Abstract
The function of the hepatitis B virus (HBV) wild-type (WT) polymerase (pol) expressed alone or in the context of the intact genome when interacting with HBV rtM204I in HepG2 cells was compared. We show that WT pol expression from a packaging-defective RNA can complement defective rtM204I pol activity resulting in increased levels of HBV replicative intermediates (RI). Analysis of the genetically marked genomes showed that this restoration resulted from trans-complementation, rather than recombination. In contrast, we demonstrate that enhanced levels of total HBV RI observed when cells were cotransduced with both WT and rtM204I baculoviruses were predominantly WT RI. In this case, WT pol was produced from a full-length pregenomic RNA (pgRNA). We conclude that the WT pol has the capacity to trans-complement the replication defect of rtM204I; however, when expressed from an authentic pgRNA, in a mixed infection, WT pol may not trans-complement efficiently.
Keywords: HBV, Drug-resistant, rtM204I, Polymerase, Reverse transcriptase, trans-complementation, Viral interaction, Baculovirus
Introduction
Hepatitis B virus (HBV) causes acute and chronic infections of the liver and is responsible for 1.2 million deaths annually. Approximately 0.5% of acute infections terminate in fatal, fulminant hepatitis. The WHO currently estimates that 350 million people are chronically infected with HBV.
The approved agents for the treatment of HBV chronically infected individuals include α-interferon and five nucleoside/tide analogues: lamivudine (3TC, Epivir-HBV), adefovir dipivoxil (Hepsera), entecavir (Baraclude), telbivudine (Tyzeka), and tenofovir (Viread). Lamivudine treatment has been very useful in reducing HBV DNA levels in patient sera and shows very limited side effects (Dienstag et al., 1999; Jarvis and Faulds, 1999; Santantonio et al., 2000); however, the emergence of drug-resistant mutants occurs at high frequency, (Allen et al., 1998; Bartholomew et al., 1997; de Man et al., 1999; Honkoop et al., 1997; Liaw et al., 1999; Peters et al., 1999) with the incidence of resistance after 1 year of treatment ranging from 17% (Chang et al., 2004) to 32% (Lai et al., 2003; Mauss and Wedemeyer, 2008). The most common mutations occur within the catalytic YMDD motif of the HBV pol, resulting in the replacement of methionine with either valine or isoleucine at amino acid 204 (Allen et al., 1998; Ono-Nita et al., 1999).
In vitro, the rtM204I lamivudine-resistant mutant replicates to significantly lower levels compared to wild-type (WT) HBV (Fu and Cheng, 1998; Gaillard et al., 2002; Melegari, Scaglioni, and Wands, 1998; Ono-Nita et al., 1999). We recently reported that the rtM204I mutant has a defect in replication, resulting in diminished levels of HBV intracellular replicative intermediates (RI) and extracellular (EC) DNA compared to WT HBV after day 5 post-transduction (p.t.) with HBV recombinant baculovirus (Heipertz et al., 2007). In contrast, in vivo, as drug-resistant mutants emerge, there is an increase in viral load and serum alanine aminotransferase levels as well as a deterioration of liver histology. Indeed, lamivudine-resistant mutants have also been associated with death in several case studies (Kagawa et al., 2004; Kim et al., 2001; Suzuki et al., 2007; Wang et al., 2002).
Interestingly, it appears that the rtM204I and rtM204V mutants can preexist in naïve patients (Pallier et al., 2006; Zhang et al., 2003). This is most likely due to the fact that HBV pol lacks proofreading function, leading to a relatively high frequency of mutations/site/year (Girones and Miller, 1989). Therefore, WT and rtM204I HBV viruses can potentially be present in the same hepatocyte, which could lead to an interaction between WT virus and lamivudine-resistant mutants. The interplay of HBV viral components, specifically, the rescue of a pol-deficient mutant virus by the expression of a WT pol in trans, has been demonstrated previously. trans-complementation has been demonstrated when the mutant virus encodes a pol containing a missense codon, rendering the viral pol nonfunctional for RNA encapsidation (Blum et al., 1991), and when the mutant virus encodes a nonsense mutation, preventing the expression of the pol (Bartenschlager, Junker-Niepmann, and Schaller, 1990; Chiang et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Okamoto et al., 1993; Radziwill, Tucker, and Schaller, 1990; Yaginuma et al., 1987). Other mutant viral proteins such as the core protein (HBcAg) and the surface protein (HBsAg) can also be rescued through trans-complementation (Chiang et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Okamoto et al., 1993; Radziwill, Tucker, and Schaller, 1990; Yaginuma et al., 1987).
The goal of the current study was to compare the function of the HBV WT pol expressed alone or in the context of the intact HBV genome in HepG2 cells transduced with the lamivudine resistant rtM204I. To achieve this goal, we took advantage of the recombinant baculovirus-mediated HBV transduction of HepG2 cells, which allows highly efficient transduction of WT and mutant HBV genomes or individual genes and detailed analyses of all post-entry steps of HBV replication.
Results
Generation of recombinant baculovirus-expressing functional HBV pol
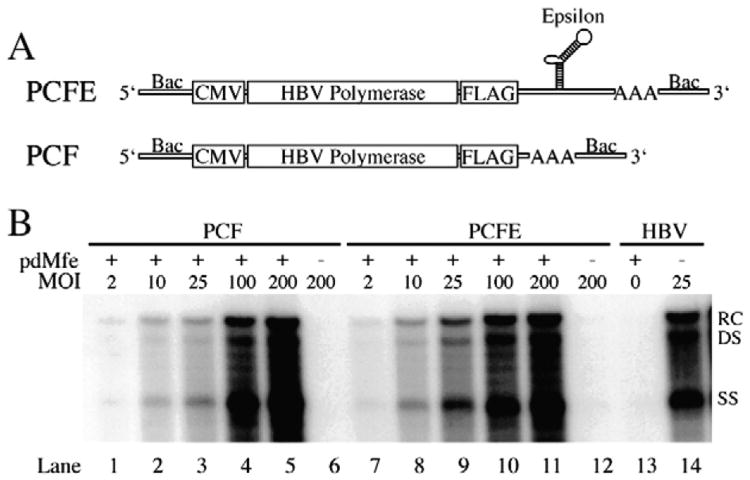
Two WT HBV pol recombinant baculoviruses designated PCF and PCFE were generated (Fig. 1A). PCF and PCFE are C-terminally FLAG-tagged, CMV promoter-driven, HBV pol-expressing recombinant baculoviruses lacking the 5′ epsilon and differing from each other only in the presence of the 3′ epsilon contained within PCFE. The ability of PCF and PCFE to express a functional HBV pol was tested in the context of the replication-deficient HBV expression plasmid, pdMfe, which lacks a functional pol. Twenty-four hours following transfection with 3 μg of pdMfe, HepG2 cells were transduced with pol-expressing baculoviruses, PCF or PCFE, at various MOIs. HBV RI were harvested from infected cells 4 days p.t. and visualized by Southern blot analysis. As shown in Fig. 1B, both PCF and PCFE were able to rescue the replication-deficient pdMfe in a MOI-dependent manner up to a MOI of 100 pfu/cell. The efficacy of PCF and PCFE appear equal, suggesting that the presence or absence of an epsilon sequence does not influence the ability of the pol to rescue the deficient pdMfe. It is important to note that PCF and PCFE (Fig. 1B, lanes 6 and 12, respectively), as well as pdMfe (Fig. 1B, lane 13) are incapable of producing a detectable signal when individually expressed in HepG2 cells, indicating that these constructs are replication-deficient. Due to the unique arrangement of the HBV genome, PCF and PCFE could have the potential to produce HBsAg; however, neither PCFE nor PCF produced detectable levels of HBsAg (data not shown).
Fig. 1.
