Abstract
Human enamel development of the permanent teeth takes place during childhood and stresses encountered during this period can have lasting effects on the appearance and structural integrity of the enamel. One of the most common examples of this is the development of dental fluorosis after childhood exposure to excess fluoride, an elemental agent used to increase enamel hardness and prevent dental caries. Currently the molecular mechanism responsible for dental fluorosis remains unknown; however, recent work suggests dental fluorosis may be the result of activated stress response pathways in ameloblasts during the development of permanent teeth. Using fluorosis as an example, the role of stress response pathways during enamel maturation is discussed.
Keywords: enamel, amelogenesis, fluorosis, ameloblast, endoplasmic reticulum stress, unfolded protein response
1. Introduction
Dental enamel is produced by specialized epithelial-derived cells known as ameloblasts, which are a layer of tall columnar polarized cells, and is the product of ameloblast progression through the various stages of their life cycle. In order to produce enamel, ameloblasts must synthesize and secrete large amounts of different proteins. This makes enamel especially susceptible to perturbations in ameloblast protein synthesis, both from internal and external sources. Perturbations in protein synthesis and secretion from trauma or illness can be observed visually in the teeth of affected individuals as unusually prominent Striae of Retzius, which are visible manifestations of the ameloblast growth cycle analogous to the growth rings of a tree [1]. Both fever lines on the permanent teeth, caused by high fever during childhood, and the neonatal line on the deciduous teeth, caused by the trauma associated with being born, are two very common examples of bodily stressors causing interruptions in enamel development [1].
Fluoride is used to increase enamel hardness and prevent the formation of caries by incorporation into the hydroxyapatite (HA) crystals that make up the bulk of dental enamel. Fluoride is not produced by the body and so must be provided as a supplement, either from community water programs or in the form of gels, coatings, or rinses supplied by dental professionals. The dose required for beneficial effects of fluoride is quite low with community water guidelines stating that 0.7 parts per million (ppm) is sufficient [2]. At doses higher than 0.7 ppm, the risk of the development of dental or skeletal fluorosis increases and the detriments begin to outweigh the benefits. Dental fluorosis is the result of excess fluoride during enamel development, which for the permanent teeth occurs in childhood between the ages of 2 to 8 years old, and affects roughly one quarter of the American population [3]. Once enamel has fully matured, the risk of developing dental fluorosis is eliminated. Dental fluorosis produces areas of weakened enamel that appear as opaque white spots or lines, with severity proportional to the affected surface area. In the most severe cases, enamel can become discolored and brittle, leading to chipping. These spots or lines are areas of weakened enamel that have higher than normal protein content [4,5,6] and it is the increased protein content in these areas that results in the observed enamel weakness of fluorosed enamel. Current work suggests the involvement of the protein synthesis machinery in the development of dental fluorosis [7,8,9] and this review uses fluorosis to illustrate the importance of the stress response pathways on protein production during amelogenesis.
2. Enamel Development
In order to better understand the role of protein synthesis during amelogenesis, a brief review of ameloblast function and enamel development follows. Amelogenesis, or enamel development, occurs over five stages but two stages are arguably the most important: the secretory stage and the maturation stage (reviewed in [10]). In the secretory stage, enamel proteins are secreted, which are required to support and organize the nascent HA crystals as the enamel layer grows to its full thickness [10]. In the maturation stage, the matrix proteins are degraded by a stage-specific protease and the fragments resorbed, allowing for further HA precipitation and crystal growth as the enamel matures into its final hardened form [10].
2.1. The Secretory Stage
In the secretory stage, the enamel reaches full thickness but is composed of a soft, cheese-like substance easily separated from the dentin by mechanical techniques. This is due to the high levels of protein still found in the enamel matrix (reviewed in [11]). Due to the high amounts of proteins produced and secreted, secretory ameloblasts are tall with well developed endoplasmic reticulum (ER) and golgi bodies [12]. Ameloblasts also develop a specialized conical structure on the apical surface known as a Tomes' process, an easily recognizable landmark from which the enamel rod will grow and various proteins required for enamel formation are secreted. These proteins include amelogenin (AMELX), ameloblastin (AMBN), enamelin (ENAM), and the secretory-staged protease enamelysin or matrix metalloproteinase 20 (MMP20). AMELX, AMBN, and ENAM are scaffold proteins required to support rod formation and HA crystallization. These proteins are broken down by MMP20 as they are secreted into the enamel matrix, meaning the full-length proteins are located closest to the Tomes’ process and the smallest fragments closer to the dentino-enamel junction, and this breakdown is necessary for enamel development [13,14,15] as well as HA crystal maturation [16,17]. Once the enamel has reached full thickness, ameloblasts transition to the maturation stage [11].
2.2. The Maturation Stage
By the beginning of the maturation stage, the enamel has reached full thickness [11] and, in rodents, has developed the characteristic decussating enamel rod pattern; however, the enamel has not yet hardened [11]. During the maturation stage, the remaining proteins in the enamel matrix will be degraded by the stage-specific serine protease kallikrein-related peptidase-4 (KLK4), also known as enamel matrix serine protease 1 (EMSP1), and the protein fragments generated are absorbed and further degraded by the ameloblasts [18,19]. This decreases the amount of enamel matrix protein from approximately 30% by weight to roughly 1% by the end of maturation stage. While the enamel matrix is undergoing proteolysis, HA precipitates increasing the density of the enamel rods and filling the spaces created during matrix degradation [11]. Rat ameloblasts undergo cyclic oscillations during this stage, with one cycle taking approximately 8 hours [20]. For roughly 50% of a cycle ameloblasts exist in their ruffle-ended (RE) form, and spend the next 25% in their smooth-ended (SE) form; the remaining 25% of a cycle is spent transitioning between these two forms [20] with the transition from RE to SE taking more time than the SE to RE transition [21]. Rat incisor ameloblasts will undergo the RE-SE cycle approximately 3 times per day and at least 45 times by the end of the maturation stage [20].
