Significance
The concentration of Ca2+ ions is kept low in cells by specialized ion-pumping proteins at the membrane. We show that in cardiac cells, cytoplasm also has an intrinsic ability to pump Ca2+. Histidyl dipeptides and ATP are diffusible cytoplasmic buffer molecules. By exchanging Ca2+ for H+, they act like local “pumps,” producing uphill Ca2+ movement within cytoplasm in response to H+ ion gradients. Intracellular H+ ions are generated locally by metabolism and competitively inhibit many Ca2+-activated biochemical processes. Recruiting Ca2+ to acidic zones facilitates these processes. Cytoplasmic histidyl dipeptides and ATP thus act like a biological pump without a membrane.
Keywords: calcium, heart, mobile buffer, pH, dual microperfusion
Abstract
Ca2+ signaling regulates cell function. This is subject to modulation by H+ ions that are universal end-products of metabolism. Due to slow diffusion and common buffers, changes in cytoplasmic [Ca2+] ([Ca2+]i) or [H+] ([H+]i) can become compartmentalized, leading potentially to complex spatial Ca2+/H+ coupling. This was studied by fluorescence imaging of cardiac myocytes. An increase in [H+]i, produced by superfusion of acetate (salt of membrane-permeant weak acid), evoked a [Ca2+]i rise, independent of sarcolemmal Ca2+ influx or release from mitochondria, sarcoplasmic reticulum, or acidic stores. Photolytic H+ uncaging from 2-nitrobenzaldehyde also raised [Ca2+]i, and the yield was reduced following inhibition of glycolysis or mitochondrial respiration. H+ uncaging into buffer mixtures in vitro demonstrated that Ca2+ unloading from proteins, histidyl dipeptides (HDPs; e.g., carnosine), and ATP can underlie the H+-evoked [Ca2+]i rise. Raising [H+]i tonically at one end of a myocyte evoked a local [Ca2+]i rise in the acidic microdomain, which did not dissipate. The result is consistent with uphill Ca2+ transport into the acidic zone via Ca2+/H+ exchange on diffusible HDPs and ATP molecules, energized by the [H+]i gradient. Ca2+ recruitment to a localized acid microdomain was greatly reduced during intracellular Mg2+ overload or by ATP depletion, maneuvers that reduce the Ca2+-carrying capacity of HDPs. Cytoplasmic HDPs and ATP underlie spatial Ca2+/H+ coupling in the cardiac myocyte by providing ion exchange and transport on common buffer sites. Given the abundance of cellular HDPs and ATP, spatial Ca2+/H+ coupling is likely to be of general importance in cell signaling.
Most cells are exquisitely responsive to calcium (Ca2+) (1) and hydrogen (H+) ions (i.e., pH) (2). In cardiac myocytes, Ca2+ ions trigger contraction and control growth and development (3), whereas H+ ions, which are generated or consumed metabolically, are potent modulators of essentially all biological processes (4). By acting on Ca2+-handling proteins directly or via other molecules, H+ ions exert both inhibitory and excitatory effects on Ca2+ signaling. For example, in the ventricular myocyte, H+ ions can reduce Ca2+ release from sarcoplasmic reticulum (SR) stores, through inhibition of the SR Ca2+ ATPase (SERCA) pump and ryanodine receptor (RyR) Ca2+ channels (5, 6). In contrast, H+ ions can enhance SR Ca2+ release by stimulating sarcolemmal Na+/H+ exchange (NHE), which raises intracellular [Na+] and reduces the driving force for Ca2+ extrusion on Na+/Ca2+ exchange (NCX), leading to cellular retention of Ca2+ (7, 8). Ca2+ signaling is thus subservient to pH.
Cytoplasmic Ca2+ and H+ ions bind avidly to buffer molecules, such that <1% of all Ca2+ ions and <0.001% of all H+ ions are free. Some of these buffers bind H+ and Ca2+ ions competitively, and this has been proposed to be one mechanism underlying cytoplasmic Ca2+/H+ coupling (9). Reversible binding to buffers greatly reduces the effective mobility of Ca2+ and H+ ions in cytoplasm (10, 11) and can allow for highly compartmentalized ionic microdomains, and hence a spatially heterogeneous regulation of cell function. In cardiac myocytes under resting (diastolic) conditions, the cytoplasm-averaged concentration of free [Ca2+] ([Ca2+]i) and [H+] ([H+]i) ions is kept near 10−7 M by membrane transporter proteins. Thus, [H+]i is regulated by the balance of flux among acid-extruding and acid-loading transporter proteins at the sarcolemma [e.g., NHE and Cl−/OH− (CHE) exchangers, respectively] (4). Similarly, the activity of SERCA and NCX proteins returns [Ca2+]i to its diastolic level after evoked signaling events (3, 12). Despite these regulatory mechanisms, cytoplasmic gradients of [H+]i and [Ca2+]i do occur in myocytes and are an important part of their physiology. Gradients arise from local differences in transmembrane fluxes that alter [H+]i or [Ca2+]i. For example, spatial [H+]i gradients are produced when NHE transporters, expressed mainly at the intercalated disk region, are activated (4, 13) or when membrane-permeant weak acids, such as CO2, are presented locally (14). Similarly, release of Ca2+ through a cluster of RyR channels in the SR produces [Ca2+]i nonuniformity in the form of Ca2+ sparks (15). Given the propensity of cytoplasm to develop ionic gradients, it is important to understand their underlying mechanism and functional role.
The present work demonstrates a distinct form of spatial interaction between Ca2+ and H+ ions. We show that cytoplasmic [H+] gradients can produce stable [Ca2+]i gradients, and vice versa, and that this interaction is mediated by low-molecular-weight (mobile) buffers with affinity for both ions. We demonstrate that the diffusive counterflux of H+ and Ca2+ bound to these buffers comprises a cytoplasmic Ca2+/H+ exchanger. This acts like a “pump” without a membrane, which can, for instance, recruit Ca2+ to acidic cellular microdomains. Cytoplasmic Ca2+/H+ exchange adds a spatial paradigm to our understanding of Ca2+ and H+ ion signaling.
Results
Acidosis Evokes Intracellular Ca2+ Release.
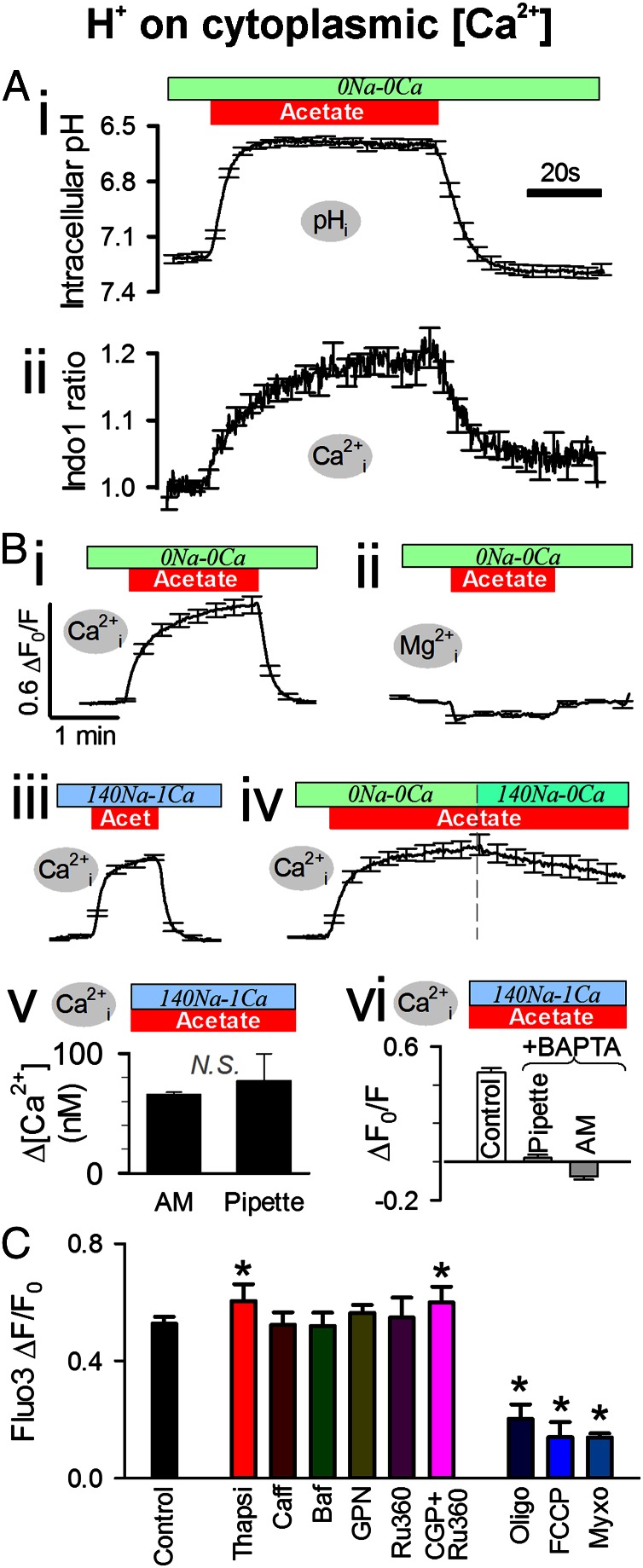
Coupling between the cytoplasmic concentrations of H+ and Ca2+ ions was first studied in unpaced myocytes superfused with Na+-free/Ca2+-free (0Na-0Ca) solutions to block major sarcolemmal routes of Ca2+ transport into and out of the cell (including L-type Ca2+ channels and NCX). Myocytes were acetoxymethyl (AM)-loaded with cSNARF1 [5-(and-6)-carboxyseminaphtharhodafluor-1] and Indo1 to monitor intracellular pH (pHi) and [Ca2+]i simultaneously. Whole-cell superfusion with 80 mM acetate acidified the cytoplasm by means of passive entry of acetic acid (Fig. 1 A, i). This evoked a reversible rise in resting [Ca2+]i that could be abolished by AM-loading cells with the Ca2+ buffer, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 100 μM). The [Ca2+]i response (Fig. 1 A, ii) was delayed relative to the pHi change, suggesting that the interaction was not an artifact of Indo1 pH-sensitivity.
Fig. 1.
