Abstract
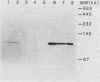
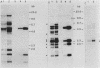
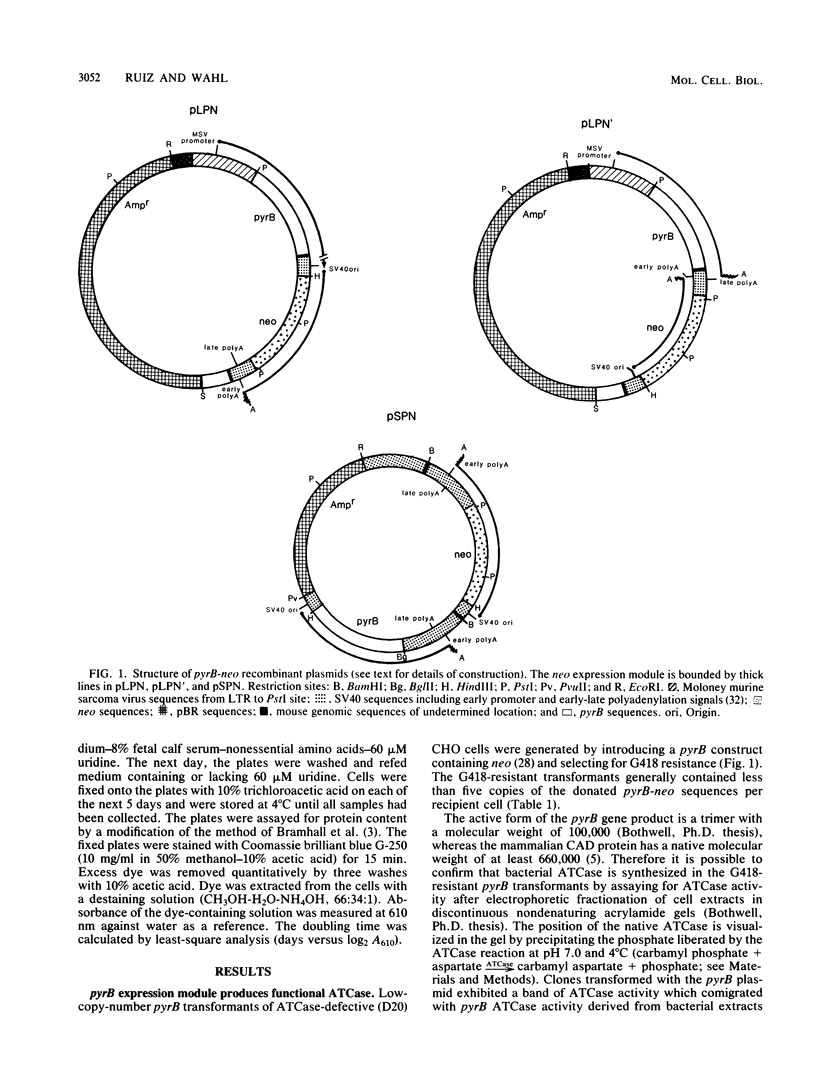
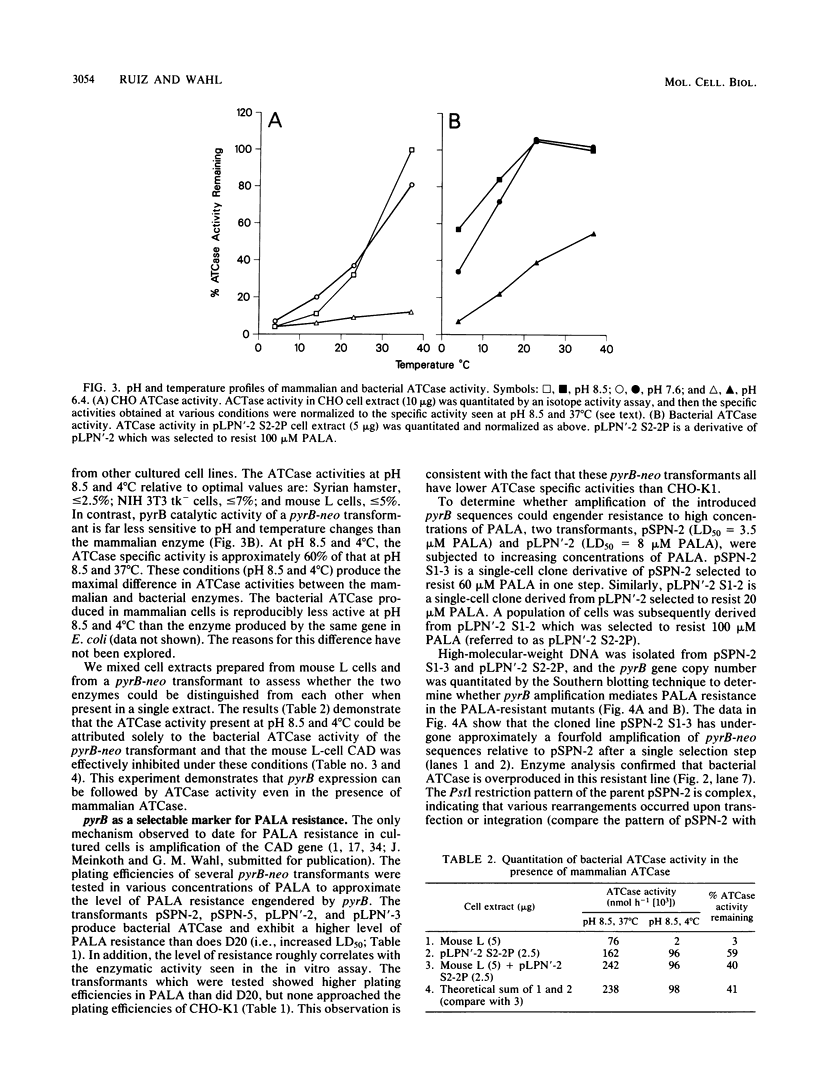
Eucaryotic expression vectors containing the Escherichia coli pyrB gene (pyrB encodes the catalytic subunit of aspartate transcarbamylase [ATCase]) and the Tn5 phosphotransferase gene (G418 resistance module) were transfected into a mutant Chinese hamster ovary cell line possessing a CAD multifunctional protein lacking ATCase activity. G418-resistant transformants were isolated and analyzed for ATCase activity, the ability to complement the CAD ATCase defect, and the ability to resist high concentrations of the ATCase inhibitor N-(phosphonacetyl)-L-aspartate (PALA) by amplifying the donated pyrB gene sequences. We report that bacterial ATCase is expressed in these lines, that it complements the CAD ATCase defect in trans, and that its amplification engenders PALA resistance. In addition, we derived rapid and sensitive assay conditions which enable the determination of bacterial ATCase enzyme activity in the presence of mammalian ATCase.
Full text
PDF


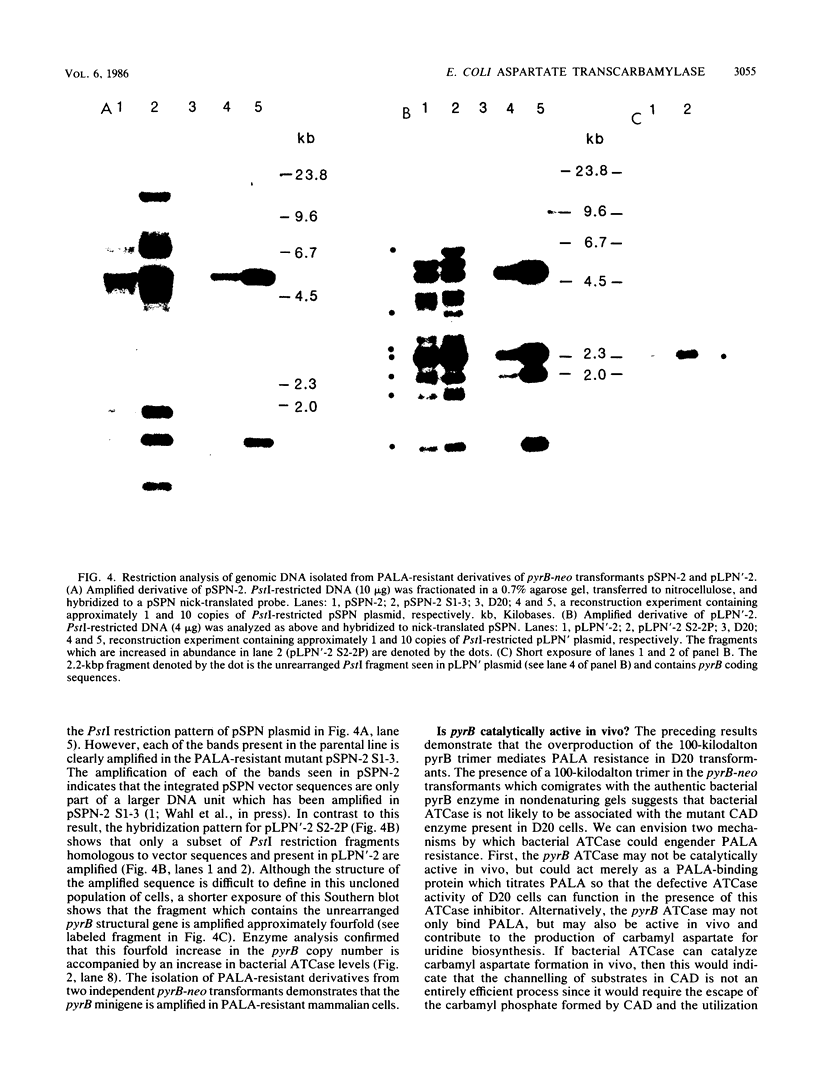
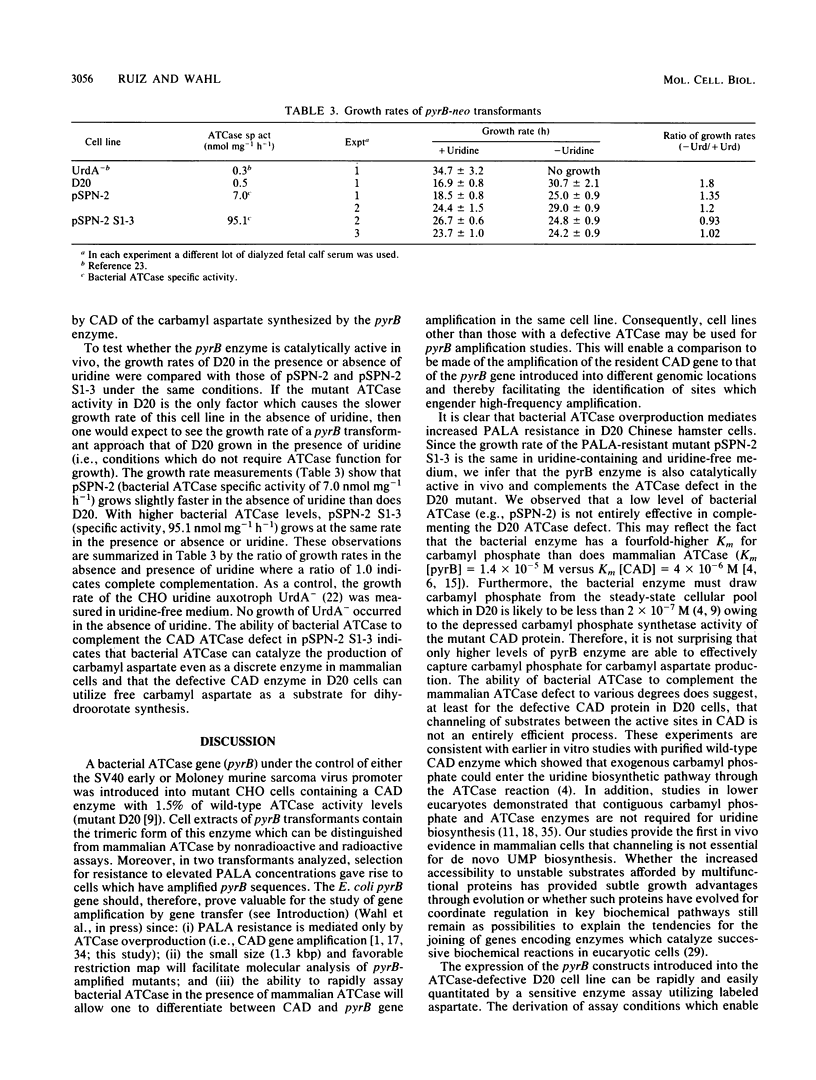