Generation and screen of HBV pol-expressing recombinant baculoviruses. (A) Schematic representation of CMV promoter-driven, C-terminally FLAG-tagged HBV pol-expressing recombinant baculoviruses PCF and PCFE. PCF and PCFE differ only in the presence of the 3′ epsilon sequence contained within PCFE. (B) Southern blot analysis of HBV RI DNA extracted from HepG2 cells transfected with 3 μg of the replication-deficient HBV plasmid, pdMfe, 4 days p.t. with pol-expressing recombinant baculoviruses PCF or PCFE at various MOIs. HBV RI DNA extracted from HBV recombinant baculovirus-transduced HepG2 cells 4 days p.t. was included as a positive control. MOI, multiplicity of infection (pfu/cell); RC, relaxed-circular; DS, double-stranded linear; SS, single-stranded; +, presence of pdMfe; −, absence of pdMfe.
pdMfe is a HBV expression plasmid that is replication-deficient due to a frameshift mutation within the pol open reading frame caused by a four-nucleotide insertion, which also results in the loss of the single Mfe I restriction enzyme site present in the HBV genome. To determine whether the rescue of the replication-deficient pdMfe was indeed due to trans-complementation by the recombinant baculoviruses PCF and PCFE and not due to recombination of the introduced HBV DNAs, restriction enzyme digestions of the HBV RI were conducted (Fig. 2). Equal concentrations of RI DNA from either WT HBV recombinant baculovirus-transduced HepG2 cells and PCF or PCFE recombinant baculovirus-transduced, pdMfe transfected HepG2 cells were digested with EcoR I and Mfe I or undigested and visualized by Southern blot analysis. As shown in Fig. 2, RI DNA from WT HBV baculovirus-transduced HepG2 cells was digested with both Mfe I and EcoR I, resulting in the conversion of the relaxed-circular (RC) RI species to the double-stranded linear (DS) form and a downward shift in the digested DS form due to the single presence of each restriction enzyme site within the WT HBV genome. However, RI DNA from PCF and PCFE baculovirus-transduced, pdMfe transfected HepG2 cells was only digested by EcoR I, indicating that the single Mfe I site contained within the HBV genome was not restored. Taken together, the data indicate that both PCF and PCFE express a functional HBV pol that is capable of complementing the replication-deficient pdMfe in trans. In addition, the epsilon sequence does not need to be present in the construct to produce functional HBV pol. The remaining experiments were carried out with only the 3′ epsilon-containing construct, PCFE.
Fig. 2.
Trans-complementation of a replication-deficient HBV expression plasmid by HBV pol-expressing recombinant baculoviruses. Southern blot analysis of restriction enzyme digested HBV RI DNA. HepG2 cells were transduced with HBV pol-expressing recombinant baculoviruses, PCF or PCFE, at a MOI of 200 pfu/cell 24 h post-transfection with 3 μg of the replication-deficient HBV expression plasmid, pdMfe. HepG2 cells were transduced with WT HBV recombinant baculovirus at a MOI of 100 pfu/cell as a positive control. Four days p.t., HBV RI DNA was extracted and equal concentrations were left undigested or digested with EcoR I or Mfe I at 37°C for 1 h and visualized by Southern blot analysis. U, Undigested; E, EcoR I digested; M, Mfe I digested, MOI, multiplicity of infection (pfu/cell); RC, relaxed-circular; DS, double-stranded linear; SS, single-stranded; +, presence of pdMfe; −, absence of pdMfe.
Effect of the WT pol construct PCFE on the replication of the lamivudine-resistant mutant rtM204I
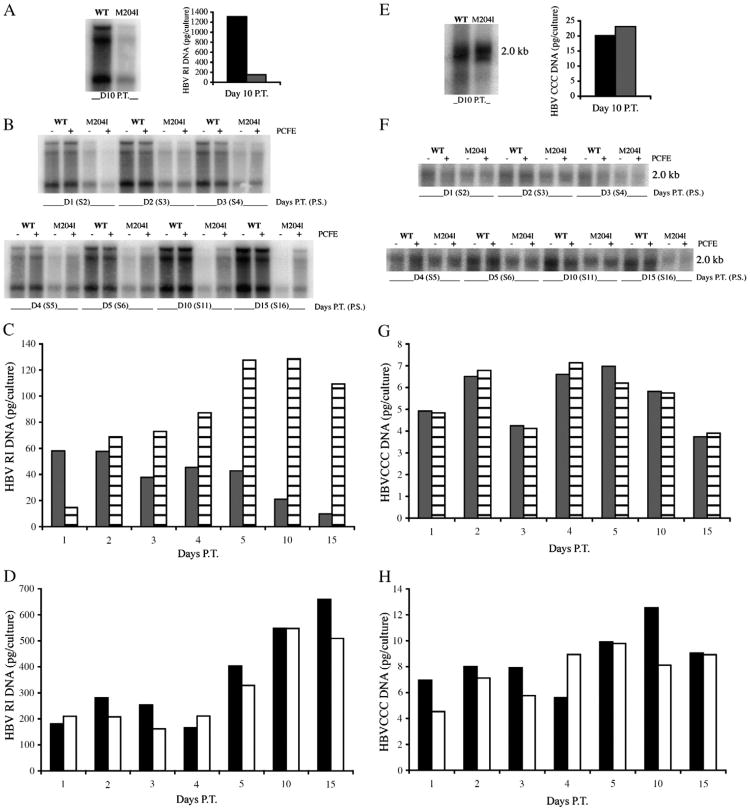
The goal of these experiments was to examine the effect of the WT pol construct PCFE on the replication of the lamivudine-resistant mutant rtM204I. Examining the effect of the WT pol construct PCFE on the replication of the WT HBV was included as a control. HepG2 cells were seeded in 60-mm dishes, grown for 16 to 24 h, and then transduced with 100 pfu/cell of WT or rtM204I HBV recombinant baculovirus. Cells were fed every-other day and maintained until day 10 p.t. At day 10 p.t., two 60-mm dishes that had been transduced with either WT or rtM204I recombinant baculovirus were harvested for HBV EC DNA as well as intracellular HBV RI and nuclear covalently closed circular (CCC) DNA. A digital image of HBV RI DNA is shown in Fig. 3A and HBV CCC DNA is shown in Fig. 3E. Quantification of HBV RI DNA and HBV CCC DNA are shown in Figs. 3A and 3E, respectively. These data agree with those previously reported. Specifically, at day 10 p.t., there is a marked decrease in HBV RI DNA when comparing rtM204I and WT viruses and no differences in the levels of CCC DNA (Heipertz et al., 2007). The data obtained for HBV EC DNA paralleled that for HBV RI, also in agreement with data previously published [data not shown] (Heipertz et al., 2007). In more recent studies, we have also shown that at day 10 p.t. the levels of HBV nucleocapsids are the same for both WT and rtM204I [Heipertz and Isom, unpublished data] (Fig. 5). Therefore, at day 10 p.t., rtM204I replication proceeds at WT levels through the encapsidation step but very low levels of HBV RI compared to WT are produced, suggesting that rtM204I pol function is defective in some manner.
Fig. 3.
Effect of the WT pol construct, PCFE, on the replication of WT HBV and the lamivudine-resistant mutant rtM204I. HepG2 cells were transduced on day 0 with 100 pfu/cell of either WT or rtM204I HBV recombinant baculovirus. At day 10 p.t., cells were harvested for HBV RI and CCC DNA. Digital images and quantification are shown for day 10 RI (A) and day 10 CCC DNA (E). Cells not harvested were subcultured 1:4 at day 10 p.t., grown for 16 to 24 h, and mock- or PCFE-transduced. HBV RI and CCC DNA were extracted on the indicated days p.t. (now post-mock- or PCFE-transduced). Digital images of HBV RI DNA are shown (B) along with quantification of rtM204I (C) and WT (D) HBV. Digital images of CCC DNA are shown (F) along with quantification of rtM204I (G) and WT (H) HBV. PCFE, WT pol construct; P.T., post-transduction; P.S., post-subculture. Black bars, WT HBV alone; White bars, WT HBV + PCFE 100 pfu/cell; Gray bars, rtM204I HBV alone; Striped bars, rtM204I HBV + PCFE 100 pfu/cell.
Fig. 5.
Trans-complementation of rtM204I is not due to an increase in the total amount of pgRNA packaging. HepG2 cells were transduced with 100 pfu/cell of rtM204I HBV recombinant baculovirus. At day 10 p.t., cells were harvested for HBV intracellular nucleocapsids (B) and HBV RI DNA (C). A digital image for day 10 is shown for WT and rtM204I nucleocapsids (B; far left image) and for WT and rtM204I RI DNA (C; far left image). Cells not harvested were subcultured 1:4 at day 10 p.t., grown for 16 to 24 h, and mock- or PCFE-transduced. HBV intracellular nucleocapsids and HBV RI DNA were extracted on the indicated days p.t. (now post-mock- or PCFE-transduced), analyzed by Southern blot using a PhosphorImager, and quantitated using ImageQuant software. Digital image and quantitation of RNA nucleocapsid (B) and HBV RI DNA (C) are shown. Panel (A) demonstrates a dilution of day 10 p.t. WT and rtM204I nucleocapsid samples to illustrate that a Southern blot of RNA contained within HBV nucleocapsids can be accurately measured at various concentrations of sample. PCFE, WT pol construct; +, transduced with PCFE; P.T., post-transduction; P.S., post-subculture. Gray bars, rtM204I HBV alone; Striped bars, rtM204I HBV + PCFE 100 pfu/cell.
