Abstract
Over two decades have passed since the original discovery of amyloid precursor protein (APP). While physiological function(s) of APP still remain a matter of debate, consensus exists that the proteolytic processing of this protein represents a critical event in the life of neurons and that abnormalities in this process are instrumental in Alzheimer's disease (AD) pathogenesis. Specific molecular components involved in APP proteolysis have been identified, and their enzymatic activities characterized in great detail. As specific proteolytic fragments of APP are identified and novel physiological effects for these fragments are revealed, more obvious becomes our need to understand the spatial organization of APP proteolysis. Valuable insights on this process have been obtained through the study of non-neuronal cells. However, much less is known about the topology of APP processing in neuronal cells, which are characterized by their remarkably complex cellular architecture and extreme degree of polarization. In this review, we discuss published literature addressing various molecular mechanisms and components involved in the trafficking and subcellular distribution of APP and APP secretases in neurons. These include the relevant machinery involved in their sorting, the identity of membranous organelles in which APP is transported, and the molecular motor-based mechanisms involved in their translocation. We also review experimental evidence specifically addressing the processing of APP at the axonal compartment. Understanding neuron-specific mechanisms of APP processing would help illuminating the physiological roles of APP-derived proteolytic fragments and provide novel insights on AD pathogenesis.
Keywords: Alzheimer's disease, Amyloid precursor protein, Secretases, Kinesin, Axonal transport
APP processing: the players
Over the last decade, our understanding of the proteolytic processing of amyloid precursor protein (APP) has increased steadily as relevant protease activities were identified and their activities characterized in detail. The fast pace of research addressing APP processing was motivated by research indicating that abnormal production and aggregation of amyloidogenic peptides derived from APP proteolysis are instrumental in Alzheimer's disease (AD) pathogenesis (Hardy and Selkoe 2002). However, many questions remain regarding the topology, regulation, and physiological role of APP proteolysis in neuronal cells. As an introduction to the topics covered in this review, we provide a brief summary of the APP gene family, the secretases participating in APP processing, and the cleavage products derived from APP proteolysis. We then discuss evidence suggesting specific physiological roles for these APP metabolites.
The APP gene family
APP belongs to a gene family consisting of three members in humans: APP and the two APP-like proteins, APLP1 and APLP2 [reviewed in (Thinakaran and Koo 2008)]. All APP family members are type 1 transmembrane proteins, harboring a large amino-terminal extracellular domain that displays a high degree of sequence homology in three well-defined domains, namely the Kunitz protease inhibitor (KPI), E1, and E2 domains. The KPI domain is present in APLP2 and some APP splice variants. The main APP isoform expressed in neurons (APP695), and APLP1, which is only expressed in neurons, lack the KPI domain. The carboxy-terminus of all APP family members is highly conserved, corresponding to a short intracellular domain known to harbor various well-defined motifs that mediate the interaction of APP family members with a diverse repertoire of proteins [reviewed in (Tang 2009)]. Major differences in primary sequence among APP family members are found in the transmembrane and extracellular juxtamembrane domains. Specifically, only APP, but not APLP1 nor APLP2 contain the sequence encoding amyloid-β peptides (Aβ), a major component of the characteristic senile plaques found in brains of AD patients.
APP secretases and proteolytic APP products
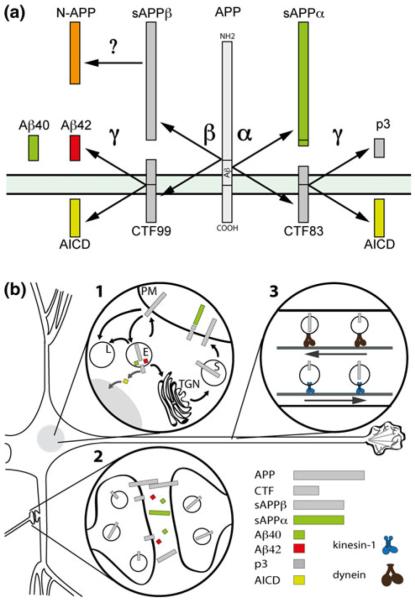
Several proteases, collectively referred to as secretases, are known to cleave APP at specific sites. Cleavage of APP by various secretases results in the generation and release of heterogeneous proteolytic fragments from various cellular membranes. Some caspases also cleave APP at specific sites within their intracellular domain, but a discussion of these findings goes beyond the scope of this review (Zhang et al. 2011). A schematic is shown in Fig. 1a), depicting the proteolytic processing of APP by secretases, as well as the main cleavage fragments derived from this processing.
Fig. 1.
Proteolytic processing of APP by secretases and the spatial arrangement of APP fragments in neurons (not drawn in proportion). a Schematic depiction of APP cleavage by secretases and the main cleavage fragments derived from APP processing. α-secretases cleave APP at a site within the Aβ peptide sequence, thus precluding cleavage by β-secretase and formation of Aβ peptides. This cleavage event gives raise to sAPPα, a soluble amino-terminal APP fragment and the C-terminal stub CTF83. BACE1 cleaves APP at the β-secretase site, yielding sAPPβ and a C-terminal fragment CTF99. sAPPβ can be processed through an unknown mechanism (question mark) to generate a 55 kDa and a 35 kDa peptide termed N-APP. CTF83 and CTF99 stubs derived from α- and β-secretase cleavages can in turn be cleaved intramembranously by γ-secretase, and their cleavage invariably results in the release of a peptide fragment comprising the intracellular domain of APP (AICD) to the cytosol. Whereas cleavage of CTF83 by γ-secretase yields a p3 peptide, cleavage of CTF99 results in the generation of Aβ peptides. b Schematic depiction of the localization and intracellular trafficking of APP and APP metabolites. 1 Major trafficking steps of APP at the biosynthetic secretory pathway. TGN Trans-Golgi network, PM plasma membrane, E endosome, L lysosome. 2 Anterograde and retrograde fast axonal transports of APP and CTFs. 3 Localization of APP and secretion of APP fragments at the synaptic compartment. See main text for a description
From several known zinc-dependent proteases, ADAM10 (a disintegrin and metalloprotease) represents the main secretase responsible for cleavage of APP at the α-cleavage site in neurons (Kuhn et al. 2010). APP cleavage by α-secretase results in the generation of a large amino-terminal soluble fragment (sAPPα) with proposed neuroprotective properties (see below). Because the α-secretase cleavage site is located within the Aβ sequence, processing of APP by α-secretases precludes the generation of Aβ peptides (Thinakaran and Koo 2008).
A transmembrane aspartyl protease termed BACE1 (β-site APP-cleaving enzyme 1) cleaves APP at β cleavage sites (β and β′), which represent the amino-terminus of Aβ peptides (Vassar et al. 2009). BACE1-mediated cleavage of APP results in the release of a soluble amino-terminal fragment termed sAPPβ. Under certain physiological conditions, sAPPβ has been reported to undergo further cleavage(s) by an unknown mechanism to yield a ~55-kDa carboxy-terminal fragment and a ~35-kDa amino-terminal fragment, termed N-APP (Nikolaev et al. 2009).