H+ ions evoke intracellular Ca2+ release. (A) Intracellular pH and [Ca2+] (measured simultaneously with cSNARF1 and Indo1) in rat ventricular myocytes superfused with 0Na-0Ca solution (to eliminate sarcolemmal Ca2+ influx and NCX activity). Eighty mM acetate rapidly acidified pHi and, more slowly, elevated [Ca2+]i (n = 10). (B) Acetate (80 mM) in 0Na-0Ca solution evoked a rise in [Ca2+]i (fluorescence from AM-loaded Fluo3) (i) but not in [Mg2+] (MagFluo4) (ii). (iii) Rise in Fluo3 fluorescence was no different with normal extracellular Na+ and Ca2+ (plus 30 μM cariporide to block sarcolemmal NHE). (iv) Activating Ca2+ extrusion on NCX during acetate exposure evoked a slow [Ca2+]i recovery (Fluo3). (v) Acetate-evoked rise in [Ca2+]i was no different when Fluo3 salt (100 μM) was pipette-loaded into cells. (vi) Rise in [Ca2+]i (AM-loaded Fluo3) was buffered out by BAPTA (100 μM AM-loaded or 5 mM pipette-loaded) (n = 5–30). (C) Rise in Fluo3 fluorescence after 2-min exposure to 80 mM acetate in 0Na-0Ca solution. The response was unaffected by 10 mM caffeine (Caff), 1.5 μM bafilomycin (Baf), 100 μM glycyl phenylalanine 2-naphthylamide (GPN), or 10 μM ruthenium-360 (Ru360). The response increased modestly with 10 μM thapsigargin (Thapsi) or 5 μM Ru360 plus 20 μM CGP-37157 (CGP), and it was decreased substantially by metabolic inhibitors: 10 μM oligomycin (Oligo), 1.5 μM FCCP (<15-s exposure), and 10 μM myxothiazol (Myxo) (n = 15–30). * denotes significant difference (P < 0.05) compared with Control.
The H+-evoked [Ca2+]i rise, in the absence of extracellular Na+ and Ca2+, could also be measured with a different Ca2+ dye, Fluo3 (Fig. 1 B, i), whereas use of an AM-loaded Mg2+ dye (MagFluo4) in other experiments indicated no simultaneous rise of intracellular [Mg2+] (Fig. 1 B, ii). The [Ca2+]i rise was not significantly different in cells superfused with normal Na+ (140 mM) and Ca2+ (1 mM) solution (ΔF/F0 = 0.44 ± 0.05 or ∼50 nM after 1 min; n = 10), and it was the same in electrically paced cells (2 Hz; rise in normalized fluorescence ΔF/F0 = 0.43 ± 0.08; n = 7), suggesting that the net activity of sarcolemmal Ca2+ transport over the measured pHi and [Ca2+]i range was not sufficient to modify the response. The response was also the same when NHE transporters were blocked with cariporide (30 μM; Fig. 3 B, iii; ΔF/F0 = 0.46 ± 0.03; n = 17). However, increasing the driving force for NCX-mediated Ca2+ extrusion (by raising extracellular Na+ from 0 to 140 mM at zero extracellular Ca2+ in the presence of 30 μM cariporide) altered the H+-evoked response (Fig. 1 B, iv), indicating that it takes place in a domain accessible to NCX.
Fig. 3.
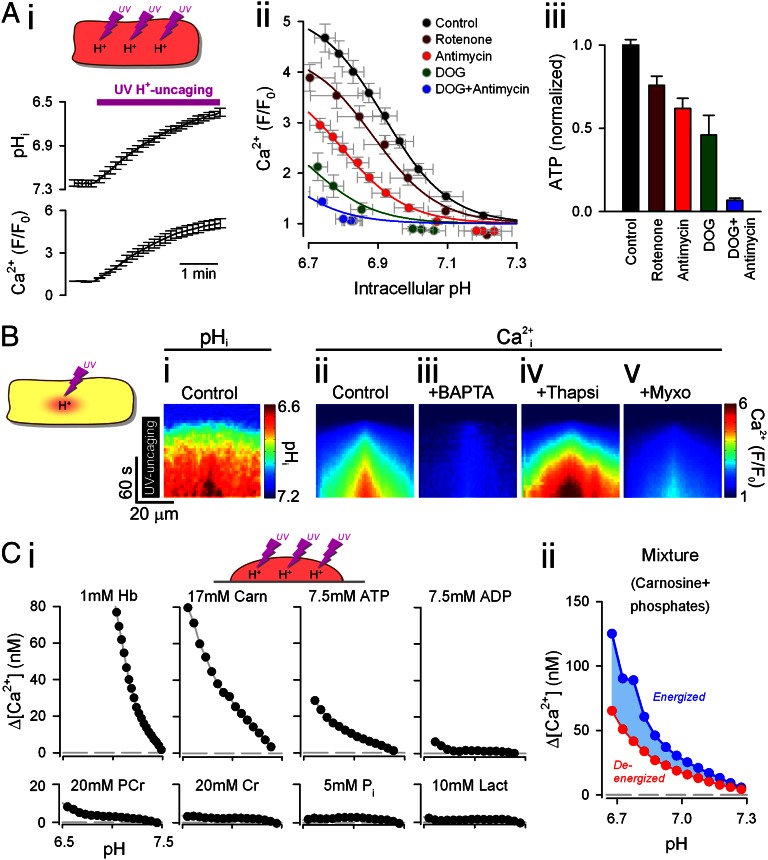
H+-evoked unloading of Ca2+ from cytoplasmic buffers depends on the metabolic state. (A) H+ uncaging by whole-field UV flash every 9 s in rat ventricular myocytes passively loaded with photolabile H+ donor NBA (1 mM) dissolved in 0Na-0Ca superfusate. (i) Intracellular pH (cSNARF1) and [Ca2+] (Fluo3) were measured after each flash (n = 35). (ii) pHi-[Ca2+]i relationship was highly sensitive to metabolic inhibition. Deoxyglucose (DOG; 5 mM) and all other drugs (10 μM) were added 3 min before the onset of H+ uncaging. (iii) ATP content in populations of isolated myocytes was assayed (luciferase bioluminescence) 20 min after exposure to drugs. Luminescence was calibrated using an ATP standard, divided by the number of myocytes staining positively for trypan blue (i.e., living cells), and subsequently normalized to the measurement under control conditions (n = 3). (B) Confocal line scans. UV flash (H+ uncaging) was confined to a central 5 × 5-μm region of intact myocytes. Lateral diffusive spread of [H+] (i) and [Ca2+] (ii). (iii) [Ca2+] response was abolished by BAPTA (100 μM AM-loaded). (iv) Diffusive spread was accelerated with thapsigargin (Thapsi; 10 μM). (v) [Ca2+] response was reduced in myxothiazol (Myxo; 10 μM). (C) (i) Uncaging of H+ ions in vitro in agarose-set mixtures of buffers (plus Hepes and BAPTA to adjust initial pH to 7.4 and [Ca2+]i to 60 nM). Hb, haemoglobin; Carn, carnosine; PCr, phosphocreatine; Cr, creatine; Pi, inorganic phosphate; Lact, lactate. (ii) Mixture of carnosine (Carn) and phosphates, representing a fully energized state (7.5 mM ATP, 20 mM PCr) or a fully deenergized state (7.5 mM ADP, 20 mM Cr, 27.5 mM Pi). Starting pH, [Mg2+] and [Ca2+]i were adjusted to 7.2, 0.75 mM and 100 nM, respectively.
Following AM loading, 18 ± 5% (n = 5) of cellular Fluo3 fluorescence and 15 ± 10% (n = 6) of cellular cSNARF1 fluorescence is attributable to dye partitioning into mitochondria (estimated by surface-membrane permeabilization with 0.005% saponin-containing high K+ buffer, which releases cytoplasmic dye only). To eliminate the possibility that AM-loaded Fluo3 was reporting an H+-evoked [Ca2+] rise in mitochondria, the membrane-impermeant (salt) form of Fluo3 was delivered to the cytoplasm via a cell-attached patch pipette. Acetate exposure evoked a rise in the Fluo3 signal (Fig. 1 B, v) comparable to that seen with AM-loaded dye (calibration is provided in Fig. S1). Furthermore, pipette loading of the salt form of BAPTA (5 mM) into the cytoplasm abolished the H+-evoked [Ca2+]i rise (Fig. 1 B, vi), indicating that the H+-evoked rise in Fluo3 fluorescence is not mitochondrial. Reducing pHi thus triggers an intracellular release of Ca2+ into the cytoplasm.
Ca2+ Is Not Sourced from Organelles.
Experiments were performed to determine the source of the H+-evoked [Ca2+]i rise. Preincubation of cells with the SERCA inhibitor thapsigargin (10 μM) emptied the SR (10 mM caffeine did not release Ca2+) but did not abolish the H+-evoked response (Fig. 1C). Thus, SR stores are not the Ca2+ source. Indeed, after thapsigargin treatment, a modest increase in the response was observed, possibly because of the inactivation of SERCA as a “Ca2+ buffer” (16). Acidic stores (e.g., lysosomes) are not the Ca2+ source, because disrupting them with glycyl phenylalanine 2-naphthylamide (100 μM) or bafilomycin (1.5 μM), confirmed to reduce Lysotracker fluorescence by 48 ± 3% (n = 12), did not affect the H+-evoked response (Fig. 1C), even after several consecutive acetate exposures (which would have involved cycles of Ca2+ release and reuptake). Inhibiting the mitochondrial Ca2+ uniporter by preincubation with ruthenium-360 (Ru360; 10 μM) or blocking mitochondrial NCX with CGP-37157 (20 μM) in the presence of Ru360 (5 μM) also had no effect on the H+-evoked response (Fig. 1C), suggesting that mitochondria are not the Ca2+ source either. The response, however, was reduced following treatment with the metabolic inhibitors oligomycin A, myxothiazol, and rotenone (all at 10 μM) or FCCP (carbonylcyanide-p-trifluoromethoxyphenylhydrazone, 1 μM for <15 s to avoid excessive cytoplasmic acidification) (Fig. 1C). These effects argue that mitochondrial activity may somehow facilitate the H+-evoked response, for example, by supporting metabolism. Hyperpolarization of mitochondrial membrane potential (ψm) with oligomycin A is opposite to that induced by the other drugs (myxothiazol, rotenone, and FCCP all depolarize), indicating that ψm is unlikely to be energizing a mitochondrial Ca2+ release (e.g., by driving electrogenic Ca2+ flux across the inner mitochondrial membrane). Furthermore, ψm (measured with the fluorescent dye JC-1) did not change during cell acidification with acetate (JC-1 ratio was 98 ± 0.03% of control; n = 6).