At day 10 p.t., 60-mm dishes that were transduced with either rtM204I or WT (as a control) recombinant baculovirus and not harvested for day 10 analysis, were split 1:4 into new 60-mm dishes. The HBV baculovirus subculture system, which has been previously described and characterized(Starkey, Chiari, and Isom, 2009), provides HepG2 cells in which HBV replication is ongoing and are at the appropriate cell density for quantitative transduction with a recombinant baculovirus. Subcultured cells were transduced at 100 pfu/cell with the WT pol construct, PCFE, or were mock-transduced. On the indicated days p.t., cells were harvested for HBV RI (Figs. 3B–D) and CCC DNA (Figs. 3F–H) and media was harvested for HBV EC DNA (data not shown). It is important to note that days p.t. now refers to post-transduction with PCFE- or mock-transduction of subcultured, WT or rtM204I-expressing HepG2 cells. The digital images of HBV RI DNA are shown in Fig. 3B. Quantification of rtM204I HBV RI DNA is shown in Fig. 3C. The addition of PCFE caused an increase in the amount of RI DNA when comparing PCFE-transduced (striped bars) to mock-transduced (gray bars) cells (Fig. 3C) through the time course studied. A similar increase was observed for rtM204I EC DNA (data not shown). The maximum level of HBV RI and EC DNAs when comparing rtM204I + 100 pfu/cell PCFE to WT HBV alone was approximately 50% of the WT level at day 4 p.t. As expected, there was no significant difference in the levels of WT HBV RI DNA whether the cells were mock-transduced (black bars) or PCFE-transduced (white bars) at an MOI of 100 pfu/cell (Fig. 3D).
The levels of HBV CCC DNA were examined using the same experimental approach. Intact nuclei were separated from the cytoplasm, which was used for HBV RI isolation, from a single 60-mm dish and used for preparation of CCC DNA, thereby enabling a direct comparison of RI and CCC DNA from the same culture. The level of HBV CCC DNA was compared after the 1:4 split at the indicated time points post PCFE- (MOI of 100 pfu/cell) or mock-transduction. The digital image of HBV CCC DNA is shown in Fig. 3F. Quantification of rtM204I HBV CCC DNA (Fig. 3G) shows no difference between mock-transduced (gray bars) and PCFE-transduced (striped bars) cells. This finding for rtM204I CCC DNA is in contrast to what was observed for rtM204I RI which was enhanced by transduction with 100 pfu/cell of PCFE. However, this result for CCC DNA was expected because the levels of rtM204I and WT CCC DNA remain comparable throughout the time course (Heipertz et al., 2007). Quantification of WT HBV CCC DNA (Fig. 3H) also indicates that there is no difference between mock-transduced (black bars) and PCFE-transduced (white bars) cells as expected.
Increase in rtM204I replication following the addition of PCFE is accomplished by trans-complementation
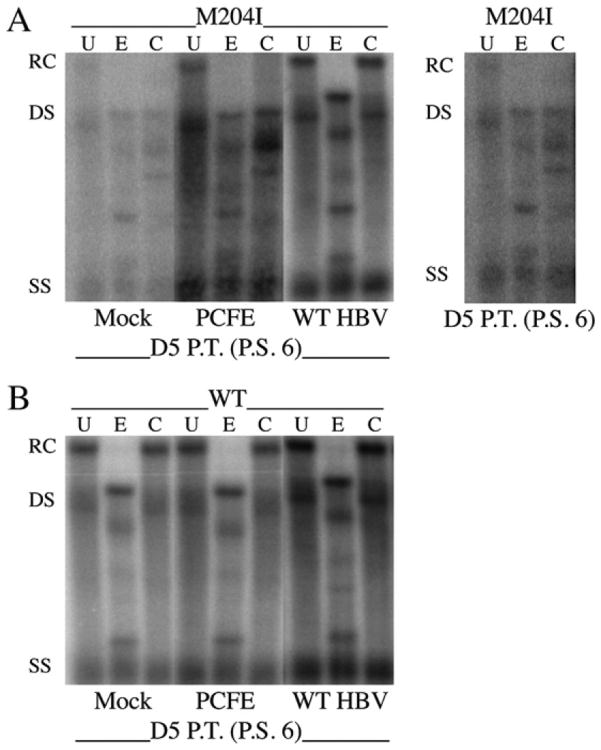
Restriction enzyme digestion analysis was utilized to determine whether the increase in rtM204I replication observed after the addition of the WT pol construct, PCFE, was due to recombination or trans-complementation. The incorporation of the rtM204I mutation in the WT HBV genome adds a unique Cla I restriction enzyme site, allowing for the differential digestion of the rtM204I and WT HBV genomes. HepG2 cells transduced with rtM204I or WT (control) HBV baculovirus were maintained until day 10 p.t. At that time point, cells were split 1:4 into 60-mm dishes and grown overnight. Twenty-four hours post-subculture, the cells were either mock-treated or transduced with PCFE (MOI of 100) or WT HBV recombinant baculovirus (MOI of 100). Cells were harvested for all conditions at day 6 post-subculture (Figs. 4A, B). RI was digested under the following conditions: Uncut [U], cut with EcoR I [E] as a single-cut control, or cut with Cla I [C]. When rtM204I expressing HepG2 cells were mock-transduced or transduced with PCFE, there was a clear shift downward of the RI DNA digested with Cla I (Fig. 4A). This shift is due to the conversion of HBV RC DNA to the DS DNA form. A longer exposure of HepG2 cells expressing rtM204I and subsequently mock transduced is shown in the panel to the right so as to more clearly indicate the downward shift that occurs following Cla I digestion of rtM204I RI. When rtM204I expressing HepG2 cells were transduced with WT HBV baculovirus, no shift occurred as expected (Fig. 4A). When WT HBV expressing HepG2 cells were mock-, PCFE-, or WT HBV-treated, RI was only digested by EcoR I and not Cla I (Fig. 4B), regardless of the condition as expected. The data in Fig. 4A clearly indicate that the RI generated when rtM204I replication was increased by transduction with PCFE is rtM204I DNA and not WT DNA and as such, the increase in HBV replication observed is accomplished through trans-complementation of rtM204I HBV by WT HBV pol.
Fig. 4.
Partial restoration of rtM204I replication in the presence of PCFE is accomplished through trans-complementation. HepG2 cells were transduced at day 0 with 100 pfu/cell of either WT or rtM204I HBV recombinant baculovirus and maintained until day 10 p.t. when the transduced cells were subcultured 1:4. The cells were either mock-transduced or transduced with PCFE (MOI = 100) or WT HBV (MOI = 100). Cells were harvested at day 5 p.t., day 6 post-subculture. HBV RI DNA was digested under the following conditions: Uncut [U], EcoR I cut [E], Cla I cut [C]. RtM204I is represented in (A) and WT HBV is represented in (B). The far right panel of (A) is a darker exposure of rtM204I Mock to illustrate the digestion pattern of rtM204I HBV RI DNA. PCFE, WT pol construct; P.T., post-transduction; P.S., post-subculture; U, Uncut; E, EcoR I cut; C, Cla I cut; RC, relaxed-circular; DS, double-stranded linear; SS, single-stranded.
Trans-complementation of rtM204I is not due to an increase in the total amount of pgRNA packaging
Given the cis-preference of RNA packaging by the pol, the ability of WT pol to trans-complement a mutant pol such as rtM204I that is fully functional in RNA packaging was quite unexpected. One possibility is that trans-complementation is due to an increase in the total amount of pregenomic RNA (pgRNA) packaging when PCFE is trans-complementing rtM204I replication. This would suggest that a single molecule of pol is contained within nucleocapsids and that the increase in HBV RI DNA observed during PCFE trans-complementation is due to PCFE recognizing free (excess) rtM204I pgRNA that is not being encapsidated by the rtM204I pol. To determine the level of encapsidation during PCFE trans-complementation, HepG2 cells were seeded in 60-mm dishes, grown for 16 to 24 h, and then transduced with 100 pfu/cell of rtM204I or WT recombinant baculovirus. Transduced cells were fed every-other day until day 10 p.t. when they were harvested for intracellular nucleocapsids and HBV RI DNA. Day 10 WT and rtM204I nucleocapsids were serially diluted to evaluate the ability of the nucleocapsid assay to distinguish two fold differences. The data clearly show that the output signal varies with the input (Fig. 5A). Parallel cultures of rtM204I-transduced HepG2 cells, not harvested on day 10, were subcultured 1:4 and the following day mock-transduced or transduced with the WT pol construct PCFE. On the indicated days post-mock or PCFE transduction, HepG2 cells were harvested for intracellular nucleocapsids (Fig. 5B) or HBV RI DNA (Fig. 5C). WT levels of intracellular nucleocapsids and HBV RI DNA at day 10 p.t. are also provided for comparison. No increase in the level of pgRNA encapsidation was observed when cells were transduced with PCFE (Fig. 5B). The addition of PCFE did cause an increase in rtM204I RI levels as observed previously. By day 14 p.t., RI levels from cells transduced with PCFE were approximately 9-fold higher than for rtM204I alone. These data indicate that trans-complementation of rtM204I by PCFE was not caused by an increase in total pgRNA packaging.
