Abstract
Previous studies demonstrated that low-level postnatal and early life exposure to the environmental contaminant, trichloroethylene (TCE), in the drinking water of MRL+/+ mice altered glutathione redox homeostasis and increased biomarkers of oxidative stress indicating a more oxidized state. Plasma metabolites along the interrelated transmethylation pathway were also altered indicating impaired methylation capacity. Here we extend these findings to further characterize the impact of TCE exposure in mice exposed to water only or two doses of TCE in the drinking water (0, 2, and 28 mg/kg/day) postnatally from birth until 6 weeks of age on redox homeostasis and biomarkers of oxidative stress in the cerebellum. In addition, pathway intermediates involved in methyl metabolism and global DNA methylation patterns were examined in cerebellar tissue. Because the cerebellum is functionally important for coordinating motor activity, including exploratory and social approach behaviors, these parameters were evaluated in the present study. Mice exposed to 28 mg/kg/day TCE exhibited increased locomotor activity over time as compared with control mice. In the novel object exploration test, these mice were more likely to enter the zone with the novel object as compared to control mice Similar results were obtained in a second test when an unfamiliar mouse was introduced into the testing arena. The results show for the first time that postnatal exposure to TCE causes key metabolic changes in the cerebellum that may contribute to global DNA methylation deficits and behavioral alterations in TCE-exposed mice.
Keywords: cerebellum, trichloroethylene, locomotor behavior, oxidative stress, methylation
Introduction
Trichloroethylene (TCE) is an organic solvent used as an industrial degreasing agent. Human TCE exposure can occur at all stages of life. TCE crosses the placenta and can be detected in breast milk (Beamer et al., 2012). Studies of school-aged children found detectable blood levels of TCE in approximately 6% of the subjects (Adgate et al., 2004;Sexton et al., 2005). One of the predominant non-cancer health effects associated with exposure to TCE is neurotoxicity (Bale et al., 2011;Chiu et al., 2006). However, the mechanisms underlying this toxicity, as well as the behavioral effects resulting from this exposure remain elusive.
Xenobiotics are known to impart long-lasting effects on organ systems especially if exposure occurs during development (Heindel, 2008). Epidemiologic studies documenting human exposure reported motor function deficits in occupationally exposed adults (Rasmussen et al., 1993). Individuals who ingested water from TCE-contaminated water wells and municipal water supplies showed higher mean scores for depression and mood disorders, and lower intelligence scores as compared to subjects who did not ingest contaminated water (Kilburn et al., 1993;Reif et al., 2003). In terms of developmental exposure, impaired motor coordination and behaviors characterized by inattention and hyperactivity in children of mothers exposed to solvents occupationally have been documented (Laslo-Baker et al., 2004;Till et al., 2001). Rodent studies by our lab and others have shown that during the perinatal and postnatal period of development, the brain is particularly vulnerable to oxidative stress, impaired glutathione redox balance, and inflammation from exposure to environmental toxicants. (Blossom et al., 2012;Stringari et al., 2006).
Oxidative stress and impaired glutathione anti-oxidant capacity can result from toxicant exposure and is linked to neurologic disorders. Glutathione is a tripeptide that functions as the major intracellular antioxidant against oxidative stress and plays an important role in the detoxification of reactive oxygen species in the brain (Biswas et al., 2006;Jain et al., 1991). Pro-oxidant environmental exposures have the potential to decrease the active reduced form of glutathione (GSH) and to increase the inactive oxidized disulfide form (GSSG) leaving the cell vulnerable to oxidative damage. Alterations in glutathione redox potential have been shown to modulate the fate of oligodendrocyte precursor cells (Noble et al., 2005), and maturing neurons (Maffi et al., 2008;McLean et al., 2005) suggesting that altered brain redox status and increased oxidative stress could hinder neural development and promote behavioral pathology.
We recently demonstrated that mice exposed to TCE in the drinking water postnatally from birth postnatal day (PND) 0 through PND 42 showed decreased GSH and an increase in the GSH/GSSG ratio commensurate with elevated levels 3-nitrotyrosine, a biomarker of oxidative protein damage, in the hippocampus (Blossom et al., 2012). These metabolic changes in the hippocampus were accompanied by alterations in the inter-related transmethylation pathway metabolites in the plasma. As shown in Fig. 1 one crucial pathway that intersects with the transsulfuration pathway leading to glutathione synthesis is the methionine cycle (Selhub, 2002). From a functional standpoint, deficits in transmethylation metabolites could lead to global DNA hypomethylation and ultimately impact cell differentiation, gene expression, and chromatin structure (Castro et al., 2003;Caudill et al., 2001;Feil, 2006;Yi et al., 2000)
Fig. 1.
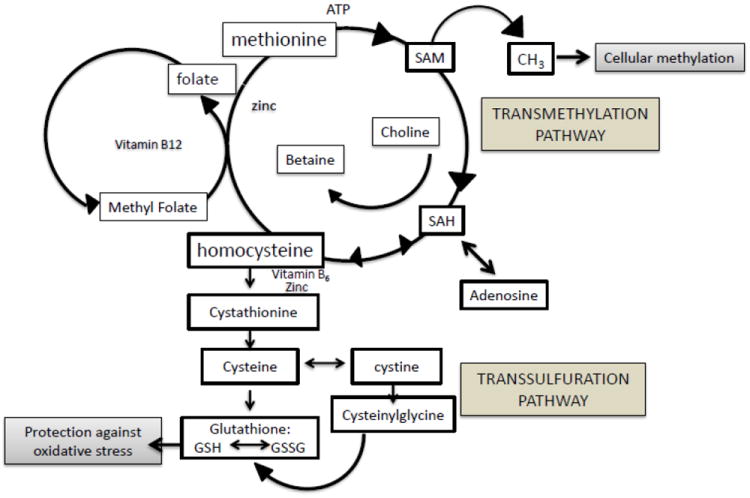
Folate-dependent methionine transmethylation and transsulfuration pathways involved in redox potential and cellular methylation.
