Abstract
Human herpesvirus-6B (HHV-6B) is a T lymphotropic β-herpesvirus that is clearly distinct from human herpesvirus-6A (HHV-6A) according to molecular biological features. The International Committee on Taxonomy of Viruses recently classified HHV-6B as a separate species. The primary HHV-6B infection causes exanthem subitum and is sometimes associated with severe encephalopathy. More than 90% of the general population is infected with HHV-6B during childhood, and the virus remains throughout life as a latent infection. HHV-6B reactivation causes encephalitis in immunosuppressed patients. The cellular receptor for HHV-6A entry was identified as human CD46, but the receptor for HHV-6B has not been clear. Here we found that CD134, a member of the TNF receptor superfamily, functions as a specific entry receptor for HHV-6B. A T-cell line that is normally nonpermissive for HHV-6B infection became highly susceptible to infection when CD134 was overexpressed. CD134 was down-regulated in HHV-6B–infected T cells. Soluble CD134 interacted with the HHV-6B glycoprotein complex that serves as a viral ligand for cellular receptor, which inhibited HHV-6B but not HHV-6A infection in target cells. The identification of CD134 as an HHV-6B specific entry receptor provides important insight into understanding HHV-6B entry and its pathogenesis.
Keywords: viral entry, gQ1, gQ2
Human herpesvirus-6B (HHV-6B) is a T lymphotropic β-herpesvirus (1) and is clearly distinct from human herpesvirus-6A (HHV-6A) according to their genetic and antigenic differences and their cell tropism (2–5). Recently the International Committee on Taxonomy of Viruses classified HHV-6B as a separate species.
The primary HHV-6B infection causes exanthem subitum (6) and is sometimes associated with severe encephalopathy, whereas the diseases caused by HHV-6A are still unknown. More than 90% of the general population is infected with HHV-6B during childhood, and the virus remains throughout life as a latent infection (7). HHV-6B reactivation causes encephalitis in immunosuppressed patients. HHV-6B reactivation is also associated with drug-induced hypersensitivity syndrome, and recent studies have suggested that it could be related to the severity of this disease (8, 9).
HHV-6A can infect a broader variety of human cells than HHV-6B (10), although the homology between HHV-6A and -6B is almost 90% over their entire genome (11–13). Human CD46 has been shown to be a cellular receptor of HHV-6 (14), and its viral ligand is a glycoprotein (g) complex made up of viral glycoprotein H (gH)/glycoprotein L (gL)/glycoprotein Q1 (gQ1)/glycoprotein Q2 (gQ2) (15). However, the HHV-6A gH/gL/gQ1/gQ2 complex binds to its human cellular receptor, CD46, whereas the corresponding complex of HHV-6B does not bind to it (10, 15). Moreover, anti-CD46 antibody does not block HHV-6B infection into the cells, whereas it does HHV-6A infection, indicating that the cellular receptor exists specific for HHV-6B infection. Because HHV-6B remains as a lifelong latent infection in more than 90% of the population and causes severe disease, it is important to identify its specific cellular receptor.
Here we show that CD134, a member of the TNF receptor superfamily, functions as a specific entry receptor for HHV-6B. A T-cell line that is normally nonpermissive for HHV-6B infection became highly susceptible to infection when CD134 was overexpressed. CD134 was down-regulated in HHV-6B–infected T cells. Soluble CD134 interacted with the HHV-6B glycoprotein complex that serves as a viral ligand, which inhibited HHV-6B but not HHV-6A infection in target cells. The identification of CD134 as an HHV-6B entry receptor provides important insight into understanding HHV-6B entry and its pathogenesis and for finding new targets for antiviral drug development.
Results
Construction of Soluble Glycoprotein Complex That Is a Viral Ligand for the Cellular Receptor.
