Abstract
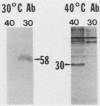
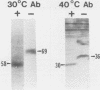
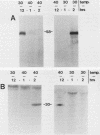
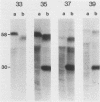
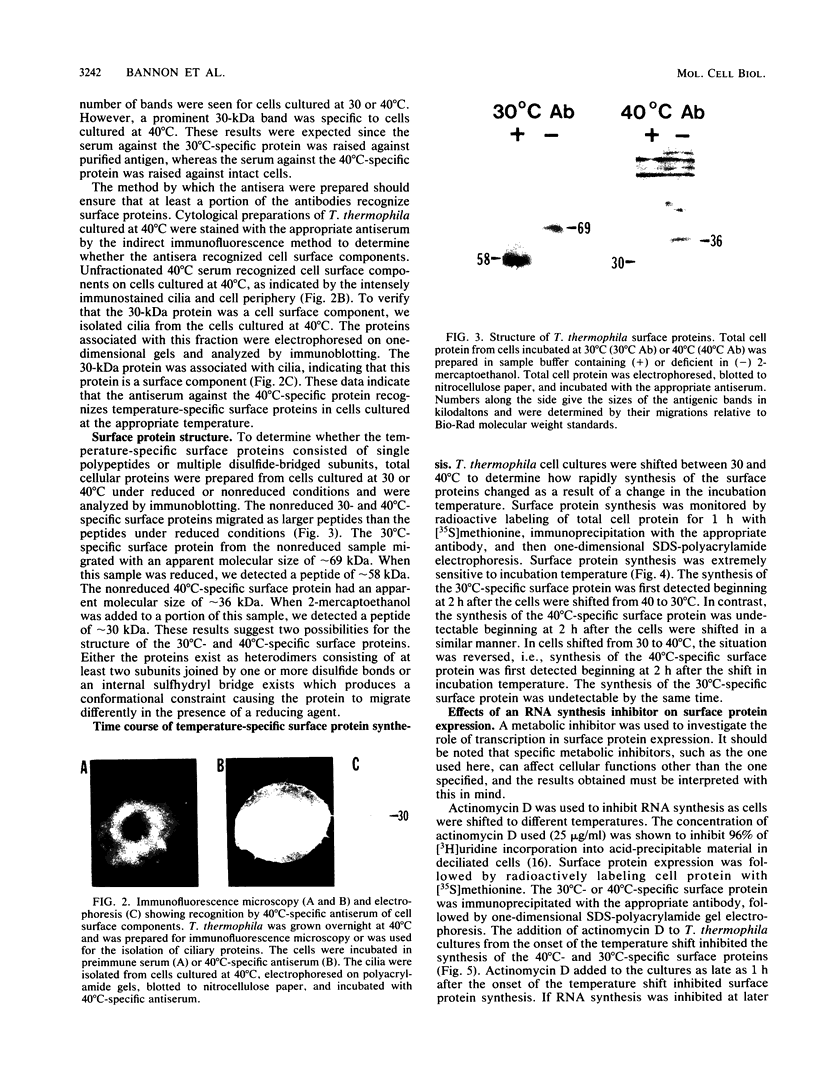
The presence of specific proteins (known as immobilization antigens) on the surface of the ciliated protozoan Tetrahymena thermophila is under environmental regulation. There are five different classes (serotypes) of surface proteins which appear on the cell surface when T. thermophila is cultured under different conditions of temperature or incubation medium; three of these are temperature dependent. The appearance of these proteins on the cell surface is mutually exclusive. We used polyclonal antibodies raised against 30 degrees C (designated SerH3)- and 40 degrees C (designated SerT)-specific surface antigens to study their structure and expression. We showed that these surface proteins contain at least one disulfide bridge. On sodium dodecyl sulfate-denaturing polyacrylamide gels, the nonreduced 30 degrees C- and 40 degrees C-specific surface proteins migrated with molecular sizes of 69 and 36 kilodaltons, respectively. The reduced forms of the proteins migrated with molecular sizes of 58 and 30 kilodaltons, respectively. The synthesis of the surface proteins responded rapidly and with a time course similar to that of the incubation temperature. The synthesis of each surface protein was greatly reduced within 1 h and undetectable by 2 h after a shift to the temperature at which the protein is not expressed. Surface protein synthesis resumed by the end of 1 h after a shift to the temperature at which the protein is expressed. The temperature-dependent induction of these surface proteins appears to be dependent on the synthesis of new mRNA, as indicated by a sensitivity to actinomycin D. Surface protein syntheses were mutually exclusive except at a transition temperature. At 35 degrees C both surface proteins were synthesized by a cell population. These data support the potential of this system as a model for the study of the effects of environmental factors on the genetic regulation of cell surface proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffret C. A., Turner M. J. Variant specific antigens of Trypanosoma brucei exist in solution as glycoprotein dimers. Biochem J. 1981 Feb 1;193(2):647–650. doi: 10.1042/bj1930647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Gorovsky M. A. Cilia regeneration in Tetrahymena. A simple reproducible method for producing large numbers of regenerating cells. Exp Cell Res. 1982 Aug;140(2):471–476. doi: 10.1016/0014-4827(82)90144-6. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S. Purification and partial characterization of the H immobilization antigens of Tetrahymena thermophila. J Protozool. 1986 May;33(2):204–208. doi: 10.1111/j.1550-7408.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S., Skalican-Crowe J. Isolation and genetic analysis of mutations at the SerH immobilization antigen locus of Tetrahymena thermophila. Genetics. 1985 Oct;111(2):273–286. doi: 10.1093/genetics/111.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P. Regulatory Serotype Mutations in TETRAHYMENA PYRIFORMIS, Syngen 1. Genetics. 1973 May;74(1):81–106. doi: 10.1093/genetics/74.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J. Antigenic variation in the salivarian trypanosomes. Adv Exp Med Biol. 1977;93:31–63. doi: 10.1007/978-1-4615-8855-9_4. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Hirumi H., Hirumi K., Lupton E. N., Cross G. A. Antigenic variation in clones of animal-infective Trypanosoma brucei derived and maintained in vitro. Parasitology. 1980 Apr;80(2):359–369. doi: 10.1017/s0031182000000810. [DOI] [PubMed] [Google Scholar]