RE ameloblasts are readily identified by large invaginations on their apical surface, which are believed to simultaneously secrete the enamel protease KLK4, absorb degraded enamel matrix for intracellular breakdown [22,23,24,25], and secrete large amounts of calcium ions into the enamel matrix to facilitate HA precipitation [26]. Due to the efflux of calcium and phosphate ions, which causes an increase in HA precipitation and subsequently the release of hydrogen ions, the pH of the enamel matrix associated with RE ameloblasts drops [27]. Precipitation of HA releases 7–14 moles of hydrogen ions per mole of HA, which acidifies the enamel matrix, and both RE and SE ameloblasts secrete bicarbonate to neutralize the enamel matrix. SE ameloblasts also provide a resting phase for the ameloblast, allowing time to process the degraded enamel matrix and reset for the next RE phase [11]. These modulations occur in waves around the developing tooth though the signal to switch phases remains unknown [11].
2.3. Enamel Defects
To illustrate the importance of protein secretion during the development of healthy enamel, defects associated with secretion or production of any of these important proteins results in malformed enamel in both mice and humans (reviewed in [28]). For example, AMELX-deficient mice display disorganized (lacks the characteristic decussating rod pattern), thin enamel [29]. In humans, there are currently 16 different mutations associated with Amelx which result in X-linked amelogenesis imperfecta (AI) [28,30]. AI is a term for a collection of non-syndromic hereditary enamel defects which vary in severity and appearance. Ambn−/− mice have a severe phenotype presenting severely malformed, very thin enamel [31]. No AI-causing mutation in the AMBN gene has been identified in humans to date [32]. Enam−/− mice lack true enamel [33] and in humans mutations result in autosomal dominant AI [28], in which only one allele has to be affected in order for AI to develop. Humans with autosomal dominant AI have extremely discolored, small teeth with very thin, weak enamel [34]. Afflicted individuals may also display prominent banding of malformed enamel [35] or caries-like lesions [36].
Mutations affecting the enamel matrix proteases, MMP20 and KLK4, can also produce the AI enamel phenotype in an autosomal recessive manner [32,37,38]. A complete loss of MMP20 expression results in thin, weak, disorganized enamel in mice [13]. Aberrant MMP20 in humans, via mutations producing either a premature stop codon, disrupted active site, or inactive splice variant, also show enamel defects [39,40,41,42]. Affected individuals present with weakened, discolored enamel susceptible to chipping though, unlike the murine phenotype, enamel is often full thickness [39,40]. To date only one AI-causing KLK4 mutation has been identified in humans, producing a truncated protein, lacking an important component of the active site, which produces weak immature enamel of normal thickness [43]. This is due to a lack of mineralization during the maturation phase and, subsequently, an increased enamel protein content [43]. In mice, Klk4-knockout produces enamel of normal thickness showing the decussating rod pattern; however, this enamel is quite brittle, breaking close to the dentino-enamel junction (DEJ) [38]. Additionally, the individual HA crystallites, which normally fuse to become a single enamel rod, fail to fuse and can be individually isolated from neighboring crystallites, indicating KLK4 is required for proper rod formation and enamel maturation [38]. AI resulting from mutation of these two proteases show that both enzymes are required for proper amelogenesis in humans as well as rodents [13,39,40,41,42,43].
In addition to the canonical enamel proteins, defects in members of other cellular processes can also produce a dysplastic enamel phenotype. AI has also been linked to mutations in two members of the family with sequence similarity (FAM) gene family, FAM83H and FAM20A, as well as WD repeat containing domain 72 (WDR72) [44,45,46]. Despite the linkage of these genes with the AI phenotype, their function in enamel development remains unclear [44,45,46]. Furthermore, mutations in the cystic fibrosis transporter gene (Cftr), in addition to causing Cystic Fibrosis, produces thin malformed enamel in both mice and humans [47,48,49], underscoring the importance of ion management during enamel maturation. The CFTR-related enamel phenotype does not fall under the AI "umbrella" and therefore is not included in a list of known AI-causing genes despite its influence on enamel development [50]. Though several AI-causing genes have been identified, there are still a number of cases in which no mutation or genetic defect has been identified indicating that there is still much work to done in this field. The variety, and in some cases severity, of enamel defects associated with proper protein production underscores its importance in ameloblasts during enamel development.
3. Fluoride and Ameloblasts
Fluoride is a widely used supplement that increases enamel hardness and reduces the incidence of dental caries [51]. In contrast to this protective effect, excess fluoride during childhood (ages 2–8; when the enamel of the permanent teeth is developing) has the opposite outcome, weakening enamel and resulting in areas of increased opacity (reviewed in [52]). In the most severe cases of dental fluorosis, the enamel can become discolored and chip resulting in jagged, uneven tooth surfaces [52]. In addition to being a cosmetic issue, fluorosed enamel is weaker [5,6], more porous [53,54], and contains higher levels of protein than healthy enamel [4,54] so dental fluorosis also has physical effects.
3.1. Fluoride does not Affect the Activity of MMP20 or KLK4
Fluorosed enamel contains more protein than normal enamel leading researchers to speculate that decreased enamel protease function in the presence of fluoride was to blame [55]. However, in the case of both recombinant MMP20 and KLK4 as well as pooled enamel organ protein extracts (containing both MMP20 and KLK4), fluoride has no effect on the activity of these two enzymes, ruling out protease inhibition as a potential mechanism [56,57]. So, while fluorosed enamel contains increased protein levels, it is not the direct result of decreased protease activity.
3.2. Fluoride Causes Oxidative Stress
Fluoride increases oxidative stress by reducing the activity of antioxidant enzymes [58,59,60] resulting in the accumulation of reactive oxygen species (ROS), which negatively affect a variety of structures and processes in the cell. Increases in ROS levels can activate the general stress response protein heat shock protein 90 (HSP 90) which arrests protein synthesis by activating the eukaryotic initiation factor 2 (eIF2) kinase heme regulated inhibitor (HRI) [61,62,63]. Treatment with antioxidant compounds such as selenium, lycopene, or α-tocopherol (vitamin E) can ameliorate the increase in ROS levels caused by excess fluoride [60,64,65,66,67,68] but whether these compounds are able to restore enamel hardness remains to be seen.
Fluoride also induces expression of genes associated with oxidative stress [69]. In osteoblasts, fluoride causes an increase in the nuclear factor erythroid 2-related factor 2 (NRF2) [8] but it is unknown whether this occurs in ameloblasts. NRF2 is a cytoprotective transcription factor involved in the response to increased ROS levels (reviewed in [70]) as well as in hematopoietic stem cell maintenance [71]. NRF2 is also required for iron deposition on murine incisors, via regulation of ferritin levels, and deficiencies in NRF2 caused premature ameloblast atrophy during late maturation stage [72]. Though there was no difference in enamel hardness between WT and Nrf2−/− mice, the enamel from null mice was more sensitive to acid, leaching greater amounts of calcium than control teeth [72]. This could result from the absence of the iron-based enamel top-layer [72], which gives murine incisors their characteristic orange coloring [73]. Fluorosed murine enamel also lacks the iron-based coating [74], suggesting fluoride may inhibit NRF2-mediated iron deposition in exposed animals.