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Giulotto E., Zieg J., Brison O., Liao W. S., Stark G. R. Structure of amplified DNA in different Syrian hamster cell lines resistant to N-(phosphonacetyl)-L-aspartate. Mol Cell Biol. 1983 Nov;3(11):2076–2088. doi: 10.1128/mcb.3.11.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Christopherson R. I., Jones M. E. The overall synthesis of L-5,6-dihydroorotate by multienzymatic protein pyr1-3 from hamster cells. Kinetic studies, substrate channeling, and the effects of inhibitors. J Biol Chem. 1980 Dec 10;255(23):11381–11395. [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., McEwan R. N., Pearson M. L. Expression and amplification of engineered mouse dihydrofolate reductase minigenes. Mol Cell Biol. 1983 Feb;3(2):257–266. doi: 10.1128/mcb.3.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. N., Carnright D. V., Patterson D. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: III. Association of carbamyl phosphate synthetase, aspartate transcarbamylase, and dihydroorotase in mutants of cultured Chinese hamster cells. Somatic Cell Genet. 1979 Mar;5(2):175–191. doi: 10.1007/BF01539159. [DOI] [PubMed] [Google Scholar]
- Davidson J. N., Niswander L. A. Partial cDNA sequence to a hamster gene corrects defect in Escherichia coli pyrB mutant. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6897–6901. doi: 10.1073/pnas.80.22.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N. Unstable expression and amplification of a transfected oncogene in confluent and subconfluent cells. Mol Cell Biol. 1985 Jun;5(6):1456–1464. doi: 10.1128/mcb.5.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Beverley S. M., Kiely M. L., Schimke R. T. Properties of an altered dihydrofolate reductase encoded by amplified genes in cultured mouse fibroblasts. J Biol Chem. 1981 Sep 25;256(18):9501–9510. [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Metabolic compartmentation at the molecular level: the function of a multienzyme aggregate in the pyrimidine pathway of yeast. Biochim Biophys Acta. 1970 Dec 16;220(3):365–372. doi: 10.1016/0005-2744(70)90268-8. [DOI] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C., Kelley W. N., Daddona P. E. Transient expression of human adenosine deaminase cDNAs: identification of a nonfunctional clone resulting from a single amino acid substitution. Mol Cell Biol. 1985 Apr;5(4):762–767. doi: 10.1128/mcb.5.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Karels M. J., Navre M., Schachman H. K. Genes encoding Escherichia coli aspartate transcarbamoylase: the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4020–4024. doi: 10.1073/pnas.79.13.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Dieckmann B., Lee F. Co-expression and amplification of dihydrofolate reductase cDNA and the Escherichia coli XGPRT gene in Chinese hamster ovary cells. J Mol Appl Genet. 1981;1(3):165–175. [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Kelleher L. E., Goutas L. J. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate-resistant L1210 clonal cell lines derived in vivo. Cancer Res. 1981 Nov;41(11 Pt 1):4447–4452. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Southern P. J. Analysis of gene expression using simian virus 40 vectors. Anal Biochem. 1983 Nov;135(1):1–15. doi: 10.1016/0003-2697(83)90723-6. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]
- Wilson D. J., Hanes S. D., Pichler M. H., Biswas D. K. 5-bromodeoxyuridine-induced amplification of prolactin gene in GH cells is an extrachromosomal event. Biochemistry. 1983 Dec 20;22(26):6077–6084. doi: 10.1021/bi00295a006. [DOI] [PubMed] [Google Scholar]