Abstract
Objective
To investigate possible correlations between the tendency towards alexithymia and the depressive state, globally and with regard to the Toronto Alexithymia scale (TAS-20) subscales and the Hamilton rating scale for depression (HAM-D) subscales.
Methods
75 patients, suffering from Unipolar Depression, were assessed through the HAM-D and the TAS-20 and compared to the control group (n = 63). Both groups were divided into two subgroups (30–60 years old; 61–80 years old). Correlations between the tendency towards alexithymia and depressive symptoms, globally and with regard to the TAS-20 subscales and the HAM-D subscales, were investigated.
Results
With regard to patients, a positive correlation was found between: the HAM-D total score and the TAS-20 total score; the HAM-D factor V (psychomotor retardation) and the TAS-20 total score; the TAS-20 subscale III (externally oriented thinking) and the HAM-D total score. In addition a positive correlation between the HAM-D factor V and the TAS-20 subscales I and III was found and confirmed among females. In patients aged 30–60 years, the HAM-D factor V was correlated with all the TAS-20 subscales. As to the control group, a positive correlation was found between: the HAM-D factor I (anxiety/somatization) and the TAS-20 total score; the TAS-20 subscale I and the HAM-D total score; the HAM-D factor I and the TAS-20 subscale. The latter was confirmed in the control group aged 30–60 years.
Conclusion
The link between alexithymia and affective symptoms has been confirmed in the patients and in the control group. An interesting data is the correlation between psychomotor retardation and externally oriented thinking among the patients. According to cognitive theories, psychomotor retardation could be related to feelings of incapacity perceived by an individual. A patient, with an externally oriented thinking, might run into a distorted perception of his own ability to function, thus causing a psychomotor “fattening”.
Keywords: alexithymia, major depression, externally oriented thinking, psychomotor retardation, correlation alexithymia and depression
Introduction
Alexithymia is a multidimensional concept that associates an emotional component, focused on the difficulty in identifying and describing feelings, with a cognitive one, centred on the use of a concrete and poorly introspective way of thinking.1 Even though alexithymia has been related to various psychiatric disorders,2,3 its correlation with major depression (MD) seems to be very strong.2,4 Such a correlation implies important practical implications; in fact, it is easy to assume that the association between alexithymia and depression may not only result in delays in the request for a psychiatric consultation (being difficult for the alexithymic patient to recognize and describe the negative emotions related to his/her depressive state), but also interfere with the effectiveness of the psychotherapeutic approaches.56 The most commonly used instrument to assess alexithymia is the 20-item Toronto Alexithymia Scale (TAS-20),7 the subscales I and II of which (respectively difficulty in identifying and in describing feelings) would seem to be the ones more related to the affective symptoms.8,9 As a matter of fact, when assessing alexithymia in the psychiatric field, for some authors, subscale II could actually represent a discriminating feature of depressive disorders.10 In addition, alexithymic patients often present other psychiatric symptoms, and exhibit a worse quality of life than the non-alexithymic ones.11 Furthermore, not only has an independent association between the subscales I, II, and the TAS-20 total score and the severity of depressive symptoms been demonstrated, but also the severity of residual symptoms has been related to alexithymia (subscale I in particular), regardless of the initial symptoms, the kind of psychotherapy and the prescribed medication.6 Indeed, while for some authors alexithymia could be a stable personality construct,12 some follow-up studies have demonstrated that changes in the affective symptoms correlate to those in the alexithymia severity13,14 Such data allow us to hypothesize that alexithymia might be a state-dependent phenomenon14 and, therefore, changeable thus giving new impetus to do research on the link between depression and alexithymia.
The aim of this study was to investigate the possible correlations between the tendency towards alexithymia and the depressive state, globally and with regard to the TAS-20 subscales and the Hamilton rating scale for depression (HAM-D) subscales.