Increase in total HBV RI DNA during a WT/rtM204I cotransduction represents predominantly wild type HBV DNA
We previously reported that when HepG2 cells were cotransduced with WT HBV recombinant baculovirus at low levels (MOI of 25 pfu/cell) and rtM204I recombinant baculovirus at high levels (MOI of 100 pfu/cell), an unexpected result was obtained (Heipertz et al., 2007). At late time points (days 10–25 p.t.), the prediction would be that the RI levels from the cotransduction should be similar to those obtained from WT alone since the RI levels from rtM204I are barely detectable at that time point. In contrast, at late time points, the RI levels observed for the cotransduction were approximately two-fold those observed for WT alone. As shown in Fig. 3, expression of HBV WT pol alone from the PCFE construct trans-complemented rtM204I and caused an increase in rtM204I RI DNA levels. The surprising results obtained from the cotransduction experimental setting led to the question of whether the observed increased HBV RI levels also resulted from trans-complementation of rtM204I by WT pol produced in this case in the context of the intact WT HBV genome. To test this hypothesis, HepG2 cells were transduced under the following conditions: WT MOI of 25 pfu/cell, rtM204I MOI of 100 pfu/cell, WT MOI of 25 pfu/cell + rtM204I MOI of 100 pfu/cell. Cells were harvested at days 20 and 25 p.t. For each condition, RI DNA was either uncut [U], digested with EcoR I [E] as a single-cut control, or digested with Cla I [C] (Fig. 6). The amount of HBV DNA in the RC and in the DS band from the uncut and Cla I cut lanes were calculated. At day 20 p.t., a 2.09 fold increase in RC plus DS HBV DNAs was observed for uncut DNA and a 2.15 fold increase for the RC plus DS HBV DNAs for Cla I cut DNA when comparing WT + rtM204I to WT (Table 1, numbers indicated in bold). At day 25 p.t., a 1.80 fold increase in RC plus DS HBV DNAs was observed for uncut DNA and a 1.67 fold increase for the RC plus DS HBV DNAs for Cla I cut DNA when comparing WT + rtM204I to WT (Table 1, numbers indicated in bold).
Fig. 6.
Increase in the total amount of HBV RI DNA during a WT (MOI = 25 pfu/cell) + rtM204I (MOI = 100 pfu/cell) cotransduction is not due to a boost in rtM204I genome replication. HepG2 cells were transduced on day 0 under the following conditions: WT HBV MOI = 25 pfu/cell, rtM204I HBV MOI = 100 pfu/cell, WT HBV MOI = 25 pfu/cell + rtM204I HBV MOI = 100 pfu/cell. Cells were harvested for HBV RI DNA at day 3 and day 5 (data now shown) as well as day 20 and day 25 p.t. At the indicated time points p.t., HBV RI DNA was extracted and enzymatically digested under the following conditions: Uncut [U], EcoR I cut [E], Cla I cut [C]. Co, cotransduction; P.T., post-transduction; U, Uncut; E, EcoR I cut; C, Cla I cut; RC, relaxed-circular. Southern blot hybridization was carried out using a [α-32P] dCTP-radiolabeled probe generated from a full-length, double-stranded HBV genome.
Table 1.
Levels of HBV RC and DS DNA in RI after cotransduction of HepG2 cells with WT and M204I baculovirusesa.
| Uncut | Cla I cut | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HBV DNA (pg) | Fold increase (WT+M204I) | HBV DNA (pg) | Fold increase (WT+M204I) | |||
|
|
|
|
|
|||
| WT | WT+M204I | WT | WT | WT+M204I | WT | |
| Day 20b | ||||||
| RC | 27.3 | 89.6 | 3.28 | 36.9 | 106.7 | 2.89 |
| DS | 43.1 | 57.6 | 1.34 | 41.6 | 61.9 | 1.49 |
| RC+DS | 70.4 | 147.2 | 2.09 | 78.5 | 168.6 | 2.15 |
| Day 25b | ||||||
| RC | 47.6 | 114.5 | 2.41 | 65.2 | 135.3 | 2.08 |
| DS | 66.9 | 92.0 | 1.38 | 76.7 | 101.2 | 1.32 |
| RC+DS | 114.5 | 206.5 | 1.80 | 141.9 | 236.5 | 1.67 |
HepG2 cells were transduced under the following three conditions: WT MOI of 25 pfu/cell, rtM204I MOI of 100 pfu/cell, and WT MOI of 25 pfu/cell+rtM204I MOI of 100 pfu/cell.
Data for rtM204I alone are not included because the DNA levels were below the limits of detection.
To further address the question of whether cotransduction of HepG2 cells with WT and rtM204I baculoviruses leads to a higher level of total HBV RI than expected by adding the amount of WT RI to the amount of rtM204I RI, a different experimental approach was taken, making use of the baculovirus subculture system (Fig. 7). HepG2 cells were transduced under the following conditions: WT MOI of 100 pfu/cell, rtM204I MOI of 100 pfu/cell, WT MOI of 100 pfu/cell + rtM204I MOI of 100 pfu/cell and harvested at day 10 p.t. A parallel set of cultures for each of the three conditions were subcultured 1:4 and harvested at days 5 and 10 post subculture. For each condition, RI DNA was either uncut [U], digested with EcoR I [E] as a single-cut control, or digested with Cla I [C] (Fig. 7). The amount of HBV DNA in the RC and in the DS band from the uncut and Cla I cut lanes were calculated for all three conditions. For cells transduced with rtM204I baculovirus alone, a low but detectable level of rtM204I DNA was measurable at day 10 p. t. (see legend to Table 2), but the rtM204I DNA levels were below the levels of detection by days 5 and 10 post subculture. At day 10 p.t., a 1.33 fold increase in RC plus DS HBV DNAs was observed for uncut DNA and a 1.43 fold increase for the RC plus DS HBV DNAs for Cla I cut DNA when comparing WT + rtM204I to WT (Table 2, numbers indicated in bold). At day 5 post subculture, a 1.95 fold increase in RC plus DS HBV DNAs was observed for uncut DNA and a 1.80 fold increase for the RC plus DS HBV DNAs for Cla I cut DNA (Table 2, numbers indicated in bold) and at day 10 post subculture, a 1.44 fold increase in RC plus DS HBV DNAs was observed for uncut DNA and a 1.88 fold increase for the RC plus DS HBV DNAs for Cla I cut DNA when comparing WT + rtM204I to WT (Table 2, numbers indicated in bold).
Fig. 7.
Increase in the total HBV RI DNA during a WT/rtM204I cotransduction represents predominately WT HBV DNA. HepG2 cells were transduced on day 0 under the following conditions: WT HBV MOI = 100 pfu/cell, rtM204I MOI = 100 pfu/cell, WT MOI = 100pfu/cell + rtM204I MOI = 100 pfu/cell. The cells were harvested at day 10 p.t. (A). A parallel set of cells that were not harvested were subcultured 1:4. The subcultured set of cells was harvested at day 5 (B) and day 10 (C) post-subculture. All samples were enzymatically digested under the following conditions: Uncut [U], Cla I cut [C]. Co, cotransduction; P.T., post-transduction; P.S., post-subculture; U, Uncut; C, Cla I cut; RC, relaxed-circular; DS, double-stranded linear. Southern blot hybridization was carried out using a positive-strand-specific [α-32P] UTP-radiolabeled riboprobe.
Table 2.
