Abstract
Previous measurements show that calcium manganese oxide nanoparticles are better water oxidation catalysts than binary manganese oxides (Mn3O4, Mn2O3, and MnO2). The probable reasons for such enhancement involve a combination of factors: The calcium manganese oxide materials have a layered structure with considerable thermodynamic stability and a high surface area, their low surface energy suggests relatively loose binding of H2O on the internal and external surfaces, and they possess mixed-valent manganese with internal oxidation enthalpy independent of the Mn3+/Mn4+ ratio and much smaller in magnitude than the Mn2O3-MnO2 couple. These factors enhance catalytic ability by providing easy access for solutes and water to active sites and facile electron transfer between manganese in different oxidation states.
Keywords: nanomaterials, thermochemistry
Research on calcium manganese oxides (CaMnOs) has been inspired by the water-oxidation centers in photosystem II (1–5), which is a manganese-calcium cluster Mn4CaO5(H2O)4 supported by a protein environment. Because the capability for efficient water oxidation is unique to photosystem II among all biological photosystems (6, 7), these CaMnO materials that mimic the elemental composition, manganese oxidation state, and particle size of the photosynthetic water oxidation center are of special interest (8–12). Although a number of other metal compounds function as water-oxidizing catalysts (13), many contain rare and expensive metals like iridium and ruthenium; the advantage of manganese oxides (Mn-oxides) is that they are earth-abundant, inexpensive, and environmentally friendly (1–5, 14).
CaMnO phases have short-range order structure and lamellar morphology (12), which are hallmarks of phyllomanganates (classically known as hydrous Mn-oxides), and they possess a layered structure (15–20). In this family of structures, manganese octahedra (and vacancies) form the layers, and charge-balancing cations (i.e., alkali and alkaline earth ions, protons) and water occupy the interlayer space.
Our previous calorimetric studies of various oxide nanoparticles, including those of manganese, iron, and cobalt (21), show that different polymorphs and phases with different oxidation states have significantly different surface energies. For particle sizes below 100 nm, these differences affect the free energies of phase transitions and of oxidation-reduction reactions, shifting the latter by as much as several log(fO2) units at a given temperature. This thermodynamic effect on redox equilibria is significant when one considers electrochemical potentials for water splitting or other catalytic reactions involving nanoparticles (14). In a charge-compensated layered structure, the Mn(III)/Mn(IV) equilibrium will be different from that of binary Mn-oxide phase assemblages, namely, Mn3O4 (hausmannite), Mn2O3 (bixbyite), and MnO2 (pyrolusite). Thus, particle size, morphology, crystal structure, and mixed valence in the more complex Mn-oxides are expected to influence the thermodynamic driving forces for various steps in water oxidation and the mechanism, kinetics, and catalytic efficiency of the process. The present work determines the enthalpies of formation of two suites of synthetic layered structure CaMnO phases, measures their surface energies, and provides insight into their redox reactions and catalytic function.
Results
Two suites of CaMnO samples with varying surface areas and Mn3+/Mn4 ratios were synthesized by slight modifications of established methods (10) from similar starting solutions with different Ca and Mn concentrations. The surface enthalpies and heats of formation were determined by calorimetry. The methodology involves high-temperature oxide-melt solution calorimetry (21–28) using a molten oxide solvent, 3Na2O⋅4MoO3, at 700 °C on a suite of nanophase samples having different surface areas determined by nitrogen adsorption measurements (29) and water contents measured by thermal analysis. Chemical analysis determined the manganese and calcium content. Thermogravimetry provided total weight loss due to oxygen and water evolution, and iodometric titration determined the Mn3+/Mn4+ ratio (Methods and SI Text).
Table 1 presents the composition (normalized to 1 mol of manganese) of both suites of CaMnO samples, along with their Brunauer–Emmett–Teller (BET) surface area. Their X-ray diffraction (XRD) patterns (Fig. S1) are consistent with layered structures showing a lack of long-range order between the layers. Sintering of the samples resulted in the same XRD pattern (Fig. S2) for all. Layered morphology was verified using transmission electron microscopy (TEM) (Fig. S3). SEM (Fig. S4) shows that the CaMnO samples consist of aggregates of nanoscale particles below 100 nm in size. Thermogravimetric (TG) differential scanning calorimetry (DSC) (Fig. S5) shows an endotherm for water loss at 142 °C and two additional endotherms at 745 °C and 887 °C, which may represent both water and oxygen loss, as well as an exothermic peak at 650 °C possibly caused by sample coarsening. Consistent with previously reported lamellar structures for other Mn-oxide materials (15–20), similar structures for these CaMnO materials are suggested by XRD (Fig. S2) and confirmed by low-resolution TEM (Fig. S3). The broad diffraction peaks and pattern of intensities suggest highly disordered materials with little long-range coherence among layers; however, because crystallography was not the focus of the present work, a detailed diffraction study was not pursued.
Table 1.