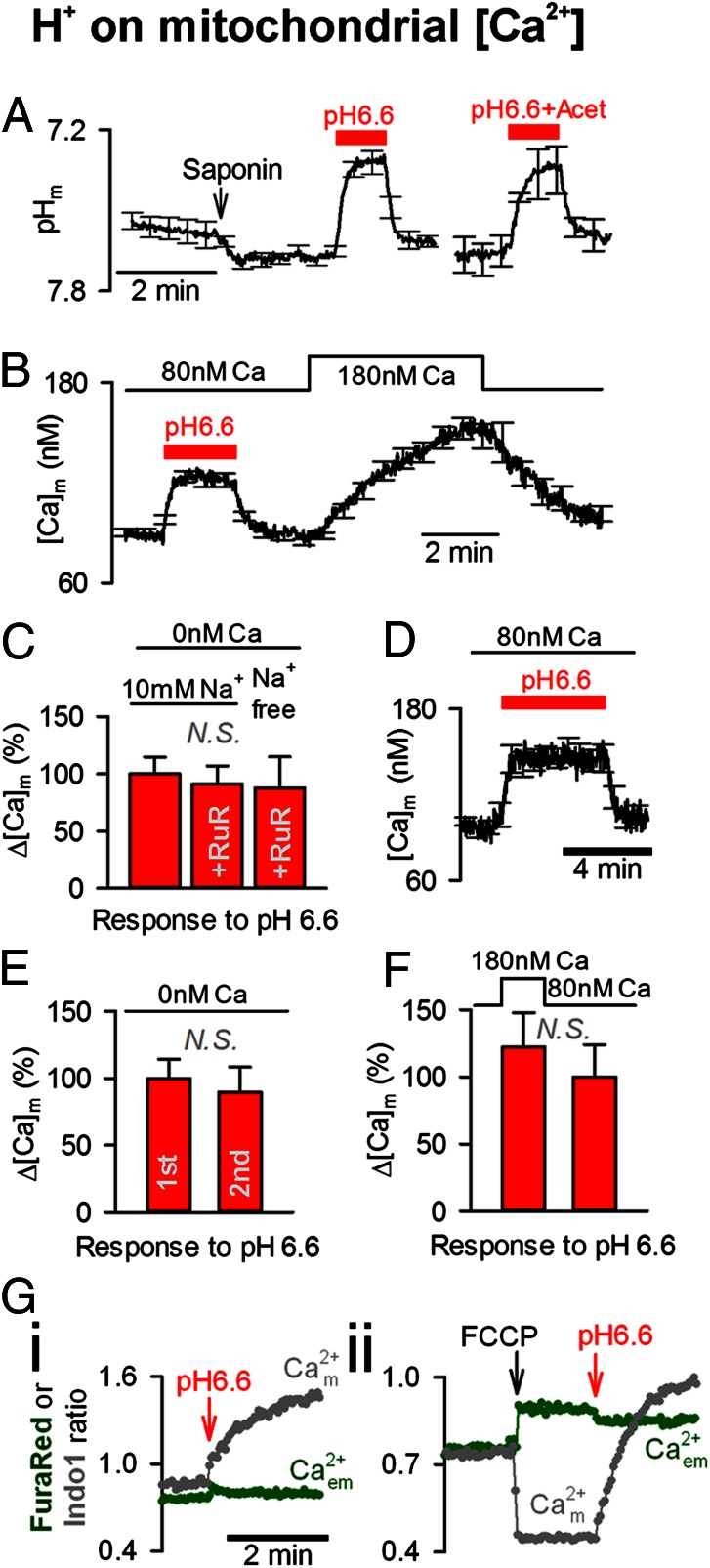
To confirm that mitochondria were not the source for H+-evoked Ca2+ release, experiments were performed on saponin-permeabilized myocytes, in which the extramitochondrial environment could be controlled directly by superfusion while recording mitochondrial matrix pH (pHm) or mitochondrial matrix Ca2+ ([Ca2+]m) with AM-loaded cSNARF1 or Rhod2, respectively. When permeabilized cells were superfused with an “intracellular” solution at pH 6.6 (i.e., the pHi in intact cells when superfused with 80 mM acetate), there was a fall in pHm (Fig. 2A), which evoked a rise in [Ca2+]m (Fig. 2B). This [Ca2+]m rise was still observed when the superfusate was Ca2+-free or contained the uniporter blocker, ruthenium red (10 μM) (Fig. 2C), suggesting that it may involve Ca2+ unloading from mitochondrial matrix buffers. If these H+-mobilized Ca2+ ions were then released from the mitochondrion [e.g., via the putative mitochondrial Ca2+/H+ transporter (17)], they would contribute to the H+-evoked rise in cytoplasmic [Ca2+]. However, four observations additional to those listed previously (Fig. 1C) argue against there being a substantial release. First, the H+-evoked [Ca2+]m rise did not relax when acid was presented for 5 min (Fig. 2D), as would be expected if substantial Ca2+ efflux were occurring. Second, in the absence of extramitochondrial Ca2+, a repeat exposure to pH 6.6 produced a comparable and reversible [Ca2+]m response, indicating that total mitochondrial Ca2+ had been conserved (Fig. 2E), again demonstrating no significant Ca2+ efflux. Third, raising extramitochondrial [Ca2+] concurrently with an acid challenge did not significantly enhance the rise of [Ca2+]m (Fig. 2F), as might have been expected if Ca2+ efflux were being impeded electrochemically. Fourth, the addition of acid to suspensions of isolated mitochondria raised [Ca2+]m (measured with Indo1) but did not evoke a substantial rise in extramitochondrial [Ca2+] (measured simultaneously with FuraRed) in a weakly Ca2+-buffered solution (containing only 10 μM BAPTA to offset dilution, which would otherwise render Ca2+ release unresolvable; Fig. 2 G, i). This finding argues for little or no Ca2+ release. In contrast, FCCP (10 μM) evoked a fall in [Ca2+]m and a prompt rise in extramitochondrial [Ca2+] (i.e., a positive control for Ca2+ release; Fig. 2 G, ii). In summary, a rise in [H+]i can evoke a rise in [Ca2+]m, but this is not the source of Ca2+ that underlies cytoplasmic Ca2+/H+ coupling.
Fig. 2.
No significant H+-evoked Ca2+ release from mitochondria. (A) pHm measured in saponin-permeabilized myocytes AM-loaded with cSNARF1. Exposure to pH 6.6 [with or without 30 mM acetate (Acet)] reduced pHm (n = 12). (B) [Ca2+]m measured in saponin-permeabilized myocytes AM-loaded with Rhod2. Exposure to pH 6.6 + 30 mM acetate at 80 nM bathing [Ca2+] reversibly raised [Ca2+]m. H+-evoked [Ca2+]m rise was faster than mitochondrial Ca2+ uptake triggered by raising bathing [Ca2+] (n = 15). (C) In Ca2+-free media, exposure to pH 6.6 in also evoked a rise in [Ca2+]m. Ruthenium red (RuR, 10 μM) did not affect this response (n = 10). (D) [Ca2+]m remained elevated during a long (5-min) exposure to pH 6.6 (n = 8). (E) Two consecutive exposures to pH 6.6 in Ca2+-free media (7 min apart) evoked comparable [Ca2+]m responses (n = 6). (F) [Ca2+]m response to pH 6.6 was not significantly larger when bathing [Ca2+] was raised to oppose potential Ca2+ efflux (n = 5). (G) Suspensions of ventricular mitochondria (1–2 g of protein per liter) in media of low Ca2+ buffering power. (i) Reducing solution pH from 7.2 to 6.55 raised [Ca2+]m but did not affect extramitochondrial [Ca2+] ([Ca2+]em) measured simultaneously. (ii) As a positive control for mitochondrial Ca2+ release, FCCP (10 μM) decreased [Ca2+]m (Indo1) and raised [Ca2+]em (FuraRed). For comparison, brief exposure of thapsigargin-treated intact myocytes to 2 μM FCCP evoked a 69 ± 9% (n = 5) rise in Fluo3 fluorescence.
Ca2+/H+ Coupling Is Weakened by ATP Depletion.
Cytoplasmic Ca2+/H+ coupling was investigated further by releasing intracellular H+ ions from the photolabile H+ donor compound 2-nitrobenzaldehyde (NBA; 1 mM), permeated passively into intact myocytes from a 0Na-0Ca superfusate (18). A whole-field UV flash rapidly unloads ∼1 mM intracellular H+ ions, most of which is buffered. Repeating the UV flash once every 9 s cumulatively induced an intracellular acid load. This is illustrated in Fig. 3 A, i, where whole-cell pHi was measured 4.5 s after each flash, at a time when intracellular H+ buffers had reequilibrated. As with acetate superfusion, the intracellular acidosis evoked a rise in cytoplasmic [Ca2+] (measured with Fluo3; Fig. 3 A, i). The [Ca2+]i rise was more than that induced by acetate superfusion (compare with Fig. 1C), most likely because rapid H+ uncaging by photolysis induces large pHi transients (Fig. S2) that briefly reach more acidic levels than those recorded after reequilibration (Fig. 3 A, i). These [H+] spikes evoke a much greater Ca2+ release than possible with slower monotonic increases in [H+]i. As with acetate superfusion experiments (Fig. 1C), the H+-evoked [Ca2+]i rise was attenuated by mitochondrial inhibitors (rotenone or antimycin-A), as well as by the glycolytic inhibitor, deoxyglucose (DOG), shown by the left and downward shifts in the pH/Ca2+ relationships (Fig. 3 A, ii). The results suggest that the H+-evoked [Ca2+]i rise is facilitated by glycolysis and mitochondrial respiration. The degree of inhibition was proportional to the reduction in intracellular ATP (ATPi) (Fig. 3 A, iii; measured with a luciferin-luciferase assay). The largest inhibition was obtained with a combination of DOG and antimycin, which also produced the largest depletion of ATPi. Metabolic stress, resulting in ATPi depletion, thus attenuates cytoplasmic Ca2+/H+ coupling.
Competitive Ca2+/H+ Binding Underlies Ca2+/H+ Coupling.