Levels of HBV RC and DS DNA in RI at day 10 post cotransduction with WT and M204I baculoviruses and post subculture of day 10 cotransduced cellsa.
| Uncut | Cla I cut | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HBV DNA (pg) | Fold increase (WT+M204I) | HBV DNA (pg) | Fold increase (WT+M204I) | |||
|
|
|
|
|
|||
| WT | WT+M204I | WT | WT | WT+M204I | WT | |
| Day 10 post cotransductionb | ||||||
| RC | 113.1 | 162.4 | 1.43 | 102.5 | 147.4 | 1.44 |
| DS | 105.6 | 129.5 | 1.23 | 98.0 | 139.1 | 1.42 |
| RC+DS | 218.7 | 291.9 | 1.33 | 200.5 | 286.5 | 1.43 |
| Day 5 post subculturec | ||||||
| RC | 15.0 | 30.7 | 2.05 | 14.4 | 34.4 | 2.39 |
| DS | 11.9 | 21.8 | 1.83 | 14.4 | 17.4 | 1.21 |
| RC+DS | 26.9 | 52.5 | 1.95 | 28.8 | 51.8 | 1.80 |
| Day 10 post subculturec | ||||||
| RC | 7.9 | 12.8 | 1.62 | 8.0 | 15.6 | 1.95 |
| DS | 8.1 | 10.3 | 1.27 | 7.6 | 13.7 | 1.80 |
| RC+DS | 16.0 | 23.1 | 1.44 | 15.6 | 24.3 | 1.88 |
HepG2 cells were transduced under the following conditions: WT MOI of 100 pfu/cell, rtM204I MOI of 100 pfu/cell, and WT MOI of 100 pfu/cell+rtM204I MOI of 100 pfu/cell and harvested at day 10 p.t. A parallel set of cultures for each of the three conditions were subcultured 1:4 and harvested at days 5 and 10 post subculture.
DNA levels for rtM204I alone were detectable but extremely low (RC: uncut = 2.5 pg, Cla I cut = 2.9 pg: DS uncut = 3.6 pg, Cla I cut = 2.1 pg) and were not included in the calculations.
Data for rtM204I alone are not included because the DNA levels were below the limits of detection.
In both experimental approaches, cotransduction with WT and rtM204I baculoviruses yielded higher levels of RC plus DS DNA than expected with fold increases ranging from 1.33 to 2.15 depending upon the time point and the experimental design (Tables 1 and 2). In all conditions, an increase in RC DNA alone and DS DNA alone when comparing WT + rtM204I to WT was observed. In addition, the fold increase for the RC band was equal to or greater than the total fold increase in the RC and DS bands for uncut DNA and this was also true for the Cla I cut DNA. If the increase in the HBV RI in the cotransduction setting was caused by trans-complementation of rtM204I by WT pol, then the expected result would be that Cla I digestion would result in a decrease in the RC band and a concomitant increase in the DS band and this was not the case. These data clearly indicate that WT HBV DNA that cannot be cut by Cla I contribute to the observed increased HBV RI levels seen in the cotransduction setting. We cannot rule out that a small portion of the increase is caused by trans-complementation of rtM204I by WT pol because of limits of the experimental approach.
Increase in HBV replication in the presence of a pol-minus HBV construct
Since the observed increase in HBV RI DNA during cotransduction of WT and rtM204I is predominantly WT HBV DNA, the question arose of what is causing the observed increase. To begin to address this issue, we asked whether the increase would also be observed if HepG2 cells were cotransduced with WT and a recombinant baculovirus encoding a pol negative HBV construct instead of a construct containing a point mutation in pol. An HBV pol negative (pol−) baculovirus was constructed and designated HBV pol−. The HBV pol− baculovirus contains an HBV pol sequence similar to the replication-deficient plasmid pdMfe, utilized in experiments described above. The HBV pol− baculovirus was created in the same backbone as the WT HBV baculovirus and utilizes endogenous HBV promoters and enhancers for expression of all HBV transcripts. Five separate conditions were utilized: rtM204I MOI of 100 + baculovirus MOI of 25 (A), WT MOI of 25 + baculovirus MOI of 100 (B), rtM204I MOI of 100 + pol−MOI of 25 (C), WT MOI of 25 + HBV pol− MOI of 100 (D), WT MOI of 25 + rtM204I MOI of 100 (E). On the indicated days p.t., HepG2 cells were harvested for HBV RI DNA (Fig. 8). Cotransduction with the HBV pol− baculovirus had no effect on the replication of rtM204I (compare A and C; gray and striped bars in F). However, contransduction with HBV pol− baculovirus increased the replication of HBV, as demonstrated in (B) and (D) [also compare black and white bars in F]. The increase in HBV replication in the presence of the HBV pol− baculovirus is similar to that observed during a WT and rtM204I cotransduction, as demonstrated in (D) and (E) [also compare white and textured bars in F]. Collectively, this data indicates that mutant pol is not playing a role in the increase in HBV replication observed during a WT and rtM204I cotransduction. In addition, it suggests the possibility that HBV proteins (HBsAg, HBeAg, HBx, HBcAg) produced by a HBV virus that does not produce RI (either because it lacks pol or possesses a replication deficient pol) can lead to increased levels of HBV RI in the cotransduction setting.
Fig. 8.
Increase in HBV RI when HepG2 cells are cotransduced with a WT and a pol-minus HBV baculovirus. HepG2 cells were transduced on day 0 under the following conditions: rtM204I HBV MOI = 100 pfu/cell + baculovirus MOI = 25 pfu/cell (A), WT HBV MOI = 25 pfu/cell + baculovirus MOI = 100 pfu/cell (B), rtM204I HBV MOI = 100 pfu/cell + HBV pol− MOI = 25 pfu/cell (C), WT HBV MOI = 25 + HBV pol− MOI = 100 pfu/cell (D), WT HBV MOI = 25 pfu/cell + rtM204I HBV MOI = 100 pfu/cell (E). Cells were harvested for HBV RI DNA at the indicated days p.t. and analyzed by Southern blot. HBV RI DNA bands were visualized using a PhosphorImager and quantitated (F) using ImageQuant software. P.T., post-transduction; RC, relaxed-circular; DS, double-stranded linear; SS, single-stranded. Gray bars, rtM204I HBV MOI = 100 pfu/cell + baculovirus MOI = 25 pfu/cell; black bars, WT HBV MOI = 25 pfu/cell + baculovirus MOI = 100 pfu/cell; striped bars, rtM204I HBV MOI = 100 pfu/cell + HBV pol− MOI = 25 pfu/cell; white bars, WT HBV MOI = 25 + HBV pol− MOI = 100 pfu/cell; textured bars, WT HBV MOI = 25 pfu/cell + rtM204I HBV MOI = 100 pfu/cell.
Discussion
Recombinant baculovirus-mediated HBV transduction of HepG2 cells, which allows highly efficient transduction of WT and mutant HBV genomes or individual genes and detailed analyses of all post-entry steps of HBV replication was used to study the effect of the HBV WT pol expressed alone or in the context of the intact WT HBV genome on rtM204I replication. We show that HBV WT pol expression can complement defective mutant rtM204I pol activity resulting in increased levels of HBV RI DNAs. Analysis of the genetically marked viral genomes verified that this restoration resulted from trans-complementation, rather than recombination. Furthermore, the WT pol functioned in trans to complement HBV replication regardless of whether or not the RNA packaging signal, epsilon, which is required to activate the pol, was provided in cis. In this case, trans-complementation was achieved by pol over-expression from a packaging-defective RNA. This finding indicates that HBV pol can function in trans, without the requirement of the epsilon RNA in cis, in either RNA packaging or DNA synthesis. In contrast, we demonstrate that enhanced levels of total HBV RI observed when HepG2 cells were cotransduced with baculoviruses expressing the WT and rtM204I genomes was not due to trans-complementation of rtM204I by the WT pol. In this case, WT pol was produced from a full-length, WT pgRNA.
An interesting result generated by this study was that both PCF (lacking a 3′ epsilon) and PCFE rescued pol-deficient HBV to equal levels, indicating that the presence of a 3′ epsilon sequence is not required for functional HBV pol activity. In previous examples of trans-complementation, a copy of epsilon was provided in cis within the pol construct (Bartenschlager, Junker-Niepmann, and Schaller, 1990; Chiang et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Okamoto et al., 1993; Radziwill, Tucker, and Schaller, 1990; Yaginuma et al., 1987); however, a direct comparison with or without epsilon was not evaluated. Ziermann and Ganem (1996), using a transfection-based approach, demonstrated that there is no requirement for epsilon in cis for the encapsidation function of HBV pol; however, DNA synthesis activity of the pol was not investigated (Ziermann and Ganem, 1996). Our data not only agree with Ziermann et al., but also indicate that the presence of epsilon in cis within the pol construct is not required for reconstitution of viral replication. Therefore, despite the well-known cis-preference of pol (see below), there is no strict requirement for epsilon in cis in order for the pol to function in either RNA packaging or reverse transcription. Our results indicate that the reported requirement for epsilon to activate the enzymatic activity of the pol (Lanford et al., 1997; Tavis and Ganem, 1996; Tavis, Massey, and Gong, 1998) previously shown in vitro to be able to be provided in trans (Pollack and Ganem, 1994; Wang et al., 1994) can also be provided in trans in vivo in cell culture.