Sample composition, average manganese oxidation state, and surface area
| Compositiona | Manganese average oxidation stateb,c | BET surface area, m2/g; and molar surface area, m2/mol |
| Ca0.39⋅Mn(3+)0.21⋅Mn(4+)0.79⋅O2.29⋅0.60H2Od | 3.79 ± 0.01 | 96.97 ± 0.56 |
| 11,461.0 ± 88.1 | ||
| Ca0.39⋅Mn(3+)0.13⋅Mn(4+)0.87⋅O2.33⋅0.48H2Od | 3.86 ± 0.01 | 88.38 ± 0.39 |
| 10,309.4 ± 79.9 | ||
| Ca0.39⋅Mn(3+)0.09⋅Mn(4+)0.91⋅O2.35⋅0.43H2Od | 3.91 ± 0.01 | 71.58 ± 0.36 |
| 8,313.7 ± 65.3 | ||
| Ca0.39⋅Mn(3+)0.06⋅Mn(4+)0.94⋅O2.37⋅0.30H2Od | 3.94 ± 0.01 | 56.64 ± 0.34 |
| 6,456.3 ± 51.8 | ||
| Ca0.39⋅Mn(3+)0.05⋅Mn(4+)0.95⋅O2.37⋅0.28H2Od | 3.95 ± 0.01 | 35.88 ± 0.29 |
| 4,081.0 ± 34.6 | ||
| Ca0.43⋅Mn(3+)0.37⋅Mn(4+)0.63⋅O2.24⋅0.43H2Oe | 3.63 ± 0.01 | 72.84 ± 0.39 |
| 8,426.5 ± 66.0 | ||
| Ca0.43⋅Mn(3+)0.39⋅Mn(4+)0.61⋅O2.23⋅0.42H2Oe | 3.61 ± 0.01 | 63.15 ± 0.34 |
| 7,287.0 ± 59.9 | ||
| Ca0.43⋅Mn(3+)0.28⋅Mn(4+)0.72⋅O2.29⋅0.35H2Oe | 3.72 ± 0.01 | 55.49 ± 0.22 |
| 6,380.9 ± 48.0 | ||
| Ca0.43⋅Mn(3+)0.29⋅Mn(4+)0.71⋅O2.28⋅0.22H2Oe | 3.71 ± 0.01 | 42.35 ± 0.32 |
| 4,768.3 ± 36.1 | ||
| Ca0.43⋅Mn(3+)0.28⋅Mn(4+)0.72⋅O2.29⋅0.15H2Oe | 3.72 ± 0.01 | 35.47 ± 0.29 |
| 3,951.1 ± 28.5 |
Sample compositions are normalized to 1 mol of manganese.
Details on the Murray titration method (41), along with calculation of manganese AOS and speciation, are provided in SI Tex. Quality control was run between different samples of CaMnO using a reference standard of manganese dioxide (Sigma–Aldrich).
Error in the manganese oxidation state titration is the standard error calculated from triplicate titrations.
CaMnO sample contains 43.06 ± 0.01 wt% manganese (0.784 mol) and 12.40 ± 0.01 wt% calcium (0.309 mol).
CaMnO sample contains 49.00 ± 0.01 wt% manganese (0.892 mol) and 15.28 ± 0.01 wt% calcium (0.381 mol).
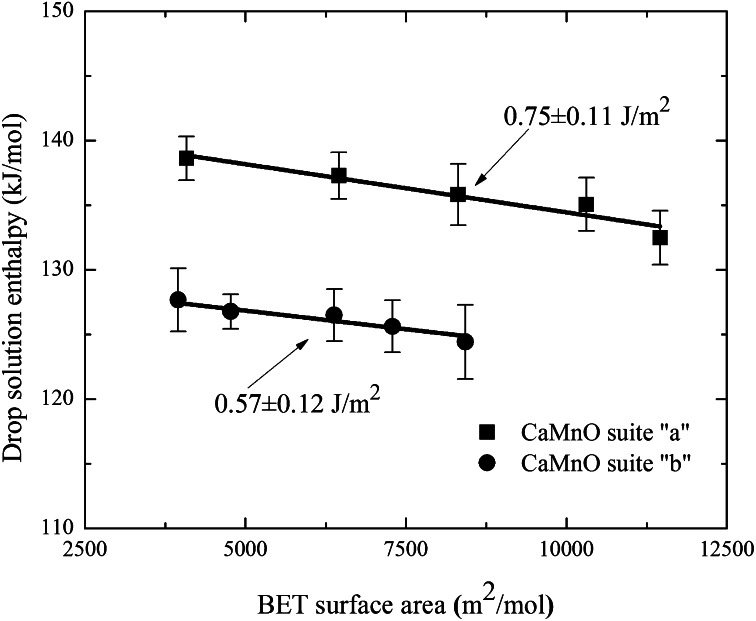
The measured enthalpy associated with dropping a sample from room temperature into the molten solvent at high temperature, where it dissolves, is called the enthalpy of drop solution (ΔHds) (more information on the thermodynamic cycle is provided in Table 2, and additional definitions and calculations are provided in SI Text). In general, the difference between the ΔHds of reactants and products (of the same composition) gives the enthalpy of reaction. Thus, the enthalpy associated with increased surface area can be obtained by comparing the ΔHds for samples with different surface areas; however, because the samples have different water contents, the ΔHds must be corrected to account for the H2O, which is released into the atmosphere above the solvent as the samples dissolve. ΔHds values are listed in Table 2, along with subsequent values corrected for water content. This correction subtracts the heat content of n moles of bulk H2O from the observed ΔHds. As discussed previously (21, 23), the slope of the line relating the corrected ΔHds (Table 2) and molar surface area (Table 1) yields the surface enthalpy (SE; essentially equivalent