To search for candidate molecules for the HHV-6B receptor, we first prepared a soluble form of the HHV-6A or -6B gH/gL/gQ1/gQ2 complex, which is expressed on the viral envelope and acts as a viral ligand for the cellular receptor (15). One component of the complex, gH, is a type I membrane protein that retains the other molecules, gQ1, gQ2, and gL, on the membrane through its interaction with them. Therefore, to prepare the soluble gH/gL/gQ1/gQ2 complex, the transmembrane domain and cytoplasmic tail of gH were removed, and the ectodomain of gH was fused in-frame with Fc (the fragment crystallizable region of the human IgG1 antibody) and a His tag. The production of the soluble complex was confirmed by immunoblot analysis using antibodies for each component of the complex (Fig. S1A). Notably, the soluble HHV-6B gH/gL/gQ1/gQ2 complex efficiently bound to the Molt-3 cell line, which is permissive for HHV-6B infection (Fig. S1B), whereas it bound poorly to the SupT1 cell line, which is nonpermissive (Fig. S1B). In contrast, the soluble HHV-6A gH/gL/gQ1/gQ2 complex bound to both the Molt-3 and SupT1 cell lines (Fig. S1B), which both express CD46 on their cell surface.
Identification of Cellular Receptor Specific for HHV-6B.
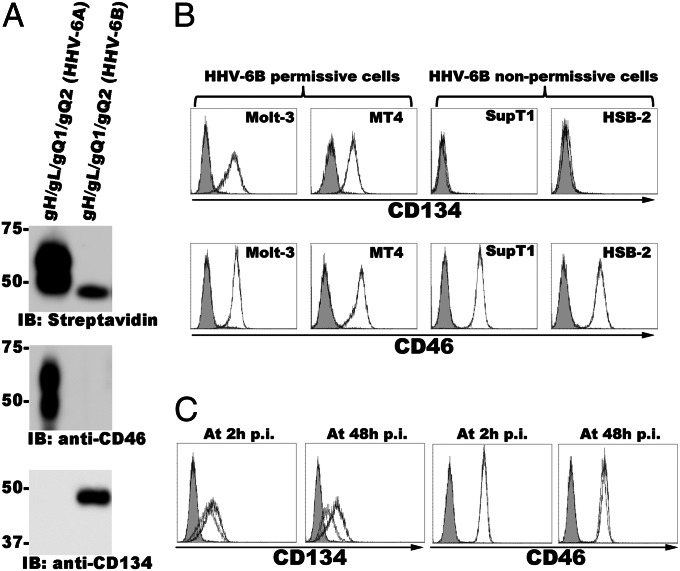
Next, to identify the cellular receptor for HHV-6B, we performed pull-down assays using the soluble HHV-6A or -6B gH/gL/gQ1/gQ2 complex and lysates of surface-biotinylated Molt-3 cells. As expected, the molecular masses of the streptavidin-labeled bands differed between HHV-6A and -6B (Fig. 1A). The band detected in the HHV-6A sample seemed to correspond to CD46, but the band in the HHV-6B sample did not (Fig. 1A). The corresponding band detected by silver staining was excised from the gel and subjected to LC-MS/MS analysis. The results identified CD134 as a candidate receptor molecule for HHV-6B.
Fig. 1.
CD134 interacts with HHV-6B gH/gLgQ1/gQ2 and is expressed on HHV-6B permissive cells. (A) Purified HHV-6A- or HHV-6B gHFc/gL/gQ1/gQ2 complex was incubated with the lysates from Molt-3 cells whose cell surface was labeled with EZ-link sulfo-NHS-Lc-Biotin. The complex and its binding proteins were immunoblotted and detected with streptavidin, anti-CD46, or anti-CD134 antibody. (B) CD134 or CD46 expression on HHV-6B permissive and nonpermissive cells. HHV-6B permissive (Molt-3 and MT4) or nonpermissive (SupT1, JJhan, and HSB-2) cells were stained with anti-CD46 or CD134 antibody, followed by staining with secondary antibody for FACS analysis. Gray shading, isotype control. (C) Down-regulation of CD134 expression from the cell surface after HHV-6B infection. CD134 or CD46 expression on the surface of HHV-6B–infected Molt-3 cells was determined as described in B. Gray shading, isotype control; dark gray line, mock infection; light gray line, 2 h or 48 h postinfection (p.i.).