Carboxy-terminal C83 (αCTF) and C99 (βCTF) stubs are generated after α- and β-secretase processing of APP, respectively. These stubs can be cleaved at various sites within their transmembrane domain by γ-secretase, a protein complex composed of four polypeptide subunits: presenilin-1 (PS1) or presenilin-2 (PS2), nicastrin, anterior pharynx-defective-1 (APH-1), and presenilin enhancer-2 (PEN-2) (De Strooper 2003). Different proteolytic fragments result from γ-secretase cleavage of APP, depending on whether APP is first cleaved by α- or β-secretases. Sequential cleavage of APP by α- and γ-secretases results in the release of a large soluble fragment termed sAPPα and a much shorter peptide termed p3 of unknown function. On the other hand, sequential cleavage of APP by β- and γ-secretase generates Aβ peptides, which range from 38 to 49 amino acid residues in length (Takami et al. 2009). From all potential Aβ peptides generated, Aβ40 represent the most abundant species in vivo (Thinakaran and Koo 2008). The less abundant Aβ42 and Aβ43 peptide variants show an increased propensity to oligomerize and aggregate, a property that correlates with their toxic effect in various experimental paradigms (Saito et al. 2011; Kim and Hecht 2005). Consistent with these properties, increased production and aggregation of Aβ42 peptides represents a major hallmark of AD (Hardy and Selkoe 2002).
Regardless of whether APP is first cleaved by α- or βsecretase, γ-secretase, cleavage of APP C83 and C99 stubs invariably results in the release of AICD, a short cytoplasmic peptide fragment corresponding to the intracellular domain of APP, for which various functions have been proposed (see below) (Chang and Suh 2010).
A wide range of functions have been proposed for APP cleavage products. While some APP-derived peptides appear to have toxic properties (i.e., Aβ42), others appear to display a neuroprotective and/or trophic role (i.e., sAPPα) (Thinakaran and Koo 2008; Zhang et al. 2011). The diversity of effects reported for the various APP cleavage fragments indicates that the subcellular distribution, intracellular trafficking, and localization of APP and secretases represent critical factors directly linked to the various pathophysiological roles of this protein.
Functional roles of APP metabolites in neurons
In simple organisms such as C. elegans or D. melanogaster, the expression of the APP orthologues is restricted to neuronal cells, arguing for important, phylogenetically conserved neuronal function(s) of APP. APP has been proposed to regulate various cellular processes including kinase-based signaling mechanisms, calcium regulation, and cell adhesion, among others (Thinakaran and Koo 2008; Zhang et al. 2011). With regard to cell adhesion, findings of homo- and heterodimerization among APP family members have led to the proposal that full-length versions of these proteins might facilitate close interactions among pre- and post-synapses (see Fig. 1b, inset 2). Additionally, receptor-like properties and trophic functions have been suggested for APP (Thinakaran and Koo 2008; Zhang et al. 2011). The diversity of functions reported for APP holoprotein could be explained as the outcome of discrete physiological effects elicited by different APP fragments. Experimental evidence indicates that such fragments can act in an autocrine and/or paracrine fashions. However, our knowledge on the specific neuronal domains where APP fragments are generated is incomplete, and little is known about the cellular location(s) where APP fragments exert their effects in vivo (Fig. 1b). A brief description of experimental data suggesting discrete functional roles for APP cleavage fragments is provided below.
Compelling evidence exists suggesting that the sAPPα fragment resulting from α-secretase cleavage displays neuroprotective and trophic properties. For example, post-traumatic administration of sAPPα reduced the number of apoptotic neurons in a mouse model of brain injury (Thornton et al. 2006). In addition, sAPPα was shown to protect neurons from proteasomal stress-induced apoptosis and to reduce the degeneration of dendrites induced by this form of cellular stress (Copanaki et al. 2010). Also, delivery of exogenous sAPPα in the brain of mice reportedly enhanced short- and long-term memory in specific experimental paradigms, an effect associated with increased synaptic density and enhanced NMDA receptor-mediated currents (Meziane et al. 1998). sAPPα was also reported to interact with the epidermal growth factor (EGF) receptor pathway and induce neuronal proliferation through a mechanisms involving the activation of specific kinase pathways (Caille et al. 2004). While production of sAPPα has not been demonstrated at the dendritic compartment of neurons, cumulative evidence indicates that presynaptic terminals represent a major site of sAPPα secretion (Nitsch et al. 1992) (Fig. 1b, inset 2). This observation suggests that sAPPα might function as a ligand for postsynaptic neurotransmitter receptors and/or yet unidentified cell adhesion or growth factor-like receptors. Consistent with this view, enhanced synaptic activity correlates with increased sAPPα secretion (Hoey et al. 2009; Nitsch et al. 1993).
Various effects have been reported for sAPPβ in the context of neuronal development. For example, sAPPβ promoted axonal elongation when added to cultured neurons, but this effect was 10 times lower than that of sAPPα (Chasseigneaux et al. 2011). However, the significance of this sAPPβ effect in vivo is unclear, since sAPPα levels in brain and primary neuronal cultures are reportedly higher than sAPPβ levels (Kuhn et al. 2010; Wu et al. 2011). Also, it was reported that sAPPβ, but not sAPPα, could be further processed by an unknown mechanism to release N-APP, a fragment that binds to death receptor 6 (DR6) protein, triggering caspase 6 activation and axonal pruning (Nikolaev et al. 2009). It is remarkably that, although only 16 additional amino acids at the carboxy-terminus distinguish sAPPα from sAPPβ, different physiological functions have been identified for these soluble APP fragments. This observation suggests structural differences among these APP-derived soluble products that depend upon the exposure and processing of APP to α- and β-secretases.
Aβ peptides are widely believed to underlie the patho-physiology of AD, but their normal physiological function remains unclear (Randall et al. 2010). Disparate results have been obtained from the use of Aβ peptides in various experimental systems [reviewed in (Lahiri and Maloney 2010)]. For example, experimental evidence exist supporting neuroprotective and physiologic effects of Aβ. These include the prevention of metal-induced oxidative damage (Baruch-Suchodolsky and Fischer 2009), the enhancement of hippocampal long-term potentiation and memory (Puzzo et al. 2008), and the modulation of synaptic activity (Hsieh et al. 2006; Abramov et al. 2009). Aβ was also found to modulate cholesterol transport and homeostasis (Grimm et al. 2005). On the other hand, a large body of reports documented toxic effects of Aβ peptides including the activation of apoptotic neuronal cell death (Nakagawa et al. 2000), as well as inhibitory effects on synaptic function (Moreno et al. 2009; Shankar and Walsh 2009), and axonal transport (Pigino et al. 2009). Factors related to specific experimental conditions likely account for the different effects reported, including the concentration of Aβ (pmolar to μmolar range), the cell type used, the source and site of Aβ action (i.e., intracellular and extracellular Aβ), and the aggregation state of Aβ (i.e., soluble, oligomeric, or fibrillar) (Busciglio et al. 1992; Lorenzo and Yankner 1994; Pigino et al. 2009).