- Forney J. D., Epstein L. M., Preer L. B., Rudman B. M., Widmayer D. J., Klein W. H., Preer J. R., Jr Structure and expression of genes for surface proteins in Paramecium. Mol Cell Biol. 1983 Mar;3(3):466–474. doi: 10.1128/mcb.3.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Gorovsky M. A. Cilia regeneration in starved tetrahymena: an inducible system for studying gene expression and organelle biogenesis. Cell. 1979 Jun;17(2):307–317. doi: 10.1016/0092-8674(79)90156-9. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Kraus K. W., Findly R. C. Starved Tetrahymena thermophila cells that are unable to mount an effective heat shock response selectively degrade their rRNA. Mol Cell Biol. 1984 Oct;4(10):2170–2179. doi: 10.1128/mcb.4.10.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Kung C. Studies of the cell surface of Paramecium. Ciliary membrane proteins and immobilization antigens. Biochem J. 1975 Dec;152(3):523–528. doi: 10.1042/bj1520523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G. The immobilization antigen of Paramecium aurelia is a single polypeptide chain. J Protozool. 1975 May;22(2):257–259. doi: 10.1111/j.1550-7408.1975.tb05861.x. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Juergensmeyer E. B. Serotype expression and transformation in Tetrahymena pyriformis. J Protozool. 1969 May;16(2):344–352. doi: 10.1111/j.1550-7408.1969.tb02280.x. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macindoe H., Reisner A. H. Adsorption titration as a specific semi-quantitative assay for soluble and bound Paramecium serotypic antigen. Aust J Biol Sci. 1967 Feb;20(1):141–152. [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney D L, Dubert J M. The Genetics of the H Serotype System in Variety 1 of Tetrahymena Pyriformis. Genetics. 1960 Oct;45(10):1335–1349. doi: 10.1093/genetics/45.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Phillips R. B. Inheritance of T serotypes in Tetrahymena. Genetics. 1967 Aug;56(4):667–681. doi: 10.1093/genetics/56.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H., Rowe J., Macindoe H. M. The largest known monomeric globular proteins. Biochim Biophys Acta. 1969;188(2):196–206. doi: 10.1016/0005-2795(69)90066-x. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Steers E., Jr, Davis R. H., Jr A reexamination of the structure of the immobilization antigen from Paramecium aurelia. Comp Biochem Physiol B. 1977;56(2):195–199. doi: 10.1016/0305-0491(77)90048-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]
- Wenkert D., Allis C. D. Timing of the appearance of macronuclear-specific histone variant hv1 and gene expression in developing new macronuclei of Tetrahymena thermophila. J Cell Biol. 1984 Jun;98(6):2107–2117. doi: 10.1083/jcb.98.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. E., Doerder F. P., Ron A. Expression of a cell surface immobilization antigen during serotype transformation in Tetrahymena thermophila. Mol Cell Biol. 1985 Aug;5(8):1925–1932. doi: 10.1128/mcb.5.8.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]