To confirm the identity of the corresponding band as CD134, immunoblotting was performed using the samples described above and an anti-CD134 antibody. The anti-CD134 antibody reacted with the eluate from the soluble HHV-6B gH/gL/gQ1/gQ2-bound resin but not with that from HHV-6A (Fig. 1A), indicating that CD134 associated specifically with the HHV-6B gH/gL/gQ1/gQ2 complex. On the other hand, an anti-CD46 antibody reacted with the eluate from the soluble HHV-6A gH/gL/gQ1/gQ2-bound resin but not with that from HHV-6B (Fig. 1A).
Expression of CD134 in Several T-Cell Lines and Down-Regulation of CD134 on the Cell Surface by HHV-6B Infection.
HHV-6B permissive T-cell lines are distinct from those of HHV-6A. Therefore, next we assessed the level of CD134 protein expression in several T-cell lines by FACS analyses. CD134 was highly expressed in the HHV-6B permissive T-cell lines MT4 and Molt-3 but rarely in the HHV-6B nonpermissive T-cell lines HSB-2 and SupT1 (Fig. 1B). In contrast, CD46 was expressed abundantly in all of these cell lines (Fig. 1B).
In general the cellular receptor at the cell surface is down-regulated after viral infection. Therefore we examined the CD134 expression on the surface of HHV-6B–infected cells (Molt-3 cells). Fig. 1C shows that CD134 on the cell surface was down-regulated after HHV-6B infection, whereas the down-regulation of CD46 on the cell surface was rarely seen in the same condition.
Inhibition of HHV-6B Infection by Soluble CD134 or Anti-CD134 Antibody.
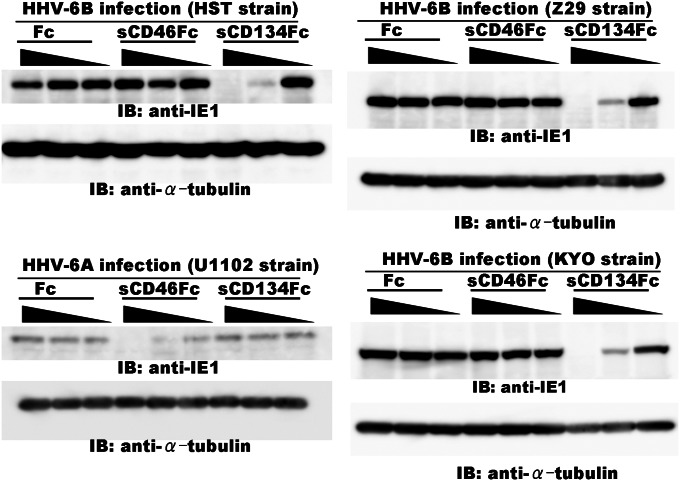
We next examined whether a soluble CD134Fc could inhibit HHV-6B infection of cells. HHV-6 entry into cells was examined by observing the expression of the HHV-6 immediate-early protein, IE1. As shown in Fig. 2, soluble CD134Fc blocked HHV-6B (HST strain) infection in a dose-dependent manner, whereas neither soluble Fc nor soluble CD46Fc did so. Notably, soluble CD134Fc did not block HHV-6A infection, although soluble CD46Fc did block it (Fig. 2, Lower Left), indicating that CD134 functions as an HHV-6B-specific receptor but not as a receptor for HHV-6A. We further examined whether CD134 also functions as the receptor for the other HHV-6B strains (Z29 and KYO), including clinical isolates. The results showed that sCD134Fc could block the other HHV-6B strains tested (Fig. 2, Right), confirming that CD134 functions as a specific cellular receptor for HHV-6B isolates.
Fig. 2.
