Abstract
Nectarivorous birds and bats have evolved highly specialized tongues to gather nectar from flowers. Here, we show that a nectar-feeding bat, Glossophaga soricina, uses dynamic erectile papillae to collect nectar. In G. soricina, the tip of the tongue is covered with long filamentous papillae and resembles a brush or mop. During nectar feeding, blood vessels within the tongue tip become engorged with blood and the papillae become erect. Tumescence and papilla erection persist throughout tongue retraction, and nectar, trapped between the rows of erect papillae, is carried into the mouth. The tongue tip does not increase in overall volume as it elongates, suggesting that muscle contraction against the tongue’s fixed volume (i.e., a muscular hydrostat) is primarily responsible for tip elongation, whereas papilla erection is a hydraulic process driven by blood flow. The hydraulic system is embedded within the muscular hydrostat, and, thus, intrinsic muscle contraction may simultaneously increase the length of the tongue and displace blood into the tip. The tongue of G. soricina, together with the tongues of nectar-feeding bees and hummingbirds, which also have dynamic surfaces, could serve as valuable models for developing miniature surgical robots that are both protrusible and have highly dynamic surface configurations.
Keywords: biomechanics, fluid dynamics, lingual papillae, feeding kinematics, soft robots
Glossophagine bats and nectar-feeding birds hover in front of flowers and use their long tongues to collect nectar (Fig. 1A). Hovering is energetically expensive, and nectar resources are limited in the wild, so birds and mammals have developed specific strategies to gather nectar efficiently from flowers—one of which is to have a long, protrusible tongue. Hummingbird and bat tongues are so long that they are housed in an elongated bill or rostrum. During nectar feeding, these tongues typically elongate to more than double their resting lengths (1, 2).
Fig. 1.
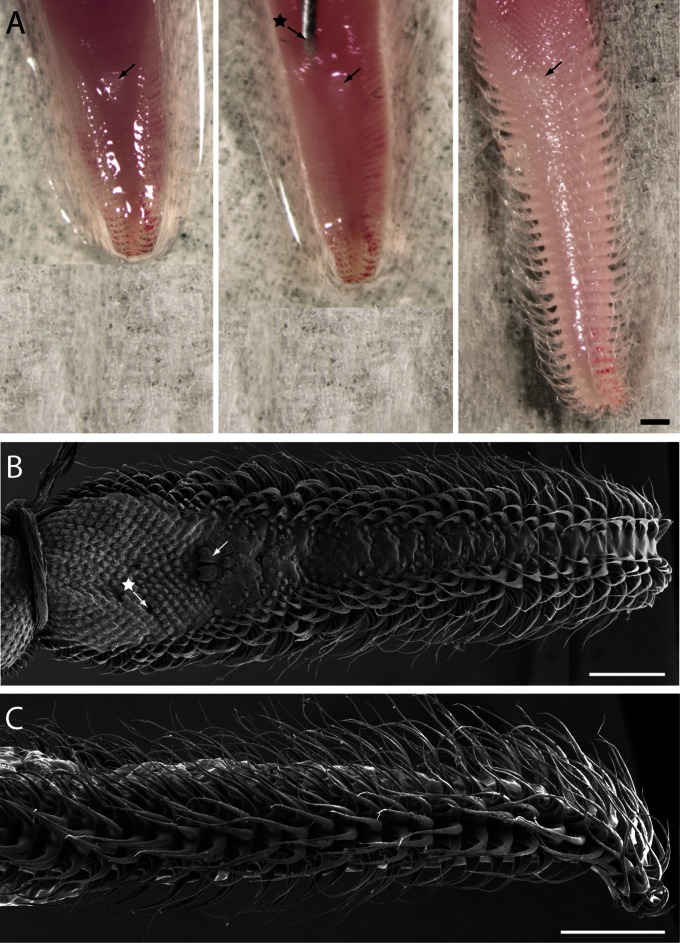
Elongated tongue of a nectar-feeding bat, G. soricina, and its characteristic hair-like papillae. (A) G. soricina hovers in front of a feeder filled with artificial nectar and laps nectar with its long tongue. The white arrow highlights the distal tip of the tongue, which is covered in hair-like papillae. (B) Scanning electron micrograph of the tongue tip, showing a mop-like structure made of elongated lingual papillae. In this micrograph, the hair-like papillae are in their resting condition. (Scale bar: 1 mm.) (C) Scanning electron micrograph of the medial surface of the hair-like papillae, demonstrating that they are arranged in horizontal rows along the tongue tip. (Scale bar: 100 µm.) (D) Scanning electron micrograph of small filiform papillae located on the middle and proximal regions of the dorsal surface of the tongue. (Scale bar: 30 µm.)
