Abstract
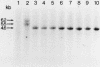
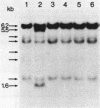
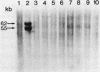
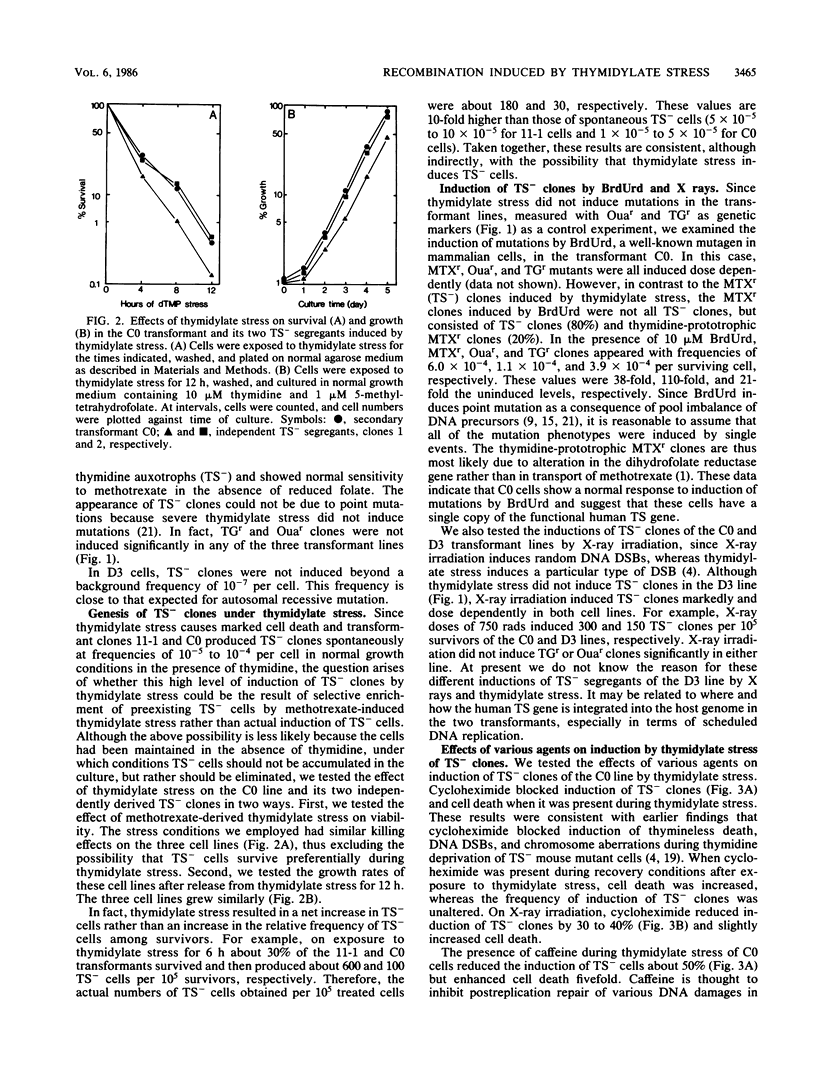
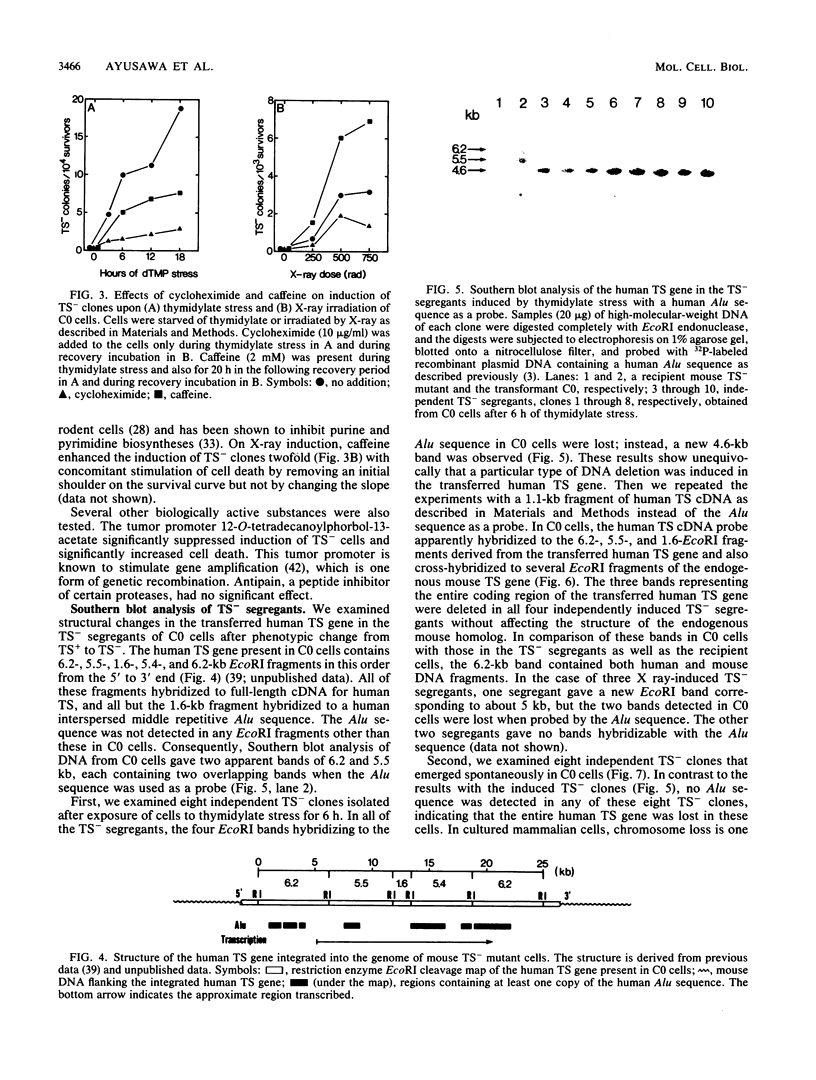
Studies were made on the genetic consequences of methotrexate-directed thymidylate stress, focusing attention on a human thymidylate synthase gene that was introduced as a heterologous genetic marker into mouse thymidylate synthase-negative mutant cells. Thymidylate stress induced thymidylate synthase-negative segregants with concomitant loss of human thymidylate synthase activity with frequencies 1 to 2 orders of magnitude higher than the uninduced spontaneous level in some but not all transformant lines. Induction of the segregants was suppressed almost completely by cycloheximide and partially by caffeine. Thymidylate stress did not, however, induce mutations, as determined by measuring resistance to ouabain or 6-thioguanine. Thymidylate synthase-negative segregants were also induced by other means such as bromodeoxyuridine treatment and X-ray irradiation. In each of the synthase-negative segregants induced by thymidylate stress, a DNA segment including almost the whole coding region of the transferred human thymidylate synthase gene was deleted in a very specific manner, as shown by Southern blot analysis with a human Alu sequence and a human thymidylate synthase cDNA as probes. In the segregants that emerged spontaneously at low frequency, the entire transferred genetic marker was lost. In the segregants induced by X-ray irradiation, structural alterations of the genetic marker were random. These results show that thymidylate stress is a physiological factor that provokes the instability of this exogenously incorporated DNA in some specific manner and produces nonrandom genetic recombination in mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Koyama H., Iwata K., Seno T. Selection of mammalian thymidine auxotrophic cell mutants defective in thymidylate synthase by their reduced sensitivity to methotrexate. Somatic Cell Genet. 1981 Sep;7(5):523–534. doi: 10.1007/BF01549656. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Koyama H., Seno T. Resistance to methotrexate in thymidylate synthetase-deficient mutants of cultured mouse mammary tumor FM3A cells. Cancer Res. 1981 Apr;41(4):1497–1501. [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Accumulation of DNA strand breaks during thymineless death in thymidylate synthase-negative mutants of mouse FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12448–12454. [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Unusual aspects of human thymidylate synthase in mouse cells introduced by DNA-mediated gene transfer. J Biol Chem. 1983 Jan 10;258(1):48–53. [PubMed] [Google Scholar]
