DOI] [PubMed] [Google Scholar]
- 23.Sandberg EM, Wallace TA, Godeny MD, VonDerLinden D, Sayeski PP. Jak2 tyrosine kinase: a true jak of all trades? Cell Biochem Biophys. 2004;41:207–32. doi: 10.1385/CBB:41:2:207. [DOI] [PubMed] [Google Scholar]
- 24.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 25.Carter-Su C, Smit LS. Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Recent Prog Horm Res. 1998;53:61–82, discussion 82-3. [discussion -3] [PubMed] [Google Scholar]
- 26.Florholmen G, Aas V, Rustan AC, Lunde PK, Straumann N, Eid H, et al. Leukemia inhibitory factor reduces contractile function and induces alterations in energy metabolism in isolated cardiomyocytes. J Mol Cell Cardiol. 2004;37:1183–93. doi: 10.1016/j.yjmcc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A. 1995;92:1142–6. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villegas S, Villarreal FJ, Dillmann WH. Leukemia Inhibitory Factor and Interleukin-6 downregulate sarcoplasmic reticulum Ca2+ ATPase (SERCA2) in cardiac myocytes. Basic Res Cardiol. 2000;95:47–54. doi: 10.1007/s003950050007. [DOI] [PubMed] [Google Scholar]
- 29.Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, et al. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270:10915–22. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–10. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 31.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–97. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 32.Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med. 2007;13:82–9. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Soond SM, Latchman DS, Stephanou A. STAT signalling in the heart and cardioprotection. Expert Rev Mol Med. 2006;8:1–16. doi: 10.1017/S1462399406000032. [DOI] [PubMed] [Google Scholar]
- 34.Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K, Kishimoto T. Signals through gp130 upregulate bcl-x gene expression via STAT1-binding cis-element in cardiac myocytes. J Clin Invest. 1997;99:2898–905. doi: 10.1172/JCI119484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23:177–82. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 36.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–9. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 37.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, et al. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–10. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 38.Pennica D, Swanson TA, Shaw KJ, Kuang WJ, Gray CL, Beatty BG, et al. Human cardiotrophin-1: protein and gene structure, biological and binding activities, and chromosomal localization. Cytokine. 1996;8:183–9. doi: 10.1006/cyto.1996.0026. [DOI] [PubMed] [Google Scholar]
- 39.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–45. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 40.Takimoto Y, Aoyama T, Iwanaga Y, Izumi T, Kihara Y, Pennica D, et al. Increased expression of cardiotrophin-1 during ventricular remodeling in hypertensive rats. Am J Physiol Heart Circ Physiol. 2002;282:H896–901. doi: 10.1152/ajpheart.00591.2001. [DOI] [PubMed] [Google Scholar]
- 41.Talwar S, Squire IB, Downie PF, O’Brien RJ, Davies JE, Ng LL. Elevated circulating cardiotrophin-1 in heart failure: relationship with parameters of left ventricular systolic dysfunction. Clin Sci (Lond) 2000;99:83–8. doi: 10.1042/CS20000002. [DOI] [PubMed] [Google Scholar]
- 42.López N, Díez J, Fortuño MA. Characterization of the protective effects of cardiotrophin-1 against non-ischemic death stimuli in adult cardiomyocytes. Cytokine. 2005;30:282–92. doi: 10.1016/j.cyto.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Brar BK, Stephanou A, Liao Z, O’Leary RM, Pennica D, Yellon DM, et al. Cardiotrophin-1 can protect cardiac myocytes from injury when added both prior to simulated ischaemia and at reoxygenation. Cardiovasc Res. 2001;51:265–74. doi: 10.1016/S0008-6363(01)00294-2. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, et al. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005;38:185–92. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–41. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- 46.Fredj S, Bescond J, Louault C, Delwail A, Lecron JC, Potreau D. Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation. J Cell Physiol. 2005;204:428–36. doi: 10.1002/jcp.20307. [DOI] [PubMed] [Google Scholar]