The purpose of this study was to investigate the impact of postnatal TCE exposure on glutathione redox potential and biomarkers of oxidative stress in the cerebellum. In this study, methionine was examined in cerebellum rather than plasma. Mice were exposed to drinking water alone or two doses of TCE in the drinking water postnatally from birth until 6 weeks of age. Global DNA methylation was assessed in cerebellar tissue to examine the potential impact of impaired methyl metabolism with TCE exposure. Because the cerebellum is functionally important for coordinating motor activity, including exploratory and social approach behaviors, these behavioral parameters were evaluated in the present study. The results show for the first time that postnatal exposure to TCE causes key metabolic changes in the cerebellum that may contribute to global DNA methylation deficits and behavioral alterations in TCE-exposed mice.
Methods
Mice
Male MRL+/+ mice were used to evaluate TCE-induced neurotoxicity as described previously (Blossom et al., 2012). The MRL+/+ strain of mice were used based on this strain’s sensitivity to TCE toxicity as described (Blossom et al., 2006;Blossom et al., 2007;Blossom et al., 2008 Blossom et al., 2008). MRL +/+ mice are by all accounts normal and are often used as the control strain for studies using MRL/lpr mice. MRL/lpr mice develop, in addition to autoimmune disease, several behavioral deficits and neuropathological changes with age and are considered to be a model of idiopathic neurological lupus (Ballok et al., 2004;Ballok, 2007;Sakic et al., 2005). Unlike control C57Bl/6 mice, young MRL+/+ mice have been used to study neurogenesis in response to pharmacological agents that target neuroplasticity (Balu et al., 2009;Hodes et al., 2010). Because many neurological disorders such as autism are accompanied by immune dysfunction and autoimmunity (Cabanlit et al., 2007;Comi et al., 1999;Molloy et al., 2006;Sweeten et al., 2003), MRL+/+ mice were used in the current study.
Only male, but not female offspring were evaluated in the current study. Females were not included based on our published findings showing male mice were much more sensitive than female mice to TCE-mediated neurologic effects involving oxidative stress (Blossom et al., 2008). In the current study, eight-week-old MRL+/+ breeder pairs were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were acclimated to the animal facility for one week before breeding cages were established. During breeding the mice were housed in standard polycarbonate cages and had free access to drinking water and standard laboratory mouse chow (Harlan 7027). The pregnant mice were housed in separate cages, provided with nesting material (Ancare, Bellmore, NY) and checked twice daily for new born pups. The date the pups were born (PND 0) was recorded and the dams were then randomly assigned to treatment groups [0 (vehicle control), 0.01, and 0.1 mg/ml TCE]. TCE (purity 99+% from Sigma, St. Louis, MO) was suspended in drinking water with an emulsifier, 1% of ethoxylated castor oil (Alkamuls EL-620™ (Rhone-Poulenc, Cranbury, NJ). Mice not receiving TCE received water with 1% ethoxylated castor oil as a vehicle control. There were a total of 10 dams per treatment group. Nine dams in the control group and 8 dams in the TCE-treated groups produced litters with sufficient numbers of male pups for our study. Dams received a freshly made solution of TCE in their drinking water every 2-3 days beginning at PND 0. At PND 21, the offspring were weaned and the male mice continued their exposure to vehicle or vehicle + TCE in the drinking water until PND 42. For each experimental study, one randomly selected male mouse from each litter was chosen thus the litter and not the individual mouse represented the experimental ‘n.’ As shown previously, water consumption in the dams and pups did not differ among treatment groups (Blossom et al., 2012). All studies were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
HPLC quantification of 3-nitrotyrosine, glutathione and cysteine redox status in cerebellum
At PND 42 mice were sacrificed. The cerebellum was dissected from whole brain tissue and flash frozen in liquid nitrogen. Samples were stored at -80°C until extraction for metabolite detection. The methodological details for metabolite detection by HPLC have been described previously (Melnyk et al., 1999). The analyses were performed using HPLC with a Shimadzu solvent delivery system (ESA model 580) and a reverse phase C18 column (3 um; 4.6 × 150 mm, MCM, Inc., Tokyo Japan) purchased from ESA, Inc. (Chemsford, MA). Cerebellar extracts were directly injected onto the column using a Beckman Autosampler (model 507E). All metabolites were quantified using a model 5200A Coulochem II and CoulArray electrochemical detection system (ESA, Inc., Chelmsford, MA) equipped with a dual analytical cell (model 5010), a 4 channel analytical cell (model 6210) and a guard cell (model 5020). The concentrations of 3-nitrotyrosine and metabolites in cerebellum were calculated from peak areas and standard calibration curves using HPLC software. Intracellular results are expressed as nmol/mg of protein using the BCA Protein Assay Kit (Pierce, Rockford, IL). The percentage of oxidized glutathione is expressed in absolute glutathione equivalents and was calculated as [2GSSG/(GSH + 2GSSG) × 100], where 2GSSG is the oxidized glutathione concentration and GSH is the glutathione concentration, both expressed in nmol/mg protein (Melnyk et al., 2011).