As discussed below, the bulk of Aβ peptides deposited in amyloid plaques appears to be derived from a pool of APP that undergoes transport along axons, rather than dendrites, suggesting differential processing of APP at pre-and postsynaptic release sites (Lazarov et al. 2002; Buxbaum et al. 1998). While most Aβ is secreted, several studies reported a fraction of intraneuronal Aβ of unknown origin, which might correlate with pathological, AD-related alterations (Oddo et al. 2006), including synaptic dys-function (Tampellini and Gouras 2010). Interestingly, as observed with extracellular Aβ (Busciglio et al. 1995; Heredia et al. 2006), intracellular Aβ has been found to modulate the activity of various protein kinases including ERK (Espana et al. 2010), GSK3 (Hernandez et al. 2010) and CK2 (Pigino et al. 2009; Moreno et al. 2009), among others (Balleza-Tapia and Pena 2009).
The AICD fragment generated after γ-secretase cleavage has been proposed to play a role in the regulation of gene transcription, cytoskeletal dynamics, and regulation of kinase-based signaling pathways (Muller et al. 2008). Surprisingly, a recent study showed that endosomal transport of APP to the perinuclear region is a prerequisite for AICD translocation to the nucleus. As endosomal processing of APP largely depends on BACE1, AICD signaling could be directly linked to BACE1 activity (Goodger et al. 2009). Again, these studies highlight the importance of considering both the subcellular localization and the use of neuronal cells for the functional analysis of APP-derived proteolytic products. In fact, the neglect of the importance of subcellular localization and cell types used might explain the current lack of consensus on specific genes regulated by AICD (Muller et al. 2008; Chang and Suh 2010).
Discrete physiological functions of APP metabolites and their relationship to the complex cellular architecture of neurons
Collectively, the information above suggests complex biological relationships among the various fragments derived from APP proteolysis. The release of soluble APP fragments to the extracellular space appears to modulate various aspects of neuronal function (i.e., axonal growth, synaptic activity, gene transcription, etc.) in a paracrine fashion that likely involves interactions with specific molecular components at the neuronal cell surface. These interactions and/or concomitantly produced APP intracellular fragments would in turn activate intracellular signaling pathways that locally regulate various cellular processes including kinase and G protein activation (Balleza-Tapia and Pena 2009; Sola Vigo et al. 2009), cytoskeletal dynamics (Busciglio et al. 1995; Heredia et al. 2006), axonal transport (Pigino et al. 2009), and APP proteolysis, thus providing a regulatory feedback mechanism for the localized control of APP processing at specific neuronal compartments. Abnormalities in these mechanisms could explain some of the well-documented pathological features of AD, including abnormal activation of kinases (Balleza-Tapia and Pena 2009), the cytoskeletal alterations (Vickers et al. 2009), the axonal transport defects (Morfini et al. 2002), and the increased secretion/accumulation of specific APP fragments characteristic of this disease. Supporting this notion, aggregated Aβ has been shown to interact with APP, promoting the accumulation of APP at the cell surface (Lorenzo et al. 2000; Heredia et al. 2006; Kedikian et al. 2010), and enhanced APP expression increases neuronal vulnerability to fibrillar Aβ (Sola Vigo et al. 2009). Moreover, Aβ deposition was shown to alter the metabolic processing of APP, leading to enhanced Aβ production (Davis-Salinas et al. 1995; Marsden et al. 2011). Together, these observations suggest a pathologic feed-forward mechanism that might contribute to Aβ plaque formation and neuronal degeneration in AD.
An understanding of mechanisms mediating APP processing at specific neuronal domains, knowledge of their local physiological concentrations, and identification of relevant molecular targets would shed light on the precise pathophysiological functions of APP and its cleavage fragments.
Intracellular trafficking and sorting of APP and secretases
Secretion of sAPPα, sAPPβ, Aβ, and p3 peptides by cultured cells indicated that APP undergoes trafficking throughout the biosynthetic secretory pathway (Haass et al. 1992). Below we provide a brief summary on the sorting and trafficking of APP and secretases, emphasizing known differences in these processes between neuronal and non-neuronal cells.
Trafficking and sorting of APP in non-neuronal cells
To date, most of the data addressing the subcellular localization of APP and secretases have been obtained from experiments based on the use of non-neuronal cells [reviewed in (Thinakaran and Koo 2008; Zhang et al. 2011)]. These data indicate that APP is co-translationally inserted into the endoplasmic reticulum (ER). From the ER, APP traffics to Golgi apparatus, where undergoes various post-translational modifications (Fig. 1a, inset 1). From the trans-Golgi network (TGN), APP is transported in secretory vesicles to the plasma membrane. At steady state levels, only a small proportion of APP in non-neuronal cells localizes to the plasma membrane [estimated to be below 20% in neurogliomal cells (Kuentzel et al. 1993)]. From the plasma membrane, a fraction of APP is internalized via clathrin- and dynamin-dependent pathways and delivered to endosomes. Once it reaches the endosomal compartment, APP can undergo sorting to the plasma membrane or be targeted to lysosomes for degradation (Thinakaran and Koo 2008). Recent experimental evidence suggests that a fraction of APP inserted at the plasma membrane can bypass the endosomal compartment, directly undergoing trafficking to lysosomes (Fig. 1b) (Lorenzen et al. 2010).
Like APP, secretases also undergo trafficking and activation along the biosynthetic secretory pathway. At the Golgi apparatus, the pro-domains of ADAM10 and BACE1 are cleaved by a furin-like proteases, resulting in enzyme activation (Huovila et al. 2005). Also, the multiple protein subunit components of the γ-secretase complex undergo assembly as they traffic throughout the ER and Golgi apparatus, eventually forming an active complex (Takasugi et al. 2003). Despite their common trafficking route, the activity of each secretase was mapped to different membrane compartments. Active ADAM10 cleaves various substrates including APP, cell adhesion molecules, growth factors, and growth factor receptors at the plasma membrane (Sisodia 1992; Huovila et al. 2005). In contrast, BACE1 activity has been predominately mapped to the TGN and the endosomal compartment (Koo and Squazzo 1994), consistent with the optimum pH requirements of this enzyme (Vassar et al. 1999). Activity of γ-secretase has been mapped to the late Golgi compartment, endosomes, and the plasma membrane [reviewed in (Kaether et al. 2006)].
Initial efforts aimed to address the targeting of APP at specific plasma membrane domains relied on the use of polarized epithelial cells (e.g., Madin-Darby canine kidney, MDCK cells), which possess functionally distinct apical and basolateral plasma membrane domains (Silverman et al. 2005). Experiments in these cells revealed that a tyrosine-based sorting signal (BaSS) in the carboxy-terminus of APP selectively targets this protein to the basolateral plasma membrane (Haass et al. 1994). ADAM10 (Haass et al. 1994) and BACE1 (Capell et al. 2002) were found to undergo sorting to the basolateral and apical membranes, respectively.