to surface energy) for the hydrous surfaces (Fig. 1). The thermochemical cycle used to compute the SE is provided in SI Text. The SE for hydrous surfaces of the samples of suite “a” with a manganese average oxidation state (AOS) of 3.89 (Table 1) is 0.75 ± 0.11 J/m2. The SE for the samples of suite “b” with a manganese AOS of 3.68 (Table 1) is 0.57 ± 0.12 J/m2. These values overlap within the experimental uncertainty, especially when one propagates the error arising from scatter in the calorimetric measurements and from uncertainty in the measured water content. One must also bear in mind that such nanophase samples may not be totally homogeneous in Ca/Mn ratio, Mn oxidation state, and water content, and that all these sources of error may combine to form a nonrandom distribution of uncertainties. Thus, we conclude that the measured surface energies of the two suites of samples are best considered as being the same within experimental error. The layered structure CaMnO samples have substantially lower SE values than those previously reported (30) for Mn3O4 (SE = 0.96 ± 0.08 J/m2), Mn2O3 (SE = 1.29 ± 0.10 J/m2), and MnO2 (SE = 1.64 ± 0.10 J/m2).
Table 2.
Sample composition, drop solution enthalpies, and formation enthalpies of the CaMnO samples.
| Compositiona,b | Measured ΔHds of the oxide⋅nH2O, kJ/molc | ΔHds,cor of the oxide, kJ/mold | ΔHf-ox, kJ/mol | ΔHf-ox*,kJ/mol | ΔH°f, kJ/mol |
| Ca0.39⋅Mn(3+)0.21⋅Mn(4+)0.79⋅O2.29⋅0.60H2Oe | 174.05 ± 1.46 (12) | 132.49 ± 1.46 | −53.57 ± 3.03 | −86.61 ± 2.85 | −934.56 ± 2.22 |
| Ca0.39⋅Mn(3+)0.13⋅Mn(4+)0.87⋅O2.33⋅0.48H2Oe | 168.20 ± 1.45 (12) | 135.05 ± 1.45 | −52.29 ± 3.02 | −88.74 ± 2.84 | −902.95 ± 2.22 |
| Ca0.39⋅Mn(3+)0.09⋅Mn(4+)0.91⋅O2.35⋅0.43H2Oe | 165.65 ± 1.67 (10) | 135.82 ± 1.67 | −50.89 ± 3.24 | −89.26 ± 3.08 | −891.05 ± 2.22 |
| Ca0.39⋅Mn(3+)0.06⋅Mn(4+)0.94⋅O2.37⋅0.30H2Oe | 158.00 ± 1.26 (10) | 137.28 ± 1.27 | −50.99 ± 2.85 | −90.56 ± 2.66 | −854.52 ± 2.22 |
| Ca0.39⋅Mn(3+)0.05⋅Mn(4+)0.95⋅O2.37⋅0.28H2Oe | 158.25 ± 1.20 (8) | 138.62 ± 1.20 | −52.13 ± 2.79 | −91.87 ± 2.59 | −850.18 ± 2.22 |
| Ca0.43⋅Mn(3+)0.37⋅Mn(4+)0.63⋅O2.24⋅0.43H2Of | 154.12 ± 2.03 (8) | 124.42 ± 2.03 | −55.89 ± 3.62 | −82.32 ± 3.48 | −899.49 ± 2.22 |
| Ca0.43⋅Mn(3+)0.39⋅Mn(4+)0.61⋅O2.23⋅0.42H2Of | 154.87 ± 1.42 (10) | 125.61 ± 1.42 | −58.18 ± 2.99 | −83.64 ± 2.81 | −896.70 ± 2.22 |
| Ca0.43⋅Mn(3+)0.28⋅Mn(4+)0.72⋅O2.29⋅0.35H2Of | 150.69 ± 1.41 (8) | 126.48 ± 1.41 | −53.60 ± 2.98 | −83.88 ± 2.80 | −880.44 ± 2.22 |
| Ca0.43⋅Mn(3+)0.29⋅Mn(4+)0.71⋅O2.28⋅0.22H2Of | 142.12 ± 0.94 (8) | 126.77 ± 0.95 | −54.41 ± 2.59 | −84.23 ± 2.37 | −843.34 ± 2.22 |
| Ca0.43⋅Mn(3+)0.28⋅Mn(4+)0.72⋅O2.29⋅0.15H2Of | 137.99 ± 1.73 (8) | 127.67 ± 1.73 | −54.63 ± 3.30 | −85.05 ± 3.14 | −823.06 ± 2.22 |
Sample compositions are normalized to 1 mol of manganese.
Quality control was run between different samples of CaMnO using a reference standard of manganese dioxide (Sigma–Aldrich) for the Murray titration method for manganese AOS and speciation.