In addition to having extraordinarily long and protrusible tongues, these animals also have elaborate structures on the tongue tip. Hummingbirds have bifurcated tubular tongue tips, formed by curled keratinous lamellae (3). During feeding, nectar is trapped within and between the tubular tongue tips and carried into the mouth (4). The tip of a nectar-feeding bat tongue is not tubular; instead, it is covered with many elongated, conical papillae. These hair-like papillae give the tongue tip a brush- or mop-like appearance (Fig. 1B). For decades, the hair-like papillae have been thought to be passive, static structures that simply increase the surface area of the tongue (5, 6).
In vivo studies on nectarivorous birds have shown that structures on the tongue tip are dynamic during feeding. In hummingbirds, the bifurcated tongue tips separate and the lamellae unfurl when the tongue is submerged in nectar (4). As the tongue is withdrawn, the lamellae roll inward and nectar is trapped within and between the tongue tips. The dynamic nectar trap in hummingbirds suggests that the hair-like papillae on nectar-feeding bat tongues may also be dynamic structures. To test this hypothesis, we investigated the anatomy and histology of the tongue tip in a nectar-feeding bat, Glossophaga soricina, and used high-speed video to visualize the movements of the tongue and papillae during nectar feeding.
Results
Tongue Morphology.
The dorsal surface of the G. soricina tongue is covered with many lingual papillae (Fig. 1). Most of the papillae are small filiform papillae, which consist of overlapping, serrated sheets of keratin. These small, pointed papillae give the middle region of the tongue a scale-like appearance (Fig. 1B). The dorsal and lateral surfaces of the tongue tip, however, are covered with elongated hair-like papillae, which are organized in discrete transverse rows along the distal third of the tongue. Each hair-like papilla is triangular in shape because it has a broad, flattened base and gradually tapers into a fine filamentous tip (Fig. 1C).
The G. soricina tongue is enveloped in fibrous connective tissue and stratified squamous epithelium, clearly seen in cross-section. At the tongue tip, the keratinized epithelium and fibrous connective tissue of the lateral tongue are elaborated into a set of finger-like projections (Fig. 2). These projections are the bases of the hair-like papillae, and they radiate from the main body of the tongue like spokes of a wheel. The core (i.e., medullary region) of the tongue is composed of orthogonally arranged muscle fibers (Fig. 2C). Horizontally and vertically oriented muscle fibers extend across the tongue’s midline and longitudinally oriented fibers around the perimeter. This orthogonal arrangement of muscle fibers within the medullary region of the tongue is typical of mammals (7, 8).
Fig. 2.
Anatomy of the G. soricina tongue tip. (A) Line tracings of three transverse sections through the proximal (Upper), middle (Middle), and distal (Lower) region of the tongue. The dorsal surface of the tongue is directed up. These drawings highlight the location of the epidermis/dermis/papillae (light gray), skeletal muscle (white), hypoglossal nerve (yellow), arteries (red), and veins (blue). (Scale bar: 1 mm.) (B) Schematic of the arteries and veins within the G. soricina tongue. Arteries are shown in red and veins in blue. The dotted lines in the tongue tip illustrate the position of the arteriovenous anastomoses. (C) Transverse section and line tracing of the tongue tip showing the direct connection between the vascular sinus and papillary vein. This micrograph shows only the left side of the tongue tip. The color scheme in the line tracing matches the schematics shown above except, here, the orthogonally arranged skeletal muscle fibers are illustrated as dark gray lines. (Scale bar: 0.1 mm.) ava, arteriovenous anastomoses; bh, base of hair-like papilla; ca, central artery; dla, deep lingual artery; dlv, deep lingual vein; f, frenulum; ft, filamentous tip of hair-like papilla; pv, papillary vein; vs., vascular sinus.
Lingual arteries and veins are interspersed within these orthogonal arrays of skeletal muscle fibers. Together, the lingual arteries and veins form a vascular loop, which brings arterial blood to the tongue tip and returns venous blood to general circulation (Fig. 2B). In the proximal part of the tongue, paired deep lingual arteries run alongside the paired hypoglossal nerves (Fig. 2A). In the middle region of the tongue, the deep lingual arteries converge to form a single midline artery that continues into the tongue tip (Fig. 2B). Distally, this central artery is enlarged. Both the deep lingual and central arteries give rise to smaller blood vessels that extend into the dorsal and lateral regions of the tongue. All of the lingual arteries described above are completely surrounded by the tongue’s horizontal and vertical muscle fibers (Fig. 2).
A specialized network of lingual veins drains blood from the tongue tip (Fig. 2). Near the base of the tongue, the deep lingual veins have large diameters and pass outside the muscular medullary region, close to the frenulum (Fig. 2A). In the middle region of the tongue, the deep lingual veins run on either side of the central artery and are embedded within the tongue’s intrinsic muscle fibers. In this region, the endothelial lining of these veins is corrugated, suggesting that it could expand when filled with blood. There are no valves in the proximal and middle portions of the deep lingual veins.