As previously mentioned, oxidative stress can decrease protein synthesis through the HSP90-eIF2 mechanism but high levels of ROS can also affect proteins in other ways. One way oxidative stress can also affect protein function is through a direct or indirect post-translational modification of amino acid side chains known as carbonylation. Carbonylation can both positively and negatively affect protein function (reviewed in [75]), depending on the protein, or can increase proteosomal degradation. NRF2 has been shown to be upregulated in cells containing high levels of carbonylated proteins, via increased dissociation from its inhibitor KEAP1 [76]. Decreased protein synthesis via HSP90 and increased protein degradation through protein carbonylation could result in decreased levels of both MMP20 and KLK4, which would lead to increased levels of enamel matrix proteins and could be a potential mechanism for fluorosis.
3.3. Fluoride Induces ER Stress and eIF2 Phosphorylation
The unfolded protein response (UPR) has evolved to sense the accumulation of misfolded proteins and halt protein synthesis until the misfolded proteins are refolded or degraded (reviewed in Hetz [77]). UPR is a three-pronged pathway which will result in the increase of chaperone and ER-assisted protein degradation (ERAD) proteins while preventing global protein translation, via phosphorylation of eIF2α and mRNA degradation [77]. Sensors of the UPR include activating transcription factor 6 (ATF6), inositol-requiring protein 1α (IRE1α), and protein kinase RNA-activated ER kinase (PERK) [77]. All three sensors are transmembrane proteins spanning the ER membrane with the sensor domains projecting into the ER lumen and the signaling domains projecting into the cytosol [77].
Fluoride has been shown to activate PERK in osteoblasts [8] and causes ER stress in ameloblasts, which decreases protein secretion [9,78]. Additionally, PERK is capable of activating NRF2 [79] and the two play an important role during redox stress (reviewed in Cullinan and Diehl [80]). Given that fluoride decreases synthesis and secretion of the enamel proteases by decreasing mRNA translation [7,81], the involvement of the UPR in dental fluorosis seems likely.
Treatment of ameloblast lineage cells with fluoride induces eIF2α phosphorylation in vitro, which is increased under mildly acidic conditions, but the kinase responsible is currently unknown [7,82]. In addition to PERK, there are three other known kinases that act on eIF2α, the previously mentioned HRI, protein kinase RNA-activated (PKR), and glucose control nonrepressed 2 (GCN2) (reviewed in [83]). Each kinase responds to a particular set of stimuli: HRI phosphorylates eIF2α in response to heme depletion, arsenite exposure, oxidative stress, and heat shock; PKR is activated in response to increased interferon levels from viral infection; and GCN2 responds to nutrient limitation such as a depleted pool of amino acids [83]. In addition to PERK, HRI is another likely candidate for playing a role in response to fluoride. HRI is activated by oxidative stress induced by the environmental toxin arsenite [61,84], possibly via an intermediary [85], as well as oxidative stress induced by other compounds [62,63]. Interestingly, treatment with both fluoride and arsenite simultaneously does not induce a synergistic effect which implies that both fluoride and arsenite activate the same biochemical pathways [86]. These data point to HRI as being the activated eIF2α kinase; however, it is also possible that both PERK and HRI phosphorylate eIF2α in concert during exposure to fluoride, as both kinases act on the same pool of eIF2α and both have been shown to play a role during oxidative stress.
Phosphorylation of eIF2α does not halt all protein synthesis, mRNA species possessing alternate upstream open reading frames (uORFs) may still be translated [87]. Phosphorylation results in depleted pools of eIF2-Met tRNAi which can cause the regular ORF to be skipped in favor of the uORF in certain mRNA species, such as activating transcription factor 4 (ATF4) [88]. Translation of ATF4 is increased when eIF2 is phosphorylated [88], which in turn increases transcription of downstream UPR genes, aiding the cell in reestablishing homeostasis. ATF4 and NRF2 have been shown to interact and play a role in the activation of ROS resolving proteins [80,89]; however, to date fluoride has not been shown to increase ATF4 levels.
4. The Acid Hypothesis
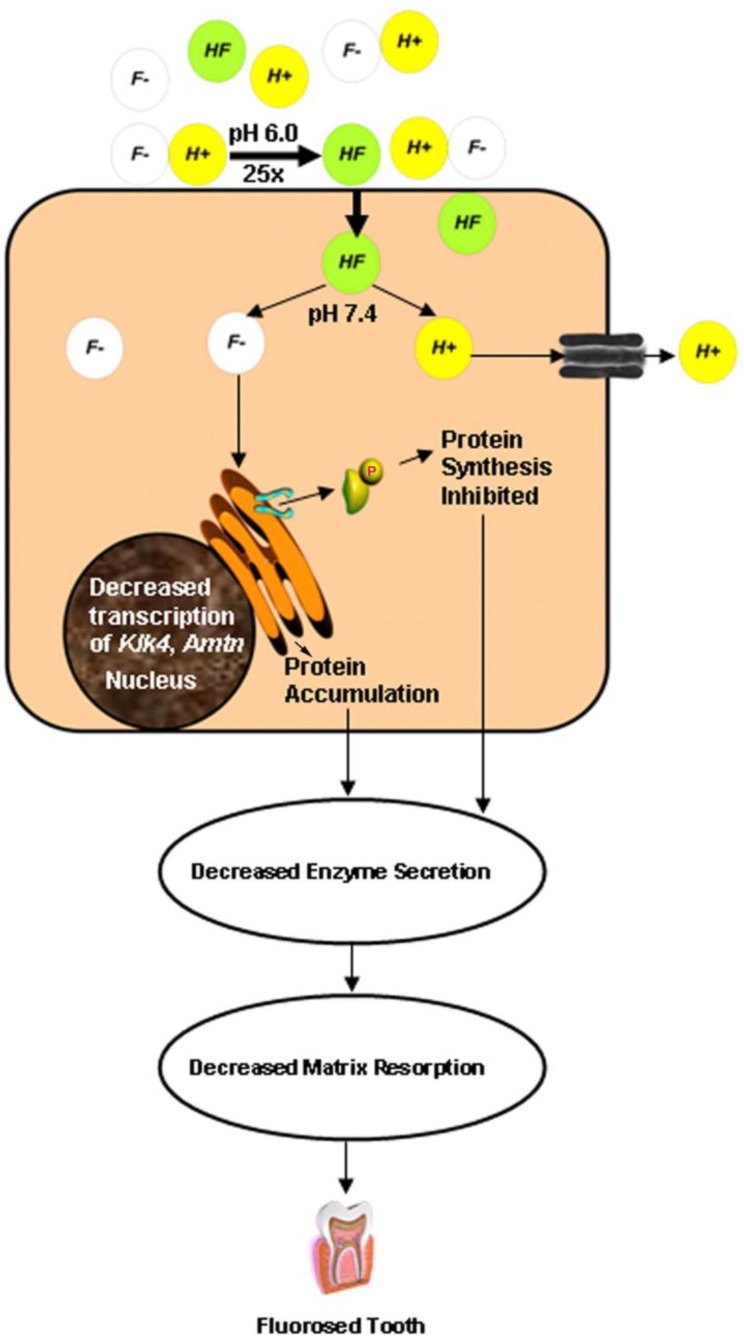