Behavioral tests
All behavioral tests were conducted and analyzed using EthoVision XT 8.0 video- tracking software (Noldus Information Technology, Inc, Leesburg, VA). This system digitizes the video signal obtained from a color LCD camera mounted on the ceiling to determine the spatial coordinates of the mouse’s location within the testing arena. From these coordinates, the distance traveled per unit time, the number of times a defined zone is entered and the amount of time within a zone can be calculated. At PND 40 behavioral tests were conducted to evaluate locomotor activity, exploratory, and social approach behaviors (open-field, novel object exploration, and novel mouse interaction tasks, respectively). One male pup per litter was chosen at random for each test. The behavioral tests were conducted in an isolated room to minimize the interference of noise. For locomotor activity, mice were placed in an unfamiliar 1m2 octagonal testing arena and the distance traveled (cm) over time was summed over 15-sec time periods for 10 mins. The novel object exploration test was performed in the same arena using a different subject chosen at random from the litter. For this test, a novel object-an inverted wire stainless steel pencil cup measuring 10 (H) × 10.5 cm (D at the rim)-was placed in the center of the testing arena. Using the EthoVision software, a virtual zone (14.5 × 14.5 cm) was defined with the cup at its center. During a 10 min test, the number of times the subject entered the zone and the subject’s latency (sec) to enter the zone was detected and recorded by the software. The third behavioral test, the novel mouse exploration test, the behavioral testing arena remained the same as the novel object exploration test, but in this case an unfamiliar mouse of the same sex, strain, and age was placed under the wire pencil cup just before the 10 min test session began. During testing the same endpoints were measured; number of times the zone was entered and latency to enter. These behavioral tests were loosely based on free exploration tests and reaction to novel object tests as described (Belzung et al., 2001;Crawley, 2007).
Global DNA methylation
The MethylFlash Methylated DNA Quantification Kit (Epigentek) was used according to manufacturer’s instruction to quantitate global DNA methylation status in cerebellum. NaOH-treated single-stranded DNA (50 ng) isolated from each sample (5 cerebella from 5 individual pups per litter in each treatment group) was used in the kit. Methodology for isolating DNA has been described previously (Gilbert et al., 2012)
Statistical Analysis
For metabolite and DNA methylation data, the statistical analysis was performed using GraphPad Prism 5.0 (LaJolla, CA). All data were expressed as mean ± standard deviation (SD). One way ANOVA with a Dunnett’s Multiple Comparison Test was used to determine significant differences between vehicle control vs. low- or high- dose TCE treatment groups for metabolite and methylation outcomes. Treatment-related differences for those outcomes were considered to be significant at p<0.05.
Behavioral outcomes were analyzed based on assumptions made of the underlying distribution of the outcome data. For time-measuring variables (latency to enter the zone, time in zone), a generalized linear model assuming a gamma distribution was utilized to account for the strictly non-negative (i.e., always greater than or equal to zero seconds) data. For count-measuring variables (number of times entering zone), Poisson regression was utilized to appropriately estimate differences in the observed counts of the behavior. Distance travelled over the duration of the behavior test was modeled using a general linear mixed model accounting for the repeated measurements made on each animal during the 10 minute test. Differences between exposure groups were subsequently estimated as a trajectory of activity. TCE-exposure related differences for the behavior outcomes were adjusted using Bonferroni’s correction and considered to be significant if p<0.017. Analysis of behavioral outcomes was completed using Strata v 12.1 (College Station, TX).
Results
Altered redox homeostasis in cerebellum of TCE exposed mice
Cerebellar tissue was harvested and processed for metabolite and oxidative stress biomarker analysis using 1 randomly selected mouse per litter in each treatment group (n=6). The data presented in Table 1 shows the concentrations of GSH, GSSG, the glutathione redox ratio, and the percentage of oxidized glutathione equivalents in cerebellar tissue of PND 42 male mice. Compared to vehicle-treated control mice, the GSH concentration in cerebellar tissue of mice exposed to 0.01 and 0.1 mg/ml TCE was significantly decreased in a dose-dependent manner by ~12% and ~20%, respectively. Similarly, the GSH/GSSG ratio was decreased significantly in the high-dose exposure group by ~27%. Although the levels of GSSG were not significantly altered with TCE exposure, the percentage of oxidized glutathione was significantly increased by ~25% in mice exposed to 0.1 mg/ml TCE. Also shown are concentrations of cysteine, cystine, and the cysteine/cystine redox ratio. There was a significant increase in cystine, the oxidized form of cysteine, with 0.1 mg/ml TCE exposure. Similarly, the cysteine/cystine redox ratio was significantly decreased in a dose- dependent manner (~31% and ~38%; 0.01 and 0.1 mg/ml TCE, respectively). Collectively these data reveal a more oxidized environment in the cerebellum of TCE-treated mice.
Table 1.
Comparison of transsulfuration metabolites in cerebellum between control and TCE-treated mice.
| Glutathione and Cysteine Redox Status in cerebellum (nmol/mg/protein) | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| GSH | 22.22 ± 1.53 | 19.58 ± 0.87* | 17.92 ± 1.21** |
| GSSG | 0.27 ± 0.05 | 0.27 ± 0.03 | 0.29 ± 0.04 |
| GSH/GSSG ratio | 86.36 ± 18.46 | 71.76 ± 7.11 | 63.14 ± 7.11* |
| Oxidized GSH (%) | 2.33 ± 0.50 | 2.73 ± 0.27 | 3.09 ± 0.39* |
| Cysteine | 3.20 ± 0.44 | 2.51 ± 0.54 | 2.39 ± 0.76 |
| Cystine | 12.52 ± 1.23 | 14.38 ± 1.87 | 14.64 ± 0.79* |
| Cysteine/Cystine ratio | 0.26 ± 0.03 | 0.18 ± 0.05* | 0.16 ± 0.04* |
Data is presented as mean ± SD.
p≤0.05 is statistically significant
p<0.001.