When the area of photolytic H+ uncaging was restricted to a central 5 × 5-μm region of the myocyte, the local H+ load (Fig. 3 B, i) evoked a local [Ca2+]i rise (Fig. 3 B, ii) that, again, could be buffered out by BAPTA (AM-loaded, 100 μM; Fig. 3 B, iii). After local release, the diffusive spread of Ca2+ was slower than for H+ ions, as expected from their cytoplasmic diffusion coefficients (10, 18, 19) and in agreement with a cytoplasmic site for Ca2+ release. Moreover, thapsigargin (10 μM) accelerated Ca2+ mobility (Fig. 3 B, iv), as expected from the inability of blocked SERCA pumps to restrict Ca2+ diffusion. Also in agreement with the previous findings, the [Ca2+]i response was substantially reduced in the presence of the mitochondrial inhibitor myxothiazol (Fig. 3 B, v). Thus, H+-induced Ca2+ release can be evoked both globally and locally within the cytoplasmic compartment, it is metabolically sensitive, and it is not due to Ca2+ release from organelles. Cytoplasmic Ca2+/H+ coupling thus appears to be an intrinsic property of cytoplasm caused by the ion-binding characteristics of its constituent buffers.
To test the possibility that Ca2+/H+ coupling is due to cytoplasmic buffers, a panel of candidate buffers was screened in vitro for Ca2+ unloading upon H+ uncaging. Hemoglobin (Hb), which is rich in H+-binding histidine residues, was chosen to represent a poorly mobile cytoplasmic protein buffer [1 mM Hb matches the intrinsic H+ buffering capacity typically measured in myocytes (4)]. Carnosine (a dipeptide composed of β-alanine and histidine) was selected to represent the group of cytoplasmic histidyl dipeptide (HDP) buffers. These comprise carnosine, anserine (β-alanine-methylhistidine) and homocarnosine (GABA-histidine), and they are collectively present in ventricular myocytes at ∼17 mM [based on biochemical analyses (11, 20, 21) and measurements of mobile H+ buffering capacity (11, 22, 23)]. Individual buffers were mixed with NBA (5 mM) in solutions containing Hepes and BAPTA and the salt form of either cSNARF1 or FuraRed to measure pH or Ca2+, respectively (Fig. 3). To reduce convective movement, solutions were mixed with low-melting-point agarose, heated to 70 °C, aliquoted in 100-μL volumes onto glass coverslips, and allowed to set. UV uncaging of H+ ions into these agarose gels evoked significant unloading of Ca2+ from Hb, carnosine, and ATP (Fig. 3 C, i). These buffer types are thus likely to contribute to cytoplasmic Ca2+/H+ coupling. Importantly, much smaller amounts of Ca2+ were unloaded from ADP or inorganic phosphate (Pi), which may explain the reduction in H+-evoked Ca2+ release observed after metabolic inhibition, when intracellular ATP levels fall and ADP and Pi levels rise. This explanation was tested further by uncaging H+ into gels containing mixtures of carnosine (17 mM) plus phosphates representing a fully energized cytoplasmic compartment [7.5 mM ATP, 20 mM phosphocreatine (PCr)] or a deenergized compartment [7.5 mM ADP, 20 mM creatine (Cr), 27.5 mM Pi] at 100 nM [Ca2+] and 0.75 mM [Mg2+]. Results show that the Ca2+ yield per uncaged H+ was reduced by ∼40% when all ATP and PCr were dephosphorylated (Fig. 3 C, ii). This situation is equivalent to full metabolic inhibition, suggesting that metabolic stress may attenuate cytoplasmic Ca2+/H+ coupling in the intact myocyte, by reducing [ATP]i, and hence the availability of common Ca2+/H+ buffer sites. The reduced coupling in vitro (40%; Fig. 3 C, ii), however, was smaller than obtained in metabolically inhibited myocytes (>90%; Fig. A, ii), implying that additional factors, such as the rise of intracellular [Mg2+] ([Mg2+]i), known to be associated with [ATP]i depletion, may also play a role in intact cells.
H+-Evoked [Ca2+] Microdomain That Does Not Dissipate.
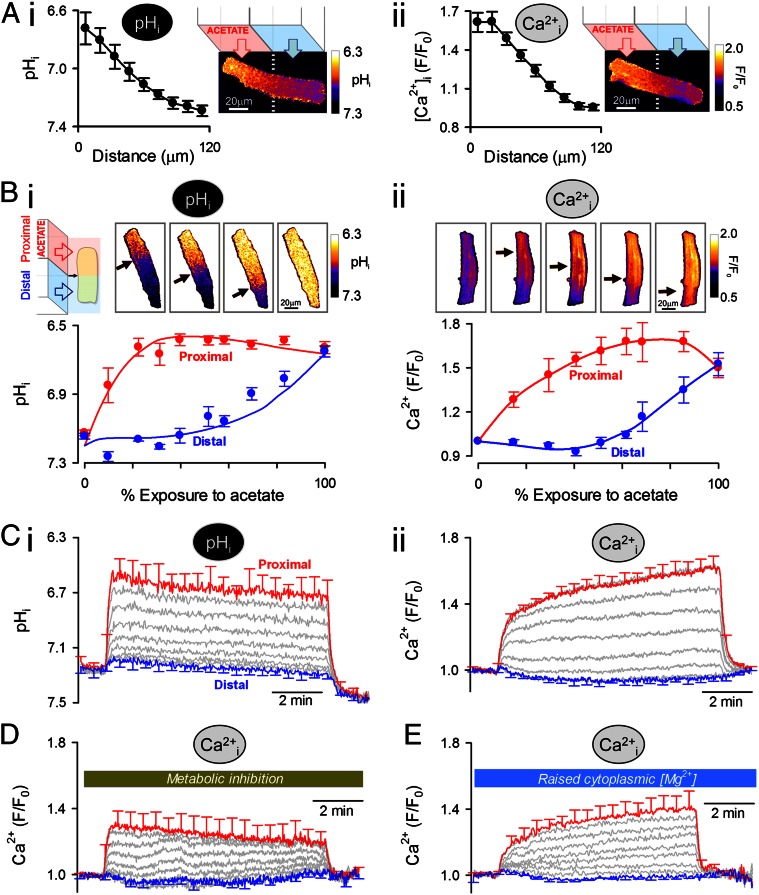
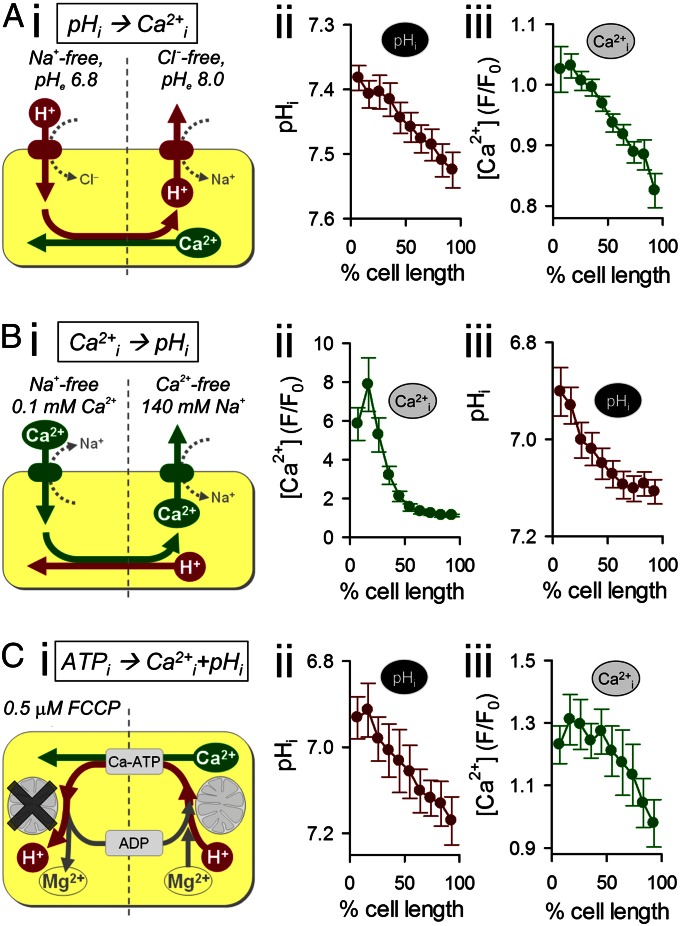
The spatial interaction between H+ and Ca2+ ions was probed by imaging [Ca2+]i in an intact myocyte while imposing a standing cytoplasmic [H+]i gradient induced by continuous exposure of one end of the cell to acetate. The maneuver was performed using a dual-microperfusion device that releases two parallel streams of solution (acetate-containing and acetate-free 0Na-0Ca) oriented perpendicular to a myocyte (14). Sustained entry of acetic acid into the exposed end of the cell, balanced by its exit at the opposite end, generates a pHi gradient of up to 0.6 pHi units (Fig. 4 A, i; imaged after 5 min of dual microperfusion). The magnitude of this gradient depended on the position of the microstream boundary across the cell (Fig. 4 B, i) and remained stable for as long as local acetate exposure was maintained (Fig. 4 C, i). In Fluo3-loaded myocytes, imposing the longitudinal pHi gradient generated a local [Ca2+]i rise in the acidified region of the cell (Fig. 4 A, ii; an end-to-end [Ca2+]i gradient of ∼80 nM). Resting [Ca2+]i thus mapped spatially onto the pHi gradient (Fig. 4B).
Fig. 4.
Stable, spatial [H+]i gradient induces a standing [Ca2+]i gradient. (A) 0Na-0Ca superfusates. A standing pHi gradient (cSNARF1) (i) and a Ca2+ gradient (Fluo3; measured in a separate experiment) (ii) were generated by regional exposure of a myocyte using a dual-microperfusion apparatus (80 mM acetate in proximal microstream). Red and blue arrows show flow direction (n = 15 and n = 18). (B) pHi (i) and Ca2+ (ii), measured separately, plotted for different degrees of acetate exposure and measured after 5 min of dual microperfusion in two regions of the myocyte. The size of regions was the cell width × one-ninth of the cell length (n = 10–20 per bin). The black arrow indicates the position of the microstream boundary. (C) Time-course of (i) pHi and (ii) [Ca2+]i (measured separately) averaged in nine adjacent regions aligned along the length of the myocyte (n = 20). (D) Time course of [Ca2+]i measured under metabolic inhibition (10 μM rotenone, 10 μM antimycin A, 5 mM deoxyglucose) (n = 15). (E) Time course of [Ca2+]i measured in myocytes loaded with fourfold raised [Mg2+]i (exposure to 0Na, 30 mM Mg2+ containing superfusate) (n = 10).