- Ayusawa D., Takeishi K., Kaneda S., Shimizu K., Koyama H., Seno T. Isolation of functional cDNA clones for human thymidylate synthase. J Biol Chem. 1984 Dec 10;259(23):14361–14364. [PubMed] [Google Scholar]
- Barbi G., Steinbach P., Vogel W. Nonrandom distribution of methotrexate-induced aberrations on human chromosomes. Detection of further folic acid sensitive fragile sites. Hum Genet. 1984;68(4):290–294. doi: 10.1007/BF00292586. [DOI] [PubMed] [Google Scholar]
- Barclay B. J., Kunz B. A., Little J. G., Haynes R. H. Genetic and biochemical consequences of thymidylate stress. Can J Biochem. 1982 Mar;60(3):172–184. doi: 10.1139/o82-023. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Sharkey N. A. Mutagenicity of thymidine to cultured Chinese hamster cells. Nature. 1978 Aug 10;274(5671):607–608. doi: 10.1038/274607a0. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Smigocki A. C., Camerini-Otero R. D. Effect of insertions, deletions, and double-strand breaks on homologous recombination in mouse L cells. Mol Cell Biol. 1985 Apr;5(4):684–691. doi: 10.1128/mcb.5.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S., Mosevitsky M., Vyacheslavov L. Complete mutagenesis in a bacterial population induced by thymine starvation on solid media. Nature. 1970 Feb 21;225(5234):764–766. doi: 10.1038/225764a0. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Segregation of recessive phenotypes in somatic cell hybrids: role of mitotic recombination, gene inactivation, and chromosome nondisjunction. Mol Cell Biol. 1981 Apr;1(4):336–346. doi: 10.1128/mcb.1.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Das K. C., Herbert V. Vitamin B12--folate interrelations. Clin Haematol. 1976 Oct;5(3):697–745. [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R. Bromodeoxyuridine mutagenesis in mammalian cells is stimulated by thymidine and suppressed by deoxycytidine. Nature. 1978 Dec 14;276(5689):722–723. doi: 10.1038/276722a0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Ayusawa D., Glover T. W., Seno T. Expression of fragile site on the human X chromosome in somatic cell hybrids between human fragile X cells and thymidylate synthase-negative mouse mutant cells. Jpn J Cancer Res. 1985 Oct;76(10):977–983. [PubMed] [Google Scholar]