- 47.Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R, Pomérance M. A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal. 2010;22:1143–52. doi: 10.1016/j.cellsig.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993;90:11924–8. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihara M, Moriya Y, Ohsugi Y. IL-6-soluble IL-6 receptor complex inhibits the proliferation of dermal fibroblasts. Int J Immunopharmacol. 1996;18:89–94. doi: 10.1016/0192-0561(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 50.Gaillard JP, Liautard J, Klein B, Brochier J. Major role of the soluble interleukin-6/interleukin-6 receptor complex for the proliferation of interleukin-6-dependent human myeloma cell lines. Eur J Immunol. 1997;27:3332–40. doi: 10.1002/eji.1830271232. [DOI] [PubMed] [Google Scholar]
- 51.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–43. doi: 10.1016/S0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 52.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O’Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–25. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A. 1995;92:4862–6. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cacciapuoti F. Molecular mechanisms of left ventricular hypertrophy (LVH) in systemic hypertension (SH)-possible therapeutic perspectives. J Am Soc Hypertens. 2011;5:449–55. doi: 10.1016/j.jash.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Crowley SD, Coffman TM. In hypertension, the kidney breaks your heart. Curr Cardiol Rep. 2008;10:470–6. doi: 10.1007/s11886-008-0074-5. [DOI] [PubMed] [Google Scholar]
- 56.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 57.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17:168–73. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 58.Varagic J, Frohlich ED. Local cardiac renin-angiotensin system: hypertension and cardiac failure. J Mol Cell Cardiol. 2002;34:1435–42. doi: 10.1006/jmcc.2002.2075. [DOI] [PubMed] [Google Scholar]
- 59.McWhinney CD, Dostal D, Baker K. Angiotensin II activates Stat5 through Jak2 kinase in cardiac myocytes. J Mol Cell Cardiol. 1998;30:751–61. doi: 10.1006/jmcc.1998.0639. [DOI] [PubMed] [Google Scholar]
- 60.McWhinney CD, Hunt RA, Conrad KM, Dostal DE, Baker KM. The type I angiotensin II receptor couples to Stat1 and Stat3 activation through Jak2 kinase in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1997;29:2513–24. doi: 10.1006/jmcc.1997.0489. [DOI] [PubMed] [Google Scholar]
- 61.Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, et al. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–36. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 62.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–23. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 63.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–84. doi: 10.1016/0092-8674(93)90541-W. [DOI] [PubMed] [Google Scholar]
- 64.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, et al. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ Res. 1995;77:258–65. doi: 10.1161/01.res.77.2.258. [DOI] [PubMed] [Google Scholar]
- 65.Tamura K, Umemura S, Nyui N, Hibi K, Ishigami T, Kihara M, et al. Activation of angiotensinogen gene in cardiac myocytes by angiotensin II and mechanical stretch. Am J Physiol. 1998;275:R1–9. doi: 10.1152/ajpregu.1998.275.1.R1. [DOI] [PubMed] [Google Scholar]
- 66.Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: opposing roles of JNK1/2 and p38alpha MAP kinases. J Mol Cell Cardiol. 2008;45:770–8. doi: 10.1016/j.yjmcc.2008.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Kesteren CA, Saris JJ, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA, et al. Cultured neonatal rat cardiac myocytes and fibroblasts do not synthesize renin or angiotensinogen: evidence for stretch-induced cardiomyocyte hypertrophy independent of angiotensin II. Cardiovasc Res. 1999;43:148–56. doi: 10.1016/S0008-6363(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 68.Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–50. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 69.Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM. Angiotensin II stimulates sis-inducing factor-like DNA binding activity. Evidence that the AT1A receptor activates transcription factor-Stat91 and/or a related protein. J Biol Chem. 1994;269:31443–9. [PubMed] [Google Scholar]