Local H+-evoked unloading of Ca2+ from buffers will release Ca2+ that should then diffuse away. In this model, the local [Ca2+]i gradient should dissipate within a minute or so. However, the observed end-to-end [Ca2+]i gradient was sustained, and actually increased in amplitude over a period of several minutes (Fig. 4 C, ii), even though the pHi gradient remained constant (Fig. 4 C, i), suggesting that Ca2+ was being transported uphill into the acidic zone at a rate that exceeded its dissipative flux in the opposite direction. A sustained acid microdomain within the cytoplasmic compartment of a ventricular myocyte thus induces a sustained Ca2+ microdomain. The [Ca2+] gradient was abolished in 100 μM BAPTA-AM–loaded cells (Fig. S3A) but was unaffected by maneuvers that alter sarcolemmal Ca2+ flux, suggesting it was maintained via an intracellular mechanism. Such maneuvers included raising microstream [Na+] from zero to 140 mM (Fig. S3B), with or without a concurrent 1 mM rise in microstream [Ca2+] (Fig. S3C), or electrically pacing cells at 2 Hz in buffer containing 140 mM Na+ and 1 mM Ca2+ (Fig. S3D). The plasmalemmal Ca2+/H+ ATPase pump (PMCA), which potentially remained functional under all the above experimental conditions, could conceivably have coupled intracellular microdomains of [H+] and [Ca2+], but this requires sarcolemmal Ca2+ influx to raise [Ca2+]i locally in the acid microdomain. Given that [Ca2+]i gradients were readily measured in the absence of extracellular Ca2+, this mechanism is unlikely. Nevertheless, although PMCA did not cause the [Ca2+]i gradient, neither did it prevent the sustained [Ca2+]i rise in acidic zones, suggesting that its activity was downregulated by the fall of pHi (24). Indeed this appears to have been the case, as inhibiting PMCA further (achieved by raising extracellular [Ca2+] to 8 mM) increased [Ca2+]i gradients only modestly (Fig. S3E). The involvement of the SR or mitochondria in maintaining the [Ca2+]i gradient was tested pharmacologically. Thapsigargin (10 μM) or the combination of CGP-37157 (20 μM) plus Ru360 (5 μM) did not collapse the Ca2+ microdomain (Fig. S3F), suggesting that uphill Ca2+ delivery to the acidic domain was neither SR- nor mitochondrion-dependent. Uphill Ca2+ transport into the acidic microdomain (i.e., Ca2+ flux up the spatial [H+]i gradient) must have involved endogenous cytoplasmic molecules.
Cytoplasmic H+ ion mobility is mediated through reversible H+ binding to small diffusible buffers, such as HDPs (11, 25). The imposition of a stable [H+]i gradient using dual microperfusion will therefore result in a large and continuous diffusive flux of unprotonated mobile buffer molecules into the acidic region, where they will be loaded with H+ ions. If an unprotonated molecule were able reversibly to bind Ca2+ ions more readily than the protonated molecule, [Ca2+]i would build up in the acidic zone (Fig. S4). In effect, H+ ions would displace Ca2+ from the incoming buffer. Buffer molecules that had discharged Ca2+ and acquired H+ ions would then diffuse to more alkaline regions of the cell, where the reverse process would occur (i.e., discharge of H+ ions in exchange for Ca2+ binding). The flux of Ca2+-loaded buffer into the acidic microdomain would thus be maintained indefinitely, allowing a local elevation of Ca2+ that did not dissipate spatially. On the basis of their low molecular weight and pH-sensitive Ca2+-binding properties (Fig. 3C), HDPs and ATP could mediate this Ca2+/H+ exchange. Performing dual microperfusion during metabolic inhibition of a myocyte (with a combination of rotenone, antimycin, and DOG) did not affect the size of the imposed [H+]i gradient [0.52 ± 0.05 pHi units (n = 5) vs. 0.56 ± 0.04 control; Fig. S5A], but it collapsed the end-to-end [Ca2+]i gradient by ∼60% (Fig. 4D). Metabolic inhibition did not affect total cellular HDP content (in vitro assay of lysates of control and DOG-treated hearts: 1.09 ± 0.12 and 1.04 ± 0.15 mM HDPs per gram of protein, respectively) (25), but it reduced [ATP]i by up to 95% (Fig. 3 A, iii). Collapse of the H+-induced [Ca2+]i gradient may result from the depletion of diffusible ATP molecules, or from a reduction in the Ca2+-carrying capacity of diffusible HDPs.
To test the effect of reducing the Ca2+-binding capacity of HDPs, advantage was taken of their similar affinity for Ca2+ and Mg2+. This feature is not evident among other intracellular Ca2+ buffer molecules, such as troponin or calmodulin. Because the Mg2+ affinity of HDPs is ∼0.7 mM (26), any rise in [Mg2+]i from its resting level of 0.75 mM will decrease HDP Ca2+ occupancy. Myocytes (in Ca2+-free media) were Mg2+-loaded by raising extracellular [Mg2+] from 1 to 30 mM and removing extracellular Na+ (27). This maneuver increased MagFluo4 fluorescence fourfold with a time constant of 3 min (Fig. S5B), without changing pHi or greatly altering [Ca2+]i (which increased by ∼10%). Applying dual microperfusion to a myocyte with raised [Mg2+]i did not change the imposed pHi gradient (0.53 ± 0.1 pH units; n = 4), but it reduced the overlying end-to-end [Ca2+]i gradient by ∼40% (Fig. 4E), suggesting that when the Ca2+-carrying capacity of HDPs is compromised, so is the uphill recruitment of Ca2+ to an acidic microdomain. The observation provides evidence for the participation of HDPs in sustaining a Ca2+ microdomain. Metabolic inhibition is known to raise [Mg2+]i and deplete ATPi (by liberating Mg2+ during net MgATP hydrolysis); thus, these changes now provide a mechanism for the attenuation of the Ca2+ microdomain by metabolic inhibitors (Fig. 4D), through reduction of available mobile Ca2+/H+-binding sites.
Testing the Cytoplasmic Ca2+/H+ Exchange Hypothesis.
The cytoplasmic Ca2+/H+ exchange hypothesis was tested further with three experimental maneuvers that did not involve regional acetate exposure. In the first maneuver, a stable [H+]i gradient was generated in a myocyte by stimulating sarcolemmal acid/base transporters. Using dual microperfusion (Fig. 5 A, i), net acid extrusion via NHE was stimulated at one end of the cell (with a Cl−-free superfusate of high pH) and net acid influx via CHE was stimulated at the other end (with a Na+-free superfusate of low pH). This maneuver resulted in a stable, end-to-end [H+]i gradient of 0.14 pHi units (Fig. 5 A, ii). The [H+]i gradient then induced a stable [Ca2+]i gradient of 0.2 F/F0 units (∼25 nM Ca2+; Fig. 5 A, iii). Thus, as with local acetate exposure, cytoplasmic [Ca2+] maps stably onto a transporter-induced pHi gradient.
Fig. 5.
Testing the cytoplasmic Ca2+/H+ exchanger hypothesis. (A) Longitudinal, standing [H+] gradient established by activating acid influx on Cl−/OH− exchange and acid efflux on Na+/H+ exchange, at opposite ends of the myocyte, using dual microperfusion. The [H+]i gradient produces a standing [Ca2+] gradient (measured in separate experiments; n = 12). (B) Longitudinal, standing [Ca2+] gradient established by activating Na+/Ca2+ exchange in inward and outward modes at opposite ends of cell using dual microperfusion. The [Ca2+]i gradient produces a standing [H+]i gradient (measured in separate experiments; n = 8). (C) Metabolic inhibition confined to one end of the cell produces a local, sustained rise in [H+]i and [Mg2+]i, which then drives longitudinal ATP/ADP exchange in the cytoplasm. This generates a localized rise of [Ca2+]I in the metabolically challenged end of the cell (pHi and [Ca2+]i measured separately; n = 7).
A second maneuver was used to investigate whether cytoplasmic Ca2+/H+ exchange could work in reverse and generate an [H+]i gradient in response to an experimentally imposed cytoplasmic [Ca2+] gradient (Fig. 5 B, i). Dual microperfusion delivered Na+-free solution containing 100 μM Ca2+ to one end of the cell (stimulating net Ca2+ influx by NCX) and Ca2+-free solution containing 140 mM Na+ to the other end (stimulating net Ca2+ efflux by NCX). This led, in the steady state, to a large longitudinal [Ca2+]i gradient of ∼7 F/F0 units (equivalent to ∼5 μM Ca2+; Fig. 5 B, ii), which, in turn, led to an end-to-end pHi gradient of 0.2 units (Fig. 5 B, iii). Thus, under appropriate conditions, pHi maps stably onto a transporter-induced [Ca2+]i gradient.
Given that global metabolic inhibition collapses an H+-evoked Ca2+ microdomain (Fig. 4D), a third maneuver was devised to test if a spatially localized metabolic microdomain could induce microdomains for [Ca2+] and [H+]. Using dual microperfusion, one-half of a myocyte was exposed to FCCP (0.5 μM) to trigger net ATP hydrolysis locally and drive the reverse reaction at the opposite end of the cell. ATP diffusion into the FCCP-treated end, in exchange for ADP, completes the cycle. This experimental maneuver produced stable overlying gradients of [H+]i and [Ca2+]i (Fig. 5C). The [H+]i gradient may be explained by H+ release during ATP hydrolysis at one end of the cell and H+ consumption by the reverse reaction at the other. The [Ca2+] gradient is explained by two processes. First, diffusion of CaATP (and MgATP) will deliver Ca2+ (and Mg2+) to the metabolically inhibited end of the cell, whereupon ATP is hydrolyzed and the divalent cations are released. Second, the local elevation of [H+]i and [Mg2+]i resulting from ATP hydrolysis will cause H+-loaded and Mg2+-loaded HDPs to diffuse out toward the metabolically functional end of the cell, in exchange for Ca2+-bound buffer. Thus, a localized zone of metabolic stress is signified by a localized and sustained rise of both [Ca2+]i and [H+]i.
Therefore, three very different experimental maneuvers support predictions of a mobile cytoplasmic Ca2+/H+ exchange.