Increased cerebellar cysteinylglycine in TCE-exposed mice
Cysteinylglycine (CysGly), one of the major precursors of neuronal glutathione, was measured in cerebellum. As shown in Fig. 2, CysGly levels significantly increased with TCE treatment in a dose-dependent manner; ~27% and ~46% increase with 0.01 and 0.1 mg/ml TCE, respectively over that of controls.
Fig. 2.
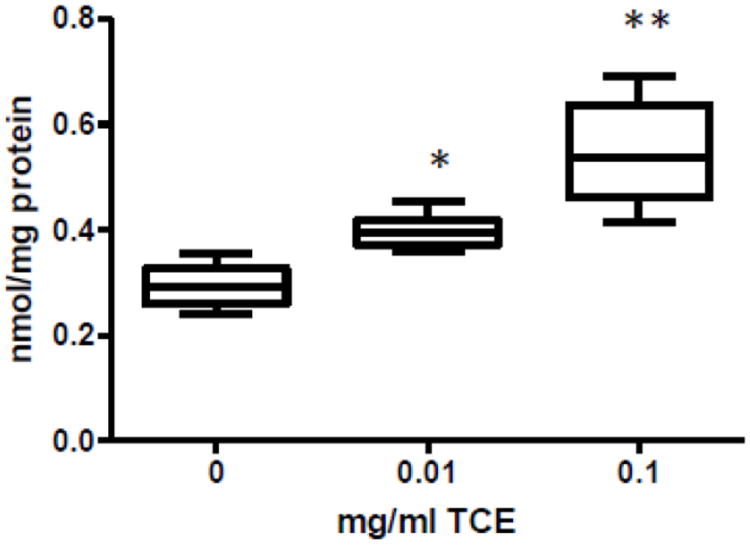
Cysteinylglycine levels were elevated dose-dependently in cerebellum of TCE-exposed mice. Metabolites in the transsulfuration pathway were measured in cerebellum by HPLC and electrochemical detection as described in the materials and methods section. Presented is mean ± SD nmol/mg protein of cerebellar Cysteinylglycine levels in control and TCE-exposed male offspring at PND42 (n=6 per treatment group). **Statistically different from the results obtained from controls (p<0.001); and *p<0.05.
TCE exposure enhances a biomarker of protein reactive nitrogen species in the cerebellum
Tyrosine nitration is a common protein modification that occurs in disease associated with oxidative stress (Andreazza et al., 2009). To determine whether or not impaired intracellular anti-oxidant capacity potential was associated with oxidative protein damage, 3-nitrotyrosine was measured in cerebellar tissue of control and TCE-treated mice. As shown in Fig. 3, cerebellar 3-nitrotyrosine concentrations were significantly elevated in mice exposed to 0.1 mg/ml TCE.
Fig. 3.
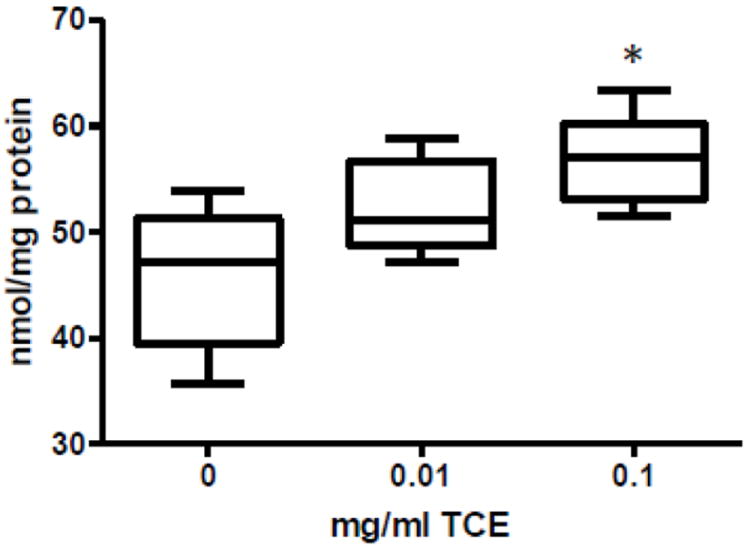
TCE increased a biomarker of oxidative stress in TCE-exposed mice. 3-Nitrotyrosine was measured in cerebellum by HPLC and electrochemical detection. Presented is mean ± SD nmol/mg protein of 3-Nitrotyrosine levels in control and TCE-exposed male offspring at PND42 (n=6 per treatment group). *Statistically different from the results obtained from control mice (p<0.05).
TCE decreases methionine levels in cerebellum
Plasma levels of transmethylation metabolites in TCE-exposed mice were previously reported (Blossom et al., 2012). TCE exposure was associated with a significant decrease in plasma methionine and S-adenosylmethionine (SAM), the major cellular methyl donor. These metabolic alterations along with an increase in S-adenosylhomocysteine (SAH) and homocysteine suggested that TCE promoted metabolic deficits in cellular methylation capacity. In this study, cerebellar tissue from control and TCE-exposed mice were evaluated for methionine, one of the crucial metabolites in the transmethylation pathway. As shown in Fig. 4, methionine levels significantly decreased by ~23% with the highest dose of TCE.