- Hori T., Ayusawa D., Seno T. Thymidylate stress and sister chromatid exchanges. Basic Life Sci. 1984;29(Pt A):149–159. doi: 10.1007/978-1-4684-4889-4_12. [DOI] [PubMed] [Google Scholar]
- Hori T., Ayusawa D., Shimizu K., Koyama H., Seno T. Chromosome breakage induced by thymidylate stress in thymidylate synthase-negative mutants of mouse FM3A cells. Cancer Res. 1984 Feb;44(2):703–709. [PubMed] [Google Scholar]
- Hori T., Lark K. G. Effect of puromycin on DNA replication in Chinese hamster cells. J Mol Biol. 1973 Jul 5;77(3):391–404. doi: 10.1016/0022-2836(73)90446-4. [DOI] [PubMed] [Google Scholar]
- Koyama H., Ayusawa D., Tsuji M., Seno T. Thymidineless death and mutation induction in cultured mouse FM3A cell mutants deficient in thymidylate synthase. Mutat Res. 1982 Dec;105(6):433–438. doi: 10.1016/0165-7992(82)90190-7. [DOI] [PubMed] [Google Scholar]
- Koyama H., Kodama H. Adenine phosphoribosyltransferase deficiency in cultured mouse mammary tumor FM3A cells resistant to 4-carbamoylimidazolium 5-olate. Cancer Res. 1982 Oct;42(10):4210–4214. [PubMed] [Google Scholar]
- Kucherlapati R. S., Eves E. M., Song K. Y., Morse B. S., Smithies O. Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3153–3157. doi: 10.1073/pnas.81.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R. S., Spencer J., Moore P. D. Homologous recombination catalyzed by human cell extracts. Mol Cell Biol. 1985 Apr;5(4):714–720. doi: 10.1128/mcb.5.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Barclay B. J., Game J. C., Little J. G., Haynes R. H. Induction of mitotic recombination in yeast by starvation for thymine nucleotides. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6057–6061. doi: 10.1073/pnas.77.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Haynes R. H. Phenomenology and genetic control of mitotic recombination in yeast. Annu Rev Genet. 1981;15:57–89. doi: 10.1146/annurev.ge.15.120181.000421. [DOI] [PubMed] [Google Scholar]
- LeBeau M. M., Rowley J. D. Heritable fragile sites in cancer. Nature. 1984 Apr 12;308(5960):607–608. doi: 10.1038/308607a0. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsby P. C., Kato H., Waldren C. A., Patterson D. Effects of caffeine on pyrimidine biosynthesis and 5-phosphoribosyl 1-pyrophosphate metabolism in Chinese hamster cells. J Biol Chem. 1982 Oct 10;257(19):11364–11367. [PubMed] [Google Scholar]