As mentioned above, normal enamel development includes oscillations in pH during the maturation stage, cycling between neutral (pH 7.4) and mildly acidic (pH < 6.0) [90], which results from proton release during HA precipitation [91,92]. According to the Henderson-Hasselbalch equation, approximately 25 times more hydrogen fluoride (HF) is present at pH 6.0 compared to pH 7.4. Fluoride ions are unable to enter the cell whereas HF is readily able to cross the plasma membrane meaning that, under acidic conditions, there is more HF available to cross the cell membrane [93]. Additionally, studies show that a pH gradient is required in order to drive cellular fluoride absorption [94]. This makes maturation stage ameloblasts uniquely sensitive to fluoride toxicity, which has been observed experimentally [82,95,96,97,98]. Greater levels of dental fluorosis were observed in rats under acidic conditions when compared to basic or neutral conditions [99,100]. Additionally, induction of severe acidosis in rats produces an enamel phenotype similar to dental fluorosis [101]. In this study, Whitford and Agmar-Mansson observed elevated enamel fluoride levels in acidotic rats compared to control [101], which may result from increased fluoride mobilization from mineralized tissue under acidic conditions [102]. Taken together, these data illustrate the importance of pH in influencing the effects of fluoride.
In cultured ameloblast-derived cells treated with fluoride, proteins associated with cellular stress (JNK and c-jun) were more readily phosphorylated under acidic conditions compared to neutral conditions [82]. These cells also showed signs of increased ER stress, including phosphorylation of eIF2α, under these same conditions which contributed to a general decrease in protein secretion in these cells [82]. Decreased protein production in maturation stage ameloblasts could lead to decreased production and secretion of KLK4, which would result in decreased enamel matrix breakdown and lead to increased protein content characteristic of fluorosed enamel. In fact, decreased protein production has already been shown in cultured ameloblasts exposed to fluoride [7,9,81] as well as in enamel isolated from fluoride-treated rats [98]. Maturation-staged ameloblasts also undergo fewer RE-SE modulation cycles in the presence of fluoride [97], fewer cycles could correspond to less time for matrix resorption further contributing to the increased protein levels observed in fluorosed enamel. This Acid Hypothesis states that the mildly acidic enamel matrix drives HF down a concentration gradient into the cytosol of maturation-staged ameloblasts where it dissociates. This both strengthens the HF concentration gradient and releases highly reactive fluorine ions into the cytoplasm leading to phosphorylation of eIF2α thereby decreasing overall protein production, including the secretion of the maturation stage protease KLK4. Consequently, there is less break down of the enamel matrix and this leads to increased levels of matrix proteins (Figure 1). DenBesten showed increased protein levels in fluorosed maturation-staged enamel and postulated it was the result of decreased protein removal [103]; these observations are consistent with our hypothesis. Current work by the Bartlett lab is focussed on validating this hypothesis.
Figure 1.
Schematic showing a postulated mechanism for maturation stage ameloblast sensitivity to fluoride. During the maturation stage, massive precipitation of hydroxyapatite occurs, releasing H+ ions. F- can reversibly associate with H+ ions to form HF. Approximately 25-fold more HF is formed at pH 6.0 as compared to pH 7.4. HF diffuses into the cell more easily than F- and flows down a steep concentration gradient from the acidic maturation stage enamel matrix into the neutral cytosol of the ameloblast. The neutral pH inside the cell causes reversion of HF to F-. Excess F- within the cell interferes with ER homoestasis that may result in the dimerization and phosphorylation of PERK and its substrate, eIF2α. Consequently, protein synthesis is attenuated. ER stress can also lead to increased degradation of transcripts encoding secreted proteins such as KLK4. Collectively, decreased secretion of matrix-degrading enzymes such as KLK4 can lead to delayed resorption of enamel matrix proteins, resulting in the higher protein content observed in fluorosed enamel. ER, endoplasmic reticulum. Reproduced with permission from [82].
5. Conclusions
The importance of producing correctly folded and functional enamel proteins is demonstrated by the wide variety and abundance of enamel defects that are traceable back to a single aberrant protein (AI) or to disrupted protein synthesis (neonatal line). Likewise, understanding how particular compounds affect proper ameloblast function is equally important for two reasons: Firstly, for providing additional insight into the mechanism of enamel development, and secondly for developing methods to avoid the development of, or for the treatment of, existing enamel formation disorders.
Acknowledgments
Portions of the research reported here were supported by a grant (DE018106) from the National Institute of Dental and Craniofacial Research. We thank Charles E. Smith for his insights into the transition from ruffle-ended ameloblasts to smooth-ended ameloblasts and back again.
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Avery J.K. Oral Development and Histology. 3rd. Thieme; New York, NY, USA: 2002. pp. 165–166. [Google Scholar]
- 2.HHS.gov Home Page. [(accessed on 2 May 2012)]. Available online: http://www.hhs.gov/news/press/2011pres/01/20110107a.html.