The expression of WT pol in HepG2 cells previously transduced with either WT or rtM204I HBV recombinant baculovirus resulted in two separate observations. For WT HBV, expression of WT pol had no effect on the replication as measured by HBV RI, EC, and CCC DNA levels. WT HBV has a fully functional pol that replicates to high levels in the HBV recombinant baculovirus system, so this was not an unexpected result. These findings also suggest that levels of HBV replication in HepG2 cells are not limited by the amount of pol and not inhibited by addition of excess, functional pol within the limits of the system used. For rtM204I HBV, the expression of WT pol partially restored virus replication at the level of RI and EC DNA at late time points p.t. However, there was no change at the level of CCC DNA for rtM204I. No change in CCC DNA levels was expected since the levels of CCC DNA remain elevated even at late time points p.t. when rtM204I RI and EC DNA are reduced compared to WT (Heipertz et al., 2007). Using restriction digests, it was demonstrated that this restoration of rtM204I replication was through trans-complementation of the WT pol protein functioning to replicate the rtM204I genome. Although complementation by WT pol did not restore rtM204I replication to WT replication levels, the restored level was approximately 10-fold higher than that seen for rtM204I replication in the absence of complementation.
It is well established that the HBV pol preferentially recognizes the epsilon of the pgRNA from which it is translated [cis-preference] (Bartenschlager, Junker-Niepmann, and Schaller, 1990; Hirsch et al., 1990; Hirsch et al., 1991; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Knaus and Nassal, 1993; Pollack and Ganem, 1993). In the study presented here, for rtM204I replication to be occurring at the levels seen, WT pol has to recognize, in trans, epsilon on the rtM204I pgRNA. Complementation by WT HBV pol in trans has been previously demonstrated, by showing that a functional pol protein can trans-complement a mutant virus that produces no pol (Bartenschlager, Junker-Niepmann, and Schaller, 1990; Chiang et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Okamoto et al., 1993; Radziwill, Tucker, and Schaller, 1990; Yaginuma et al., 1987) or a packaging-defective pol (Blum et al., 1991). The current study is unique in that it addresses the question of the ability of WT HBV pol to complement the reverse transcriptase activity of a drug-resistant pol mutant that arises in chronic HBV patients, yet remains functional in pgRNA packaging. We conclude from these studies that WT pol, at least when over-expressed from a RNA deficient in packaging, can partially restore, through trans-complementation, the replication of rtM204I at late time points p.t. in the HBV recombinant baculovirus system.
Given the cis-preference of RNA packaging by the pol, the ability of WT pol to trans-complement a mutant pol such as rtM204I that is fully functional in RNA packaging was quite unexpected. We can suggest at least three possibilities for how WT pol could trans-complement rtM204I replication. The first possibility is that trans-complementation is due to an increase in the total amount of pgRNA packaging when PCFE is trans-complementing rtM204I replication. This would suggest that a single molecule of pol is contained within nucleocapsids and that the increase in HBV RI DNA observed during PCFE trans-complementation is due to pol from PCFE recognizing free (excess) rtM204I pgRNA that is not being encapsidated by the rtM204I pol. We have tested this possibility and the data clearly show that there is no increase in packaging when PCFE is trans-complementing rtM204I replication. The second possibility, based on current dogma, is that although the total amount of RNA packaging remains equal, over-expression of WT pol can out-compete the mutant pol for the mutant pgRNA. This is unlikely because trans-recognition of pgRNA is much less efficient than cis-recognition (Bartenschlager, Junker-Niepmann, and Schaller, 1990; Hirsch et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990). The third possibility is that more than one pol molecule is packaged within a capsid such that both the WT and rtM204I polymerases are co-packaged into the same nucleocapsid. This would be in contrast to a previous report where it was determined that duck HBV (DHBV) virions contain only monomers of pol covalently attached to DHBV DNA (Zhang and Tavis, 2006). However, our results raise the possibility that more than one pol molecule may be packaged under specific circumstances.
We also wanted to determine whether trans-complementation of rtM204I by WT pol was possibly responsible for the unexpected enhanced levels of total HBV RI observed at late time points when HepG2 cells were cotransduced with WT HBV and rtM204I recombinant baculoviruses (Heipertz et al., 2007). Given the fact that WT pol can trans-complement the rtM204I virus, it was plausible that the increase in replication observed previously during the cotransduction might be due to the same event. Our data indicate that increased HBV RI produced at late time points from the cotransduction are predominantly WT DNA. We cannot rule out that a low rate of trans-complementation is occurring during cotransduction that is below the limits of detection. The failure of the WT pol, which in this case is translated from a fully functional, packaging-competent pgRNA, to effectively complement the rtM204I mutant as compared to the addition of PCFE, is possibly due to the lower levels of pol expression in the context of the entire WT genome and also the sequestration of the WT pol by its own pgRNA in cis into nucleocapsids, due to the cis-preference. Thus, whether the pol, either WT or variants, expressed in the context of a full-length pgRNA, can function efficiently in trans in a natural infection remains to be established. In fact, it has been suggested that any contribution from trans-complementation of a WT and mutant virus in a mixed infection would be minimal (Litwin et al., 2005). One complication is the fact that without the selection pressure provided by lamivudine treatment, WT HBV is the more fit virus when compared to rtM204I, and is therefore preferentially replicated, thus out-competing rtM204I when both viruses are present within the same hepatocyte. This event has been observed in patients whose serum HBV DNA levels have rebounded because of the presence of lamivudine-resistant mutants. When lamivudine therapy is stopped after the resistant mutants predominate the population in serum, WT HBV overtakes the mutants after only a few months following cessation, indicating that without the selection pressure of lamivudine treatment, there is a disappearance of resistant mutants in patient sera (Cane et al., 1999; Chayama et al., 1998).
We also show that HBV RI levels are increased similar to those seen for cotransduction with WT and rtM204I when HepG2 cells were cotransduced with a WT baculovirus and a pol negative baculovirus indicating that the rtM204I mutant pol protein or any variant HBV proteins modified as a result of overlapping effects of the rtM204I mutation are not causing the increase. These studies suggest the possibility that HBV proteins (HBsAg, HBeAg, HBx, HBcAg), expressed from an HBV virus that does not produce RI (either because it lacks pol or possesses a replication deficient pol), present in the cotransduction setting are either directly or indirectly involved in increasing the production of RI. This explanation is feasible because HBxAg can boost HBV replication by trans-activation (reviewed by Bouchard and Schneider, 2004) and because HBcAg can also function in trans (Chiang et al., 1990; Junker-Niepmann, Bartenschlager, and Schaller, 1990; Okamoto et al., 1993; Radziwill, Tucker, and Schaller, 1990; Yaginuma et al., 1987). Addressing the question of which HBV protein(s) may be functioning to increase RI in the cotranduction setting is beyond the scope of the current studies.
We conclude from the initial transfection/transduction experiments that a WT pol has the capacity to function to trans-complement a pol-minus HBV virus without the requirement of an epsilon in cis for its RNA packaging or enzymatic activity. The pol also has the capacity to trans-complement the defect in replication of the rtM204I mutant; however, trans-complementation appears to require pol over-expression from a packaging-defective RNA. In contrast, when expressed from an authentic pgRNA, WT pol may not be able to efficiently trans-complement a mutant pol that is not defective in RNA packaging (such as those arising during current antiviral therapy). Viral proteins, other than pol may be able to function in trans more effectively in a mixed infection setting. Given the high prevalence of drug-resistant HBV variants arising during current antiviral therapy (Chang et al., 2006; Lai et al., 2003; Lai et al., 2007; Lee et al., 2006; Mauss and Wedemeyer, 2008), it is important to understand interactions between the different HBV variants that may occur within infected hepatocytes, e.g. through trans-complementation, as show here, in order to develop more effective antivirals and therapeutic strategies.