CD134 is necessary for HHV-6B infection. HHV-6B (HST, KYO, or Z29 strain) or HHV-6A (U1102 strain) was incubated with the indicated amounts (diluted 10-fold from 2.5 µg) of soluble CD134Fc, CD46Fc, or Fc, and then Molt-3 cells were infected with these viruses. The cell lysates were immunoblotted for HHV-6 immediate-early protein (BIE1 or AIE1) and α-tubulin.
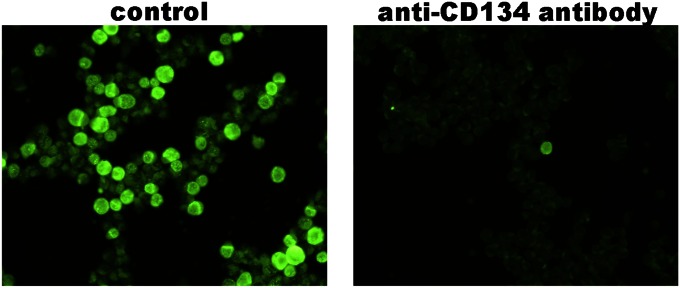
Furthermore, we analyzed whether the anti-CD134 antibody blocks HHV-6B infection into target cells. When MT4 cells were infected with HHV-6B in the presence of anti-CD134 antibody, infection was blocked by the anti-CD134 antibody (Fig. 3). By contrast, control antibody did not affect HHV-6B infection into the cells (Fig. 3).
Fig. 3.
Anti-CD134 antibody blocks HHV-6B infection. MT4 cells were infected with HHV-6B in the presence of anti-CD134 antibody (guinea pig serum) or control antibody (preimmune serum). The infection was examined by indirect immunofluorescence antibody assay using BIE1 antibody.
CD134-Expressing SupT1 Cells Become Susceptible for HHV-6B Infection.
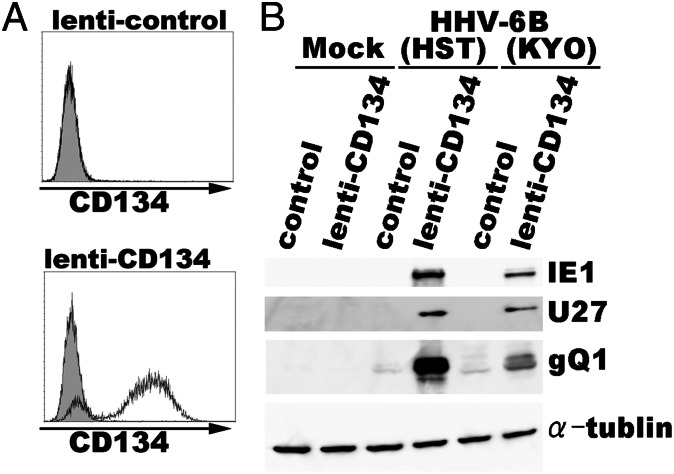
Next, the CD134 gene was introduced into SupT1 cells, which are nonpermissive for HHV-6B infection, by a nonreplicative lentivirus, and HHV-6B was used to infect the CD134-overexpressing cells. The expression of CD134 on the SupT1 cells was confirmed by FACS (Fig. 4A). Notably, the CD134-expressing SupT1 cells were highly susceptible to HHV-6B entry (Fig. 4B), supporting the identification of CD134 as an HHV-6B receptor.
Fig. 4.
SupT1 cells overexpressing CD134 become permissive for HHV-6B infection. (A) SupT1 cells were transduced with lentivirus or CD134-expressing lentivirus. CD134 expression on the SupT1 cells was confirmed by FACS analysis using an anti-CD134 antibody before HHV-6B infection. Gray shading, isotype control. (B) Four days later, the cells were infected with HHV-6B (HST or KYO strain), followed by immunoblotting with anti-HHV-6B IE1, -U27, -gQ1, and -α-tubulin antibodies.
Specific Interaction of HHV-6B Glycoprotein Complex and CD134.