The observations above imply that alterations in the intracellular trafficking of APP at different stages of the biosynthetic pathway could influence its exposure to different secretases, thereby directly affect the generation and relative levels of APP-derived proteolytic products. Accordingly, multiple cellular factors and specific molecular components that influence APP trafficking appear to impact the proteolytic processing of this protein [reviewed in (Tang 2009; Lee et al. 2005; Uryu et al. 2007)].
Unique trafficking and sorting mechanisms of APP in neurons
Neurons display a cellular architecture far more complex than non-neuronal cells, representing the most striking example of cellular polarization. The development and maintenance of such architecture require numerous specializations of the biosynthetic secretory pathway (Pigino et al. 2006). Accordingly, several lines of evidence suggest neuron-specific features relevant to the subcellular distribution and intracellular trafficking of APP and its secretases. For example, although the bulk of cellular APP is processed by α-secretase in both neuronal and non-neuronal cells (Sisodia et al. 1990), a much larger fraction of APP is processed by β-secretase and γ-secretase in neurons (Simons et al. 1996). Higher levels of Aβ secretion in neurons as compared to non-neuronal cells confirm this observation and also imply cell-type-specific specializations of APP processing in these cells.
Most neuronal cells have a cell body, multiple dendrites, and a single axon. Selective enrichment of specific membrane proteins at each of these domains can be achieved by several known mechanisms, including selective transport of specific membranous cargoes by motor proteins, selective endocytosis, and retention/anchoring mechanisms (Burack et al. 2000). Given the functional analogies between the apical and basolateral plasma membrane domains of epithelial cells with the axonal and somatodendritic compartments of neurons (Silverman et al. 2005), it was expected that APP and ADAM10 would have a somatodendritic distribution in neurons. However, many independent studies reported localization of APP at both the somatodendritic and the axonal compartments (Schubert et al. 1991; Ferreira et al. 1993; Simons et al. 1995; Back et al. 2007). Further, deletion of the BaSS motif, identified as a critical sorting signal for APP in MDCK cells, did not affect the localization of APP in neuronal cells (Back et al. 2007; Rusu et al. 2007). Instead, the Aβ-domain of APP appeared to modulate its initial sorting to the axonal compartment (Tienari et al. 1996). Other studies showed that, after initial delivery to axons, APP is transported to dendrites via transcytosis (Simons et al. 1995). Like APP, BACE1 is also targeted to axons and dendrites (Capell et al. 2002). These and other observations suggest neuronal specializations for the sorting of APP and APP secretases in neuronal cells. Therefore, caution should be taken before conclusions obtained from experiments in non-neuronal cells are extrapolated to neurons.
The widespread localization of APP in neurons raised several questions: Where does APP processing take place? In axons, in dendrites, or in both compartments? To help address these questions, Lazarov et al. (2002) performed unilateral lesions of the perforant pathway, an axonal route connecting neurons in the entorhinal cortex to the dentate gyrus and other areas of the hippocampus. Significantly, a pronounced reduction in the amyloid burden was detected in the dentate gyrus ipsilateral to the lesion, indicating that axonally transported APP gives rise to the bulk of secreted Aβ peptides. Therefore, Aβ peptides deposited in amyloid plaques are mainly derived from axonally transported APP (Lazarov et al. 2002). This and other studies also indicated that presynaptic terminals represent important sites of Aβ production and secretion (Buxbaum et al. 1998). Consistent with this view, synaptic activity has been shown to modulate levels of secreted Aβ (Cirrito et al. 2008). The effects of various APP fragments, including Aβ, on the integrity and function of dendrites suggest local processing of APP at this compartment (Wei et al. 2010). However, studies directly addressing the processing of APP at the dendritic compartment are lacking.
Axonal transport of APP
The enormous size and complex functional architecture of neurons depend upon exquisitely regulated transport and delivery of materials to hundreds or thousands of discrete heterogeneous subcellular compartments, including pre- and postsynaptic terminals, nodes of Ranvier, axon hillock, etc. (Morfini et al. 2001). The intracellular transport of lipids and membrane protein components to these neuronal compartments represents a specialized form of trafficking collectively referred to as fast axonal transport (FAT) [reviewed in (Morfini et al. 2006)]. Within axons, the polarized organization of microtubules allows for vectorial FAT of membrane-bounded organelles (MBOs) in the anterograde and retrograde direction by microtubule-based molecular motors. Molecular motors of the kinesin superfamily of proteins are responsible for anterograde FAT, transporting various MBO cargoes from their sites of synthesis and packaging in the neuronal cell body to their final sites of utilization within axons and dendrites. On the other hand, the execution of retrograde FAT is carried out by cytoplasmic dynein (CDyn). CDyn transports MBOs containing signaling complexes and degradation products from various subcellular compartments to the neuronal cell body. The unique dependence of neuronal function on correct FAT is highlighted by recent genetic data (Morfini et al. 2009). Indeed, loss of function mutations in ubiquitously expressed motor protein subunits results in human neurological diseases featuring loss of synaptic function and axonal connectivity (Morfini et al. 2009; Roy et al. 2005). Relevant to the main topic covered in this review, experimental evidence has emerged addressing different aspects of APP transport within neurons. A summary of these findings is discussed below.
Sorting and packaging of APP in transport vesicles
Specific protein coats on the cytosolic face of the TGN help gather selected protein and lipid components into a discrete membrane domain, ultimately facilitating the packaging of these components into post-Golgi transport vesicles [reviewed in (Derby and Gleeson 2007)]. In this context, recent studies indicate that the μ4 subunit of the heterotetrameric adaptor protein (AP) complex AP4, though to be part of a non-clathrin coat, interacts with a YKFFE sequence within the cytosolic tail of APP to facilitate the recruitment of APP into specific transport vesicles (Burgos et al. 2010). Until recently, the identity of membrane cargoes co-assembled with APP in discrete transport vesicles remained unknown. Consistent with a role of APP in the regulation of synaptic function and vesicle fusion (Wang et al. 2011), membrane immunoisolation experiments identified a subset of presynaptic, but not postsynaptic proteins co-assembled with APP in synaptic vesicles (Groemer et al. 2011) and discrete transport vesicles. Presynaptic components identified in transport vesicles included synapsin-I, SNAP25, syntaxin-1B, VAMP2, Munc13-1, and RIM2, but not synaptophysin (Szodorai et al. 2009). Interestingly, the small GTPase Rab3, and the Rab3-GTPase-associated proteins (Rab3GAP) p130 and p150 were also associated with these vesicles (Szodorai et al. 2009). This finding was consistent with the long-recognized role of Rab GTPases in the regulation of intracellular trafficking (Schmid 1997). For example, after vesicles are released from the donor membrane (i.e., the TGN), GTP hydrolysis by selected GTPases promotes the disassembly of vesicles coats. This uncoating process, which is facilitated by chaperones of the Hsp70 family, presumably promotes the exposure of selected membrane proteins on the surface of transport vesicles and the engagement of specific molecular motors to these vesicles (Tsai et al. 2000). Consistent with this notion, functional and biochemical evidence indicates that Rab3A plays a role in the recruitment of microtubule-based molecular motors to APP-containing transport vesicles (see below) (Szodorai et al. 2009).