Error is 2 SDs of the mean. Value in parentheses is number of experiments performed. Uncertainties are propagated in subsequent calculations.
ΔHds,cor formula compositions are normalized to 1 mol of manganese. The enthalpy of formation (ΔHf-ox) from the oxides for the reaction mCaO + 0.5(1-y)Mn2O3 + yMnO2 + nH2O = CamMnO(1.5+m+0.5y)⋅nH2O and the enthalpy of formation (ΔHf-ox*) from the oxides for the reaction mCaO + 0.5Mn2O3 + 0.25yO2 + nH2O = CamMnO(1.5+m+0.5y)·nH2O were computed using appropriate thermochemical cycles that included the use of measured drop solution enthalpies (ΔHds) of the CaMnO samples and of ΔHdsCaO = (−90.70±1.69) kJ/mol (32), ΔHdsMn2O3 = 154.87±1.00 kJ/mol (30), and ΔHdsMnO2 = 124.92±1.03 kJ/mol (30). Heats of formation (ΔHof) were computed using standard formation enthalpies (33) of binary oxides from the elements and heat content (33) of O2.
CaMnO sample contains 43.06 ± 0.01 wt% manganese (0.784 mol) and 12.40 ± 0.01 wt% calcium (0.309 mol).
CaMnO sample contains 49.00 ± 0.01 wt% manganese (0.892 mol) and 15.28 ± 0.01 wt% calcium (0.381 mol).
Fig. 1.
Water-corrected ΔHds (kJ/mol) in molten 3Na2O-4MoO3 (sodium molybdate) at 700 °C vs. BET surface area (m2/mol) of both sample series. The slopes of the lines represent the SE (J/m2) of the hydrous surfaces of the nanophase layered structure CaMnO samples: SE of suite a = 0.75 ± 0.11 J/m2, and SE of suite b = 0.57 ± 0.12 J/m2. Measured error is 2 SDs of the mean for several performed experiments. Uncertainties are propagated in subsequent calculations.
Formation enthalpies (Table 2) for the CaMnO samples were computed using thermochemical cycles (SI Text). The enthalpies of formation from the binary oxides (thermochemical cycles are discussed in SI Text) are calculated directly from differences in the appropriate ΔHds of reactants and products; those from the elements added the stoichiometrically weighted enthalpies of formation of the binary oxides from the elements (Table S1) as given in standard tabulations. The enthalpies of formation from the binary oxides for layered structure CaMnO samples are exothermic, which indicates the strongly basic nature of the CaO that combines with the relatively acidic Mn-oxides. Enthalpy of formation from CaO, Mn2O3, and O2 (ΔHf-ox*) is more exothermic than that from CaO, Mn2O3, and MnO2 (ΔHf-ox), which reflects the exothermic oxidation of trivalent to tetravalent manganese. Likewise, the standard formation enthalpies from the elements, ΔH°f, of the CaMnO samples (Table 2) are strongly exothermic. The formation enthalpies (Table 2) include the effects of calcium incorporation and structural change, as well as the variable manganese oxidation state, in the suite of samples. To focus on the energetic effect of the manganese oxidation state within the layered structure, Fig. 2 shows the enthalpy of formation from CaO, Mn2O3, O2, and H2O for the reaction:
plotted vs. the number of moles of oxygen (0.25y) in the reaction. The enthalpy of formation becomes more exothermic linearly with the number of moles of O2 used, independent of the Ca/Mn ratio (different in the two suites) or the average oxidation state. The slope then gives the change in enthalpy of formation per mole of O2 added, which represents the enthalpy of internal oxidation (internal in the sense of being within the layered structure with no phase change) and yields a value of −100.0 ± 8.4 kJ per mole of O2. This enthalpy is exothermic but significantly less so than the enthalpy of oxidation of Mn2O3 to MnO2 (−168.1 kJ per mole of O2) (30). The intercept at zero excess oxygen (−67.0 ± 1.7 kJ/mol) is the enthalpy of formation of the hypothetical layered CaMnO phase containing only trivalent manganese and is a measure of the stabilizing interaction of CaO, Mn2O3, and H2O to form the layered structure. For a hypothetical CaMnO with only Mn4+, the corresponding enthalpy of formation would be −92.0 ± 8.6 kJ/mol from CaO, Mn2O3, O2, and H2O or −28.3 ± 8.9 kJ/mol from CaO, MnO2, and H2O. This suggests that a fully oxidized layered material would be stable relative to CaO, MnO2, and H2O. However, we did not try to extend the range of manganese oxidation states in these materials to lower or higher values than those achieved in the indicated syntheses.
Fig. 2.
Enthalpy of formation, ΔH°f-ox* for the reaction mCaO + 0.5Mn2O3 + 0.25yO2 + nH2O = CamMnO(1.5+m+0.5y)⋅nH2O vs. moles of O2 (0.25y) reacted to form the CaMnO. ■, suite a; ●, suite b.