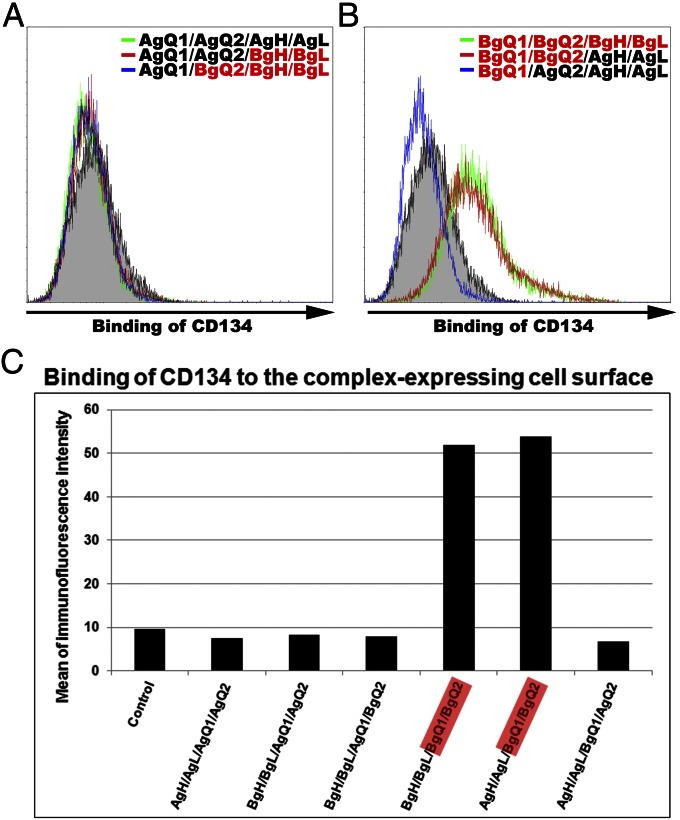
HHV-6A and -6B share 90% identity in their nucleic acid sequence. However, the amino acid sequences of gQ1 and gQ2 show low identity, with 78% and 69%, respectively, between HHV-6A and -6B compared with the sequences of gH and gL (11–13). Therefore, the gQ1 and gQ2 components of the gH/gL/gQ1/gQ2 complex may contribute to the virus specificity for different cellular receptors. To examine this possibility, several chimeric complexes were expressed in 293T cells. The cell-surface expression of each component in the complexes was confirmed. The binding of soluble CD134Fc to the surface of these cells was then measured by FACS. No CD134Fc binding was detected when AgH/AgL/AgQ1/AgQ2 (Fig. 5 A and C), AgH/AgL/BgQ1/AgQ2 (Fig. 5 B and C), or BgH/BgL/AgQ1/AgQ2 (Fig. 5 A and C) was expressed in 293T cells, whereas CD134Fc bound to 293T cells expressing BgH/BgL/BgQ1/BgQ2 (Fig. 5 B and C) or AgH/AgL/BgQ1/BgQ2 (Fig. 5 B and C), indicating that CD134 specifically bound to complexes containing HHV-6B gQ1 and gQ2. These data suggest that gQ1 and gQ2 of HHV-6B but not of HHV-6A in the complex are essential for binding to CD134.
Fig. 5.
HHV-6B-gQ1 and -gQ2 are the key molecules for HHV-6B’s binding with CD134. (A and B) 293T cells were transfected with plasmids harboring individual molecules (indicated as different colors in histograms) or control plasmid (gray shading) and harvested 24 h later. The cells were incubated with soluble CD134Fc at 4 °C for 2 h and stained with Alexa Fluor 488 goat anti-human IgG antibody at 4 °C for 1 h for FACS analysis. (C) The intensity of immunofluorescence of the stained cells shown in A and B was quantified. A, HHV-6A; B, HHV-6B.
Discussion
Recently HHV-6B was classified as a separate species. Although its entry receptor has not been clear, here we found that CD134 is a cellular receptor specific for HHV-6B. Although HHV-6B and -6A share 90% identity in their nucleic acid sequence, they show distinct pathogenesis and cell tropism. The discovery of an HHV-6B–specific receptor supports the idea that the use of different receptors by HHV-6A and -6B is an important biological feature underlying their different characteristics and disease manifestations.