The deep lingual veins are continuous with two vascular sinuses located in the tongue tip (Fig. 2). These enlarged sinuses extend longitudinally along the lateral margins of the tongue tip, just beneath the rows of hair-like papillae (Fig. 2B). The sinuses are thin-walled and irregularly shaped, suggesting that they are venous structures. The vascular sinuses communicate directly with veins in the base of each hair-like papilla (Fig. 2C). These papillary veins are found only in the base of each papilla and do not extend into the filamentous tip. Red blood cells are visible within the lumina of the papillary veins, confirming that these spaces are vascular.
Observations from High-Speed Movies.
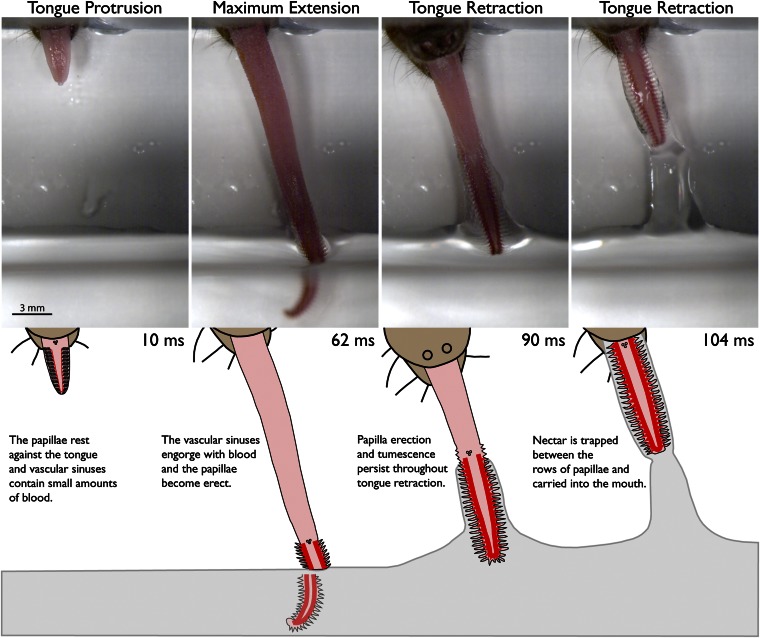
A monochrome high-speed movie of nectar gathering in G. soricina shows that the hair-like papillae are not simple, static structures. Instead, the papillae become erect during nectar feeding, dynamically extending off the surface of the tongue with each lap (Movie S1). In the initial phases of tongue protrusion, the papillae are at rest; the bases of the papillae curve posteriorly and the filamentous tips lie flat against the surface of the tongue. As the tongue approaches maximum extension, the filamentous tips change their orientation to become perpendicular to the tongue’s long axis (Movie S1). The hair-like papillae remain in their erected state throughout tongue retraction, and nectar is trapped between the transverse rows of papillae. Papilla erection always occurs just before maximum extension of the tongue and does not occur during initial tongue protrusion.
Our high-speed movies also show that papilla erection occurs in air when the tongue misses the nectar (Movie S2). When the tongue does not contact the nectar, the bases of the papillae still extend off the surface of the tongue. This observation shows that surface tension release does not drive the changes in tongue surface configuration in G. soricina, as it does in the hummingbird tongue (4). Therefore, a different mechanism must be responsible for papilla erection in bats. Based on the vascular morphology of the G. soricina tongue, we hypothesize that rapid blood flow into the vascular sinuses and papillary veins causes the papillae to become erect during nectar feeding.
A color high-speed movie shows increased blood flow to the vascular sinuses and engorgement of the papillary veins during nectar feeding (Fig. 3 and Movie S3). As the tongue first protrudes from the mouth and the papillae are at rest, the lateral margins of the tongue tip overlying the vascular sinuses are pale pink, indicating that relatively little blood is contained within these vessels. As the tongue reaches maximum extension, the vascular sinuses and papillary veins engorge with blood and become bright red as the papillae become erect. Blood is temporarily trapped within these vessels and the papillae remain erect throughout tongue retraction (Fig. 3).
Fig. 3.
Blood flow and papilla erection in actively feeding G. soricina. (Upper) Frames from a color high-speed movie. (Lower) Line tracings of the rostrum, tongue, and nectar. The bat hovered in front of an acrylic feeder filled with sugar water and only the tip of the rostrum and dorsal surface of the tongue are in the camera’s field of view. In the line tracings, the tongue is shown in pink, the vascular sinuses and papillary veins in red, and the sugar water in light gray.
The papillae alternate between their rest and erect postures during each tongue cycle. At the beginning of tongue protrusion, the papillae rest flat against the tongue’s surface, and as the vascular sinuses and papillary veins engorge with blood, the papillae extend off the tongue’s surface. At the start of the second tongue cycle, the papillae have reverted to their flattened position, and only small amounts of blood are visible in the vascular sinuses (Movie S3). Near maximum extension, the vessels reengorge with blood, the papillae become erect, and the cycle continues.