- 70.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci U S A. 1998;95:5590–4. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nyui N, Tamura K, Mizuno K, Ishigami T, Kihara M, Ochiai H, et al. gp130 is involved in stretch-induced MAP kinase activation in cardiac myocytes. Biochem Biophys Res Commun. 1998;245:928–32. doi: 10.1006/bbrc.1998.8548. [DOI] [PubMed] [Google Scholar]
- 72.Ausoni S, Sartore S. The cardiovascular unit as a dynamic player in disease and regeneration. Trends Mol Med. 2009;15:543–52. doi: 10.1016/j.molmed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, et al. Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem. 2004;279:7917–24. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 74.Willis MS, Patterson C. Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease. Circulation. 2010;122:1740–51. doi: 10.1161/CIRCULATIONAHA.110.942250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edwards HV, Cameron RT, Baillie GS. The emerging role of HSP20 as a multifunctional protective agent. Cell Signal. 2011;23:1447–54. doi: 10.1016/j.cellsig.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Mathew S, Mascareno E, Siddiqui MA. A ternary complex of transcription factors, Nishéd and NFATc4, and co-activator p300 bound to an intronic sequence, intronic regulatory element, is pivotal for the up-regulation of myosin light chain-2v gene in cardiac hypertrophy. J Biol Chem. 2004;279:41018–27. doi: 10.1074/jbc.M403578200. [DOI] [PubMed] [Google Scholar]
- 77.Karmazyn M, Kilicá A, Javadov S. The role of NHE-1 in myocardial hypertrophy and remodelling. J Mol Cell Cardiol. 2008;44:647–53. doi: 10.1016/j.yjmcc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 78.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003;35:871–86. doi: 10.1016/S0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 79.Avkiran M, Haworth RS. Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance. Cardiovasc Res. 2003;57:942–52. doi: 10.1016/S0008-6363(02)00782-4. [DOI] [PubMed] [Google Scholar]
- 80.Penna C, Rastaldo R, Mancardi D, Cappello S, Pagliaro P, Westerhof N, et al. Effect of endothelins on the cardiovascular system. J Cardiovasc Med (Hagerstown) 2006;7:645–52. doi: 10.2459/01.JCM.0000242996.19077.ba. [DOI] [PubMed] [Google Scholar]
- 81.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, et al. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Izumi T, Kihara Y, Sarai N, Yoneda T, Iwanaga Y, Inagaki K, et al. Reinduction of T-type calcium channels by endothelin-1 in failing hearts in vivo and in adult rat ventricular myocytes in vitro. Circulation. 2003;108:2530–5. doi: 10.1161/01.CIR.0000096484.03318.AB. [DOI] [PubMed] [Google Scholar]
- 83.Manukyan I, Galatioto J, Mascareno E, Bhaduri S, Siddiqui MA. Cross-talk between calcineurin/NFAT and Jak/STAT signalling induces cardioprotective alphaB-crystallin gene expression in response to hypertrophic stimuli. J Cell Mol Med. 2010;14(6B):1707–16. doi: 10.1111/j.1582-4934.2009.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurdi M, Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–56. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dostal DE, Hunt RA, Kule CE, Bhat GJ, Karoor V, McWhinney CD, et al. Molecular mechanisms of angiotensin II in modulating cardiac function: intracardiac effects and signal transduction pathways. J Mol Cell Cardiol. 1997;29:2893–902. doi: 10.1006/jmcc.1997.0524. [DOI] [PubMed] [Google Scholar]
- 86.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272:23382–8. doi: 10.1074/jbc.272.37.23382. [DOI] [PubMed] [Google Scholar]