Previously we found that HHV-6A gH/gL/gQ1/gQ2 complex on the viral envelope is a viral ligand for CD46 (10, 16), which is a cellular receptor of HHV-6A (14). Here by using the soluble form of gH/gL/gQ1/gQ2 complex, we identified that the CD134 was a cellular receptor specific for HHV-6B. As predicted, the soluble form of HHV-6B gH/gL/gQ1/gQ2 complex bound to CD134, whereas that of HHV-6A did not. In addition, soluble CD134 could inhibit HHV-6B infection into target cells, whereas it could not inhibit HHV-6A infection, thus indicating that CD134 is a specific receptor for HHV-6B.
gQ1 and gQ2 in the complex are unique genes that are encoded specifically in HHV-6 and human herpesvirus-7 (HHV-7). In addition, HHV-6A and HHV-6B share low identity of them. Therefore we made several chimeric complexes of gQ1 and gQ2 and examined the interaction of chimeric complexes with CD134. Only the complex with HHV-6B gQ1 and gQ2 could bind to CD134, showing that gQ1 and gQ2 in the complex are crucial for the different receptor use between HHV-6B and HHV-6A.
CD134, which is also called OX40, is a member of the TNF receptor superfamily and is present on activated T lymphocytes, but it is rarely expressed on glial cells. HHV-6B is well known to have a cellular tropism for T lymphocytes, as shown in vivo during viremia from acute infection as well as in vitro (17, 18). Activated CD4+ T lymphocytes are the preferential target of the fully permissive infection in vivo (18). Therefore, the present findings that strongly indicate CD134 is a functional HHV-6B entry receptor are consistent with the in vivo observations.
As described above, HHV-6B causes exanthem subitum in infants, and its reactivation causes encephalitis, especially in immunocompromised patients.
Because effective therapeutic agents for HHV-6B have not been developed, these findings may lead to new prophylactic and therapeutic approaches for HHV-6B–associated diseases, through the development of drugs that target CD134 and its regulators.
Materials and Methods
Plasmids.
An Fc fragment of human IgG1 with L266A and L267E mutations to reduce its binding affinity to cellular Fc receptors was used (19). The IL-2 signal sequence (amplified from pFuse-hIgG1-Fc2; Invivogen) was used in place of the original signal sequence in each of the Fc fusion protein-expressing plasmids. In the CD46-expressing plasmid, the original signal sequence was used. To generate gHFcHis-expressing plasmids, the nucleic sequence for the ectodomain of gH (base pairs 46–2067) was amplified from the HHV-6A and -6B genomes by PCR, ligated with the Fc fragment containing a 6× histidine sequence at its 3′ end, and cloned into the pCAGGS-MCS plasmid (20). Similarly, FcHis, CD46FcHis (base pairs 1–1029 of the CD46 sequence), and CD134FcHis (base pairs 85–642 of the CD134 sequence) were cloned into pCAGGS-MCS (provided by J. Miyazaki, Osaka University, Suita, Japan). We cloned the full-length CD134 sequence into CS-CA-MCS (provided by RIKEN: the Institute of Physical and Chemical Research; Japan) plasmid. The plasmids for expressing gQ1, gQ2, gH, and gL of HHV-6A and -6B were described previously (21, 22).
Antibodies.
Mouse monoclonal antibodies to CD46 (J4. 48) and CD134 (Ber-ACT35) were purchased from Immunotech and BioLegend, respectively. The antibodies to IE1, U27, gH/gL, gH, gL, gQ1, and gQ2 of HHV-6A and HHV-6B were described previously (21–23). Anti-CD134 antibody was obtained by immunizing the purified CD134 protein to guinea pigs. Preimmune sera of guinea pigs were used as control antibody.
Fc-Fusion Protein.