Intermittent blood flow through the vascular sinuses and papillary veins occurs in synchrony with dynamic changes in the shape of the tongue tip. During lapping, the length of the tongue tip increases by more than 50%, and the papillae transform from their resting to erect postures, thereby increasing the surface area of the elongated tip. We determined tongue tip length changes in vivo by measuring the distance between the horny papillae and distal tip of the tongue. The horny papillae are large, keratinized papillae at the proximal end of the tongue tip that can be consistently visualized in high-speed movies during lapping (Figs. 3 and 4). In the early stages of tongue protrusion (Fig. 3; 10 ms), the mean length of the tongue tip was 5.0 ± 0.13 mm (n = 3 animals; 11–37 tongue cycles per animal) and increased to a mean length of 8.0 ± 0.27 mm in the early phases of tongue retraction (Fig. 3, 90 ms).
Fig. 4.
Artificial papilla erection produced by saline injection in a G. soricina tongue (postmortem). (A) Photographs taken with a dissecting microscope before, during, and after saline injection into the vascular sinus. The papillae become erect and the tongue tip lengthens when saline is injected into the vascular spaces. (B and C) Scanning electron micrographs of the injected tongue in dorsal (B) and lateral (C) views. In both micrographs, the tongue tip is on the right and the proximal end of the tongue is on the left. The proximal tongue was ligated with suture after inflation to prevent saline from draining out of the vascular spaces. In A and B, a star demarcates the saline injection site and small arrows point to the horny papillae. (Scale bars: 1 mm.)
Using the same movie frames, we also measured changes in the diameter of the tongue tip during lapping. Diameter was measured as the distance between the right and left sides of the tongue at the location of the horny papillae. In the early stages of tongue protrusion, the mean diameter was 2.2 ± 0.12 mm and decreased by 27% to a mean diameter of 1.6 ± 0.07 mm in the early phases of tongue retraction. To estimate the volume of the tongue tip at rest and at maximum extension, we modeled the tongue tip as a cylinder. The estimated volume decreased from 18 mm3 at rest to 16 mm3 when elongated (P < 0.0001).
Tongue movements and dynamic shape changes of the tongue tip occur rapidly in G. soricina. The mean duration of a single tongue cycle, from the start of protrusion to the end of retraction, was 0.118 ± 0.002 s (n = 3 animals and 7–31 tongue cycles per animal). The mean duration of the complete feeding bout, including multiple tongue cycles, was 0.33 ± 0.009 s (n = 3 animals and 29 feeding bouts). G. soricina extends and retracts the tongue at a rate of eight cycles per second. Mean time for tongue extension was 0.057 ± 0.001 s (n = 3 animals and 7–31 tongue cycles per animal), and mean time from the start of tongue extension to the appearance of blood in the bases of the papillae was 0.04 ± 0.0002 s.
Postmortem Experiments.
We conducted postmortem experiments with three excised G. soricina tongues to determine whether papilla erection could be produced by blood flow alone. In these specimens, the muscles of the tongue are no longer functional and therefore they cannot perform work. We injected saline into the vascular sinuses of three tongues postmortem and produced papilla erection (Fig. 4). In their resting state, the bases of the papillae are compressed and flattened against the sides of the tongue (Fig. 1B), but after saline injection, they rapidly inflate and extend off the lateral margins of the tongue tip (Fig. 4).
Saline injection into the vascular sinuses also produced tongue tip elongation (Fig. 4). However, during these experiments, the diameter of the tongue tip did not decrease. Saline injection caused the length of the tongue tip to increase by 33–100% of its resting length and diameter to either increase by 11% of the resting tongue diameter (n = 2) or remain constant (n = 1).
Discussion
The tip of the G. soricina tongue is a hemodynamic nectar mop that increases in both surface area and length during nectar feeding. We combined histology, high-speed video, and postmortem experiments to determine that blood flow through the vascular sinuses is responsible for these dynamic changes in surface area. Our histological sections show vascular sinuses at the lateral margins of the tongue tip and a direct connection between the vascular sinuses and papillary veins (Fig. 2). Color high-speed video provides compelling visual evidence that the vascular sinuses and papillary veins rapidly engorge with bright red blood during nectar feeding, causing the hair-like papillae to become erect (Fig. 3). In the postmortem experiments, saline injection alone was sufficient to cause the papillae to become erect (Fig. 4). These results show that papilla erection is a hydraulic process, driven by the rapid influx of blood into the vascular sinuses and papillary veins.
The length of the tongue tip also increased by more than 50% during nectar feeding. According to the muscular hydrostat model for elongation (9), this increase in tongue length is likely driven by contraction of the tongue’s orthogonally arranged muscle fibers. Muscular hydrostats, including the mammalian tongue, elongate when horizontally and vertically oriented muscle fibers contract, which decreases the tongue’s diameter and causes a corresponding increase in tongue length (9). However, this mechanism for elongation is valid only if the volume of the tongue does not increase when it elongates. We modeled the tongue tip as a cylinder and used our measured lengths and diameters to determine whether the elongated and engorged tongue tip increased in volume. The volume of the tongue tip decreased slightly, but statistically significantly, during elongation. We suspect that the true volume remained constant even as the tongue tip elongated and the papillae engorged with blood, and the small decrease in our estimate of tongue tip volume may be attributable to our rough approximation of the tongue tip as a cylinder. Thus, the in vivo tongue measurements, coupled with the orthogonal arrangement of muscle fibers, suggest that the G. soricina tongue tip is acting as a muscular hydrostat.