Anterograde axonal transport of APP
Nerve ligation experiments in the rat peripheral nervous system first demonstrated that APP is transported along axons by anterograde FAT (Koo et al. 1990), a finding confirmed by results of pulse-chase labeling experiments (Morin et al. 1993; Amaratunga et al. 1995). Consistent with results from immunoisolation experiments (Szodorai et al. 2009), microscopic experiments documented FAT of APP in association with MBOs distinct in morphology and transport kinetics from synaptophysin-containing ones (Kaether et al. 2000). Gene silencing experiments indicated that conventional kinesin is the main molecular motor involved in the anterograde FAT of MBOs containing APP (Yamazaki et al. 1995; Ferreira et al. 1993; Koo et al. 1990). Conventional kinesin is a heterotetrameric protein complex composed of two kinesin heavy chain (KHCs, kinesin-1s) and two kinesin light chain (KLCs) subunits (Bloom et al. 1988). Three KHCs (kinesin-1a, b, and c) and two KLC (KLC1 and KLC2) isoforms are expressed in the mammalian nervous tissue (Miki et al. 2003). Experimental evidence indicates that the tandem repeat (TR) domain of KLCs plays a role in the tight binding of conventional kinesin to MBOs (Stenoien and Brady 1997), whereas the alternatively spliced carboxy-terminus of KLCs appears to mediate the targeting of conventional kinesin to selected MBOs (Cyr et al. 1991; Khodjakov et al. 1998; Gyoeva et al. 2000). Kinesin-1s on the other hand, are responsible for the mechanochemical properties of the conventional kinesin holoenzyme, containing both MT binding and ATPase domains (Bloom et al. 1988).
Biochemically heterogeneous form of conventional kinesin exists, which results from different combinations of kinesin-1 and KLC homodimers (DeBoer et al. 2008). Initial studies using antisense oligos suggested that FAT of APP was mediated by kinesin-1B (Ferreira et al. 1992; Kaether et al. 2000). However, neither a specific reduction in kinesin-1B levels nor the analyses of other kinesin-1 isoforms were performed in these studies. More recently, the availability of antibodies recognizing specific kinesin-1 isoforms facilitated the identification of kinesin-1C as the main KHC isoform associated with APP-containing transport vesicles (Szodorai et al. 2009). This information appeared consistent with the divergent carboxy-terminal tail of kinesin-1s playing a role in the targeting of conventional kinesin holoenzymes to specific MBO cargoes (DeBoer et al. 2008).
The mode of connection between conventional kinesin and APP-containing transport vesicles
Several models have been proposed to explain the mode of connection between APP-containing transport vesicles and conventional kinesin. Over a decade ago, immunoprecipitation studies by Kamal et al. reported a direct, high-affinity interaction between the intracellular domain of APP and the TR domain of KLCs, fueling the proposal that APP functions as a cargo receptor for conventional kinesin (Kamal et al. 2000; Kamal and Goldstein 2002). An independent report supported this model, showing that a 15 amino acid sequence within the intracellular domain of APP was sufficient to drive anterograde FAT of exogenous beads in isolated squid axoplasm (Satpute-Krishnan et al. 2006).
More recently, an alternative model was proposed, where c-Jun N-terminal kinase-interacting protein 1 (JIP1b) acted as scaffold protein, simultaneously binding both APP and the TR domain of KLC1, as well as c-Jun amino-terminal kinase (JNK) (Matsuda et al. 2003; Inomata et al. 2003).
Despite its initial popularity, a large body of cumulative data appeared inconsistent with these models above. For example, multiple independent studies indicated that FAT of APP occurs independently of the carboxy-terminus of APP domain. Indeed, APP constructs lacking the carboxy-terminus domain were efficiently delivered to axons when expressed in cultured neurons (Tienari et al. 1996) or motor neurons of Drosophila larva (Rusu et al. 2007). Such deletion constructs also undergo FAT in cells lacking both APLP1 and APLP2, ruling out compensatory mechanisms involving other APP family members (Szodorai et al. 2009). In addition, mild detergent treatment of immunoisolated APP-containing membranes resulted in the dissociation of kinesin-1 from APP, arguing against direct or indirect interactions between conventional kinesin and APP (Szodorai et al. 2009). Moreover, a study conducted by several independent groups failed to reproduce key findings from the studies by Kamal et al. including a direct interaction between KLCs and APP, and the reductions in conventional kinesin levels in nerves of APP knock-out mice reported in this work (Lazarov et al. 2005). Thus, a large body of evidence indicates that neither APP nor JIP1b fulfills the criteria expected for a conventional kinesin receptor. Instead, cumulative data suggest that APP represents one of several membrane proteins present in MBOs that undergo FAT by means of conventional kinesin variants containing kinesin-1C (Szodorai et al. 2009).
A finding of particular interest is the identification of Rab3GAP p130 and Rab3GAP p150 in association with APP-containing transport vesicles (Szodorai et al. 2009). These proteins are known to interact with and stimulate the GTPase activity of Rab3 family members (Rab3A–Rab3D), prompting investigations on the role that Rab3s might play on APP FAT. Interestingly, it was found that anterograde FAT of APP-containing vesicles depends upon the activity of Rab3A, but nor Rab3B, Rab3C, or Rab3D (Szodorai et al. 2009). Accordingly, analysis of Rab3GAP p130 knock-out mice, which display reduced levels of Rab3A-GTPase activity, indicated that Rab3A participates in the assembly/packaging of specific proteins into APP-containing transport vesicles, as well as the engagement of conventional kinesin to these vesicles (Szodorai et al. 2009). The exact mechanisms by which Rab3A fulfills this function are currently unknown.
Retrograde axonal transport of APP
Experimental evidence indicates that a fraction of cellular APP undergoes retrograde FAT. For example, anti-APP antibodies were internalized at distal axons of cultured neurons and retrogradely transported to cell bodies in MBOs that co-localized with fluid-phase endocytic markers (Yamazaki et al. 1995). This observation appeared consistent with a report showing retrograde transport of APP from endosomes to the TGN (Ferreira et al. 1993). Findings of APP transcytosis in neurons (Simons et al. 1995; Yamazaki et al. 1995) imply that the retrograde FAT machinery plays a critical role in the subcellular distribution of APP and its proteolytic products in dendrites. Finally, regulation of gene transcription by AICD fragments implies that these cleavage fragments might need to undergo retrograde FAT from their site of generation to the neuronal cell body. The execution of retrograde FAT is largely carried out by cytoplasmic dynein (CDyn), a multi-subunit molecular motor that generates processive movement toward the minus end of microtubules (Morfini et al. 2006; Vallee 1993). However, specific CDyn subunits mediating retrograde FAT of APP-containing organelles remain unknown. Further work is required to illuminate mechanisms underlying retrograde FAT of APP, including the biochemical identity of relevant MBOs and the intracellular signals that regulate the commitment of a fraction of APP to cell bodies.