- 3.Beltrán-Aguilar E.D., Barker L., Dye B.A. Prevalence and severity of dental fluorosis in the United States, 1999–2004. NCHS Data Brief. 2010;53:1–8. [PubMed] [Google Scholar]
- 4.DenBesten P.K., Crenshaw M.A. The effects of chronic high fluoride levels on forming enamel in the rat. Arch. Oral Biol. 1984;29:675–679. doi: 10.1016/0003-9969(84)90171-7. [DOI] [PubMed] [Google Scholar]
- 5.Shearer T.R., Britton J.L., DeSart D.J., Suttie J.W. Microhardness of molar teeth in cattle with fluorosis. Am. J. Vet. Res. 1980;41:1543–1545. [PubMed] [Google Scholar]
- 6.Newbrun E. Studies on the physical properties of fluorosed enamel. II. Microhardness. Arch. Oral Biol. 1960;12:21–27. doi: 10.1016/0003-9969(60)90033-9. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R., Tsuchiya M., Bartlett J.D. Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ. Health Persp. 2008;116:1142–1146. doi: 10.1289/ehp.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H., Zhou Y.L., Zhang X.Y., Lu P., Li G.S. Activation of PERK signaling through fluoride-mediated endoplasmic reticulum stress in OS732 cells. Toxicology. 2010;277:1–5. doi: 10.1016/j.tox.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Wei W., Gao Y., Wang C., Zhao L., Sun D. Excessive fluoride induces endoplasmic reticulum stress and interferes enamel proteinases secretion. Environ. Toxicol. 2011 doi: 10.1002/tox.20724. [DOI] [PubMed] [Google Scholar]
- 10.Hu J.C., Chun Y.H., Al Hazzazzi T., Simmer J.P. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 2007;186:78–85. doi: 10.1159/000102683. [DOI] [PubMed] [Google Scholar]
- 11.Smith C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 12.Warshawsky H. The fine structure of secretory ameloblasts in rat incisors. Anat. Rec. 1968;161:211–229. doi: 10.1002/ar.1091610207. [DOI] [PubMed] [Google Scholar]
- 13.Caterina J.J., Skobe Z., Shi J., Ding Y., Simmer J.P., Birkedal-Hansen H., Bartlett J.D. Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2002;277:49598–49604. doi: 10.1074/jbc.M209100200. [DOI] [PubMed] [Google Scholar]
- 14.Fukae M., Tanabe T., Uchida T., Lee S.K., Ryu O.H., Murakami C., Wakida K., Simmer J.P., Yamada Y., Bartlett J.D. Enamelysin (matrix metalloproteinase-20): Localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J. Dent. Res. 1998;77:1580–1588. doi: 10.1177/00220345980770080501. [DOI] [PubMed] [Google Scholar]
- 15.Nanci A., Zalzal S., Lavoie P., Kunikata M., Chen W., Krebsbach P.H., Yamada Y., Hammarström L., Simmer J.P., Fincham A.G., et al. Comparative immunochemical analyses of the developmental expression and distribution of ameloblastin and amelogenin in rat incisors. J. Histochem. Cytochem. 1998;46:911–934. doi: 10.1177/002215549804600806. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett J.D., Skobe Z., Nanci A., Smith C.E. Matrix metalloproteinase 20 promotes a smooth enamel surface, a strong dentino-enamel junction, and a decussating enamel rod pattern. Eur. J. Oral Sci. 2011;119:199–205. doi: 10.1111/j.1600-0722.2011.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak S.Y., Green S., Wiedemann-Bidlack F.B., Beniash E., Yamakoshi Y., Simmer J.P., Margolis H.C. Regulation of calcium phosphate formation by amelogenins under physiological conditions. Eur. J. Oral Sci. 2011;119:103–111. doi: 10.1111/j.1600-0722.2011.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmer J.P., Fukae M., Tanabe T., Yamakoshi Y., Uchida T., Xue J., Margolis H.C., Shimizu M., DeHart B.C., Hu C.C., et al. Purification, characterization, and cloning of enamel matrix serine proteinase 1. J. Dent. Res. 1998;77:377–386. doi: 10.1177/00220345980770020601. [DOI] [PubMed] [Google Scholar]
- 19.Nanci A., Slavkin H.C., Smith C.E. Immunocytochemical and radioautographic evidence for secretion and intracellular degradation of enamel proteins by ameloblasts during the maturation stage of amelogenesis in rat incisors. Anat. Rec. 1987;217:107–123. doi: 10.1002/ar.1092170202. [DOI] [PubMed] [Google Scholar]
- 20.Smith C.E., McKee M.D., Nanci A. Cyclic induction and rapid movement of sequential waves of new smooth-ended ameloblast modulation bands in rat incisors as visualized by polychrome fluorescent labeling and GBHA-staining of maturing enamel. Adv. Dent. Res. 1987;1:162–175. doi: 10.1177/08959374870010020401. [DOI] [PubMed] [Google Scholar]
- 21.Josephsen K., Fejerskov O. Ameloblast modulation in the maturation zone of the rat incisor enamel organ. A light and electron microscopic study. J. Anat. 1977;124:45–70. [PMC free article] [PubMed] [Google Scholar]
- 22.Salama A.H., Zaki A.E., Eisenmann D.R. Tubular lysosomes in ruffle-ended ameloblasts associated with enamel maturation in rat incisor. J. Histochem. Cytochem. 1989;37:801–811. doi: 10.1177/37.6.2542393. [DOI] [PubMed] [Google Scholar]
- 23.Smith C.E. Ameloblasts: Secretory and resorptive functions. J. Dent. Res. 1979;58:695–707. doi: 10.1177/002203457905800221011. [DOI] [PubMed] [Google Scholar]
- 24.Takano Y., Ozawa H. Ultrastructural and cytochemical observations on the alternating morphologic changes of the ameloblasts at the stage of enamel maturation. Arch. Histol. Jpn. 1980;43:385–399. doi: 10.1679/aohc1950.43.385. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T. Endocytotic pathways at the ruffled borders of rat maturation ameloblasts. Histochemistry. 1984;80:263–268. doi: 10.1007/BF00495775. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto T., Shimizu M. Pathway and speed of calcium movement from blood to mineralizing enamel. J. Histochem. Cytochem. 1997;45:213–230. doi: 10.1177/002215549704500207. [DOI] [PubMed] [Google Scholar]
- 27.Smith C.E., Issid M., Margolis H.C., Moreno E.C. Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv. Dent. Res. 1996;10:159–169. doi: 10.1177/08959374960100020701. [DOI] [PubMed] [Google Scholar]