Fig. 4.
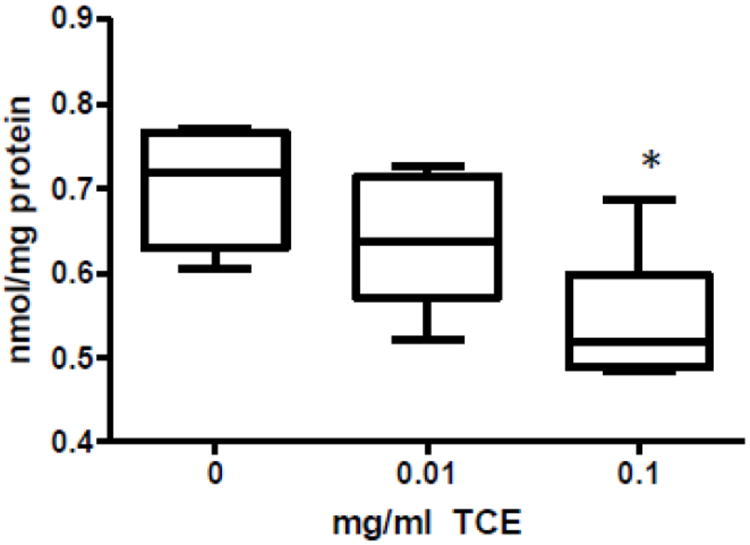
TCE decreased methionine levels in TCE-exposed mice. Methionine was measured in cerebellum by HPLC and electrochemical detection. Presented is mean ± SD nmol/mg protein of methionine in control and TCE-exposed male offspring at PND42 (n=6 per treatment group). *Statistically different from the results obtained from control mice (p<0.05).
Decreased Global DNA Methylation in cerebellum with TCE Exposure
Collectively the transsulfuration and transmethylation metabolite results in cerebellum indicated a reduction of methyl donors available for cellular methylation and would thereby predict a decrease in global DNA methylation. Cerebellar tissue was harvested and DNA isolated from 1 randomly selected mouse per litter in each treatment group (n=5). A quantification kit suitable for detecting global DNA methylation patterns showed that DNA isolated from cerebellum of mice exposed to 0.1 mg/ml TCE was significantly less methylated than DNA that was isolated from cerebellum of vehicle control mice (Fig. 5). This finding provided novel evidence that TCE altered DNA methylation in brain tissue.
Fig. 5.
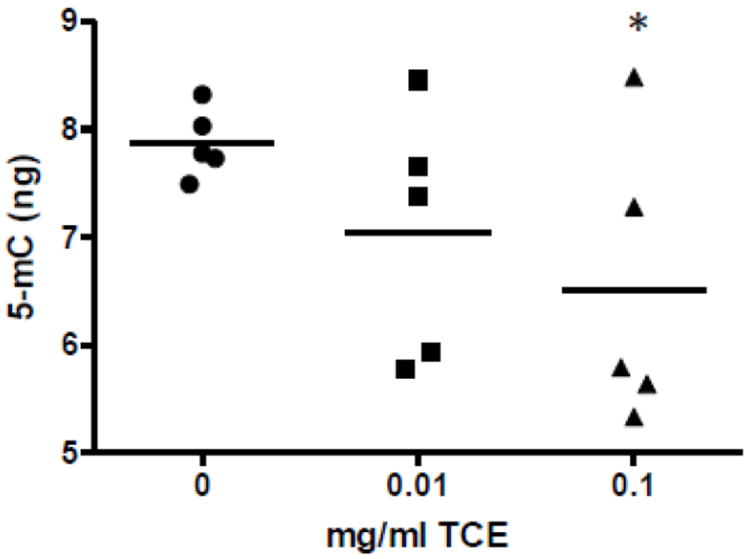
TCE inhibited global DNA methylation in cerebellum. Global DNA methylation was quantified using DNA from extracted from the tissues as described in the methods section (n=5 per treatment group). *Statistically different from the results obtained from controls.
TCE exposure alters behavior
Next we examined whether or not behavioral changes were associated with altered metabolites in the transsulfuration and transmethylation pathway. One randomly selected mouse in each litter was selected to undergo behavioral testing (n=9 for controls and n=8 for low and high TCE groups and PND40. Fig. 6 depicts the effects of postnatal/early life TCE exposure on spontaneous locomotor activity in an open-field task. The highest dose of TCE, unlike the lower dose, significantly increased locomotor activity over time as compared with control mice. In addition, there was a statistically significant increase in locomotor activity in with 0.1 mg/ml TCE exposure compared with 0.01 mg/ml TCE. In the novel object exploration test, the mice exposed to the highest concentration of TCE (0.1 mg/ml) were more likely to enter the zone with the novel object as compared to control mice (Fig 7A). Similar results were obtained in a second test when an unfamiliar mouse was introduced into the arena as the highest TCE dose led to more total zone entrances than control (Fig 7B). The TCE-treated mice also appeared to demonstrate a reduced latency to approach the novel object and the novel mouse (Fig 7C and 7D, respectively). However, this result did not reach statistical significance. These results showed that mice exposed to TCE exhibited increased locomotor activity and exploratory activity toward novel objects and conspecifics that may be linked with TCE-induced metabolic alterations in the cerebellum.