Discussion
The present work establishes that the spatial homogeneity of resting (diastolic) Ca2+ in ventricular myocytes is labile, being readily disturbed by changes in [H+]i. Both local and global elevations of cytoplasmic [H+]i increase cytoplasmic [Ca2+]i, but the surprising observation is that when localized acidosis is sustained, it produces an equally localized and sustained rise of [Ca2+]i that does not dissipate spatially. Although this latter reaction requires the uphill transport of Ca2+ ions, it is an intrinsic property of cytoplasm. Local pHi nonuniformity is known to occur within cells, as discussed elsewhere in this paper; thus, spatial Ca2+/H+ interaction is likely to be a frequent event. The coupling is not dependent on Ca2+ movement at organelles like the SR, acidic stores, or mitochondria. It occurs within seconds; as a result, cytoplasmic Ca2+ in the myocyte maps spatially onto pHi. The reaction is physiologically relevant because nanomolar displacements of [H+]i (equivalent to fractions of a pHi unit) lead to nanomolar changes in [Ca2+]i. The H+-evoked rise of [Ca2+]i is caused by these ions sharing common intracellular buffer sites on proteins and other molecules. However, the ability of Ca2+ to map stably onto spatially varied [H+]i is dependent on a subclass of small molecules that display competitive Ca2+/H+ binding but are also diffusible. Notable among these are HDPs and ATP. By exchanging Ca2+ for H+, these molecules act like local pumps within cytoplasm, producing spatial uphill Ca2+ transport in response to local [H+]i gradients. We evaluate here the cytoplasmic source of Ca2+/H+ coupling, its spatial properties, and its physiological significance.
Cytoplasmic Buffers Underlie Cytoplasmic Ca2+/H+ Coupling.
Resting [Ca2+]i (normally ∼100 nM) sets the background level of Ca2+ signaling in a cell and determines the threshold for triggering more elaborate spatiotemporal patterns of signaling. In the present work, we have shown that reducing resting pHi by about 0.6 units using acetate superfusion (equivalent to an [H+]i elevation of 190 nM; Fig. 1A), raises resting [Ca2+]i by about 60 nM (Fig. 1C). Both Ca2+ release from mitochondria and functional coupling between sarcolemmal NHE and NCX transporter activities have previously been postulated as important underlying mechanisms (9, 28). We find, however, that although a rise of mitochondrial matrix [Ca2+] occurs in response to low cytoplasmic pH, significant Ca2+ efflux does not ensue (Fig. 2). Similarly, Ca2+ release from the SR or acidic stores, or [Ca2+]i elevation via sarcolemmal NHE/NCX coupling, has also been excluded, although the latter mechanism is known to enhance electrically evoked Ca2+ transients during acidosis (7, 8). Instead, diastolic Ca2+/H+ coupling in the ventricular myocyte relies on buffer molecules with H+-sensitive Ca2+ affinity. As a result, diastolic Ca2+ and H+ levels rise and fall in tandem. This coupling (9, 29, 30) was first mooted more than 30 y ago, but with no evidence for the nature or identity of the Ca2+/H+-binding sites. We now show that Ca2+ unloading from histidyl molecules is likely to be a key component. Histidine residues are sited on many proteins within the cytoplasmic compartment, including known Ca2+ buffers like troponin, calmodulin, and SERCA, as well as the smaller, more readily diffusible HDP molecules like carnosine, anserine, and homocarnosine (∼250 Da). In addition, we have demonstrated ATP to be an important H+-releasable source of Ca2+ (31) (Fig. 3C). Ca2+ release from proteins and HDPs may involve binding of H+ and Ca2+ ions to common sites (e.g., at imidazole groups), or it may involve a more sophisticated intramolecular reorganization of the buffer molecule that changes binding affinities.
The effective Ca2+ affinity of HDPs is modulated by Mg2+ as well as H+ ions. A rise in [Mg2+]i will displace Ca2+ because, unusually among cytoplasmic mobile Ca2+ buffers, both ions have similar binding affinities. Displacement will then reduce the functional expression of cytoplasmic Ca2+/H+ coupling in a manner analogous to the effects of competitive inhibitors on membrane transporter proteins. Metabolic inhibition also reduces the H+-sensitivity of diastolic [Ca2+] (Fig. 3 A, ii), an effect caused partly by the fall in [ATP]i (which reduces the availability of Ca2+/H+-binding sites) and partly by the rise of [Mg2+]i under these conditions (which reduces Ca2+ binding by HDPs). Thus, cytoplasmic Ca2+/H+ coupling exhibits plasticity through its sensitivity to the cell’s energetic status.
Cytoplasmic Ca2+/H+ Exchange: A Pump Without a Membrane.
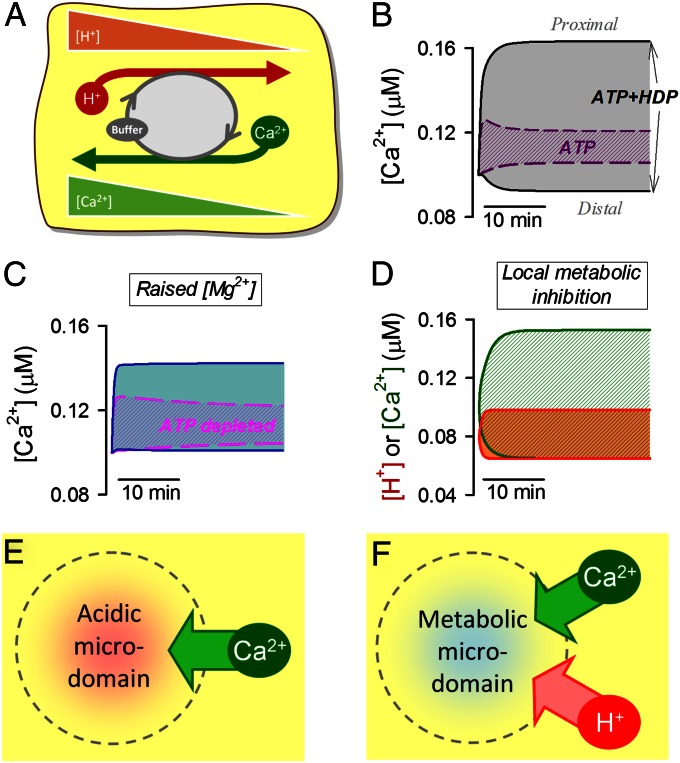
The present work has demonstrated that a spatial cytoplasmic [Ca2+] microdomain maps automatically onto an H+ microdomain, and vice versa. Although the H+-evoked Ca2+ response occurs within seconds, the spatially confined Ca2+ microdomain is sustained for the lifetime of the H+ microdomain (tested here for several minutes). Theoretically, an 80-nM longitudinal [Ca2+]i gradient, such as that observed during dual microperfusion (Fig. 4 A, ii), could be generated by sarcolemmal Ca2+ influx (at 10 μM/min) into the acidic end of the cell, coupled with an equal Ca2+ efflux from the nonacidic end. However, because the same [Ca2+] nonuniformity is observed under different bathing conditions (varying extracellular [Na+] and [Ca2+]; Fig. S3), such a sarcolemmal flux arrangement is highly unlikely, particularly because the [Ca2+]i gradient persists in Ca2+-free media. Instead, spatial Ca2+/H+ coupling must involve a cytoplasmic mechanism. This requires cytoplasmic Ca2+/H+ buffer molecules of adequate mobility (i.e., of low molecular weight). The [H+]i gradient drives a diffusive flux of protonated buffer out of the acidic region, and a counterflux of unprotonated buffer (Fig. 6A). Due to competitive Ca2+/H+ binding, the counterflux delivers Ca2+ to the acidic microdomain, thus offsetting the tendency for Ca2+ to diffuse away. For the process to occur optimally, the concentrations of protonated and unprotonated buffer must be similar (i.e., the mobile buffer’s acid dissociation constant pKH must be in the physiological pH range) and the Ca2+ affinity of the unprotonated buffer must be sufficient to carry a significant Ca2+ cargo. All these conditions are satisfied for cytoplasmic HDPs (26), which spatially traffic most of the diffusive H+ flux within the cell (11, 14, 25), and for ATP. Although Fluo3 is technically a Ca2+/H+ buffer (32), it is not capable of generating the observed Ca2+ gradients because of its low cytoplasmic concentration, low intracellular diffusion coefficient (19), and low H+ affinity (32) (Mathematical Supplement). The uphill recruitment of Ca2+ ions via HDP and ATP molecules to an acidic microdomain is thus coupled to a downhill counterflux of H+ ions via the same buffers. Provided the spatial gradient for H+ ions is maintained by some other source, so is the uphill recruitment of Ca2+, financed thermodynamically by the energy stored in the spatial [H+]i gradient. The phenomenon is therefore an example of a simple Ca2+/H+ exchange ion transporter, operating spatially within the cytoplasmic compartment. The diffusible Ca2+/H+ carriers act like a pump without a membrane (Fig. 6A).
Fig. 6.
Modeling cytoplasmic Ca2+/H+ exchange. Acidic microdomains actively recruit Ca2+ ions, and metabolic microdomains recruit both Ca2+ and H+ ions. (A) Cartoon shows the role of mobile Ca2+/H+ buffers (i.e., HDPs, ATP) in producing and sustaining [Ca2+]i nonuniformity, generated by a longitudinal [H+] gradient (or vice versa). (B) Mathematical model simulates [Ca2+]i at either end of a model myocyte during an imposed [H+] gradient (pH 6.5 at the proximal end and pH 7.2 at the distal end). Simulation with 7.5 mM ATP (dashed line) or 7.5 mM ATP and 17 mM HDP (solid line). HDP and ATP are able to support a standing [Ca2+] gradient during imposed [H+] nonuniformity. (C) Simulation with 7.5 mM ATP plus 17 mM HDP when [Mg2+]i is raised fourfold (solid line) or when ATP is depleted and [Mg2+]i is raised by 7 mM (dashed line). [Ca2+] gradients are greatly reduced. (D) Simulation with 17 mM HDP. One-half of the cell is a net consumer of ATP (15 mM/min) and the other half a net producer of ATP (15 mM/min); the cell-averaged concentration [ATP] is 7.5 mM. Standing [Ca2+] and [H+] gradients develop. (E) Local acidic microdomain will recruit Ca2+ via cytoplasmic Ca2+/H+ exchange. (F) Metabolic microdomain (i.e., local zone of net MgATP hydrolysis) will recruit both Ca2+ and H+ ions.