Materials and methods
Patient assessment
Patients, suffering from Unipolar Depression, who were referred to our Psychiatry Unit, were consecutively enrolled and compared to a healthy control group with no familial or personal history of psychiatric illnesses. Each enrolled subject signed a written informed consent and was assessed through the Italian version of the following scales:
– Hamilton rating scale for depression (HAM-D) consisting of 21 items, each of which provides answers with a 5, 4 or a 3-point rating scale. The severity of depression is evaluated as follows: ≥25 severe depression; 18–24 moderate depression; 8–17 mild depression; ≤7 absence of depression. Six subscales (or factors) can be isolated from the HAM-D: factor I (anxiety/somatization), factor II (weight), factor III (cognitive disturbance), factor IV (diurnal variation), factor V (psychomotor retardation) and factor VI (sleep).15
– TAS-20 consisting of 3 subscales: subscale I assessing the difficulty in identifying feelings; subscale II assessing the difficulty in describing feelings; subscale III assessing externally oriented thinking and the lack of introspective capacities. Each item provides answers from a Likert-scale specifying levels of “strong disagreement” or “strong agreement”. The TAS-20 total score ranges from 20–100 points. Scores of 61 and above indicate an alexithymic state.7,16
Hence, the possible correlations between the tendency towards alexithymia and the depressive state, globally and with regard to the TAS-20 subscales and the HAM-D subscales, were investigated in the depressed patients. In order to investigate possible correlations (not related to depression) between affective symptoms and alexithymia, even if obviously the HAM-D scores did not indicate a depressive state (HAM-D total score ≤7), the same statistical analysis was performed in the control group. The study was approved by the Ethics Committee of the University Hospital “Policlinico-Vittorio Emanuele” of Catania (Italy).
Statistical analysis
Data were analyzed using SPSS version 13.0 (SPSS Inc, Chicago, IL, USA). Scalar measures were presented as mean ± standard deviation (SD). A parametric t-test was used for the mean comparison between the groups. Correlations were evaluated using Pearson’s coefficient and, in the case of a positive correlation, a linear regression model was applied. Statistical significance was considered if P ≤ 0.05.
Results
Characteristics of the depressed patients
Seventy-seven patients were eligible for the study. Two of them refused to participate. At the end of the study, 75 depressed patients were enrolled (25 males and 50 females; aged 54.8 ± 15.3 years). No significant differences in age (57.0 ± 14.5 years old among males versus 53.8 ± 15.7 years old among females, P = 0.6) and years of education (9.8 ± 4.6 years among males versus 8.8 ± 4.9 years among females, P = 0.7) were recorded. See Tables 1 and 2 for more details.
Table 1.
Baseline characteristics of the patients
| Number | 75 |
| Gender (female/male) | 50/25 |
| Age (years) | 54.8 ± 15.3 |
| Education (years) | 9.1 ± 4.8 |
| HAM-D total score | 25.8 ± 8.2 |
| HAM-D factor I | 8.0 ± 3.2 |
| HAM-D factor II | 1.1 ± 1.4 |
| HAM-D factor III | 6.0 ± 3.5 |
| HAM-D factor IV | 2.0 ± 1.5 |
| HAM-D factor V | 6.3 ± 2.8 |
| HAM-D factor VI | 3.0 ± 2.0 |
| TAS-20 total score | 57.3 ± 12.8 |
| TAS-20 subscale I | 21.8 ± 6.9 |
| TAS-20 subscale II | 14.6 ± 4.8 |
| TAS-20 subscale III | 20.8 ± 5.5 |
Notes: The baseline characteristics of the whole sample of the patients (n = 75) are shown. All values are presented as mean ± standard deviation, except for number and gender.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
Table 2.