Fig. 6.
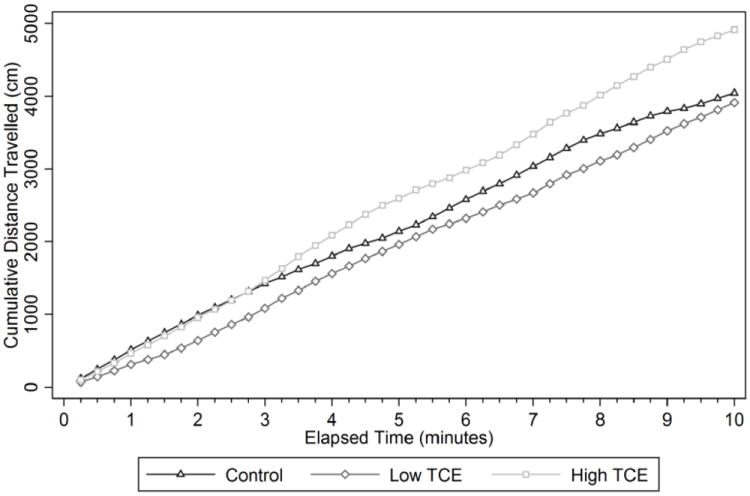
TCE promoted increased spontaneous locomotor activity in an open field. Details about the methodologies and statistics used on the behavioral tests are described in Materials and Methods. Presented in the graph is cumulative distance over time (n=9 for controls and n=8 for TCE-treated groups) measured by EthoVision behavioral tracking software. *Statistically significant comparing 0.1 mg/ml TCE group vs. controls (p=0.016) and 0.01 vs. 0.1 mg/ml TCE (p=0.007)
Fig. 7.
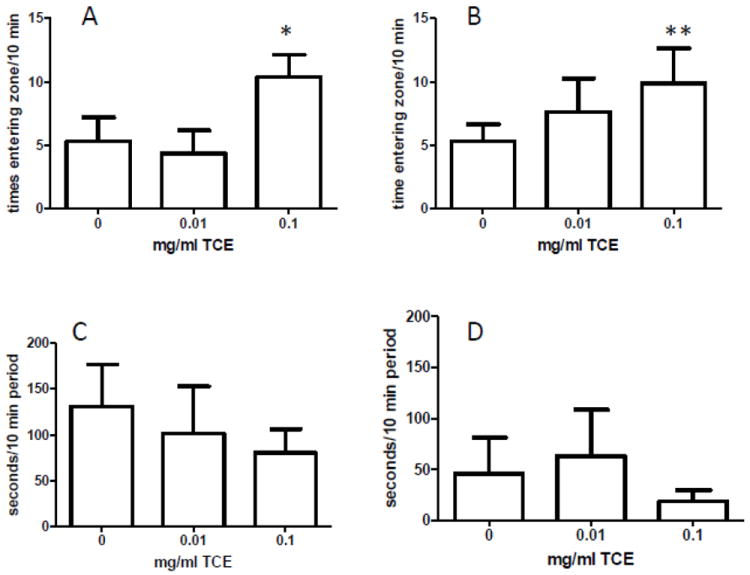
TCE exposure increased exploratory behavior. Methodological and statistical methods are described in the Methods section. The number of times entering the zone with the novel object (A) or novel mouse (B) were counted in a 10 min period using EthoVision behavioral tracking software. The latency to approach the zone with the novel object (C) or mouse (D) was measured in seconds within a 10 min period using EthoVision. Graphs represent the mean ± SEM. * (A) statistically significant comparing 0.1 mg/ml TCE vs. controls (p=0.006) and 0.01 mg/ml TCE (0.008). **(B) statistically significant comparing 0.1 mg/ml TCE vs. controls (p<0.001).
Discussion
Our previous studies have pointed to the postnatal period including lactation (PND 1-20) and early life (PND 21-42) as a crucial phase where the mouse hippocampus is a vulnerable target for the pro-oxidative effects of TCE. Neurotoxic effects of TCE were observed at concentrations commensurate with possible human exposure. Based on water consumption, the mice given water at 0.01 and 0.1 mg/ml were exposed to TCE at levels of approximately 2 and 28 mg/kg/day, respectively, as previously reported in detail (Blossom et al., 2012). Here, we showed that TCE caused significant neurotoxic effects related to oxidative stress and altered glutathione-related anti-oxidant metabolites in the cerebellum. Novel information regarding global DNA hypomethylation and abnormal behavior with low-level TCE exposure were also reported.
The brain is particularly susceptible to oxidative stress due to its high oxygen consumption and inherently low anti-oxidant defense (Fernandez-Fernandez et al., 2012). The developing brain reportedly has only a fraction of the anti-oxidant capacity of the adult brain and is therefore more vulnerable to neurotoxicity brought on by oxidative stress and pro-oxidant exposures. (Noble et al., 2005;Sato et al., 2010). Our results clearly show that TCE dose-dependently alters glutathione redox status in the brain by decreasing GSH and the GSH/GSSG ratio and increasing the percentage of GSSG. Levels of oxidized cystine were significantly elevated, and the cysteine/cystine redox pair was significantly decreased in cerebellar tissue of TCE-exposed mice indicating a generalized oxidized state. Interestingly, similar to what was observed in hippocampal tissues in our previous study, CysGly was significantly elevated in cerebella of TCE-exposed mice. Although the meaning of this result is not clear, an increase in CysGly could reflect a compensatory mechanism for observed TCE-mediated glutathione depletion. Alternatively, TCE may somehow inhibit the uptake or impact the exchange systems responsible for cystine or CysGly transport into cerebellar neurons as has been demonstrated with methylmercury exposure (Shanker et al., 2001a;Shanker et al., 2001b). Such a mechanism could lead to glutamate toxicity, glutathione depletion, and oxidative stress. Further study is needed to determine whether TCE or its metabolites modulate this process in astrocytic and neuronal cultures in order to determine the mechanism of TCE-induced neurotoxicity.