- 28.Wright J.T. The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am. J. Med. Genet. A. 2006;140:2547–2555. doi: 10.1002/ajmg.a.31358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson C.W., Yuan Z.A., Hall B., Longenecker G., Chen E., Thyagarajan T., Sreenath T., Wright J.T., Decker S., Piddington R., et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2001;276:31871–31875. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- 30.Lee K.E., Lee S.K., Jung S.E., Song S.J., Cho S.H., Lee Z.H., Kim J.W. A novel mutation in the AMELX gene and multiple crown resorptions. Eur. J. Oral Sci. 2011;119:324–328. doi: 10.1111/j.1600-0722.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukumoto S., Kiba T., Hall B., Iehara N., Nakamura T., Longenecker G., Krebsbach P.H., Nanci A., Kulkarni A.B., Yamada Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J. Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright J.T., Hart T.C., Hart P.S., Simmons D., Suggs C., Daley B., Simmer J., Hu J., Bartlett J.D., Li Y., et al. Human and mouse enamel phenotypes resulting from mutation or altered expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs. 2009;189:224–229. doi: 10.1159/000151378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J.C., Hu Y., Smith C.E., McKee M.D., Wright J.T., Yamakoshi Y., Papagerakis P., Hunter G.K., Feng J.Q., Yamakoshi F., et al. Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice. J. Biol. Chem. 2008;283:10858–10871. doi: 10.1074/jbc.M710565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajpar M.H., Harley K., Laing C., Davies R.M., Dixon M.J. Mutation of the gene encoding the enamel-specific protein, enamelin, causes autosomal-dominant amelogenesis imperfecta. Hum. Mol. Genet. 2001;10:1673–1677. doi: 10.1093/hmg/10.16.1673. [DOI] [PubMed] [Google Scholar]
- 35.Mårdh C.K., Bäckman B., Holmgren G., Hu J.C., Simmer J.P., Forsman-Semb K. A nonsense mutation in the enamelin gene causes local hypoplastic autosomal dominant amelogenesis imperfecta (AIH2) Hum. Mol. Genet. 2002;11:1069–1074. doi: 10.1093/hmg/11.9.1069. [DOI] [PubMed] [Google Scholar]
- 36.Chan H.C., Mai L., Oikonomopoulou A., Chan H.L., Richardson A.S., Wang S.K., Simmer J.P., Hu J.C. Altered enamelin phosphorylation site causes amelogenesis imperfecta. J. Dent. Res. 2010;89:695–699. doi: 10.1177/0022034510365662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma R., Tye C.E., Arun A., Macdonald D., Chatterjee A., Abrazinski T., Everett E.T., Whitford G.M., Bartlett J.D. Assessment of Dental Fluorosis in Mmp20+/− Mice. J. Dent. Res. 2011;90:788–792. doi: 10.1177/0022034511398868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmer J.P., Hu Y., Lertlam R., Yamakoshi Y., Hu J.C. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J. Biol. Chem. 2009;284:19110–19121. doi: 10.1074/jbc.M109.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.W., Simmer J.P., Hart T.C., Hart P.S., Ramaswami M.D., Bartlett J.D., Hu J.C. MMP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J. Med. Genet. 2005;42:271–275. doi: 10.1136/jmg.2004.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozdemir D., Hart P.S., Ryu O.H., Choi S.J., Ozdemir-Karatas M., Firatli E., Piesco N., Hart T.C. MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J. Dent. Res. 2005;84:1031–1035. doi: 10.1177/154405910508401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papagerakis P., Lin H.K., Lee K.Y., Hu Y., Simmer J.P., Bartlett J.D., Hu J.C. Premature stop codon in MMP20 causing amelogenesis imperfecta. J. Dent. Res. 2008;87:56–59. doi: 10.1177/154405910808700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S.K., Seymen F., Kang H.Y., Lee K.E., Gencay K., Tuna B., Kim J.W. MMP20 hemopexin domain mutation in amelogenesis imperfecta. J. Dent. Res. 2010;89:46–50. doi: 10.1177/0022034509352844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart P.S., Hart T.C., Michalec M.D., Ryu O.H., Simmons D., Hong S., Wright J.T. Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis imperfecta. J. Med. Genet. 2004;41:545–549. doi: 10.1136/jmg.2003.017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J.W., Lee S.K., Lee Z.H., Park J.C., Lee K.E., Lee M.H., Park J.T., Seo B.M., Hu J.C., Simmer J.P. FAM83H mutations in families with autosomal-dominant hypocalcified amelogenesis imperfecta. Am. J. Hum. Genet. 2008;82:489–494. doi: 10.1016/j.ajhg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Sullivan J., Bitu C.C., Daly S.B., Urquhart J.E., Barron M.J., Bhaskar S.S., Martelli-Júnior H., dos Santos Neto P.E., Mansilla M.A., Murray J.C., et al. Whole-Exome sequencing identifies FAM20A mutations as a cause of amelogenesis imperfecta and gingival hyperplasia syndrome. Am. J. Hum. Genet. 2011;88:616–620. doi: 10.1016/j.ajhg.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Sayed W., Parry D.A., Shore R.C., Ahmed M., Jafri H., Rashid Y., Al-Bahlani S., Al Harasi S., Kirkham J., Inglehearn C.F., et al. Mutations in the beta propeller WDR72 cause autosomal-recessive hypomaturation amelogenesis imperfecta. Am. J. Hum. Genet. 2009;85:699–705. doi: 10.1016/j.ajhg.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Primosch R.E. Tetracycline discoloration, enamel defects, and dental caries in patients with cystic fibrosis. Oral Surg. Oral Med. Oral Pathol. 1980;50:301–308. doi: 10.1016/0030-4220(80)90411-9. [DOI] [PubMed] [Google Scholar]
- 48.Wright J.T., Kiefer C.L., Hall K.I., Grubb B.R. Abnormal enamel development in a cystic fibrosis transgenic mouse model. J. Dent. Res. 1996;75:966–973. doi: 10.1177/00220345960750041101. [DOI] [PubMed] [Google Scholar]
- 49.Sui W., Boyd C., Wright J.T. Altered pH regulation during enamel development in the cystic fibrosis mouse incisor. J. Dent. Res. 2003;82:388–392. doi: 10.1177/154405910308200512. [DOI] [PubMed] [Google Scholar]