Because the oxidation state in these CaMnO materials can be varied over a significant range at constant calcium content, charge balance in the structure must involve species in addition to calcium. Indeed, the Ca/Mn(III) stoichiometry exceeds 1:1. Given that calcium is divalent, this seems to imply that it cannot only be stabilizing excess charge due to Mn(III). The other well-known cause for excess structural charge is the presence of Mn(IV) vacancies (31–33). Thus, these highly disordered materials may contain both Mn(III) “defects” and Mn(IV) vacancies. More detailed crystallographic and spectroscopic studies, outside the scope of the present study, are needed to investigate these structural features.
The important thermodynamic observation is that because the enthalpies of the two suites of samples fall on the same line in Fig. 2, the formation and redox energetics in the layered structure are not sensitive to the Ca/Mn ratio (or to the water content) and are independent of the Mn3+/Mn4+ ratio. This result indicates that all the layered CaMnO samples provide a similar energetic environment for manganese redox reactions.
Discussion
Previous experiments (2, 4) have shown that the efficiency of CaMnO water oxidation catalysis decreases in the following order: CaMnO >> Mn2O3 > MnO2, Mn3O4. The existence of mixed valence (Mn3+ and Mn4+) is probably critical to catalysis and efficient electron transfer in solution and at the solid–solution interface. This point has been made before (8), but several unique observations in the present study give further insight into these processes. In contrast to the two-phase Mn2O3/MnO2 assemblage with limited redox capability within each phase, the CaMnO materials allow a much wider range of Mn3+/Mn4+ ratios within a single structure. The less exothermic oxidation enthalpy for the layered structure CaMnO phases than in the binary oxides (the Mn2O3-MnO2 couple) means that the equilibrium between Mn3+ and Mn4+ is more closely balanced and oxidation or reduction does not require a profound structural change but can occur, with energetics independent of the Mn3+/Mn4+ ratio, within this constant layered environment. Furthermore, the thermodynamic stability of the Ca-containing layered structure phases permits these redox reactions to proceed without phase change or decomposition.
The low SE of the layered structure CaMnO materials (significantly smaller than for Mn3O4, Mn2O3, or MnO2) suggests that water is not strongly bound to the surface sites, because, for many oxides, the enthalpy of H2O chemisorption becomes more exothermic with increasing SE (28, 34–36). In comparing TiO2 and SnO2 (rutile structure), Ma et al. (35) have argued that the better gas-sensing ability of the latter stems from a lower surface energy, lower water coverage on the surface, and weaker binding of surface water, enabling energetically easier access of other gas molecules to the SnO2 surface. Indeed, Castro and Quach (36) proposed recently that measurement of water adsorption enthalpy can be used as a proxy to estimate surface energy. Loosely bound water on CaMnOs may be readily displaced, making sites on the catalyst surface more accessible to other species. Such accessibility also connects the solution chemistry to the catalyst performance. During the water-splitting reaction, reactive intermediates are presumed to form and bind to the surface of the catalytic oxide, with the strength of binding depending on the nature of the transition metal and the structure of the oxide (37). A low binding energy and high mobility for water and counterions may make surface sites near the transition metal more accessible and favor the binding of these activated oxygen species. A simplistic and strictly local view of the O2-generation reaction would have two nearby terminal oxo groups link as bonded atoms are oxidized to form peroxide or O2. However, the layered structure suggests a more nuanced view that emphasizes interlayer chemistry and delocalized charges on the layers, which are only a few nanometers in dimension. Increases in layer charge that accompany O2 generation require proton transfer and charge adjustment. These are best accomplished via reactions involving solutes in the interlayer region, including Brønsted acids/bases that allow rapid proton transfers. Thus, we speculate that the catalytic performance of the Mn-oxides will be favored in the layered structure phyllomanganates, such as birnessites, which facilitates reversibility and transport by allowing rapid charge compensation. It also has a point-of-zero charge near the pH conditions, where sacrificial oxidants like cerium ammonium nitrate are used (pH 1).
Additionally, the nanoparticle size and disorder of the layered structure CaMnO materials increase the ability of the solid structure to support Mn(III) defects and to have a large number of nonbridging oxygen sites for ligand exchange and catalysis. The major role of calcium in the CaMnO materials may be to stabilize the layered structure rather than to take part in the catalytic reactions, although one can imagine incorporation of acids/bases that facilitate proton transfer in the interlayer. At highly acidic pH, such as near the pH at which sacrificial oxidants like cerium ammonium nitrate are used (pH 1), much of the Ca2+ in the structure may be progressively replaced by H+, with calcium released to aqueous solution. However, although the Ca2+ is slowly leached out of the material in acid (8), manganese is conserved by repeated reductive dissolution, followed by reoxidation at the solid surface. Takashima et al. (38, 39) argue that the strong pH dependence of catalytic activity can be explained by the effect of pH on the disproportionation of Mn3+ to Mn2+ and Mn4+, with the divalent manganese dissolving in the aqueous solution. For such equilibrium to be maintained during catalysis, adsorption of manganese species on the surface and their transport into, or out of, the structure must be rapid and reversible, as is likely to be the case here.