Baseline characteristics and the gender differences of the patients
| Female (n = 50) | Male (n = 25) | P-value | |
|---|---|---|---|
| Age (years) | 53.8 ± 15.7 | 57 ± 14.5 | 0.6 |
| Education (years) | 8.8 ± 4.9 | 9.8 ± 4.6 | 0.7 |
| HAM-D total score | 27.3 ± 8.1 | 22.9 ± 7.6 | 0.7 |
| HAM-D factor I | 8.6 ± 3 | 6.8 ± 3.2 | 0.6 |
| HAM-D factor II | 0.8 ± 1.3 | 1.7 ± 1.7 | 0.1 |
| HAM-D factor III | 6.5 ± 3.5 | 5.3 ± 3.1 | 0.5 |
| HAM-D factor IV | 1.6 ± 1.4 | 1.6 ± 1.7 | 0.2 |
| HAM-D factor V | 6.9 ± 2.5 | 5.3 ± 3.1 | 0.2 |
| HAM-D factor VI | 3 ± 2 | 2.4 ± 1.9 | 0.8 |
| TAS-20 total score | 57 ± 13.8 | 58 ± 10.4 | 0.1 |
| TAS-20 factor I | 21.8 ± 6.8 | 21.6 ± 7.1 | 0.7 |
| TAS-20 factor II | 14.4 ± 5 | 15.1 ± 4.5 | 0.5 |
| TAS-20 factor III | 20.7 ± 5.8 | 20.8 ± 4.9 | 0.2 |
Notes: The baseline characteristics of the whole sample of the patients (n = 75) are shown with regard to gender. All values are presented as mean ± standard deviation.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
Results of the HAM-D and the TAS-20 in the depressed group
With regard to the whole sample, the HAM-D total mean score was 25.8 ± 8.2 and the TAS-20 total mean score was 57.3 ± 12.8 (see Table 1). No statistically significant differences were found either in the HAM-D total scores (P = 0.7) or in the TAS-20 scores (P = 0.1), when comparing females and males (see Table 2 for more details).
Correlations between the HAM-D and the TAS-20 in the depressed patients
Taking into consideration the whole sample of patients, Pearson’s correlation coefficient between the HAM-D total mean score and the TAS-20 total mean score was 0.29 (P = 0.01). A positive correlation between the HAM-D total score and the TAS-20 total score (r = 0.29; P = 0.01) was found and, when taking into consideration the gender, this correlation was confirmed only in females (r = 0.34; P = 0.01).
The statistical analysis then examined the possible correlations between: (a) the HAM-D factors and the TAS-20 total score; (b) the TAS-20 subscales and the HAM-D total score; and (c) the TAS-20 subscales and the HAM-D factors. A positive correlation between the HAM-D factor V (psychomotor retardation) and the TAS-20 total score was found (r = 0.3; P < 0.001). Furthermore, analyzing the TAS-20 subscales and their possible correlations with the HAM-D total score, the TAS-20 subscale I (difficulty in identifying feelings) and III (externally oriented thinking and lack of introspective capacities) were found to be positively correlated with the HAM-D total score (r = 0.25; P = 0.03; r = 0.23; P = 0.04). See Table 3 for more details.
Table 3.
The correlations between depression and the tendency towards alexithymia among the whole sample of the patients
| Pearson’s coefficient (r) | P-value | |
|---|---|---|
| HAM-D total/TAS-20 total | 0.29 | 0.01** |
| HAM-D total/TAS-20 total female | 0.34 | 0.01** |
| HAM-D total/TAS-20 total male | 0.23 | 0.2 |
| HAM-D factors/TAS-20 total | ||
| HAM-D factor I/TAS-20 total | 0.1 | 0.3 |
| HAM-D factor II/TAS-20 total | −0.01 | 0.9 |
| HAM-D factor III/TAS-20 total | 0.1 | 0.1 |
| HAM-D factor IV/TAS-20 total | 0.1 | 0.3 |
| HAM-D factor V/TAS-20 total | 0.3 | <0.001*** |
| HAM-D factor VI/TAS-20 total | 0.1 | 0.2 |
| TAS-20 subscales/HAM-D total | ||
| TAS subscale I and HAM-D total | 0.25 | 0.03* |
| TAS subscale II and HAM-D total | 0.19 | 0.09 |
| TAS subscale III and HAM-D total | 0.23 | 0.04* |
Notes: The correlations between the results of the Hamilton depression rating scale and of the Toronto Alexithymia scale, taking into consideration the total scores and the subscales of both instruments of assessment, are shown. Statistical significance is represented by: *P ≤ 0.05, **P < 0.01, ***P < 0.001.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
In addition, the investigation of the possible correlations between the HAM-D factors and the TAS-20 subscales revealed a positive correlation between the HAM-D factor V and the TAS-20 subscale I (r = 0.276; P < 0.01), and the TAS-20 subscale III (r = 0.359; P = 0.002).