- 50.Bailleul-Forestier I., Molla M., Verloes A., Berdal A. The genetic basis of inherited anomalies of the teeth. Part 1: Clinical and molecular aspects of non-syndromic dental disorders. Eur. J. Med. Genet. 2008;51:273–291. doi: 10.1016/j.ejmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Tubert-Jeannin S., Auclair C., Amsallem E., Tramini P., Gerbaud L., Ruffieux C., Schulte A.G., Koch M.J., Rège-Walther M., Ismail A. Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database Syst. Rev. 2011;12:CD007592. doi: 10.1002/14651858.CD007592.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aoba T., Fejerskov O. Dental fluorosis: chemistry and biology. Crit. Rev. Oral. Biol. Med. 2002;13:155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- 53.Fejerskov O., Thylstrup A., Larsen M.J. Clinical and structural features and possible pathogenic mechanisms of dental fluorosis. Scand. J. Dent. Res. 1977;85:510–534. doi: 10.1111/j.1600-0722.1977.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 54.Wright J.T., Chen S.C., Hall K.I., Yamauchi M., Bawden J.W. Protein characterization of fluorosed human enamel. J. Dent. Res. 1996;75:1936–1941. doi: 10.1177/00220345960750120401. [DOI] [PubMed] [Google Scholar]
- 55.DenBesten P.K., Yan Y., Featherstone J.D., Hilton J.F., Smith C.E., Li W. Effects of fluoride on rat dental enamel matrix proteinases. Arch. Oral. Biol. 2002;47:763–770. doi: 10.1016/S0003-9969(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 56.Tye C.E., Antone J.V., Bartlett J.D. Fluoride does not inhibit enamel protease activity. J. Dent. Res. 2011;90:489–494. doi: 10.1177/0022034510390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerlach R.F., de Souza A.P., Cury J.A., Line S.R. Fluoride effect on the activity of enamel matrix proteinases in vitro. Eur. J. Oral. Sci. 2000;108:48–53. doi: 10.1034/j.1600-0722.2000.00735.x. [DOI] [PubMed] [Google Scholar]
- 58.Varol E., Icli A., Aksoy F., Bas H.A., Sutcu R., Ersoy I.H., Varol S., Ozaydin M. Evaluation of total oxidative status and total antioxidant capacity in patients with endemic fluorosis. Toxicol. Ind. Health. 2011 doi: 10.1177/0748233711428641. in press. [DOI] [PubMed] [Google Scholar]
- 59.Mittal M., Flora S.J. Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem. Biol. Interact. 2006;162:128–139. doi: 10.1016/j.cbi.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Jin X.Q., Xu H., Shi H.Y., Zhang J.M., Zhang H.Q. Fluoride-induced oxidative stress of osteoblasts and protective effects of baicalein against fluoride toxicity. Biol. Trace. Elem. Res. 2007;116:81–89. doi: 10.1007/BF02685921. [DOI] [PubMed] [Google Scholar]
- 61.Lu L., Han A.P., Chen J.J. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kulkarni A.P., Mittal S.P., Devasagayam T.P., Pal J.K. Oxidative stress perturbs cell proliferation in human K562 cells by modulating protein synthesis and cell cycle. Free Radic. Res. 2009;43:1090–1100. doi: 10.1080/10715760903179673. [DOI] [PubMed] [Google Scholar]
- 63.Kulkarni A.P., Mittal S.P., Devasagayam T.P., Pal J.K. Hsp90 mediates activation of the heme regulated eIF-2 alpha kinase during oxidative stress. Indian J. Biochem. Biophys. 2010;47:67–74. [PubMed] [Google Scholar]
- 64.Mittal M., Flora S.J. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem. Toxicol. 2007;30:263–281. doi: 10.1080/01480540701380075. [DOI] [PubMed] [Google Scholar]
- 65.Mansour H.H., Tawfik S.S. Efficacy of lycopene against fluoride toxicity in rats. Pharm. Biol. 2011;50:707–711. doi: 10.3109/13880209.2011.618994. [DOI] [PubMed] [Google Scholar]
- 66.Feng P., Wei J.R., Zhang Z.G. Influence of selenium and fluoride on blood antioxidant capacity of rats. Exp. Toxicol. Pathol. 2010;64:565–568. doi: 10.1016/j.etp.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Izquierdo-Vega J.A., Sanchez-Gutierrez M., Del Razo L.M. NADPH oxidase participates in the oxidative damage caused by fluoride in rat spermatozoa: Protective role of alpha-tocopherol. J. Appl. Toxicol. 2010;31:579–588. doi: 10.1002/jat.1600. [DOI] [PubMed] [Google Scholar]
- 68.Feng P., Wei J., Zhang Z. Intervention of Selenium on Chronic Fluorosis-Induced Injury of Blood Antioxidant Capacity in Rats. Biol. Trace Elem. Res. 2011;144:1024–1031. doi: 10.1007/s12011-011-9087-9. [DOI] [PubMed] [Google Scholar]
- 69.Lacruz R.S., Smith C.E., Chen Y.B., Hubbard M.J., Hacia J.G., Paine M.L. Gene-expression analysis of early- and late-maturation-stage rat enamel organ. Eur. J. Oral. Sci. 2011;119:149–157. doi: 10.1111/j.1600-0722.2011.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tkachev V.O., Menshchikova E.B., Zenkov N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry. 2011;76:407–422. doi: 10.1134/s0006297911040031. [DOI] [PubMed] [Google Scholar]
- 71.Merchant A.A., Singh A., Matsui W., Biswal S. The redox-sensitive transcription factor, Nrf2, regulates murine hematopoietic stem cell survival independent of ROS levels. Blood. 2011;118:6572–6579. doi: 10.1182/blood-2011-05-355362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yanagawa T., Itoh K., Uwayama J., Shibata Y., Yamaguchi A., Sano T., Ishii T., Yoshida H., Yamamoto M. Nrf2 deficiency causes tooth decolourization due to iron transport disorder in enamel organ. Genes Cells. 2004;9:641–651. doi: 10.1111/j.1356-9597.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- 73.Ratner S. The Iron Content of Teeth of Normal and Anemic Rats. J. Dent. Res. 1935;15:89–92. doi: 10.1177/00220345350150020601. [DOI] [Google Scholar]
- 74.Lindemann G. Pigment alterations and other disturbances in rat incisor enamel in chronic fluorosis and in recovery. Acta Odontol. Scand. 1967;25:525–539. doi: 10.3109/00016356709028752. [DOI] [PubMed] [Google Scholar]
