Abstract
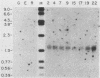
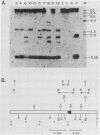
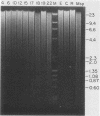
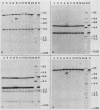
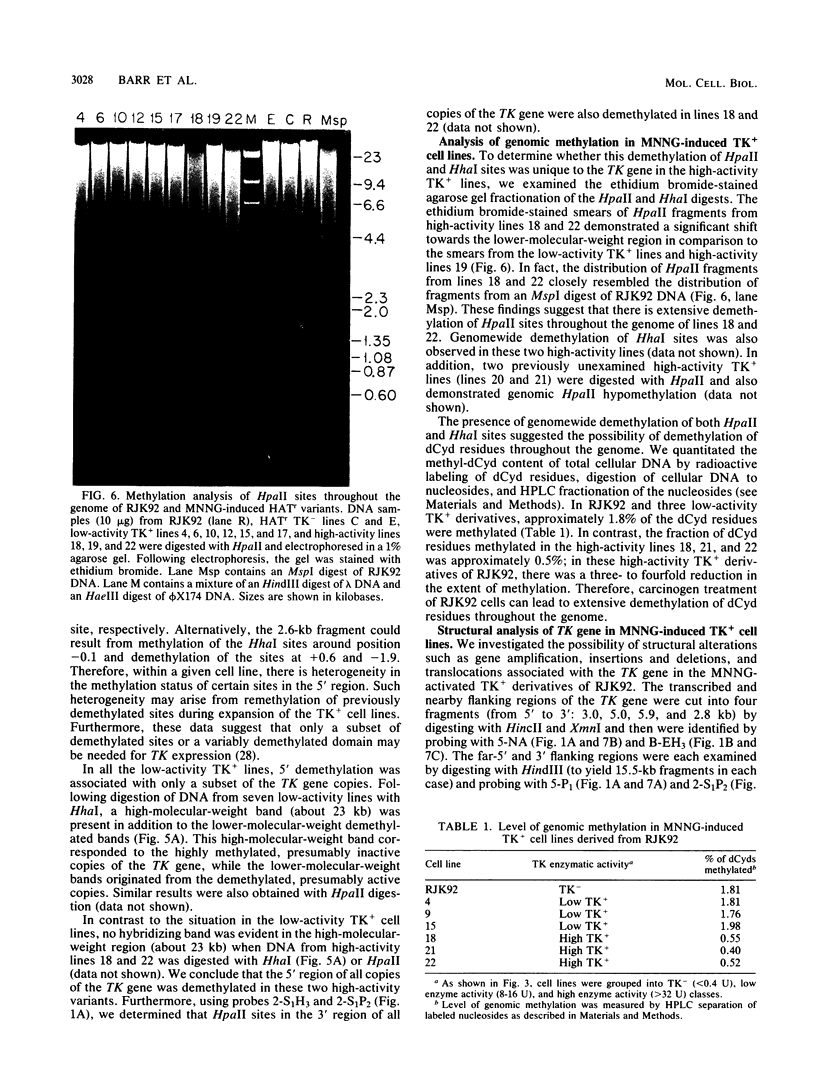
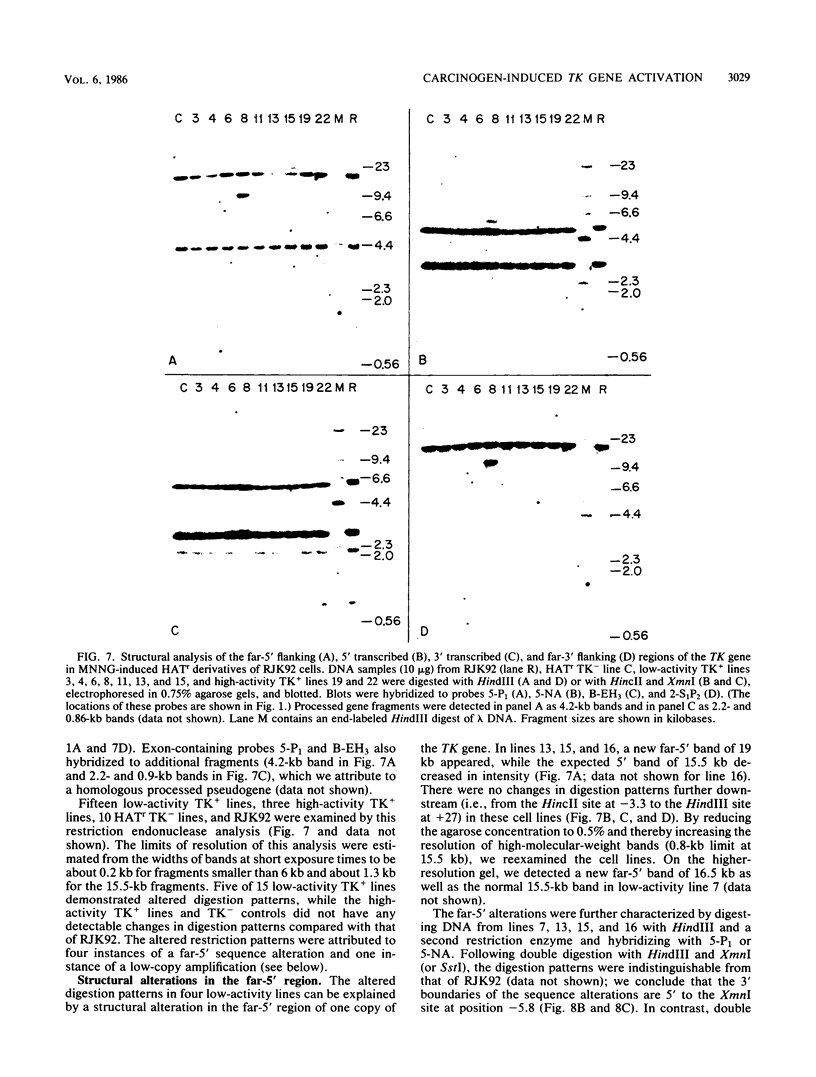
We have investigated the mechanism of activation of an inactive but functionally intact hamster thymidine kinase (TK) gene by the chemical carcinogen N-methyl-N'-nitro-N-nitrosoguanidine. Following carcinogen treatment of TK- RJK92 Chinese hamster cells, aminopterin-resistant (HATr) colonies appeared at a frequency 50-fold higher than in untreated controls. More than 80% of these HATr variants expressed TK enzymatic activity and were divided into high- and low-activity classes. In all TK+ variants, TK expression was correlated with demethylation in the 5' region of the TK gene and the appearance a 1,400-nucleotide TK mRNA. Using high-performance liquid chromatography to measure the level of genomic methylation, we found that four of five high-activity lines demonstrated extensive genomic hypomethylation (approximately 25% of normal level) that was associated with demethylation of all TK gene copies. Restriction endonuclease analysis of 15 low-activity lines revealed four instances of sequence alterations in the far-5' region of the TK gene and one instance of a tandem low-copy amplification. In these lines, the structurally altered gene copy was demethylated. Thus, we propose that a chemical carcinogen can activate TK expression by several different mechanisms. Focal demethylation with or without gene rearrangement was associated with low TK activity, whereas demethylation throughout the genome was associated with high TK activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Barr F. G., Kastan M. B., Lieberman M. W. Distribution of 5-methyldeoxycytidine in products of staphylococcal nuclease digestion of nuclei and purified DNA. Biochemistry. 1985 Mar 12;24(6):1424–1428. doi: 10.1021/bi00327a021. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis. N Engl J Med. 1981 Dec 3;305(23):1379–1389. doi: 10.1056/NEJM198112033052304. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Caskey C. T. Mutant chinese hamster cells with a thermosensitive hypoxanthine-guanine phosphoribosyltransferase. Cell. 1975 Jun;5(2):115–122. doi: 10.1016/0092-8674(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Flatau E., Gonzales F. A., Michalowsky L. A., Jones P. A. DNA methylation in 5-aza-2'-deoxycytidine-resistant variants of C3H 10T1/2 C18 cells. Mol Cell Biol. 1984 Oct;4(10):2098–2102. doi: 10.1128/mcb.4.10.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Harris M., Collier K. Phenotypic evolution of cells resistant to bromodeoxyuridine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4206–4210. doi: 10.1073/pnas.77.7.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Induction of thymidine kinase in enzyme-deficient Chinese hamster cells. Cell. 1982 Jun;29(2):483–492. doi: 10.1016/0092-8674(82)90165-9. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Bailey B. D., White J. C., Goldman I. D. Characteristics of transport of 4-amino antifolates and folate compounds by two lines of L5178Y lymphoblasts, one with impaired transport of methotrexate. Cancer Res. 1979 Jul;39(7 Pt 1):2440–2446. [PubMed] [Google Scholar]
- Ivarie R., Morris J. A. Activation of a nonexpressed hypoxanthine phosphoribosyltransferase allele in mutant H23 HeLa cells by agents that inhibit DNA methylation. Mol Cell Biol. 1986 Jan;6(1):97–104. doi: 10.1128/mcb.6.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Gowans B. J., Lieberman M. W. Methylation of deoxycytidine incorporated by excision-repair synthesis of DNA. Cell. 1982 Sep;30(2):509–516. doi: 10.1016/0092-8674(82)90248-3. [DOI] [PubMed] [Google Scholar]
- King H. W., Brookes P. On the mechanism of induction of resistance to 6-thioguanine in Chinese hamster V79 cells by 3-methylcholanthrene-diolepoxide. Carcinogenesis. 1985 Oct;6(10):1471–1476. doi: 10.1093/carcin/6.10.1471. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Krawisz B. R., Lieberman M. W. Methylation of deoxycytidine in replicating cells treated with ultraviolet radiation and chemical carcinogens. Carcinogenesis. 