- 87.Adiarto S, Heiden S, Vignon-Zellweger N, Nakayama K, Yagi K, Yanagisawa M, et al. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci. 2012 doi: 10.1016/j.lfs.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Beckles DL, Mascareno E, Siddiqui MA. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascul Pharmacol. 2006;45:350–7. doi: 10.1016/j.vph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 89.Akazawa H, Zou Y, Komuro I. A novel mechanism of mechanical stress-induced hypertrophy. Novartis Found Symp. 2006;274:20–31, discussion 31-40, 152-5, 272-6. doi: 10.1002/0470029331.ch3. [DOI] [PubMed] [Google Scholar]
- 90.Ghatpande S, Goswami S, Mathew S, Rong G, Cai L, Shafiq S, et al. Identification of a novel cardiac lineage-associated protein(cCLP-1): A candidate regulator of cardiogenesis. Dev Biol. 1999;208:210–21. doi: 10.1006/dbio.1998.9180. [DOI] [PubMed] [Google Scholar]
- 91.Huang F, Wagner M, Siddiqui MA. Structure, expression, and functional characterization of the mouse CLP-1 gene. Gene. 2002;292:245–59. doi: 10.1016/S0378-1119(02)00596-6. [DOI] [PubMed] [Google Scholar]
- 92.Rice AP. Dysregulation of positive transcription elongation factor B and myocardial hypertrophy. Circ Res. 2009;104:1327–9. doi: 10.1161/CIRCRESAHA.109.200485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Espinoza-Derout J, Wagner M, Salciccioli L, Lazar JM, Bhaduri S, Mascareno E, et al. Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1. Circ Res. 2009;104:1347–54. doi: 10.1161/CIRCRESAHA.108.191726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell. 2003;12:971–82. doi: 10.1016/S1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]
- 95.Dey A, Chao SH, Lane DP. HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy. Cell Cycle. 2007;6:1856–63. doi: 10.4161/cc.6.15.4556. [DOI] [PubMed] [Google Scholar]
- 96.Michels AA, Nguyen VT, Fraldi A, Labas V, Edwards M, Bonnet F, et al. MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol Cell Biol. 2003;23:4859–69. doi: 10.1128/MCB.23.14.4859-4869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, et al. RNA polymerase is poised for activation across the genome. Nat Genet. 2007;39:1507–11. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet. 2007;39:1512–6. doi: 10.1038/ng.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Espinoza-Derout J, Wagner M, Shahmiri K, Mascareno E, Chaqour B, Siddiqui MA. Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy. Cardiovasc Res. 2007;75:129–38. doi: 10.1016/j.cardiores.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giraud S, Hurlstone A, Avril S, Coqueret O. Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene. 2004;23:7391–8. doi: 10.1038/sj.onc.1207972. [DOI] [PubMed] [Google Scholar]
- 101.Hou T, Ray S, Brasier AR. The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression. J Biol Chem. 2007;282:37091–102. doi: 10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 102.Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O. Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem. 2002;277:8004–11. doi: 10.1074/jbc.M111486200. [DOI] [PubMed] [Google Scholar]
- 103.Duan HO, Simpson-Haidaris PJ. Cell type-specific differential induction of the human gamma-fibrinogen promoter by interleukin-6. J Biol Chem. 2006;281:12451–7. doi: 10.1074/jbc.M600294200. [DOI] [PubMed] [Google Scholar]
- 104.Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–68. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- 105.Paulson M, Pisharody S, Pan L, Guadagno S, Mui AL, Levy DE. Stat protein transactivation domains recruit p300/CBP through widely divergent sequences. J Biol Chem. 1999;274:25343–9. doi: 10.1074/jbc.274.36.25343. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Cheng MB, Zhang YJ, Zhong X, Dai H, Yan L, et al. A switch from hBrm to Brg1 at IFNγ-activated sequences mediates the activation of human genes. Cell Res. 2010;20:1345–60. doi: 10.1038/cr.2010.155. [DOI] [PubMed] [Google Scholar]
- 107.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–85. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Bolli R, Stein AB, Guo Y, Wang OL, Rokosh G, Dawn B, et al. A murine model of inducible, cardiac-specific deletion of STAT3: its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol. 2011;50:589–97. doi: 10.1016/j.yjmcc.2011.01.002. [DOI] [PubMed] [Google Scholar]