Materials and Methods
Cell culture
HepG2 cells were maintained at 37°C in a humidified incubator at 5% CO2 (Knowles, Howe, and Aden, 1980) in minimal medium (MEM; Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone) [MEM-FBS]. Sf21 insect cells were maintained in Grace's insect medium supplemented with yeastolate (Mediatech, Inc.), lactalbumin hydrolase (Mediatech, Inc.), and 10% FBS in a nonhumidified incubator without CO2 at 28°C.
Plasmids
The replication-deficient HBV expression plasmid pCMV-HBV/POL−, referred to here as dMfe, is unable to make any pol protein due to a frame shift mutation, as previously described (Gao and Hu, 2007). PCFE and PCF WT HBV pol-expressing plasmids were generated as follows. The WT HBV pol open reading frame, modified at the carboxyterminus by the addition of a “Flag-tag” peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys), was inserted into vector pCl (Promega) immediately downstream of the CMV promoter element generating PCF. For PCFE, an additional 341-bp segment encompassing the DR1 and epsilon regions of HBV transcripts was cloned directly downstream of the “Flag-tag” of PCF.
The entire CMV promoter and modified HBV pol open reading frame of PCFE and PCF was isolated by partial Bgl II digestion and complete Cla I digestion and subsequently cloned into the Bgl II – BstB I sites of pBlueBac4.5 (Invitrogen), thus generating the transfer vectors pBBPCFE and pBBPCF, respectively.
Generation of WT HBV pol-expressing baculoviruses
pBBPCFE and pBBPCF were used to generate the recombinant baculoviruses PCFE and PCF, respectively, according to methods described previously (Delaney and Isom, 1998).
Generation of HBV pol-baculovirus
The HBV pol− baculovirus was generated from the WT HBV baculovirus transfer vector pBB4.5HBV1.3 through site-directed mutagenesis using the QuickChange II XL Kit (Stratagene). The sequence of the forward primer used for mutagenesis was: 5′ CAGTTAATGAGAAAAGAAGATTGTAATTGATTATGCCTGCCAGGTTT 3′ and the reverse primer: 5′ AAACCTGGCAGGCATAATCAATTACAATCTTCTTTTCTCATTAACTG 3′. The single base substitution at nt 2626, as highlighted by the bold face, introduced a stop codon in the pol gene after codon 107. The same substitution also eliminated the Mfe I restriction site. The mutation was confirmed by restriction enzyme digestion and transfection check for HBV replication in the presence or absence (lack of replication) of the pol-expressing plasmid pBBPCFE. The transfer vector pBB4.5HBVpol− was used to generate the recombinant baculovirus HBV pol− according to methods previously described (Delaney and Isom, 1998).
Transfection and baculovirus transduction
Subconfluent HepG2 cells were transfected with 3 μg of plasmid pdMfe using Effectene transfection reagent (Qiagen) 20 h after seeding into 60-mm plates according to manufacturer's instructions. Baculovirus transductions were carried out as previously described (Delaney and Isom, 1998; Heipertz et al., 2007). Briefly, HepG2 cells were seeded in 60 mm plates at a confluency of 20–40% 24 h prior to baculovirus transduction. An average cell count was determined the day of transduction, and used to calculate the appropriate volume of high-titer baculovirus stock required to obtain the desired MOI. The baculovirus stock was diluted in 0.5 mL MEM-FBS, added to HepG2 cells drop-wise, and allowed to absorb for 1 h at 37°C with gentle rocking every 15 min. The baculovirus inoculum was removed by gently washing the cells two times with PBS, and were re-fed and maintained as described above.
Restriction enzyme digestion
An appropriate amount of HBV RI DNA was digested with restriction enzymes EcoR I, Mfe I, or Cla I for the appropriate amount of time at 37°C.
HBV DNA extraction and Southern blot analysis
Intracellular HBV RI and nuclear CCC DNA were extracted from HepG2 cells as previously described in detail (Abdelhamed et al., 2003; Heipertz et al., 2007; Starkey, Chiari, and Isom, 2009), separated on 1% agarose gels and Southern blotted. Membranes were hybridized with a [α-32P] dCTP-radiolabeled probe generated from a full-length, double-stranded HBV genome or a positive-strand-specific [α-32P] UTP-radiolabeled riboprobe, generated by an in vitro transcription label (Hu et al., 2004; Nguyen and Hu, 2008). Southern blots were visualized with a PhosphorImager and DNA bands were quantified using ImageQuant (Molecular Dynamics).
Intracellular nucleocapsid extraction and Southern blot analysis
Cytoplasmic preparations of HBV nucleocapsids were obtained by using a modified protocol that has been previously described (Nguyen and Hu, 2008). HBV recombinant baculovirus-transduced HepG2 cells were harvested in a lysis buffer (50 mM Tris-HCl, 1 mM EDTA, 1% IGEPAL, and protease inhibitor cocktail) followed by centrifugation to remove the nuclei. The supernatant was treated with DNase I and RNase for 1 h at 37°C followed by another centrifugation. Cytoplasmic nucleocapsids were precipitated by the addition of 35% PEG in 1.75 M NaCl and incubating at 4°C for 1 h. Nucleocapsids were then centrifuged, thoroughly resuspended in TNE (pH = 8.0), and stored at −80°C. Nucleocapsid preparations were separated on 1% agarose gels by running overnight and then transferred to nitrocellulose membranes. The following day, membranes were denatured for 10 s and hybridized with a positive-strand-specific [α-32P] UTP-radiolabeled riboprobe, generated by an in vitro transcription label and used for Southern blot (Hu et al., 2004; Nguyen and Hu, 2008). Southern blots were visualized with a PhosphorImager and DNA bands were quantified using ImageQuant.
Acknowledgments
This work was supported by research grant RO1-CA 023931 to H.C.I. and RO1-AI 043453 to J.H. from the National Institutes of Health. R.A.H. was supported in part by training grant CA60395-12 from the National Cancer Institute.
The molecular imaging of the Southern blots and PhosphorImager Analysis used in this study were carried out in the Macromolecular Core Facility of The Pennsylvania State University College of Medicine.
We also thank Susan Kocher for expert technical assistance.