Processing of APP at the axonal compartment
In neurons, the various secretases involved in APP processing must be assembled, transported, and activated at specific subcellular compartments before they can encounter APP and process it to generate various APP fragments. In this context, significant research effort has been made to determine whether APP is co-transported with one or more secretases within the same transport vesicles. Based on results from sciatic nerve ligations and biochemical experiments, Kamal et al. (2001) postulated that APP, BACE1, and γ-secretase are co-transported in the same type of vesicles and that APP undergoes processing by these secretases during transit along the axon to generate Aβ. Extending their model of APP acting as a receptor for conventional kinesin (Kamal et al. 2000), these authors suggested that cleavage of APP within axons could represent a mechanism to detach conventional kinesin from APP-containing transport vesicles (Kamal et al. 2001). However, steady state levels of PS1 in sciatic nerves were unchanged in APP knock-out mice, suggesting this critical γ-secretase component and APP are transported in different MBOs (Lazarov et al. 2005). Also, independent reports failed to detect Aβ within the axonal compartment (Lazarov et al. 2005; Goldsbury et al. 2006). Instead, APP carboxy-terminal stubs, likely generated by β-secretase processing in the TGN, appeared to undergo transport along axons (Buxbaum et al. 1998). Complementing these studies, analysis of APP-containing membrane immunoisolates failed to detect BACE1, PS1, nicastrin, and the α-secretase ADAM17 (Szodorai et al. 2009). Further, live-cell-imaging analysis revealed that APP and BACE are transported in distinct vesicles along the axon (Goldsbury et al. 2006). From all secretase components analyzed, only active ADAM10 was detected in APP-containing MBOs, suggesting the possibility that small amounts of sAPPα could be generated as APP is transported along the axon (Szodorai et al. 2009). Taken together, cumulative evidence indicates that APP, PS1, and BACE1 are transported in different membrane compartments along axons. A molecular basis for the selective delivery of these membranes at specific subcellular domains is currently unknown. However, recent evidence illuminated phosphorylation-dependent mechanisms for the regulation of molecular motors that would facilitate the delivery of MBOs containing APP at specific subcellular domains (Morfini et al. 2002, 2009).
Concluding remarks
Illuminating molecular mechanisms underlying the sub-cellular distribution and trafficking of APP, as well as the spatial regulation of APP proteolytic processing in neuronal cells, represents a major future challenge to investigators in the APP field. An understanding of such mechanisms would shed light on the normal physiological roles of APP-derived fragments, provide important insights on AD pathogenesis, and help the design and development of novel therapeutic strategies for this disease.
Acknowledgments
This work was supported by NIH RO1 NS066942A, ALS/CVS Therapy Alliance and Brain Research Foundation grants to G.M.; grants form CONICET, SECyT-UNC, and ANPCyT06-01941 to A. L.; Cure Alzheimer's Fund, Adler Foundation, and NIH RO1 AG021494 grants to S.S; and Deutsche Forschungsgemeinschaft FOR1332 (KI 819/5–1 and/6–1) grants to S. K. SS discloses that he is a paid Consultant of Noscira, Inc and Eisai Research Labs Inc, but is not a shareholder in any company that is a maker or owner of a FDA-regulated drug or device.
References
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12(12):1567–1576. doi: 10.1038/nn.2433. doi:10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Amaratunga A, Leeman SE, Kosik KS, Fine RE. Inhibition of kinesin synthesis in vivo inhibits the rapid transport of representative proteins for three transport vesicle classes into the axon. J Neurochem. 1995;64(5):2374–2376. doi: 10.1046/j.1471-4159.1995.64052374.x. [DOI] [PubMed] [Google Scholar]
- Back S, Haas P, Tschape JA, Gruebl T, Kirsch J, Muller U, Beyreuther K, Kins S. Beta-amyloid precursor protein can be transported independent of any sorting signal to the axonal and dendritic compartment. J Neurosci Res. 2007;85(12):2580–2590. doi: 10.1002/jnr.21239. doi:10.1002/jnr.21239. [DOI] [PubMed] [Google Scholar]
- Balleza-Tapia H, Pena F. Pharmacology of the intracellular pathways activated by amyloid beta protein. Mini Rev Med Chem. 2009;9(6):724–740. doi: 10.2174/138955709788452784. [DOI] [PubMed] [Google Scholar]
- Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48(20):4354–4370. doi: 10.1021/bi802361k. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26(2):465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell. 2010;18(3):425–436. doi: 10.1016/j.devcel.2010.01.015. doi:10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yankner BA. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992;13(5):609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yeh J, Yankner BA. Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14(4):879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O'Callahan J, Slunt HH, Price DL, Sisodia SS. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci. 1998;18:9629–9637. doi: 10.1523/JNEUROSCI.18-23-09629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. doi:10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- Capell A, Meyn L, Fluhrer R, Teplow DB, Walter J, Haass C. Apical sorting of beta-secretase limits amyloid beta-peptide production. J Biol Chem. 2002;277(7):5637–5643. doi: 10.1074/jbc.M109119200. doi:10.1074/jbc.M109119200. [DOI] [PubMed] [Google Scholar]
- Chang KA, Suh YH. Possible roles of amyloid intracellular domain of amyloid precursor protein. BMB Rep. 2010;43(10):656–663. doi: 10.5483/BMBRep.2010.43.10.656. doi:10.3858/BMBRep.2010.43.10.656. [DOI] [PubMed] [Google Scholar]
- Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B. Secreted amyloid precursor protein beta and secreted amyloid precursor protein alpha induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS One. 2011;6(1):e16301. doi: 10.1371/journal.pone.0016301. doi:10.1371/journal.pone.0016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. doi:10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copanaki E, Chang S, Vlachos A, Tschape JA, Muller UC, Kogel D, Deller T. sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Mol Cell Neurosci. 2010;44(4):386–393. doi: 10.1016/j.mcn.2010.04.007. doi:10.1016/j.mcn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Cyr JL, Pfister KK, Bloom GS, Slaughter CA, Brady ST. Molecular genetics of kinesin light chains: generation of isoforms by alternative splicing. Proc Nat Acad Sci USA. 1991;88:10114–10118. doi: 10.1073/pnas.88.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Salinas J, Saporito-Irwin SM, Cotman CW, Van Nostrand WE. Amyloid beta-protein induces its own production in cultured degenerating cerebrovascular smooth muscle cells. J Neurochem. 1995;65(2):931–934. doi: 10.1046/j.1471-4159.1995.65020931.x. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-secretase complex. Neuron. 2003;38(1):9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- DeBoer SR, You Y, Szodorai A, Kaminska A, Pigino G, Nwabuisi E, Wang B, Estrada-Hernandez T, Kins S, Brady ST, Morfini G. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry. 2008;47(15):4535–4543. doi: 10.1021/bi702445j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby MC, Gleeson PA. New insights into membrane trafficking and protein sorting. Int Rev Cytol. 2007;261:47–116. doi: 10.1016/S0074-7696(07)61002-X. doi: 10.1016/S0074-7696(07)61002-X. [DOI] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol Psychiatry. 