During the postmortem experiments, the length of the tongue tip increased when the muscles of the tongue were no longer functional (Fig. 4). This observation suggests that fluid flow into the vascular sinuses could potentially also contribute to tongue tip elongation. However, tongue diameter increased or remained constant in the inflated postmortem tongues, whereas diameter decreased in live bats. Thus, we hypothesize that muscle contraction is primarily responsible for tongue tip elongation in G. soricina, but we cannot completely rule out the possibility that blood flow into the tongue tip may also contribute, producing an increase in volume that was too small to detect in our high-speed movies and crude cylindrical volume modeling.
Hemodynamic papilla erection and hydrostatic tongue elongation likely increase the nectar gathering ability of G. soricina. The volume of nectar collected during lapping is directly related to the length and radius of the tongue tip, as well as the thickness of the adhered-nectar layer (10). Hydrostatic tongue elongation increases the length of the tongue tip and hemodynamic papilla erection increases the thickness of the adhered-nectar layer. This adhered-nectar layer is quite thick for G. soricina because it is determined by the length of the elongated hair-like papillae and, therefore, extends beyond the surface of the tongue. It is likely that the hemodynamic nectar mop helps G. soricina take advantage of limited nectar resources to fuel its energy intensive lifestyle (11, 12).
Biomechanical Hypothesis for Papilla Erection.
Here, we propose a mechanistic hypothesis for the dynamic changes in tongue surface area during nectar feeding. We combine histological and in vivo evidence to hypothesize about how blood flows within the lingual vessels and produces papilla erection.
Before the first lapping cycle, the tongue resides in the mouth and the hair-like papillae rest flat against the sides of the tongue. At this stage, blood is present in the lingual arteries and veins to supply oxygen and nutrients to the tissues in the tongue. In the initial phases of tongue elongation, the horizontal and vertical muscle fibers contract and the tongue hydrostatically elongates. The deep lingual arteries, central artery, and middle portion of the deep lingual veins are completely surrounded by the tongue’s intrinsic muscle fibers (Fig. 2). When the tongue begins to elongate, the horizontally and vertically oriented muscle fibers contract, which not only decreases the diameter of the tongue but also compresses these arteries and veins and displaces blood into the tongue tip. Our observation that tumescence occurred when the tongue was maximally extended supports this hypothesis.
We suspect that blood enters the vascular sinuses from two locations: the central artery and the deep lingual veins. We identified small blood vessels that arise from the central artery and extend into the dorsal and lateral margins of the tongue (Fig. 2B). We suspect that these small blood vessels connect the central artery to the vascular sinuses. These blood vessels could act as vascular shunts (i.e., arteriovenous anastomoses), which allow blood to flow directly from the central artery into the vascular sinuses without passing through capillary beds (13). Arteriovenous anastomoses are abundant in the tongues of other mammals and supply blood to the arteries and veins within the lingual papillae (13).
Blood may also enter the vascular sinuses from the deep lingual veins. In the middle region of the tongue, the deep lingual veins are contiguous with the proximal end of the vascular sinuses and are completely surrounded by orthogonally arranged muscle fibers (Fig. 2). We hypothesize that when the horizontal and vertical muscle fibers contract to elongate the tongue, blood within these veins is squeezed into the vascular sinuses. We did not find valves in the deep lingual veins, which suggests that they permit bidirectional flow.
As blood moves into the vascular sinuses, the papillary veins become engorged, and the bases of the hair-like papillae become erect. The tongue is plunged into the nectar and the release of surface tension allows the filamentous tips of the hair-like papillae to orient perpendicular to the long axis of the tongue (Movie S3). As the tongue is withdrawn, nectar is trapped between the transverse rows of erect papillae. During retraction, the proximal end of the tongue shortens and withdraws the engorged tongue tip from the nectar. Blood remains in the vascular sinuses and papillary veins for the entire duration of tongue retraction, which maintains the papillae in their erect posture and allows a large bolus of nectar to be carried into the mouth (Fig. 3 and Movie S3). During retraction, the proximal end of the tongue shortens, whereas the tip remains extended. This observation suggests that the orthogonally arranged muscle fibers in the proximal and distal regions of the tongue contract at different times.
At the beginning of the second lapping cycle, the hair-like papillae have reverted to their resting position and only small amounts of blood are present in the vascular sinuses. We hypothesize that the hard palate and upper lip return the erect papillae to their resting position at the beginning of the second tongue cycle. When the tongue tip protrudes from the mouth a second time, it is ratcheted across the palatal rugae and upper lip, which could flatten the erect papillae against the dorsal surface of the tongue and squeeze blood into the proximal tongue. Also, at this stage in the lapping cycle, nectar could be stripped off the tongue as the papillae are folded back into their resting position.