The findings above provide insight into why the poorly ordered crystalline-layered structure CaMnO materials are better catalysts than well-ordered crystalline binary Mn-oxide minerals (Mn3O4, Mn2O3, and MnO2). However, it is possible that the well-ordered materials may evolve under repeated electrochemical cycling to resemble birnessites more closely (with charge balance coming from various cations, including H+) (40). One must also ask whether calcium is unique or whether birnessites with other strong electrolyte cations, particularly Na+ and K+, behave analogously. Further study of the effect of different exchangeable interlayer cations on thermodynamics and catalytic activity is in progress.
Conclusion
The present study suggests that the high surface area, thermodynamic stability, constant and closely balanced redox energetics over a large variation in the Mn3+/Mn4+ ratio, low surface energy, and weakly bound surface water act together to provide a highly favorable environment for water oxidation catalysis in layered CaMnOs and possibly other birnessite-related structures. The findings in this work can be generalized to guide the search for new and better oxide catalysts. One should look for structures providing easy accessibility and a low binding energy for water (often associated with a low surface energy) in which the redox-active cations can exist, with modest and constant energy difference, over a wide range of Mn+/M(n+1)+ ratios without disrupting the structure.
Methods
The CaMnOs had been synthesized previously using a minor modification of published methods (10). Overall, the synthesis involved reaction of potassium permanganate, Mn(II) acetate, and calcium chloride in the presence of potassium hydroxide. The first change was in the use of a very highly concentrated solution of potassium hydroxide, and the second was the discontinuation of the hydrothermal conditions. It is believed that this assisted the formation of small particles because it promoted rapid precipitation. Conversely, hydrothermal treatment, which promotes crystal growth, was removed from the procedure to maintain nanoparticles. The two suites of CaMnO samples are similar but vary in their calcium, manganese, oxygen, and water content; average particle size; and surface area. The syntheses are detailed in SI Text.
All phases were confirmed by powder XRD using a Bruker D8 Advance X-ray diffractometer (Cu Kα radiation) operated at 45 kV and 40 mA. The XRD patterns were collected with a 0.02° two-θ step size and 10-s dwell time, and they were analyzed by Jade software (version 6.11, 2002, Materials Data, Inc.) to evaluate the phase(s) present and to estimate the average nanoparticle size. All samples displayed XRD patterns appropriate to a layered structure phase, with significant peak broadening particularly evident in the low-angle region (Fig. S1). The samples were measured as received as well as after heating to 1,000 °C during TG analysis. Heated samples crystallized to an ∼50:50 mixture of CaMn2O4 (marokite) and Mn3O4 (hausmannite) as shown in Fig. S2.
The samples were heated to 1,000 °C at a rate of 10 K/min under a flow of argon (75 mL/min) in platinum crucibles using the Setaram Labsys Evo instrument and associated Calisto software. The TG data were corrected for buoyancy by running an empty crucible. H2O content was determined from the weight difference (obtained on a microbalance for highest accuracy) before and after annealing the samples overnight at 1,000 °C. Samples heated at 1,000 °C lost all their water and crystallized to a mixture of CaMn2O4 (marokite) and Mn3O4. Part of the measured total weight loss was due to loss of oxygen, because Mn4+ in the original sample was reduced on heating, and part was due to dehydration. The former was calculated from the difference in manganese oxidation state between the pristine sample and annealed sample measured by Murray (41) titration. This was subtracted from the total weight loss to give the weight loss due to water. Other than serving as reference material for determining the oxygen and water content of the initial samples, these sintered samples were not considered further because they no longer possessed the layered structures characteristic of the CaMnO catalysts.
Surface area was measured by N2 adsorption at −196 °C using a five-point BET (29) technique on a Micromeritics ASAP 2020 in the P/Po (relative pressure) range of 0.05–0.3. Before analysis, the samples were degassed under nitrogen gas at 200 °C for 12 h. XRD patterns for each phase after similar temperature treatment indicated no coarsening or phase change.
TEM images were obtained using an FEI Tecnai G2 F30 Super-Twin TEM system, which operates at 300 kV. The 2D morphology was explored using bright-field mode. Preparation of the CaMnO samples involved 5 min of sonication in a Branson 2200 sonicator in which 3 mg of sample was suspended in a glass beaker containing 10 mL of 2-propanol. Two drops of the resulting mixture were added to a holey carbon-coated support grid (no. 01800-F; Ted Pella, Inc.). A typical TEM image is shown in Fig. S3. A JEOL JSM-5610 scanning electron microscope was operated at 15 kV accelerating voltage for secondary electron imaging (SEI) topography studies using the SEI detector. The samples were mounted onto aluminum stubs with conductive tape and then gold-coated. A typical SEM image is shown in Fig. S4. TEM and SEM provided information about sample morphology.
The custom-built Calvet twin calorimeter and techniques for measurement of enthalpy of formation by solution calorimetry in a molten salt solvent at high temperature have been described previously (24, 25). Before calorimetric measurements, samples were equilibrated with the controlled temperature and humidity conditions of the calorimetry suite. Sample pellets (∼5 mg) were dropped into 20 g of molten sodium molybdate (3Na2O4-MoO3) at 700 °C with oxygen flushing through the calorimeter at 30 mL/min and also bubbling through the solvent at 5 mL/min. Flushing and bubbling maintain oxidizing conditions, remove evolving water vapor, and aid dissolution. Contribution of bulk H2O was subtracted from the experimental enthalpy using the appropriate thermochemical cycle. The slope of the line relating the water-corrected heat of solution to the surface area provided the SE. Full details of the method and thermochemical cycles used in the calculations are provided in SI Text.