The formalism more generally used to describe the kinetics of membrane transport can also be applied to cytoplasmic Ca2+/H+ exchange. Vmax can be approximated from the mobile buffer’s concentration (C), diffusion coefficient (D), and distance (l) as 1/2 × C × D/l2. At resting cytoplasmic pH and Ca2+ levels, the H+:Ca2+ stoichiometry (SH:Ca) is ∼2,400:1 for HDPs and ∼40:1 for ATP (derived from the buffer’s H+ and Ca2+ occupancy). The size of [Ca2+] nonuniformity (Δ[Ca2+]) can be predicted from the [H+] gradient (Δ[H+]) using an approximation featuring SH:Ca, H+, and Ca2+ buffering ratio (βH ∼ 1:200,000; βCa ∼ 1:200), as well as a variable (αH or αCa) that describes the fraction of H+ or Ca2+ flux carried by mobile Ca2+/H+ buffer (as opposed to noncompetitively binding mobile buffers):
Thus, a rise in [Mg2+]i, by increasing SH:Ca, reduces [H+]-evoked [Ca2+] gradients (Fig. 4D). In response to an [H+] gradient, HDPs can produce a large [Ca2+] gradient because the majority of diffusive H+ flux is carried by these buffers (i.e., αH close to 1). Although ATP is a minor H+ buffer, it contributes toward [Ca2+] gradient formation because of its lower SH:Ca. In contrast, [Ca2+] gradients will typically produce substantially smaller [H+] gradients because Ca2+ buffers with high SH:Ca (e.g., HDPs) carry only a small fraction of diffusive Ca2+ flux (i.e., αCa << 1; HDPs hold 1% of total cytoplasmic Ca2+).
We have further tested the Ca2+/H+ buffer hypothesis by comparing experimental data with predictions of a diffusion-reaction model (Mathematical Supplement). This features pH-sensitive Ca2+ binding to large, essentially immobile Ca2+ buffers (troponin, calmodulin, and SERCA), plus smaller mobile ATP and HDP molecules (Table S1). The model can be used to simulate [Ca2+]i within a myocyte during an imposed longitudinal [H+]i gradient of 0.6 pHi units, as attained experimentally (Fig. 4). The presence of immobile buffers alone predicts an initial [Ca2+]i rise in the acidic zone of the cell, but this dissipates by means of diffusion. A sustained Ca2+ rise, however, occurs when ATP (7.5 mM) is included in the model and, to a much greater extent, when HDPs (17 mM) are included (Fig. 6B). A combination of the two mobile buffers accurately simulates the experimentally observed sustained [Ca2+]i gradient (Fig. 6B). The diffusion-reaction model predicts attenuation of the Ca2+ gradient on elevation of [Mg2+]i (Fig. 6C) or on adding metabolic inhibitors that deplete ATPi with a concurrent rise in [Mg2+]i (Fig. 6C). Finally, the model predicts that a localized cytoplasmic region of metabolic stress will induce a sustained local rise of both [Ca2+]i and [H+]i (Fig. 6D), again as observed experimentally (Fig. 5C). The localization and stabilization of H+ and Ca2+ microdomains via spatial cytoplasmic Ca2+/H+ exchange are thus supported both experimentally and theoretically.
Physiological Importance of Ca2+/H+ Coupling and the Cytoplasmic Ca2+/H+ Exchanger.
The ability of cytoplasmic buffers to raise diastolic [Ca2+]i rapidly in response to a rise of [H+]i has important implications for cardiac function. Because H+ ions are end products of metabolism, cytoplasmic Ca2+/H+ coupling may provide an efficient means of matching metabolic activity with an appropriate basal Ca2+ signal. Many Ca2+-activated cellular process, such as excitation, contraction, and gene transcription, are inhibitable by H+ ions; thus, a positive coupling between [H+] and diastolic [Ca2+] will help to sustain Ca2+ activation in the face of a metabolic challenge. Additional effects of acidosis on dynamic Ca2+ signaling, routed through NHE and NCX activity, have been well characterized (7, 8), but these latter effects will be superimposed on the H+-activated control of diastolic Ca2+ described here.
The strong spatial coupling between [H+]i and [Ca2+]i in cardiac myocytes implies that any localized H+ microdomain has the potential to generate an overlying Ca2+ microdomain (shown schematically in Fig. 6E). Spatial pHi gradients of 0.1–0.2 pHi units can be generated in myocytes by sarcolemmal NHE activity (4, 13, 33), and Eq. 1 suggests that these will be associated with a [Ca2+]i gradient of 30–60 nM (note that the cytoplasmic Ca2+/H+ exchanger’s Vmax increases with sharper pHi gradients over shorter distances). Cytoplasmic Ca2+/H+ exchange could enhance the transport of Ca2+ toward mitochondria, where a local [H+] gradient [∼0.5 pH units (34)] stretches from the acidic intermembrane space (IMS) to the more alkaline cytoplasmic domain. The IMS is readily accessed by ATP and other small buffer molecules (35), implying that diffusible Ca2+/H+ exchange may promote an uphill flux of cytoplasmic Ca2+ into this region, thereby facilitating mitochondrial Ca2+ uptake via the uniporter. Spatial gradients of pHi in the heart may also arise from regional differences in metabolic rate and capillary perfusion (4). For example, under localized ischemia, extracellular gradients of membrane-permeant weak acids (CO2 and lactic acid) will generate large and sustained standing gradients of pHi (14), which are likely to extend over many cell lengths in the myocardium. Because HDPs and ATP permeate gap junctions (36), pHi gradients between electrically coupled cells would then be expected to drive uphill, cell-to-cell Ca2+ flux into the acidic region, via cytoplasmic Ca2+/H+ exchange over a wider spatial range.
Cytoplasmic domains of elevated [Ca2+] may drive an uphill H+ flux, provided the local [Ca2+] gradient is of sufficient magnitude and persistence. Cyclical Ca2+ release events locally raise dyadic [Ca2+] into the high micromolar range (37), which is predicted to reduce [MgATP] and [ATP2−] and to raise [CaATP] (37, 38). A similar redistribution of buffer states is expected for HDPs. Consequently, SR Ca2+ release, by raising dyadic [Ca2+], may drive local Ca2+/H+ exchange, thereby elevating dyadic [H+] and possibly influencing RyR gating. Because rapid pH responses will be smoothed by high overall cytoplasmic H+ buffering, cyclical changes in dyadic [Ca2+] may translate into a more tonic dyadic [H+] rise. For example, a time-averaged dyadic [Ca2+] of 50 μM could raise [H+] by 1 μM if HDPs were responsible for mediating 1% of passive Ca2+ efflux out of the dyad (Eq. 2).
A further degree of complexity is introduced by the sensitivity of cytoplasmic Ca2+/H+ exchange to the cell’s metabolic state, through dynamic changes in ATP concentration and related changes in [Mg2+]i. Indeed, localized ATPi depletion (metabolic stress, either within a cell or regionally within the myocardium), by acting on the Ca2+/H+ exchange mechanism (compare with Fig. 5C), may contribute to the elevated levels of intracellular Ca2+ and H+ typically observed in localized ischemia (Fig. 6F).
Mobile Ca2+/H+ buffers are not unique to the heart; thus, many other cell types are likely to show spatial Ca2+/H+ exchange reactions. ATP is a universal molecule present in all cells, and HDPs are present at high concentrations in skeletal muscle (20), glia, and neurons (39). Furthermore, in addition to cardiac myocytes (4, 13, 14), spatial pHi nonuniformity has been reported, at least transiently, in epithelial cells (40), glia (41), migrating tumor cells (42), and molluscan and mammalian neurons (43, 44), whereas membrane acid transport has been reported to generate pHi microdomains in heterologous expression systems (45). In some cases, these pHi microdomains have been associated with local Ca2+ gradients (43). We have thus demonstrated a spatial paradigm linking the behavior of intracellular H+ and Ca2+, likely to be of fundamental importance in the control of cell signaling and function.
Materials and Methods
Isolation of Ventricular Myocytes and Mitochondria.
Enzymic isolation of myocytes from rat heart ventricles was performed using a previously published method (14). Animals were killed by means of schedule I killing, as approved by the UK Home Office and departmental guidelines (Department of Physiology, Anatomy and Genetics, Oxford). Mitochondrial isolation was performed according to the method of Bednarczyk et al. (46).
Solutions.
Normal Tyrode solution: 140 mM NaCl, 4.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 11 mM glucose, 10 mM Hepes (pH 7.4) at 37 °C. 0Na-0Ca solution: Na+ replaced with 140 mM N-methyl-d-gluconate and CaCl2 replaced with 0.5 mM EGTA. Pipette-filling solution: 110 mM KCl, 5 mM NaCl, 5 mM MgATP, 5 mM phosphocreatine, 1 mM NaGTP, and 10 mM Hepes (titrated to pH 7.2 with 1 M KOH). High-K+ “intracellular solution” for superfusing permeabilized cells: 100 mM K-aspartate, 30 mM KCl, 15 mM Hepes, 1 or 5 mM EGTA, 0.5 mM pyruvate, 0.5 mM malate, 0.25 mM K-ADP, 5 mM Mg-ATP, 5 mM Na-phosphocreatine, 0.5 mM K-phosphate, and 4% (wt/vol) dextran (pH at 6.6 or 7.2), and [Ca2+] and [Mg2+] were adjusted appropriately using CaBuf software (G. Droogmans, Leuven, Belgium). Acetate-containing solutions had equivalently reduced Cl−. Total Ca2+ in Ca2+-containing acetate solutions was raised ∼20% to compensate for Ca2+-acetate binding ([Ca2+]i measured by electrode). Bathing solution for mitochondria: 140 mM KCl, 2 mM malate, 2 mM glutamate, 10 mM Hepes (pH 7.2), 1 mM MgCl2, and 10 μM BAPTA (sparingly low Ca2+ buffering to improve resolving power of dye to detect Ca2+ release), and measured [Ca2+]i at pH 7.2 was 130 nM (based on calibration of FuraRed fluorescence). High K+ buffer for resuspending mitochondria: 140 mM KCl, 10 mM Hepes, 1 mM EGTA, and 1 mM MgCl2 (pH 7.2).
Superfusion, Dual Microperfusion, and Fluorescence Imaging of Myocytes.