Between successive tongue cycles, blood is likely displaced into temporary reservoirs within the lingual arteries and veins. On the arterial side, we hypothesize that the branches of the deep lingual arteries, as they converge to form the central artery (Fig. 2B), act as a temporary storage site for blood. On the venous side, we hypothesize that the veins themselves act as blood reservoirs. Along their entire length, from the frenulum to the horny papillae, the deep lingual veins have a corrugated, irregular shape, suggesting that these veins could expand to act as a temporary storage site for blood between subsequent lapping cycles.
Tumescence and papilla erection occurred rapidly in G. soricina. The vascular sinuses and papillary veins become engorged with blood and papillae become erect in 0.04 s. This study raises the question: how does blood flow cause such rapid changes in tongue tip surface area? We identified three morphological features of the tongue that could contribute to the rapidity of papilla erection. First, the hydraulic system responsible for papilla erection is housed within a muscular hydrostat. Intrinsic muscle contraction decreases tongue diameter and produces rapid changes in tongue length. The tongue’s intrinsic muscle fiber systems may also be directly involved in pressurizing the tongue tip and inflating the hair-like papillae. Because tongue elongation is driven by contraction of the orthogonal muscle fibers within the core of the tongue, internal pressure will likely drive blood into the vascular sinuses and papillary veins, which are not under pressure. Thus, the mechanism that drives rapid papillary filling is internal pressurization caused by rapid hydrostatic elongation. Second, we identified portions of the lingual arteries and veins that could act as temporary storage sites for blood between sequential tongue cycles. These storage sites contribute to the rapidity of papilla erection because they prevent blood from exiting the tongue entirely between tongue cycles. Third, there are no valves in the deep lingual veins; therefore, blood could quickly move between the proximal and distal portions of the lingual veins during lapping.
The tongue’s speed, combined with its miniature size and flexibility, make it a potentially valuable model for developing robots for endoluminal surgeries such as angioplasty and gastric endoscopy. During these procedures, physicians must access target organs in tight working spaces by inserting rigid catheters and trocars through small incisions (14, 15). Biologically inspired soft robots, such as inchworm devices, are useful during endoluminal surgeries because of their infinite degrees of freedom, ability to elongate, and high dexterity (16, 17). We propose that surgical instrument design inspired by the G. soricina tongue could be especially useful because of the tongue tip’s flexible structure and small size and its ability to rapidly increase in length and simultaneously change its surface configuration. Surgical instruments modeled after the G. soricina tongue tip could access small, remote regions of blood vessels or the gastrointestinal tract and, once inflated, could maintain the patency of such biological tubes.
Comparisons Among Nectar-Feeding Bats, Birds, and Insects.
Nectar provides a major component of the diet in all bat species within the Tribe Glossophagini. All have hair-like papillae at the distal tip of the tongue and enlarged lingual vessels (18, 19). We have shown here that vascular sinuses and papillary veins are important morphological characteristics for hemodynamic papilla erection and tongue tip elongation. To date, these structures have not been examined in other nectar-feeding phyllostomid bats, nor have the detailed kinematics of the tongue been studied at high temporal and spatial resolution, so it remains unclear whether all nectar-feeding bats use a hemodynamic nectar mop to gather food.
In addition to glossophagine bats, long-tongued bees and the honey possum also have elaborate brushes on the ends of their tongues. In long-tongued bees, as in G. soricina, the hair-like projections are dynamic during nectar feeding. In bees, the bristles become erect when the tunic surrounding the tongue is stretched longitudinally during tongue protrusion (20), whereas we found that in G. soricina, the hair-like papillae are actively inflated by blood flow into the papillary veins. Although lapping behavior has not been studied in detail in the honey possum, these mammals also have enlarged blood vessels and a single midline artery in the tongue tip (21, 22), suggesting that they may also hemodynamically inflate their lingual papillae.
Erection of hair-like projections, as in G. soricina and bees, produces rapid changes in tongue surface area during nectar lapping. Hummingbird tongues also undergo rapid changes in tongue surface area during lapping, but in this case, the changes are driven by release of surface tension when the tongue enters the nectar and elastic recoil of the keratinous lamellae (4). Thus, hummingbirds, long-tongued bees, and bats appear to have converged on rapid changes in the tongue surface during nectar collection, but the morphology and biomechanics of their tongue tips differ fundamentally. Together, these three systems could serve as valuable models for the development of miniature surgical robots that are flexible, can change length, and have dynamic surface configurations.
Materials and Methods
Tongue Morphology.
To examine the external morphology of the lingual papillae, whole tongues were removed from three G. soricina and preserved in Karnovsky’s fixative. Each tongue was dehydrated, critical point dried, and mounted on an aluminum stub. The tongues were coated in gold palladium and imaged with a Hitachi 2700 scanning electron microscope.