The plasmids for expressing Fc-fusion proteins were transfected into 293T cells using Lipofectamine 2000 (Invitrogen), according the manufacturer’s instructions. Two days after transfection, the soluble Fc-fusion proteins in the culture medium were purified by Ni-NTA (Qiagen) affinity chromatography.
Identification of Proteins Associated with the BgH/BgL/BgQ1/BgQ2 Complex.
293T cells were transfected with BgHFcHis-, BgL-, BgQ1-, and BgQ2-expressing plasmids or with AgHFcHis-, AgL-, AgQ1-, and AgQ2-expressing plasmids. The culture medium was harvested 48 h after transfection, incubated with Ni-NTA at 4 °C for 8 h, and then the Ni-NTA was spun down. Molt-3 cells (T-cell line) (1 × 108 cells per sample) were labeled with EZ-link sulfo-NHS-Lc-Biotin (Thermo Scientific) according to the manufacturer’s protocol and lysed with TNE buffer [10 mM Tris·HCl (pH 7.8), 0.15 M NaCl, 1 mM EDTA, and 1% Nonidet P-40 (Nacalai Tesque)]. After centrifugation, the supernatant was incubated with the glycoprotein complex-bound Ni-NTA described above at 4 °C for 16 h. The proteins that bound to the Ni-NTA were eluted with 250 mM imidazole, and the buffer was changed to PBS using centrifugal filter devices (Millipore). Finally, the protein solution was incubated with protein G Sepharose at 4 °C for 8 h. The eluates were prepared for immunoblot analysis [using streptavidin-HRP (GE Healthcare) and silver staining (Invitrogen)]. The positive bands in the silver-stained gel (corresponding to the position of the positive band in the Western blot) were excised for in-gel digestion and LC-MS/MS analysis (24).
Preparation of Virus Solution.
To prepare virus stocks, the viruses (HHV-6A and -6B strains) were propagated in umbilical cord blood mononuclear cells [CBMCs; provided by K. Adachi (Minoh Hospital, Minoh, Japan) and H. Yamada (Kobe University Graduate School of Medicine, Kobe, Japan) and purchased from the Cell Bank of the RIKEN Bioresource Center], which had been stimulated with 5 µg/mL phytohemagglutinin and 2 ng/mL IL-2 for 3 d. When more than 80% of the cells showed cytopathic effects, the cultures were frozen and thawed twice, then centrifuged at 1,500 × g for 5 min. The supernatants were collected and stored at −80 °C as cell-free virus stocks. We used CBMCs to titer the viruses by the 50% tissue culture infectious dose assay (25).
Infection Inhibition Assay.
Cell-free HHV-6A or HHV-6B virus was incubated with soluble Fc, CD46Fc, or CD134Fc (diluted 10-fold from 2.5 µg) at 37 °C for 30 min, and then the virus was used to infect Molt-3 cells (5 × 105) at 37 °C for 1 h. The cells were cultured in 1 mL of medium for 24 h and then lysed with RIPA buffer [50 mM Tris (pH 7.4), 150 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS] and used for immunoblotting analysis.
Cell-Surface Expression Assay.
Cells were incubated with isotype control, anti-CD46, or anti-CD134 antibody at 4 °C for 1 h, followed by a secondary antibody. The cells were fixed with 4% (wt/vol) paraformaldehyde for 10 min before being analyzed on a FACSCalibur (BD).
Cell-Surface Binding Assay.
Fc or soluble Fc-fusion proteins were incubated with a T-cell line (Molt-3 or SupT1) or with 293T cells transfected with glycoprotein-expressing plasmids (24 h after transfection) at 4 °C for 2 h, then the cells were washed with 3% (wt/vol) BSA/PBS and stained with an Alexa Fluor 488 goat anti-human IgG antibody (Invitrogen) at 4 °C for 1 h. The cells were washed with PBS, fixed with 4% (wt/vol) paraformaldehyde for 10 min, and then subjected to FACS analysis.
Overexpression of CD134 in SupT1 Cells and Infection with HHV-6B.