Titration of Mn(III) and Mn(IV) oxidation states used a modified Murray method (41, 42). The method directly titrates iodine produced by the reaction of iodide with dissolved manganese and can be performed at room temperature. Full details of the method and calculations are provided in SI Text.
Details of methodology; thermochemical cycles; figures for XRD, TEM, SEM, and TG-DSC; and calculation details of the manganese average oxidation state, manganese speciation, water-corrected ΔHds, and formation enthalpies are provided in SI Text.
Supplementary Material
Acknowledgments
The calorimetry, sample characterization, and data interpretation performed at the University of California, Davis, and at the Nanomaterials in Environment, Agriculture, and Technology Organized Research Unit received support from US Department of Energy Grant DE-FG02-97ER14749 (to A.N.). S.N., B.P., and M.M.N. thank the Institute for Advanced Studies in Basic Sciences and the National Elite Foundation for financial support. Support to W.H.C. was provided via National Science Foundation Grant EAR-1231322.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306623110/-/DCSupplemental.
References
- 1.Iyer A, et al. Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures. J Phys Chem C. 2012;116:6474–6483. [Google Scholar]
- 2.Jiao F, Frei H. Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Chem Commun (Camb) 2010;46:2920–2922. doi: 10.1039/b921820c. [DOI] [PubMed] [Google Scholar]
- 3.Dismukes GC, et al. Development of bioinspired Mn4O4-cubane water oxidation catalysts: Lessons from photosynthesis. Acc Chem Res. 2009;42(12):1935–1943. doi: 10.1021/ar900249x. [DOI] [PubMed] [Google Scholar]
- 4.Harriman A, Pickering IJ, Thomas JM, Christensen PA. Metal oxides as heterogenous catalysts for oxygen evolution under photochemical condition. J Chem Soc Faraday Trans 1. 1988;84(8):2795–2806. [Google Scholar]
- 5.Shafirovich VY, Khannanov NK, Shilov AE. Inorganic models of photosystem II of plant photosynthesis. Catalytic and photocatalytic oxidation of water with participation of manganese compounds. J Inorg Biochem. 1981;15:113–129. [Google Scholar]
- 6.Umena Y, Kawakami K, Shen J-R, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473(7345):55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303(5665):1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 8.Najafpour MM, Ehrenberg T, Wiechen M, Kurz P. Calcium manganese(III) oxides (CaMn2O4.xH2O) as biomimetic oxygen-evolving catalysts. Angew Chem Int Ed Engl. 2010;49(12):2233–2237. doi: 10.1002/anie.200906745. [DOI] [PubMed] [Google Scholar]
- 9.Shevela D, Koroidov S, Najafpour MM, Messinger J, Kurz P. Calcium manganese oxides as oxygen evolution catalysts: O2 formation pathways indicated by 18O-labelling studies. Chemistry. 2011;17(19):5415–5423. doi: 10.1002/chem.201002548. [DOI] [PubMed] [Google Scholar]
- 10.Zaharieva I, et al. Synthetic manganese–calcium oxides mimic the water-oxidizing complex of photosynthesis functionally and structurally. Energy & Environmental Science. 2011;4:2400–2408. [Google Scholar]
- 11.Najafpour MM, Nayeri S, Pashaei B. Nano-size amorphous calcium-manganese oxide as an efficient and biomimetic water oxidizing catalyst for artificial photosynthesis: Back to manganese. Dalton Trans. 2011;40(37):9374–9378. doi: 10.1039/c1dt11048a. [DOI] [PubMed] [Google Scholar]
- 12.Wiechen M, Zahariev I, Dau H, Kurz P. Layered manganese oxides for water-oxidation: Alkaline earth cations influence catalytic activity in a photosystem II-like fashion. Chem Sci. 2012;3:2330–2339. [Google Scholar]
- 13.Liu X, Wang F. Transition metal complexes that catalyze oxygen formation from water: 1979–2010. Coord Chem Rev. 2012;256:1115–1136. [Google Scholar]
- 14.Najafpour MM, Rahimi F, Aro EM, Lee CH, Allakhverdiev SI. Nano-sized manganese oxides as biomimetic catalysts for water oxidation in artificial photosynthesis: A review. J R Soc Interface. 2012;9(75):2383–2395. doi: 10.1098/rsif.2012.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drits VA, Lanson B, Gorshkov AI. Substructure and superstructure of 4-layer Ca-exchanged birnessite. Am Mineral. 1998;83:97–118. [Google Scholar]
- 16.Essington ME. Soil and Water Chemistry: An Integrative Approach. Boca Raton, FL: CRC; 2003. p. 86. [Google Scholar]
- 17.Villalobos M, Lanson B, Manceau A, Toner B, Sposito G. Structural model for the biogenic Mn oxide produced by Pseudomonas putida. Am Mineral. 2006;91:489–502. [Google Scholar]