Consistent with our observation of altered redox status, we also observed increased 3-nitrotyrosine levels with TCE exposure. The reaction of superoxide anion and nitric oxide to produce peroxynitrite modifies protein tyrosine residues to generate 3-nitrotyrosine. Formation of reactive nitrogen species is presumed to play a major role in neuronal cell death and the presence of 3-nitrotyrosine is presumed to be a biomarker of this event (Nakazawa et al., 2000;Butterfield et al., 2008). This result provides additional functional evidence of a redox imbalance in the cerebellum resulting from TCE exposure and is consistent with our previous report of elevated plasma and hippocampal 3-nitrotyrosine levels. The significance of this finding is underscored by the presence of this biomarker in brain tissue from patients with neurologic disorders including Parkinson’s disease (Mythri et al., 2011), Alzheimer’s disease (Butterfield et al., 2006) and autism (Sajdel-Sulkowska et al., 2011).
Cerebellar tissue was examined for methionine, an important methylation pathway intermediate. TCE significantly and dose-dependently decreased methionine levels in cerebellum as compared with controls. This difference may be due to increased transit of homocysteine from methionine via SAM and SAH through the transsulfuration pathway which makes cysteine (and cystine). Cystine makes up the vast majority of total (cystine and cysteine) that was measured in the cerebellum in the current study. Thus, a decrease in cerebellar methionine may result from oxidative stress promoting the transsulfuration pathway (Melnyk et al., 2000;Vitvitsky et al., 2006). These results in cerebellum are in the same direction as those we reported previously for plasma in TCE-treated mice (Blossom et al., 2012).
The decreased methionine with TCE exposure could indicate a decrease in methyl donors available for cellular methylation. In particular, DNA methylation levels on specific genes in the brain can have wide-ranging and long-term impacts on health and behavior (Onishchenko et al., 2008;Roth et al., 2011). Consistent with this result, TCE exposure promoted global DNA hypomethylation in the current study. This result is similar to what we recently demonstrated in CD4+ T cells isolated from mice chronically exposed to TCE (Gilbert et al., 2012). Further studies to evaluate the DNA methylation patterns in specific genes altered by TCE exposure in brain regions as well as in specific cell populations (e.g., microglia) are currently underway.
TCE-induced cerebellar metabolic and DNA methylation alterations were associated with behavioral alterations. We observed increased locomotor activity in TCE-exposed mice in an open-field test. The mechanism by which the postnatal exposure to TCE induces hyperlocomotor activity is still not known. Studies to investigate behavior in CD-1 mice exposed to TCE in utero have shown no differences in locomotor activity (Jones et al., 1996). The discrepancy between this study and ours could be explained by the route of exposure (inhalation vs. drinking water), the exposure period (gestation only vs. postnatal period), duration of exposure (6 days vs. 40 days), and the different doses used (2,000-8,000 ppm vs. 2 and 28 mg/kg/day). Furthermore, mouse strain and procedural differences could account for these differences. Studies to address gestational exposure only on parameters described here are currently underway. In addition to locomotor activity we observed what appeared to be increased novelty/exploratory behavior with TCE exposure. In this regard, the presence of attention deficits and increased hyperactivity with gestational TCE exposure that has been reported in human studies points to the relevance of the present phenomenon in terms of human health concerns (Laslo-Baker et.al, 2004; Till et al., 2001).
The present results show that the postnatal period caused metabolic, methylation and behavioral changes in mice and point to this phase as a critical stage of life where mouse cerebellum is a vulnerable target for the neurotoxic effects of TCE. Further knowledge of the functional role of TCE in promoting these behaviors is warranted. Additional studies to study the impact of targeted dietary intervention to circumvent these metabolic and behavioral alterations could potentially lead to novel therapies with real clinical value.
Highlights.
We exposed male mice to low-level trichloroethylene from postnatal day 1 through 42.
This exposure altered redox potential and increased oxidative stress in cerebellum.
This exposure altered metabolites important in cellular methylation in cerebellum.
This exposure promoted DNA hypomethylation in cerebellum
This exposure enhanced locomotor activity and exploratory behavior.