CD134-expressing lentivirus and its control were constructed by transfecting 293T cells with CS-CA-MCS-CD134 (or its control, CS-CA-MCS) and packaging plasmids (pCAG-HIV-gag and pCMV-VSV-G-RSV-Rev provided by RIKEN). The culture media containing the viruses were harvested 3 d after transfection. SupT1 cells were transduced with the lentiviruses for 4 d and then infected with HHV-6B viruses. The cells were harvested and prepared for immunoblot analysis.
Supplementary Material
Acknowledgments
We thank Y. Yamamoto (National Institute of Biomedical Innovation) and E. Moriishi (National Institute of Biomedical Innovation) for technical assistance, J. Miyazaki (Osaka University) for providing reagents, and K. Adachi (Minoh City Hospital) and H. Yamada (Kobe University) for the CBMCs. This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305187110/-/DCSupplemental.
References
- 1.Roizmann B, et al. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses The family Herpesviridae: An update. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 2.Salahuddin SZ, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 3.Aubin JT, et al. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol. 1991;29(2):367–372. doi: 10.1128/jcm.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Guerrini S, Liu X, Foà-Tomasi L. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J Gen Virol. 1993;74(Pt 10):2257–2262. doi: 10.1099/0022-1317-74-10-2257. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt LS, Balachandran N, Frenkel N. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J Infect Dis. 1990;162(4):852–857. doi: 10.1093/infdis/162.4.852. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 7.Okuno T, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol. 1989;27(4):651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchett JC, Nanau RM, Neuman MG. The link between hypersensitivity syndrome reaction development and human herpes virus-6 reactivation. Int J Hepatol. 2012;2012:723062. doi: 10.1155/2012/723062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tohyama M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157(5):934–940. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 10.Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 2009;11(7):1001–1006. doi: 10.1111/j.1462-5822.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez G, et al. Human herpesvirus 6B genome sequence: Coding content and comparison with human herpesvirus 6A. J Virol. 1999;73(10):8040–8052. doi: 10.1128/jvi.73.10.8040-8052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gompels UA, et al. The DNA sequence of human herpesvirus-6: Structure, coding content, and genome evolution. Virology. 1995;209(1):29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 13.Isegawa Y, et al. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J Virol. 1999;73(10):8053–8063. doi: 10.1128/jvi.73.10.8053-8063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santoro F, et al. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99(7):817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 15.Akkapaiboon P, Mori Y, Sadaoka T, Yonemoto S, Yamanishi K. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J Virol. 2004;78(15):7969–7983. doi: 10.1128/JVI.78.15.7969-7983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori Y, et al. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J Virol. 2004;78(9):4609–4616. doi: 10.1128/JVI.78.9.4609-4616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ablashi DV, et al. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184(2):545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, et al. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiratori I, Ogasawara K, Saito T, Lanier LL, Arase H. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J Exp Med. 2004;199(4):525–533. doi: 10.1084/jem.20031885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 21.Kawabata A, et al. Analysis of a neutralizing antibody for human herpesvirus 6B reveals a role for glycoprotein Q1 in viral entry. J Virol. 2011;85(24):12962–12971. doi: 10.1128/JVI.05622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang H, Hayashi M, Maeki T, Yamanishi K, Mori Y. Human herpesvirus 6 glycoprotein complex formation is required for folding and trafficking of the gH/gL/gQ1/gQ2 complex and its cellular receptor binding. J Virol. 2011;85(21):11121–11130. doi: 10.1128/JVI.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori Y, et al. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J Virol. 2002;76(13):6750–6761. doi: 10.1128/JVI.76.13.6750-6761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68(5):850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 25.Asada H, Yalcin S, Balachandra K, Higashi K, Yamanishi K. Establishment of titration system for human herpesvirus 6 and evaluation of neutralizing antibody response to the virus. J Clin Microbiol. 1989;27(10):2204–2207. doi: 10.1128/jcm.27.10.2204-2207.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.