To examine the morphology of the intrinsic tongue muscles and blood vessels, whole tongues were excised from four G. soricina and preserved in neutral buffered formalin. Each tongue was separated into proximal, middle, and distal regions. Each region was then embedded in paraffin and sectioned along the transverse or longitudinal axis with a rotary microtome. All sections were stained with hematoxylin/eosin and imaged with a compound microscope (Nikon Eclipse e600 equipped with a Nikon DXM 12000C digital camera). Brightness and contrast of the digital images were adjusted with Adobe Photoshop.
High-Speed Movies of Nectar-Feeding Bats.
Three G. soricina were filmed with a high-speed video camera (Photron Fastcam 1024 PCI or SA5; Vision Research Phantom v9 or v10). All tongue cycles were recorded at 500 frames per second, and fiber optic microscope lamps illuminated the tongue during lapping. Cardboard light shields were attached to the feeder to protect the bat’s eyes from the light. The animals fed from a rectangular feeder filled with artificial nectar (17% mass/mass sucrose concentration). The rectangular shape of the feeder does not match the circular shape of a flower corolla, but this feeder design was necessary to prevent optical distortion.
Length and diameter of the tongue tip were measured in vivo in a total of 76 tongue cycles from three individual bats. Tongue tip length was measured as the distance between the horny papillae and the distal tip of the tongue. Tongue tip diameter was measured as the distance between the right and left sides of the tongue at the location of the horny papillae. Using ImageJ software (23), all distances were measured from movie frames at two specific points in the lapping cycle. Resting length and diameter were measured at the first emergence of the horny papillae from the mouth (Fig. 3; 10 ms). Extended tongue length and diameter were measured during tongue retraction, immediately after the tip exited the nectar interface (Fig. 3; 90 ms). Mean length and diameter were calculated for the three individuals at the two phases of lapping (i.e., start of protrusion; early in retraction), and these means were used to calculate an overall mean ± SEM (n = 3 individuals) for each phase. A mixed-model ANOVA with individual and phase of the lapping cycle as factors was used to test for a significant change in tongue volume with elongation. Phase (F = 17.8; df = 1; P < 0.0001), individual (F = 70.6; df = 2; P < 0.0001), and phase by individual (F = 3.6; df = 2; P = 0.0312) were all significant at the P < 0.05 level.
Duration of events was measured by counting frames from high-speed movies. Mean durations were calculated for the three individual bats, and these means were used to calculate an overall mean ± SEM (n = 3 individuals). The time to maximum tongue extension and the time required for blood to reach the tongue tip were measured in a total of 52 tongue cycles from three individuals. The duration of tongue protrusion was measured as the time from the first appearance of the tongue to the time just before the tongue reversed directions. The time required for blood to reach the papillary veins was measured from the first appearance of the tongue to the instant when the papillary veins became engorged with blood. The duration of a single tongue cycle (lap) was measured from the first appearance of the tongue tip to the moment when the tongue tip was fully retracted back into the mouth. The duration of a feeding bout (i.e., multiple tongue cycles in rapid succession) was measured in 29 bouts from three individuals. It was defined as the time from the first appearance of the tongue tip in the first lap to the time when the tongue retracted back into the mouth at the end of the last lap.
Postmortem Saline Injection Experiments.
Neutral buffered saline was injected into the vascular sinuses of three excised tongues using a 31-gauge hypodermic needle and a 1-mL syringe. Once the papillae were erect, suture was tied around the circumference of the tongue. Photographs were taken before, during, and after saline injection with a digital camera mounted on a dissecting scope (Nikon Eclipse e600 with Nikon DXM 12000C digital camera). Adobe Photoshop was used to adjust the brightness and color balance of each photograph. Using ImageJ software, length and width of the artificially inflated tongues were measured using the same anatomical landmarks described in High-Speed Movies of Nectar-Feeding Bats.
All ligated tongues were preserved in Karnovsky’s fixative and imaged with a scanning electron microscope. The scanning electron micrographs of the inflated tongues were manually stitched together in Adobe Photoshop to form composite images, and the brightness and contrast were adjusted in the composite image. Extra empty background was added to the left and center images in Fig. 4A. This modification did not affect the tongue tip lengths, but it was necessary to make the overall length of the left and center images match that in the right image.
Animal Welfare Statement.
All activities involving live bats were approved by the Institutional Animal Care and Use Committee at Brown University (no. 1004016).
Supplementary Material
Acknowledgments
We thank K. Schwenk for histology advice and insightful comments on hydrostatic tongue elongation; M. Tschapka and B. Nowroozi for helpful discussions; E. Giblin and F. Lemieux for laboratory assistance; Vision Research and Tech Imaging Services, Inc., for loaning the color high-speed video camera equipment; P. Weston, M. Golde, G. Williams, and M. Hixon for histology advice and use of the microtome; and the Biodôme de Montréal for use of bats. Financial support was provided by a Grant-In-Aid of Research from Sigma Xi, The Scientific Research Society, the American Microscopical Society, the Bushnell Graduate Research and Education Fund, Air Force Office of Scientific Research Grant FA9550-07-1-0540, and National Science Foundation Grants 1052700 and 0723392.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.K.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222726110/-/DCSupplemental.