Acknowledgments
This study was supported by Arkansas Biosciences Institute New Investigator Funds, and the National Institutes of Health (5 R21ES017311 02) to S.J.B. We would like to gratefully acknowledge the excellent technical assistance of Meagan Kreps, Oleksandra Pavliv, and Ashley Nelson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adgate JL, Eberly LE, Stroebel C, Pellizzari ED, Sexton K. Personal, indoor, and outdoor VOC exposures in a probability sample of children. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S4–S13. doi: 10.1038/sj.jea.7500353. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Goncalves CA, Young LT, Yatham LN. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–271. [PMC free article] [PubMed] [Google Scholar]
- Bale AS, Barone S, Jr, Scott CS, Cooper GS. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol Appl Pharmacol. 2011;255:113–126. doi: 10.1016/j.taap.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Ballok DA. Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res Rev. 2007;54:67–79. doi: 10.1016/j.brainresrev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballok DA, Earls AM, Krasnik C, Hoffman SA, Sakic B. Autoimmune-induced damage of the midbrain dopaminergic system in lupus-prone mice. J Neuroimmunol. 2004;152:83–97. doi: 10.1016/j.jneuroim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer PI, Luik CE, Abrell L, Campos S, Martinez ME, Saez AE. Correction to concentration of trichloroethylene in breast milk and household water from nogales, Arizona. Environ Sci Technol. 2012;46:11483. doi: 10.1021/es301380d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC. Trichloroethylene Alters Central and Peripheral Immune Function in Autoimmune-Prone MRL(+/+) Mice Following Continuous Developmental and Early Life Exposure. J Immunotoxicol. 2007;4:129–141. doi: 10.1080/15476910701337035. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Gilbert KM. Ability of Trichloroethylene Metabolite to Promote Immune Pathology is Strain-Specific. J Immunotoxicol. 2006;3:179–187. doi: 10.1080/15476910600978046. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Hennings LJ, Jernigan S, Melnyk S, James SJ. Developmental exposure to trichloroethylene promotes CD4(+) T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice. Toxicol Appl Pharmacol. 2008 doi: 10.1016/j.taap.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Melnyk S, Cooney CA, Gilbert KM, James SJ. Postnatal exposure to trichloroethylene alters glutathione redox homeostasis, methylation potential, and neurotrophin expression in the mouse hippocampus. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Sultana R. Identification of 3-nitrotyrosine-modified brain proteins by redox proteomics. Methods Enzymol. 2008;440:295–308. doi: 10.1016/S0076-6879(07)00819-1. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de WJ. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, Blom HJ, Jakobs C, Tavares d A I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–1296. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, Santos-Guzman J, Swendseid ME, Cogger EA, James SJ. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Caldwell JC, Keshava N, Scott CS. Key scientific issues in the health risk assessment of trichloroethylene. Environ Health Perspect. 2006;114:1445–1449. doi: 10.1289/ehp.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Nelson AR, Cooney CA, Reisfeld B, Blossom SJ. Epigenetic alterations may regulate temporary reversal of CD4+ T cell activation caused by trichloroethylene exposure. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ. Animal models for probing the developmental basis of disease and dysfunction paradigm. Basic Clin Pharmacol Toxicol. 2008;102:76–81. doi: 10.1111/j.1742-7843.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 2010;484:12–16. doi: 10.1016/j.neulet.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kunko PM, Robinson SE, Balster RL. Developmental consequences of intermittent and continuous prenatal exposure to 1,1,1-trichloroethane in mice. Pharmacol Biochem Behav. 1996;55:635–646. doi: 10.1016/s0091-3057(96)00288-2. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH. Effects on neurobehavioral performance of chronic exposure to chemically contaminated well water. Toxicol Ind Health. 1993;9:391–404. doi: 10.1177/074823379300900301. [DOI] [PubMed] [Google Scholar]
- Laslo-Baker D, Barrera M, Knittel-Keren D, Kozer E, Wolpin J, Khattak S, Hackman R, Rovet J, Koren G. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158:956–961. doi: 10.1001/archpedi.158.10.956. [DOI] [PubMed] [Google Scholar]
- Maffi SK, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S, Henderson GI. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J Neurosci Res. 2008;86:1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CW, Mirochnitchenko O, Claus CP, Noble-Haeusslein LJ, Ferriero DM. Overexpression of glutathione peroxidase protects immature murine neurons from oxidative stress. Dev Neurosci. 2005;27:169–175. doi: 10.1159/000085989. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, Bellando J, Pavliv O, Rose S, Seidel L, Gaylor DW, Jill JS. Metabolic Imbalance Associated with Methylation Dysregulation and Oxidative Damage in Children with Autism. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Court Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa H, Fukuyama N, Takizawa S, Tsuji C, Yoshitake M, Ishida H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic Res. 2000;33:771–784. doi: 10.1080/10715760000301291. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castren E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Arlien-Soborg P, Sabroe S. Clinical neurological findings among metal degreasers exposed to chlorinated solvents. Acta Neurol Scand. 1993;87:200–204. doi: 10.1111/j.1600-0404.1993.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Reif JS, Burch JB, Nuckols JR, Metzger L, Ellington D, Anger WK. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environ Res. 2003;93:248–258. doi: 10.1016/s0013-9351(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- Sakic B, Hanna SE, Millward JM. Behavioral heterogeneity in an animal model of neuropsychiatric lupus. Biol Psychiatry. 2005;57:679–687. doi: 10.1016/j.biopsych.2004.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J Clin Biochem Nutr. 2010;47:224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- Sexton K, Adgate JL, Church TR, Ashley DL, Needham LL, Ramachandran G, Fredrickson AL, Ryan AD. Children’s exposure to volatile organic compounds as determined by longitudinal measurements in blood. Environ Health Perspect. 2005;113:342–349. doi: 10.1289/ehp.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001a;914:159–165. doi: 10.1016/s0006-8993(01)02791-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocyte transport. J Neurosci Res. 2001b;66:998–1002. doi: 10.1002/jnr.10066. [DOI] [PubMed] [Google Scholar]
- 51.Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res. 2006;31:563–569. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 52.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- 53.Till C, Koren G, Rovet JF. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol Teratol. 2001;23:235–245. doi: 10.1016/s0892-0362(01)00141-6. [DOI] [PubMed] [Google Scholar]
- 54.Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 55.Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]