AM loading of dyes or buffers was performed in nonsuperfused solution at room temperature. Superfusion (37 °C) was performed in Perspex chambers mounted on a Leica TCS NT confocal imaging microscope (14). Dual microperfusion was performed on myocytes at a right angle to the microstream boundary using a previously published method (14). One microstream contained, in addition, 15 mM sucrose to help visualize the interstream boundary. Intracellular fluorescence was measured with the following settings: cSNARF1 (10-min loading): excitation at 514 nm, emission at 580 nm and 640 nm; Fluo3 and MagFluo4 (5-min loading): excitation at 488 nm, emission at >520 nm; Indo1 (30-min loading): excitation at 361 nm, emission at 405 nm and 465 nm; and Rhod2: excitation at 514 nm and emission at 580 nm. The Fluo3 Kd was estimated in situ to be 0.84 μM by pipette-loading cells with 10 μM to measure maximal fluorescence (Fmax) relative to F0 (Fmax/F0 = 9.3 ± 0.3). For simultaneous Indo1/cSNARF1 measurements, cSNARF1 fluorescence was corrected for Indo1 bleed-through. The pHi in intact cells was calibrated using nigericin (10 μM). Ca2+ measured in permeabilized cells with Rhod2 was calibrated with ionomycin (10 µM).
Fluorimetry on Mitochondrial Suspensions.
Mitochondria (1–2 g of protein per liter) were AM-loaded with Indo1 (20 μM for 20 min) to measure matrix [Ca2+] and then resuspended in Indo1-free media containing FuraRed (20 μM free acid) to measure extramitochondrial [Ca2+]. The measurement artifact associated with the pH change was established in mitochondria-free solutions and subtracted from mitochondria-containing recordings. Indo1 was measured at an excitation of 340 nm and emission of 410 and 490 nm; FuraRed was measured at an excitation of 430 and 460 nm and an emission of 615 nm. Measurements performed on a Hitachi F4500 fluorescence spectrophotometer.
Myocyte Permeabilization.
Cell surface membrane permeabilization was performed by a 15-s exposure to 0.005% saponin in high-K+ internal solution. Cells were subsequently superfused with intracellular solution.
Photolytic UV Uncaging of Acid.
The photolabile donor NBA in ethanol stock was dissolved in solutions at 1–5 mM. Uncaging was triggered with 361-nm laser-line (18) scanning at 400 Hz.
In Vitro Assays for H+/Ca2+ Buffers.
Solutions were based on 140 mM KCl, 5 mM NBA, 10 mM Hepes, and 150 μM BAPTA to buffer background Ca2+. Solutions were prepared at a 200% concentration, warmed to 40 °C, and mixed 1:1 with warm 2% low-gelling-point agarose solution. They were then allowed to cool and set on coverslips (10 μL) for uncaging and pH/Ca2+ imaging.
Supplementary Material
Acknowledgments
This study was funded by British Heart Foundation Programme Grant RG/08/016 (to R.D.V.-J.), by National Institutes of Health Grant 5R37HL042873 (to K.W.S.), and by a fellowship from the Royal Society (to P.S.). We thank Dr. Alzbeta Hulikova for performing the ATP assays and Dr. Robert Wilkins for access to the spectrophotometer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222433110/-/DCSupplemental.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28(1-4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46(3):318–331. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Mandel F, Kranias EG, Grassi de Gende A, Sumida M, Schwartz A. The effect of pH on the transient-state kinetics of Ca2+-Mg2+-ATPase of cardiac sarcoplasmic reticulum. A comparison with skeletal sarcoplasmic reticulum. Circ Res. 1982;50(2):310–317. doi: 10.1161/01.res.50.2.310. [DOI] [PubMed] [Google Scholar]
- 6.Choi HS, Trafford AW, Orchard CH, Eisner DA. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J Physiol. 2000;529(Pt 3):661–668. doi: 10.1111/j.1469-7793.2000.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bountra C, Vaughan-Jones RD. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+ and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992;262(2 Pt 1):C348–C357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- 9.Gambassi G, et al. Effects of acidosis on resting cytosolic and mitochondrial Ca2+ in mammalian myocardium. J Gen Physiol. 1993;102(3):575–597. doi: 10.1085/jgp.102.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan-Jones RD, Peercy BE, Keener JP, Spitzer KW. Intrinsic H(+) ion mobility in the rabbit ventricular myocyte. J Physiol. 2002;541(Pt 1):139–158. doi: 10.1113/jphysiol.2001.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassani RA, Bassani JW, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swietach P, Vaughan-Jones RD. Spatial regulation of intracellular pH in the ventricular myocyte. Ann N Y Acad Sci. 2005;1047:271–282. doi: 10.1196/annals.1341.024. [DOI] [PubMed] [Google Scholar]
- 14.Swietach P, Leem CH, Spitzer KW, Vaughan-Jones RD. Experimental generation and computational modeling of intracellular pH gradients in cardiac myocytes. Biophys J. 2005;88(4):3018–3037. doi: 10.1529/biophysj.104.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 16.Higgins ER, Cannell MB, Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys J. 2006;91(1):151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326(5949):144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swietach P, Spitzer KW, Vaughan-Jones RD. pH-Dependence of extrinsic and intrinsic H(+)-ion mobility in the rat ventricular myocyte, investigated using flash photolysis of a caged-H(+) compound. Biophys J. 2007;92(2):641–653. doi: 10.1529/biophysj.106.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordeiro JM, et al. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol. 2001;531(Pt 2):301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Dowd JJ, Robins DJ, Miller DJ. Detection, characterisation, and quantification of carnosine and other histidyl derivatives in cardiac and skeletal muscle. Biochim Biophys Acta. 1988;967(2):241–249. doi: 10.1016/0304-4165(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 21.House JR, Miller DJ, O’Dowd JJ. Differences in the distribution of the imidazoles of rat heart between atria and ventricles. J Physiol. 1989;417:162P. [Google Scholar]
- 22.Swietach P, Vaughan-Jones RD. Relationship between intracellular pH and proton mobility in rat and guinea-pig ventricular myocytes. J Physiol. 2005;566(Pt 3):793–806. doi: 10.1113/jphysiol.2005.086165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Dowd JJ, Cairns MT, Trainor M, Robins DJ, Miller DJ. Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain. J Neurochem. 1990;55(2):446–452. doi: 10.1111/j.1471-4159.1990.tb04156.x. [DOI] [PubMed] [Google Scholar]
- 24.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 25.Swietach P, Camelliti P, Hulikova A, Kohl P, Vaughan-Jones RD. Spatial regulation of intracellular pH in multicellular strands of neonatal rat cardiomyocytes. Cardiovasc Res. 2010;85(4):729–738. doi: 10.1093/cvr/cvp343. [DOI] [PubMed] [Google Scholar]
- 26.Baran EJ. Metal complexes of carnosine. Biochemistry (Mosc) 2000;65(7):789–797. [PubMed] [Google Scholar]
- 27.Almulla HA, Bush PG, Steele MG, Ellis D, Flatman PW. Loading rat heart myocytes with Mg2+ using low-[Na+] solutions. J Physiol. 2006;575(Pt 2):443–454. doi: 10.1113/jphysiol.2006.109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: Its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985;17(11):1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM, Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- 30.Kohmoto O, Spitzer KW, Movsesian MA, Barry WH. Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res. 1990;66(3):622–632. doi: 10.1161/01.res.66.3.622. [DOI] [PubMed] [Google Scholar]
- 31.Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol. 1997;272(5 Pt 1):C1739–C1747. doi: 10.1152/ajpcell.1997.272.5.C1739. [DOI] [PubMed] [Google Scholar]
- 32.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264(14):8171–8178. [PubMed] [Google Scholar]
- 33.Garciarena CD, Ma YL, Swietach P, Huc L, Vaughan-Jones RD. J Physiol. 2013. Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3−co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. jphysiol.2012.249664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong JW, Zhu L, Jiao X, Liu SS. Evidence for DeltapH surface component (DeltapH(S)) of proton motive force in ATP synthesis of mitochondria. Biochim Biophys Acta. 2010;1800(3):213–222. doi: 10.1016/j.bbagen.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Yurkov VI, Fadeeva MS, Yaguzhinsky LS. Proton transfer through the membrane-water interfaces in uncoupled mitochondria. Biochemistry (Mosc) 2005;70(2):195–199. doi: 10.1007/s10541-005-0101-8. [DOI] [PubMed] [Google Scholar]
- 36.Swietach P, Rossini A, Spitzer KW, Vaughan-Jones RD. H+ ion activation and inactivation of the ventricular gap junction: A basis for spatial regulation of intracellular pH. Circ Res. 2007;100(7):1045–1054. doi: 10.1161/01.RES.0000264071.11619.47. [DOI] [PubMed] [Google Scholar]
- 37.Cannell MB, Kong CH. Local control in cardiac E-C coupling. J Mol Cell Cardiol. 2012;52(2):298–303. doi: 10.1016/j.yjmcc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Valent I, Zahradníková A, Pavelková J, Zahradník I. Spatial and temporal Ca2+, Mg2+ and ATP2− dynamics in cardiac dyads during calcium release. Biochim Biophys Acta. 2007;1768(1):155–166. doi: 10.1016/j.bbamem.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Biffo S, Grillo M, Margolis FL. Cellular localization of carnosine-like and anserine-like immunoreactivities in rodent and avian central nervous system. Neuroscience. 1990;35(3):637–651. doi: 10.1016/0306-4522(90)90335-2. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AK, Boyd CA, Vaughan-Jones RD. A novel role for carbonic anhydrase: Cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J Physiol. 1999;516(Pt 1):209–217. doi: 10.1111/j.1469-7793.1999.209aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ro HA, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279(35):37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- 42.Martin C, Pedersen SF, Schwab A, Stock C. Intracellular pH gradients in migrating cells. Am J Physiol Cell Physiol. 2011;300(3):C490–C495. doi: 10.1152/ajpcell.00280.2010. [DOI] [PubMed] [Google Scholar]
- 43.Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538(Pt 2):371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol. 2002;544(Pt 2):487–499. doi: 10.1113/jphysiol.2002.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson DE, Casey JR. Cytosolic H+ microdomain developed around AE1 during AE1-mediated Cl−/HCO3− exchange. J Physiol. 2011;589(Pt 7):1551–1569. doi: 10.1113/jphysiol.2010.201483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bednarczyk P, Barker GD, Halestrap AP. Determination of the rate of K(+) movement through potassium channels in isolated rat heart and liver mitochondria. Biochim Biophys Acta. 2008;1777(6):540–548. doi: 10.1016/j.bbabio.2008.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.