2010;67(6):513–521. doi: 10.1016/j.biopsych.2009.06.015. doi:10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992;117(3):595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J Neurosci. 1993;13(7):3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbury C, Mocanu MM, Thies E, Kaether C, Haass C, Keller P, Biernat J, Mandelkow E, Mandelkow EM. Inhibition of APP trafficking by tau protein does not increase the generation of amyloid-beta peptides. Traffic. 2006;7(7):873–888. doi: 10.1111/j.1600-0854.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- Goodger ZV, Rajendran L, Trutzel A, Kohli BM, Nitsch RM, Konietzko U. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci. 2009;122(Pt 20):3703–3714. doi: 10.1242/jcs.048090. doi: 10.1242/jcs.048090. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7(11):1118–1123. doi: 10.1038/ncb1313. doi:10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Groemer TW, Thiel CS, Holt M, Riedel D, Hua Y, Huve J, Wilhelm BG, Klingauf J. Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One. 2011;6(4):e18754. doi: 10.1371/journal.pone.0018754. doi: 10.1371/journal.pone.0018754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoeva FK, Bybikova EM, Minin AA. An isoform of kinesin light chain specific for the Golgi complex. J Cell Sci. 2000;113(Pt 11):2047–2054. doi: 10.1242/jcs.113.11.2047. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. doi: 10.1038/359322a0. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C, Koo EH, Teplow DB, Selkoe DJ. Polarized secretion of beta-amyloid precursor protein and amyloid beta-peptide in MDCK cells. Proc Natl Acad Sci USA. 1994;91(4):1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Heredia L, Helguera P, de Olmos S, Kedikian G, Sola Vigo F, LaFerla F, Staufenbiel M, de Olmos J, Busciglio J, Caceres A, Lorenzo A. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer's disease. J Neurosci. 2006;26(24):6533–6542. doi: 10.1523/JNEUROSCI.5567-05.2006. doi:10.1523/JNEUROSCI.5567-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 2010;223(2):322–325. doi: 10.1016/j.expneurol.2009.09.011. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29(14):4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. doi:10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30(7):413–422. doi: 10.1016/j.tibs.2005.05.006. doi:10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N. A scaffold protein JIP-1b enhances amyloid precursor protein phosphorylation by JNK and its association with kinesin light chain 1. J Biol Chem. 2003;278(25):22946–22955. doi: 10.1074/jbc.M212160200. doi:10.1074/jbc.M212160200. [DOI] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell. 2000;11(4):1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Haass C, Steiner H. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis. 2006;3(4–5):275–283. doi: 10.1159/000095267. doi:10.1159/000095267. [DOI] [PubMed] [Google Scholar]
- Kamal A, Goldstein LS. Principles of cargo attachment to cytoplasmic motor proteins. Curr Opin Cell Biol. 2002;14(1):63–68. doi: 10.1016/s0955-0674(01)00295-2. [DOI] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28(2):449–459. doi: 10.1016/s0896-6273(00)00124-0. doi:S0955067401002952. [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414(6864):643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Kedikian G, Heredia F, Salvador VR, Raimunda D, Isoardi N, Heredia L, Lorenzo A. Secreted amyloid precursor protein and holo-APP bind amyloid beta through distinct domains eliciting different toxic responses on hippocampal neurons. J Neurosci Res. 2010;88(8):1795–1803. doi: 10.1002/jnr.22347. doi:10.1002/jnr.22347. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Lizunova EM, Minin AA, Koonce MP, Gyoeva FK. A specific light chain of kinesin associates with mitochondria in cultured cells. Molec Biol Cell. 1998;9:333–343. doi: 10.1091/mbc.9.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Hecht MH. Sequence determinants of enhanced amyloidogenicity of Alzheimer A{beta}42 peptide relative to A{beta}40. J Biol Chem. 2005;280(41):35069–35076. doi: 10.1074/jbc.M505763200. doi:10.1074/jbc.M505763200. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269(26):17386–17389. [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA. 1990;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuentzel SL, Ali SM, Altman RA, Greenberg BD, Raub TJ. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem J. 1993;295(Pt 2):367–378. doi: 10.1042/bj2950367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29(17):3020–3032. doi: 10.1038/emboj.2010.167. doi:10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. Beyond the signaling effect role of amyloid-ss42 on the processing of APP, and its clinical implications. Exp Neurol. 2010;225(1):51–54. doi: 10.1016/j.expneurol.2010.04.018. doi:10.1016/j.expneurol.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22(22):9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, DeBoer SR, Koliatsos VE, Kins S, Lee VM, Wong PC, Price DL, Brady ST, Sisodia SS. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J Neurosci. 2005;25(9):2386–2395. doi: 10.1523/JNEUROSCI.3089-04.2005. doi:10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Zhang B, Liu K, Greenbaum EA, Doms RW, Trojanowski JQ, Lee VM. BACE overexpression alters the subcellular processing of APP and inhibits Abeta deposition in vivo. J Cell Biol. 2005;168(2):291–302. doi: 10.1083/jcb.200407070. doi:10.1083/jcb.200407070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen A, Samosh J, Vandewark K, Anborgh PH, Seah C, Magalhaes AC, Cregan SP, Ferguson SS, Pasternak SH. Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol Brain. 2010;3:11. doi: 10.1186/1756-6606-3-11. doi:10.1186/1756-6606-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc Natl Acad Sci USA. 1994;91(25):12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yuan M, Zhang Z, Paganetti PA, Sturchler-Pierrat C, Staufenbiel M, Mautino J, Vigo FS, Sommer B, Yankner BA. Amyloid beta interacts with the amyloid precursor protein: a potential toxic mechanism in Alzheimer's disease. Nat Neurosci. 2000;3(5):460–464. doi: 10.1038/74833. doi:10.1038/74833. [DOI] [PubMed] [Google Scholar]
- Marsden IT, Minamide LS, Bamburg JR. Amyloid-beta-induced amyloid-beta secretion: a possible feed-forward mechanism in Alzheimer's disease. J Alzheimers Dis. 2011;24(4):681–691. doi: 10.3233/JAD-2011-101899. doi:10.3233/JAD-2011-101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuda Y, D'Adamio L. Amyloid beta protein precursor (AbetaPP), but not AbetaPP-like protein 2, is bridged to the kinesin light chain by the scaffold protein JNK-interacting protein 1. J Biol Chem. 2003;278(40):38601–38606. doi: 10.1074/jbc.M304379200. [DOI] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA. 1998;95(21):12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Setou M, Hirokawa N. Kinesin superfamily proteins (KIFs) in the mouse transcriptome. Genome Res. 2003;13(6B):1455–1465. doi: 10.1101/gr.984503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci USA. 2009;106(14):5901–5906. doi: 10.1073/pnas.0900944106. doi:10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Richards B, Brady ST. Regulation of kinesin: implications for neuronal development. Dev Neurosci. 2001;23:364–376. doi: 10.1159/000048720. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer's disease. Neuro-molecular Med. 2002;2(2):89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Stenoien DL, Brady ST. Axonal transport. In: Siegel G, Albers RW, Brady S, Price D, editors. Basic neurochemistry. 