- 18.Birkner N. 2009. Manganese oxide mineral phases produced at room temperature under acidic conditions investigated with XRD, TEM, SEM, EDS, and BET. MS thesis (Univ of Nevada, Las Vegas, NV)
- 19.Grangeon S, Lanson B, Miyata N, Tani Y, Manceau A. Structure of nanocrystalline phyllomanganates produced by freshwater fungi. Am Mineral. 2010;95:1608–1616. [Google Scholar]
- 20.Manceau A, et al. Short-range and long-range order of phyllomanganate nanoparticles determined using high-energy X-ray scattering. J Appl Crystallogr. 2013;46:193–209. [Google Scholar]
- 21.Navrotsky A, Ma C, Lilova K, Birkner N. Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria. Science. 2010;330(6001):199–201. doi: 10.1126/science.1195875. [DOI] [PubMed] [Google Scholar]
- 22.Navrotsky A, Mazeina L, Majzlan J. Size-driven structural and thermodynamic complexity in iron oxides. Science. 2008;319(5870):1635–1638. doi: 10.1126/science.1148614. [DOI] [PubMed] [Google Scholar]
- 23.Mazeina L, Deore S, Navrotsky A. Energetics of bulk and nano-akaganeite, β-FeOOH: Enthalpy of formation, surface enthalpy, and enthalpy of water adsorption. Chem Mater. 2006;18(7):1830–1838. [Google Scholar]
- 24.Navrotsky A. Thermochemistry of new, technologically important inorganic materials. Mater Res Bull. 1997;22:35–41. [Google Scholar]
- 25.Navrotsky A. Progress and new directions in high temperature calorimetry revisited. Phys Chem Miner. 1997;24:222–241. [Google Scholar]
- 26.Navrotsky A. High temperature oxide melt calorimetry of oxides and nitrides. J Chem Thermodyn. 2001;33:859–871. [Google Scholar]
- 27.Navrotsky A. Calorimetry of nanoparticles, surfaces, interfaces, thin films, and multilayers. Journal of Chemical Thermodynamics. 2007;39:2–9. [Google Scholar]
- 28.Navrotsky A. Nanoscale effects on thermodynamics and phase equilibria in oxide systems. ChemPhysChem. 2011;12(12):2207–2215. doi: 10.1002/cphc.201100129. [DOI] [PubMed] [Google Scholar]
- 29.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309. [Google Scholar]
- 30.Birkner N, Navrotsky A. Thermodynamics of manganese oxides: Effects of particle size and hydration on oxidation-reduction equilibria among hausmannite, bixbyite, and pyrolusite. Am Mineral. 2012;97:1291–1298. [Google Scholar]
- 31.Cornell RM, Giovanoli R. Transformation of hausmannite into birnessite in alkaline media. Clays Clay Miner. 1988;36(3):249–257. [Google Scholar]
- 32.Helean KB, et al. Enthalpies of formation of Ce-pyrochlore, U-pyrochlore and Gd-pyrochlore: Three materials relevant to the proposed waste form for excess weapons plutonium. Journal of Nuclear Materials. 2002;303:226–239. [Google Scholar]
- 33.Robie RA, Hemingway BS. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geological Survey Bulletin. 1995;2131:200–202. [Google Scholar]
- 34.Ranade MR, et al. Energetics of nanocrystalline TiO2. Proc Natl Acad Sci USA. 2002;99(Suppl 2):6476–6481. doi: 10.1073/pnas.251534898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Castro RHR, Zhou W, Navrotsky A. Surface enthalpy and enthalpy of water adsorption of nanocrystalline tin dioxide. J Mater Res. 2011;26:848–853. [Google Scholar]
- 36.Castro RHR, Quach DV. Analysis of anhydrous and hydrated surface energies of gamma-Al2O3 by water adsorption microcalorimetry. J Phys Chem C. 2012;116:24726–24733. [Google Scholar]
- 37.Man IC, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem. 2011;3:1159–1165. [Google Scholar]
- 38.Takashima T, Hashimoto K, Nakamura R. Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. J Am Chem Soc. 2012;134(3):1519–1527. doi: 10.1021/ja206511w. [DOI] [PubMed] [Google Scholar]
- 39.Takashima T, Hashimoto K, Nakamura R. Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J Am Chem Soc. 2012;134(44):18153–18156. doi: 10.1021/ja306499n. [DOI] [PubMed] [Google Scholar]
- 40.Hocking RK, et al. Water-oxidation catalysis by manganese in a geochemical-like cycle. Nat Chem. 2011;3(6):461–466. doi: 10.1038/nchem.1049. [DOI] [PubMed] [Google Scholar]
- 41.Murray JW, Balistrieri LS, Paul B. The oxidation state of manganese in marine sediments and ferromanganese nodules. Geochim Cosmochim Acta. 1984;48:1237–1247. [Google Scholar]
- 42.Villalobos M, Toner B, Bargar J, Sposito G. Characterization of the manganese oxide produced by pseudomonas putida strain MnB1. Geochim Cosmochim Acta. 2003;67(14):2649–2662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.