References
- Abdelhamed AM, Kelley CM, Miller TG, Furman PA, Cable EE, Isom HC. Comparison of anti-hepatitis B virus activities of lamivudine and clevudine by a quantitative assay. Antimicrob Agents Chemother. 2003;47(1):324–336. doi: 10.1128/AAC.47.1.324-336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, Brown N, Condreay LD. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group Hepatology. 1998;27(6):1670–1677. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- Bartenschlager R, Junker-Niepmann M, Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol. 1990;64(11):5324–5332. doi: 10.1128/jvi.64.11.5324-5332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew MM, Jansen RW, Jeffers LJ, Reddy KR, Johnson LC, Bunzendahl H, Condreay LD, Tzakis AG, Schiff ER, Brown NA. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349(9044):20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- Blum HE, Galun E, Liang TJ, von Weizsacker F, Wands JR. Naturally occurring missense mutation in the polymerase gene terminating hepatitis B virus replication. J Virol. 1991;65(4):1836–1842. doi: 10.1128/jvi.65.4.1836-1842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Schneider RJ. The enigmatic × gene of hepatitis B virus. J Virol. 2004;78(23):12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane PA, Mutimer D, Ratcliffe D, Cook P, Beards G, Elias E, Pillay D. Analysis of hepatitis B virus quasispecies changes during emergence and reversion of lamivudine resistance in liver transplantation. Antivir Ther. 1999;4(1):7–14. [PubMed] [Google Scholar]
- Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis. B J Gastroenterol Hepatol. 2004;19(11):1276–1282. doi: 10.1111/j.1440-1746.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis. B N Engl J Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I, Arase Y, Saitoh S, Murashima N, Ikeda K, Kumada H. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27(6):1711–1716. doi: 10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- Chiang PW, Hu CP, Su TS, Lo SC, Chu MH, Schaller H, Chang CM. Encapsidation of truncated human hepatitis B virus genomes through trans-complementation of the core protein and polymerase. Virology. 1990;176(2):355–361. doi: 10.1016/0042-6822(90)90005-c. [DOI] [PubMed] [Google Scholar]
- de Man RA, Honkoop P, Janssen HL, Schalm SW. Chronic hepatitis-b-virus infections: new options for antiviral therapy. Ned Tijdschr Geneeskd. 1999;143(37):1857–1861. [PubMed] [Google Scholar]
- Delaney IV WE, Isom HC. Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology. 1998;28(4):1134–1146. doi: 10.1002/hep.510280432. [DOI] [PubMed] [Google Scholar]
- Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, Brown NA. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341(17):1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- Fu L, Cheng YC. Role of additional mutations outside the YMDD motif of hepatitis B virus polymerase in L(—)SddC (3TC) resistance. Biochem Pharmacol. 1998;55(10):1567–1572. doi: 10.1016/s0006-2952(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Gaillard RK, Barnard J, Lopez V, Hodges P, Bourne E, Johnson L, Allen MI, Condreay P, Miller WH, Condreay LD. Kinetic analysis of wild-type and YMDD mutant hepatitis B virus polymerases and effects of deoxyribonucleotide concentrations on polymerase activity. Antimicrob Agents Chemother. 2002;46(4):1005–1013. doi: 10.1128/AAC.46.4.1005-1013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Hu J. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J Virol. 2007;81(12):6164–6174. doi: 10.1128/JVI.02721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girones R, Miller RH. Mutation rate of the hepadnavirus genome. Virology. 1989;170(2):595–597. doi: 10.1016/0042-6822(89)90455-8. [DOI] [PubMed] [Google Scholar]
- Heipertz RA, Jr, Miller TG, Kelley CM, Delaney WE, IV, Locarnini SA, Isom HC. In vitro study of the effects of precore and lamivudine-resistant mutations on hepatitis B virus replication. J Virol. 2007;81(7):3068–3076. doi: 10.1128/JVI.02341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch RC, Lavine JE, Chang LJ, Varmus HE, Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as wel as for reverse transcription. Nature. 1990;344(6266):552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- Hirsch RC, Loeb DD, Pollack JR, Ganem D. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J Virol. 1991;65(6):3309–3316. doi: 10.1128/jvi.65.6.3309-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkoop P, Niesters HG, de Man RA, Osterhaus AD, Schalm SW. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997;26(6):1393–1395. doi: 10.1016/s0168-8278(97)80476-x. [DOI] [PubMed] [Google Scholar]
- Hu J, Flores D, Toft D, Wang X, Nguyen D. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J Virol. 2004;78(23):13122–13131. doi: 10.1128/JVI.78.23.13122-13131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis. B Drugs. 1999;58(1):101–141. doi: 10.2165/00003495-199958010-00015. [DOI] [PubMed] [Google Scholar]
- Junker-Niepmann M, Bartenschlager R, Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Watanabe N, Kanouda H, Takayama I, Shiba T, Kanai T, Kawazoe K, Takashimizu S, Kumaki N, Shimamura K, Matsuzaki S, Mine T. Fatal liver failure due to reactivation of lamivudine-resistant HBV mutant. World J Gastroenterol. 2004;10(11):1686–1687. doi: 10.3748/wjg.v10.i11.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, Kim CY. Fatal submassive hepatic necrosis associated with tyrosine–methionine–aspartate– aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33(3):403–405. doi: 10.1086/321879. [DOI] [PubMed] [Google Scholar]
- Knaus T, Nassal M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem–loop structure that is critical for its function. Nucleic Acids Res. 1993;21(17):3967–3975. doi: 10.1093/nar/21.17.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36(6):687–696. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, Naoumov NV, Di Bisceglie AM, Zeuzem S, Moon YM, Goodman Z, Chao G, Constance BF, Brown NA. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J Med. 2007;357(25):2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Notvall L, Lee H, Beames B. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J Virol. 1997;71(4):2996–3004. doi: 10.1128/jvi.71.4.2996-3004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43(6):1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30(2):567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- Litwin S, Toll E, Jilbert AR, Mason WS. The competing roles of virus replication and hepatocyte death rates in the emergence of drug-resistant mutants: theoretical considerations. J Clin Virol. 2005;34(1):S96–S107. doi: 10.1016/s1386-6532(05)80018-6. [DOI] [PubMed] [Google Scholar]
- Mauss S, Wedemeyer H. Treatment of chronic hepatitis B and the implications of viral resistance to therapy. Expert Rev Anti Infect Ther. 2008;6(2):191–199. doi: 10.1586/14787210.6.2.191. [DOI] [PubMed] [Google Scholar]
- Melegari M, Scaglioni PP, Wands JR. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology. 1998;27(2):628–633. doi: 10.1002/hep.510270243. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Hu J. Reverse transcriptase- and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids. J Virol. 2008;82(14):6852–6861. doi: 10.1128/JVI.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Wang Y, Tanaka T, Machida A, Miyakawa Y, Mayumi M. Trans-complementation among naturally occurring deletion mutants of hepatitis B virus and integrated viral DNA for the production of viral particles with mutant genomes in hepatoma cell lines. J Gen Virol. 1993;74(Pt 3):407–414. doi: 10.1099/0022-1317-74-3-407. [DOI] [PubMed] [Google Scholar]
- Ono-Nita SK, Kato N, Shiratori Y, Masaki T, Lan KH, Carrilho FJ, Omata M. YMDD motif in hepatitis B virus DNA polymerase influences on replication and lamivudine resistance: a study by in vitro full-length viral DNA transfection. Hepatology. 1999;29(3):939–945. doi: 10.1002/hep.510290340. [DOI] [PubMed] [Google Scholar]
- Pallier C, Castera L, Soulier A, Hezode C, Nordmann P, Dhumeaux D, Pawlotsky JM. Dynamics of hepatitis B virus resistance to lamivudine. J Virol. 2006;80(2):643–653. doi: 10.1128/JVI.80.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MG, Singer G, Howard T, Jacobsmeyer S, Xiong X, Gibbs CS, Lamy P, Murray A. Fulminant hepatic failure resulting from lamivudine-resistant hepatitis B virus in a renal transplant recipient: durable response after orthotopic liver transplantation on adefovir dipivoxil and hepatitis B immune globulin. Transplantation. 1999;68(12):1912–1914. doi: 10.1097/00007890-199912270-00017. [DOI] [PubMed] [Google Scholar]
- Pollack JR, Ganem D. An RNA stem–loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67(6):3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack JR, Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J Virol. 1994;68(9):5579–5587. doi: 10.1128/jvi.68.9.5579-5587.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwill G, Tucker W, Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990;64(2):613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32(2):300–306. doi: 10.1016/s0168-8278(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Starkey JL, Chiari EF, Isom HC. Hepatitis B virus (HBV)-specific short hairpin RNA is capable of reducing the formation of HBV covalently closed circular (CCC) DNA but has no effect on established CCC DNA in vitro. J Gen Virol. 2009;90(Pt 1):115–126. doi: 10.1099/vir.0.004408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Yotsuyanagi H, Okuse C, Nagase Y, Takahashi H, Moriya K, Suzuki M, Koike K, Iino S, Itoh F. Fatal liver failure caused by reactivation of lamivudine-resistant hepatitis B virus: a case report. World J Gastroenterol. 2007;13:964–969. doi: 10.3748/wjg.v13.i6.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavis JE, Ganem D. Evidence for activation of the hepatitis B virus polymerase by binding of its RNA template. J Virol. 1996;70(9):5741–5750. doi: 10.1128/jvi.70.9.5741-5750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavis JE, Massey B, Gong Y. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, epsilon. J Virol. 1998;72(7):5789–5796. doi: 10.1128/jvi.72.7.5789-5796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GH, Zoulim F, Leber EH, Kitson J, Seeger C. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B viruses. J Virol. 1994;68(12):8437–8442. doi: 10.1128/jvi.68.12.8437-8442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Lu SN, Lee CM, Lee JF, Chou YP. Fatal hepatic failure after emergence of the hepatitis B virus mutant during lamivudine therapy in a patient with liver cirrhosis. Scand J Gastroenterol. 2002;37(3):366–369. doi: 10.1080/003655202317284309. [DOI] [PubMed] [Google Scholar]
- Yaginuma K, Shirakata Y, Kobayashi M, Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu C, Gong Q, Zhang S, Zhang D, Lu Z, Wang Y. Evolution of wild type and mutants of the YMDD motif of hepatitis B virus polymerase during lamivudine therapy. J Gastroenterol Hepatol. 2003;18(12):1353–1357. doi: 10.1046/j.1440-1746.2003.03176.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tavis JE. The duck hepatitis B virus reverse transcriptase functions as a full-length monomer. J Biol Chem. 2006;281(47):35794–35801. doi: 10.1074/jbc.M608031200. [DOI] [PubMed] [Google Scholar]
- Ziermann R, Ganem D. Homologous and heterologous complementation of HBV and WHV capsid and polymerase functions in RNA encapsidation. Virology. 1996;219(2):350–356. doi: 10.1006/viro.1996.0260. [DOI] [PubMed] [Google Scholar]