7th edn. Elsevier Academic; Burlington: 2006. pp. 485–502. [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29(41):12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. doi:10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Abraham CR, Amaratunga A, Johnson RJ, Huber G, Sandell JH, Fine RE. Amyloid precursor protein is synthesized by retinal ganglion cells, rapidly transported to the optic nerve plasma membrane and nerve terminals, and metabolized. J Neurochem. 1993;61(2):464–473. doi: 10.1111/j.1471-4159.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Muller T, Meyer HE, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer's disease. Prog Neurobiol. 2008;85(4):393–406. doi: 10.1016/j.pneurobio.2008.05.002. doi:10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. doi:10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. doi:10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Farber SA, Growdon JH, Wurtman RJ. Release of amyloid beta-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc Natl Acad Sci USA. 1993;90(11):5191–5193. doi: 10.1073/pnas.90.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Smith IF, Green KN, LaFerla FM. A dynamic relationship between intracellular and extracellular pools of Abeta. Am J Pathol. 2006;168(1):184–194. doi: 10.2353/ajpath.2006.050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Brady ST. Intracellular trafficking. In: Siegel G, Albers RW, Brady S, Price D, editors. Basic neurochemistry. 7th edn. Elsevier Academic; Burlington: 2006. pp. 139–164. [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA. 2009;106(14):5907–5912. doi: 10.1073/pnas.0901229106. doi:10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. doi:10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AD, Witton J, Booth C, Hynes-Allen A, Brown JT. The functional neurophysiology of the amyloid precursor protein (APP) processing pathway. Neuropharmacology. 2010;59(4–5):243–267. doi: 10.1016/j.neuropharm.2010.02.011. doi:10.1016/j.neuropharm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol (Berl) 2005;109(1):5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Rusu P, Jansen A, Soba P, Kirsch J, Lower A, Merdes G, Kuan YH, Jung A, Beyreuther K, Kjaerulff O, Kins S. Axonal accumulation of synaptic markers in APP transgenic Drosophila depends on the NPTY motif and is paralleled by defects in synaptic plasticity. Eur J Neurosci. 2007;25(4):1079–1086. doi: 10.1111/j.1460-9568.2007.05341.x. doi:10.1111/j.1460-9568.2007.05341.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Suemoto T, Brouwers N, Sleegers K, Funamoto S, Mihira N, Matsuba Y, Yamada K, Nilsson P, Takano J, Nishimura M, Iwata N, Van Broeckhoven C, Ihara Y, Saido TC. Potent amyloidogenicity and pathogenicity of Abeta43. Nat Neurosci. 2011 doi: 10.1038/nn.2858. doi:10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- Satpute-Krishnan P, DeGiorgis JA, Conley MP, Jang M, Bearer EL. A peptide zipcode sufficient for anterograde transport within amyloid precursor protein. Proc Natl Acad Sci USA. 2006;103(44):16532–16537. doi: 10.1073/pnas.0607527103. doi:10.1073/pnas.0607527103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563(1–2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. doi:10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Peck R, Glover G, He C, Carlin C, Banker G. Motifs that mediate dendritic targeting in hippocampal neurons: a comparison with basolateral targeting signals. Mol Cell Neurosci. 2005;29(2):173–180. doi: 10.1016/j.mcn.2005.02.008. doi:10.1016/j.mcn.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Simons M, Ikonen E, Tienari PJ, Cid-Arregui A, Monning U, Beyreuther K, Dotti CG. Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites. J Neurosci Res. 1995;41(1):121–128. doi: 10.1002/jnr.490410114. doi:10.1002/jnr.490410114. [DOI] [PubMed] [Google Scholar]
- Simons M, de Strooper B, Multhaup G, Tienari PJ, Dotti CG, Beyreuther K. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J Neurosci. 1996;16(3):899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA. 1992;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Sola Vigo F, Kedikian G, Heredia L, Heredia F, Anel AD, Rosa AL, Lorenzo A. Amyloid-beta precursor protein mediates neuronal toxicity of amyloid beta through Go protein activation. Neurobiol Aging. 2009;30(9):1379–1392. doi: 10.1016/j.neurobiolaging.2007.11.017. doi:10.1016/j.neurobiolaging.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Stenoien DS, Brady ST. Immunochemical analysis of kinesin light chain function. Molec Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, Brady S, Morfini G, Kins S. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J Neurosci. 2009;29(46):14534–14544. doi: 10.1523/JNEUROSCI.1546-09.2009. doi:10.1523/JNEUROSCI.1546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. Gamma-secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. doi:10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422(6930):438–441. doi: 10.1038/nature01506. doi:10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tampellini D, Gouras GK. Synapses, synaptic activity and intraneuronal abeta in Alzheimer's disease. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00013. doi:10.3389/fnagi.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. Neuronal protein trafficking associated with Alzheimer disease: from APP and BACE1 to glutamate receptors. Cell Adh Migr. 2009;3(1):118–128. doi: 10.4161/cam.3.1.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–29619. doi: 10.1074/jbc.R800019200. doi:10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006;1094(1):38–46. doi: 10.1016/j.brainres.2006.03.107. doi:10.1016/j.brainres.2006.03.107. [DOI] [PubMed] [Google Scholar]
- Tienari PJ, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters CL, Van Leuven F, Beyreuther K, Dotti CG. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 1996;15(19):5218–5229. [PMC free article] [PubMed] [Google Scholar]
- Tsai M-Y, Morfini G, Szebenyi G, Brady ST. Modulation of kinesin-vesicle interactions by Hsc70: implications for regulation of fast axonal transport. Molec Biol Cell. 2000;11:2161–2173. doi: 10.1091/mbc.11.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208(2):185–192. doi: 10.1016/j.expneurol.2007.06.018. doi:10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB. Molecular analysis of the microtubule motor dynein. Proc Nat Acad Sci USA. 1993;90:8769–8772. doi: 10.1073/pnas.90.19.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29(41):12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. doi:10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers JC, King AE, Woodhouse A, Kirkcaldie MT, Staal JA, McCormack GH, Blizzard CA, Musgrove RE, Mitew S, Liu Y, Chuckowree JA, Bibari O, Dickson TC. Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res Bull. 2009;80(4–5):217–223. doi: 10.1016/j.brainresbull.2009.08.004. doi: 10.1016/j.brainresbull.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang L, Zheng H. Role of APP and Aβ in synaptic physiology. Curr Alzheimer Res. 2011 doi: 10.2174/156720512799361691. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13(2):190–196. doi: 10.1038/nn.2476. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Sankaranarayanan S, Hsieh SH, Simon AJ, Savage MJ. Decrease in brain soluble amyloid precursor protein beta (sAPPbeta) in Alzheimer's disease cortex. J Neurosci Res. 2011;89(6):822–832. doi: 10.1002/jnr.22618. doi:10.1002/jnr.22618. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface beta-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J Cell Biol. 1995;129(2):431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. doi:10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]