References
- 1.Grant V, Temeles EJ. Foraging ability of rufous hummingbirds on hummingbird flowers and hawkmoth flowers. Proc Natl Acad Sci USA. 1992;89(20):9400–9404. doi: 10.1073/pnas.89.20.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter Y, von Helversen O. Operational tongue length in phyllostomid nectar-feeding bats. J Mammal. 2003;84(3):886–896. [Google Scholar]
- 3.Weymouth RD, Lasiewski RC, Berger AJ. The tongue apparatus in hummingbirds. Acta Anat (Basel) 1964;58:252–270. doi: 10.1159/000142586. [DOI] [PubMed] [Google Scholar]
- 4.Rico-Guevara A, Rubega MA. The hummingbird tongue is a fluid trap, not a capillary tube. Proc Natl Acad Sci USA. 2011;108(23):9356–9360. doi: 10.1073/pnas.1016944108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koopman KF. The distributional patterns of New World nectar-feeding bats. Ann Mo Bot Gard. 1981;68(2):352–369. [Google Scholar]
- 6.Howell DJ, Hodgkin N. Feeding adaptations in the hairs and tongues of nectar-feeding bats. J Morphol. 1976;148(3):329–339. doi: 10.1002/jmor.1051480305. [DOI] [PubMed] [Google Scholar]
- 7. Schwenk K (2000) Feeding: Form, Function, and Evolution in Tetrapod Vertebrates, ed Schwenk K (Academic, San Diego), pp 21–61.
- 8.Schwenk K. Extrinsic versus intrinsic lingual muscles: A false dichotomy? Bull Mus Comp Zool. 2001;156(1):219–235. [Google Scholar]
- 9.Kier WM, Smith KK. Tongues, tentacles, and trunks: The biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–324. [Google Scholar]
- 10.Kim W, Gilet T, Bush JWM. Optimal concentrations in nectar feeding. Proc Natl Acad Sci USA. 2011;108(40):16618–16621. doi: 10.1073/pnas.1108642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voigt CC, Speakman JR. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Funct Ecol. 2007;21(5):913–921. [Google Scholar]
- 12.Suarez RK, Welch KC, Jr, Hanna SK, Herrera M LG. Flight muscle enzymes and metabolic flux rates during hovering flight of the nectar bat, Glossophaga soricina: Further evidence of convergence with hummingbirds. Comp Biochem Physiol A Mol Integr Physiol. 2009;153(2):136–140. doi: 10.1016/j.cbpa.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Prichard MML, Daniel PM. Arterio-venous anastomoses in the tongue of the dog. J Anat. Lond. 1953;87(1):66–74. [PMC free article] [PubMed] [Google Scholar]
- 14.Howe RD, Matsuoka Y. Robotics for surgery. Annu Rev Biomed Eng. 1999;1:211–240. doi: 10.1146/annurev.bioeng.1.1.211. [DOI] [PubMed] [Google Scholar]
- 15.De Greef A, Lambert P, Delchambre A. Towards flexible medical instruments: Review of flexible fluidic actuators. Precis Eng. 2009;33(4):311–321. [Google Scholar]
- 16.Menciassi A, Dario P. Bio-inspired solutions for locomotion in the gastrointestinal tract: Background and perspectives. Philos Transact A Math Phys Eng Sci. 2003;361(1811):2287–2298. doi: 10.1098/rsta.2003.1255. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi D, Rahn CD, Kier WM, Walker ID. Soft robotics: Biological inspiration, state of the art, and future research. Appl Bionics Biomech. 2008;5(3):99–117. [Google Scholar]
- 18.Griffiths TA. Muscular and vascular adaptations for nectar-feeding in the Glossophagine bats Monophyllus and Glossophaga. J Mammal. 1978;59(2):414–418. [Google Scholar]
- 19.Griffiths TA. Systematics of the New World nectar-feeding bats (Mammalia, Phyllostomidae), based on the morphology of the hyoid and lingual regions. Am Mus Novit. 1982;2742:1–45. [Google Scholar]
- 20.Simpson J, Riedel IBM. Discharge and manipulation of labial gland secretion by workers of Apis mellifera (L.) (Hymenoptera: Apidae) Proc R Entomol Soc Lond Ser A. 1964;39(4-6):76–82. [Google Scholar]
- 21.Richardson KC, Wooller RD, Collins BG. Adaptations to a diet of nectar and pollen in the marsupial Tarsipes rostratus (Marsupialia: Tarsipedidae) J Zool. 1986;208(2):285–297. [Google Scholar]
- 22.Rosenberg HI, Richardson KC. Cephalic morphology of the honey possum, Tarsipes rostratus (Marsupialia: Tarsipedidae); an obligate nectarivore. J Morphol. 1995;223(3):303–323. doi: 10.1002/jmor.1052230307. [DOI] [PubMed] [Google Scholar]
- 23.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.