- Small J., Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. Early events and mechanisms in the induction of bacterial SOS functions: analysis of the phage repressor inactivation process in vivo. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1657–1661. doi: 10.1073/pnas.75.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Rubnitz J. Recombination events after transient infection and stable integration of DNA into mouse cells. Mol Cell Biol. 1985 Apr;5(4):659–666. doi: 10.1128/mcb.5.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G. R. The fragile X chromosome. Int Rev Cytol. 1983;81:107–143. doi: 10.1016/s0074-7696(08)62336-0. [DOI] [PubMed] [Google Scholar]
- Szabo P., Purrello M., Rocchi M., Archidiacono N., Alhadeff B., Filippi G., Toniolo D., Martini G., Luzzatto L., Siniscalco M. Cytological mapping of the human glucose-6-phosphate dehydrogenase gene distal to the fragile-X site suggests a high rate of meiotic recombination across this site. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7855–7859. doi: 10.1073/pnas.81.24.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi K., Ayusawa D., Kaneda S., Shimizu K., Seno T. Molecular cloning of genomic DNA segments partially coding for human thymidylate synthase from the mouse cell transformant. J Biochem. 1984 May;95(5):1477–1483. doi: 10.1093/oxfordjournals.jbchem.a134755. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Kaneda S., Ayusawa D., Shimizu K., Gotoh O., Seno T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucleic Acids Res. 1985 Mar 25;13(6):2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. Phorbol ester dramatically increases incidence of methotrexate-resistant mouse cells: possible mechanisms and relevance to tumor promotion. Cell. 1981 Aug;25(2):561–572. doi: 10.1016/0092-8674(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Vock Hall L. Genetic demonstration of mitotic recombination in cultured Chinese hamster cell hybrids. Cell. 1984 Mar;36(3):697–707. doi: 10.1016/0092-8674(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. Fragile sites and predisposition to leukemia and lymphoma. Cancer Genet Cytogenet. 1984 May;12(1):85–88. doi: 10.1016/0165-4608(84)90011-6. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L. Constitutive fragile sites and cancer. Science. 1984 Dec 7;226(4679):1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Wahl G. M. Homologous recombination in mammalian cells mediates formation of a functional gene from two overlapping gene fragments. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2002–2006. doi: 10.1073/pnas.80.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]