1984 Sep;5(9):1141–1144. doi: 10.1093/carcin/5.9.1141. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Walker M. S., Becker F. F. DNA methylation and methylase levels in normal and malignant mouse hepatic tissues. Carcinogenesis. 1981;2(9):873–878. doi: 10.1093/carcin/2.9.873. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Shimizu K., Zipser D. Isolation and preliminary characterization of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1983 Oct;3(10):1815–1823. doi: 10.1128/mcb.3.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. W., Beach L. R., Palmiter R. D. Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell. 1983 Nov;35(1):207–214. doi: 10.1016/0092-8674(83)90223-4. [DOI] [PubMed] [Google Scholar]
- MacArthur C. A., Ramabhadran R., Godwin A. K., Lebovitz R. M., Lieberman M. W. Chemical carcinogens induce cadmium resistance and activate metallothionein genes in cadmium sensitive S49 mouse cells. Carcinogenesis. 1985 Jun;6(6):887–892. doi: 10.1093/carcin/6.6.887. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- Matherly L. H., Voss M. K., Anderson L. A., Fry D. W., Goldman I. D. Enhanced polyglutamylation of aminopterin relative to methotrexate in the Ehrlich ascites tumor cell in vitro. Cancer Res. 1985 Mar;45(3):1073–1078. [PubMed] [Google Scholar]
- Orlofsky A., Chasin L. A. A domain of methylation change at the albumin locus in rat hepatoma cell variants. Mol Cell Biol. 1985 Jan;5(1):214–225. doi: 10.1128/mcb.5.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Roufa D. J., Sadow B. N., Caskey C. T. Derivation of TK- clones from revertant TK+ mammalian cells. Genetics. 1973 Nov;75(3):515–530. doi: 10.1093/genetics/75.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Constantinides P. A., Jones P. A. 5-Azacytidine, DNA methylation, and differentiation. Curr Top Microbiol Immunol. 1984;108:115–127. doi: 10.1007/978-3-642-69370-0_8. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982 Dec 15;162(3):679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983 Jan;32(1):239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]