- 75.Grimsrud P.A., Xie H., Griffin T.J., Bernlohr D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levonen A.L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., Darley-Usmar V.M. Cellular mechanisms of redox cell signalling: Role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell. Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 78.Kubota K., Lee D.H., Tsuchiya M., Young C.S., Everett E.T., Martinez-Mier E.A., Snead M.L., Nguyen L., Urano F., Bartlett J. D. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J. Biol. Chem. 2005;280:23194–23202. doi: 10.1074/jbc.M503288200. [DOI] [PubMed] [Google Scholar]
- 79.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 80.Cullinan S.B., Diehl J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell. Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Riksen E.A., Kalvik A., Brookes S., Hynne A., Snead M.L., Lyngstadaas S.P., Reseland J.E. Fluoride reduces the expression of enamel proteins and cytokines in an ameloblast-derived cell line. Arch. Oral Biol. 2010;56:324–330. doi: 10.1016/j.archoralbio.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 82.Sharma R., Tsuchiya M., Skobe Z., Tannous B.A., Bartlett J.D. The acid test of fluoride: How pH modulates toxicity. PLoS One. 2010;5:e10895. doi: 10.1371/journal.pone.0010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jackson R.J., Hellen C.U., Pestova T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell. Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McEwen E., Kedersha N., Song B., Scheuner D., Gilks N., Han A., Chen J.J., Anderson P., Kaufman R.J. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005;280:16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- 85.Wehner K.A., Schutz S., Sarnow P. OGFOD1, a novel modulator of eukaryotic translation initiation factor 2alpha phosphorylation and the cellular response to stress. Mol. Cell. Biol. 2010;30:2006–2016. doi: 10.1128/MCB.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flora S.J., Mittal M., Pachauri V., Dwivedi N. A possible mechanism for combined arsenic and fluoride induced cellular and DNA damage in mice. Metallomics. 2012;4:78–90. doi: 10.1039/c1mt00118c. [DOI] [PubMed] [Google Scholar]
- 87.Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST0340007. [DOI] [PubMed] [Google Scholar]
- 88.Vattem K.M., Wek R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He C.H., Gong P., Hu B., Stewart D., Choi M.E., Choi A.M., Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 90.Sasaki S., Takagi T., Suzuki M. Cyclical changes in pH in bovine developing enamel as sequential bands. Arch. Oral Biol. 1991;36:227–231. doi: 10.1016/0003-9969(91)90090-H. [DOI] [PubMed] [Google Scholar]
- 91.Tsuji T., Onuma K., Yamamoto A., Iijima M., Shiba K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl. Acad. Sci. USA. 2008;105:16866–16870. doi: 10.1073/pnas.0804277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith C.E., Chong D.L., Bartlett J.D., Margolis H.C. Mineral acquisition rates in developing enamel on maxillary and mandibular incisors of rats and mice: Implications to extracellular acid loading as apatite crystals mature. J. Bone Miner. Res. 2005;20:240–249. doi: 10.1359/JBMR.041002. [DOI] [PubMed] [Google Scholar]
- 93.Kawase T., Suzuki A. Studies on the transmembrane migration of fluoride and its effects on proliferation of L-929 fibroblasts (L cells) in vitro. Arch. Oral Biol. 1989;34:103–107. doi: 10.1016/0003-9969(89)90133-7. [DOI] [PubMed] [Google Scholar]
- 94.He H., Ganapathy V., Isales C.M., Whitford G.M. pH-dependent fluoride transport in intestinal brush border membrane vesicles. Biochim. Biophys. Acta. 1998;1372:244–254. doi: 10.1016/s0005-2736(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 95.Zhou R., Zaki A.E., Eisenmann D.R. Morphometry and autoradiography of altered rat enamel protein processing due to chronic exposure to fluoride. Arch. Oral Biol. 1996;41:739–747. doi: 10.1016/S0003-9969(96)00078-7. [DOI] [PubMed] [Google Scholar]
- 96.Lyaruu D.M., de Jong M., Bronckers A.L., Wöltgens J.H. Ultrastructure of in-vitro recovery of mineralization capacity of fluorotic enamel matrix in hamster tooth germs pre-exposed to fluoride in organ culture during the secretory phase of amelogenesis. Arch. Oral Biol. 1987;32:107–115. doi: 10.1016/0003-9969(87)90053-7. [DOI] [PubMed] [Google Scholar]
- 97.Denbesten P.K., Crenshaw M.A., Wilson M.H. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J. Dent. Res. 1985;64:1365–1370. doi: 10.1177/00220345850640120701. [DOI] [PubMed] [Google Scholar]
- 98.Smith C.E., Nanci A., Denbesten P.K. Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat. Rec. 1993;237:243–258. doi: 10.1002/ar.1092370212. [DOI] [PubMed] [Google Scholar]
- 99.Whitford G.M., Reynolds K.E. Plasma and developing enamel fluoride concentrations during chronic acid-base disturbances. J. Dent. Res. 1979;58:2058–2065. doi: 10.1177/00220345790580110401. [DOI] [PubMed] [Google Scholar]
- 100.Reynolds K.E., Whitford G.M., Pashley D.H. Acute fluoride toxicity: the influence of acid-base status. Toxicol. Appl. Pharmacol. 1978;45:415–427. doi: 10.1016/0041-008X(78)90105-9. [DOI] [PubMed] [Google Scholar]
- 101.Whitford G.M., Angmar-Månsson B. Fluorosis-like effects of acidosis, but not NH4+, on rat incisor enamel. Caries Res. 1995;29:20–25. doi: 10.1159/000262035. [DOI] [PubMed] [Google Scholar]
- 102.Angmar-Månsson B., Lindh U., Whitford G.M. Enamel and dentin fluoride levels and fluorosis following single fluoride doses: A nuclear microprobe study. Caries Res. 1990;24:258–262. doi: 10.1159/000261279. [DOI] [PubMed] [Google Scholar]
- 103.Den Besten P.K. Effects of fluoride on protein secretion and removal during enamel development in the rat. J. Dent. Res. 1986;65:1272–1277. doi: 10.1177/00220345860650101401. [DOI] [PubMed] [Google Scholar]