The linear regression model of the correlation between the HAM-D factor V and the TAS-20 subscale III (y = 2.57 + 0.18x) is shown in Figure 1. These correlations were confirmed among females (the HAM-D factor V and the TAS-20 subscale I: r = 0.276; P = 0.01; the HAM-D factor V and the TAS-20 subscale III: r = 0.359; P = 0.002), but were lacking among males (the HAM-D factor V and the TAS-20 subscale I: r = 0.150; P = 0.4; the HAM-D factor V and the TAS-20 subscale III: r = 0.239; P = 0.2).
Figure 1.
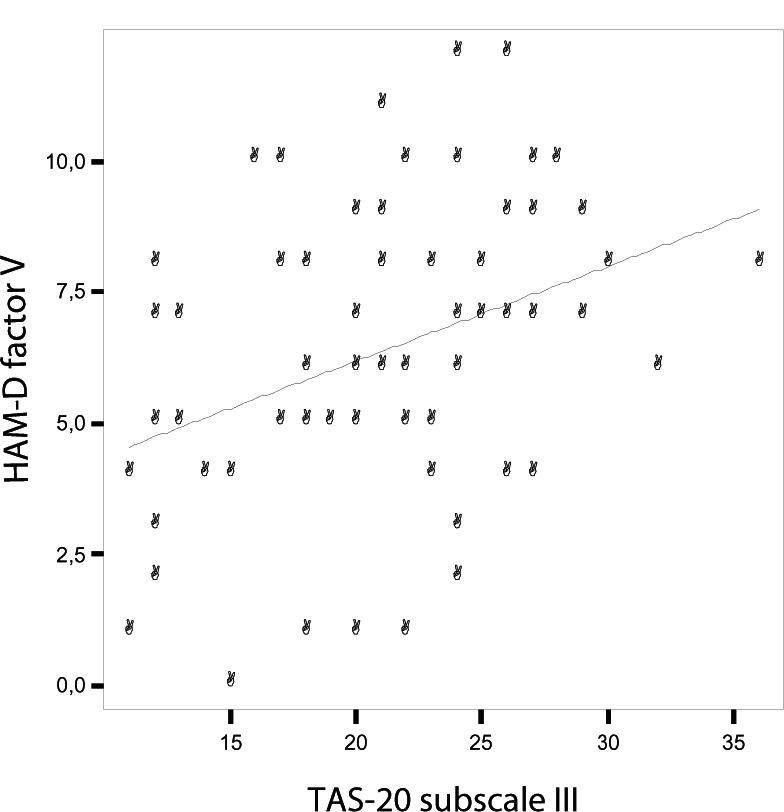
Correlation between the HAM-D factor V and the TAS-20 subscale III.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, Toronto Alexithymia scale.
Moreover, the sample was then divided into two groups: subjects aged 30–60 years and subjects aged 61–80 years. The former (aged 30–60 years) had a higher level of education (P = 0.02), presented a higher tendency towards alexithymia (P = 0.04) (see Table 4), and the HAM-D factor V was correlated with all the TAS-20 subscales (the HAM-D factor V and the TAS-20 subscale I: r = 0.285, P = 0.05; the HAM-D factor V and the TAS-20 subscale II: r = 0.362, P = 0.01; the HAM-D factor V and the TAS-20 subscale III: r = 0.490, P < 0.001). In the latter (aged 61–80 years) no correlations were found between the HAM-D factors and the TAS-20 subscales.
Table 4.
The comparison between the patients aged 30–60 years and those aged 61–80 years
| Age 30–60 years | Age 61–80 years | P-value | |
|---|---|---|---|
| Total number (female/male) | 48 (34/14) | 27 (16/11) | / |
| Age (years) | 45.4 ± 9.5 | 71.6 ± 6.7 | 0.05* |
| Education (years) | 9.3 ± 4.1 | 8.9 ± 5.9 | 0.02* |
| HAM-D total | 25.4 ± 8.4 | 26.5 ± 7.9 | 0.7 |
| HAM-D factor I | 7.9 ± 3.4 | 8.1 ± 2.9 | 0.3 |
| HAM-D factor II | 1.0 ± 1.5 | 1.3 ± 1.4 | 0.7 |
| HAM-D factor III | 6.2 ± 3.6 | 5.6 ± 3.2 | 0.5 |
| HAM-D factor IV | 1.5 ± 1.6 | 1.7 ± 1.3 | 0.2 |
| HAM-D factor V | 6.3 ± 2.7 | 6.3 ± 2.9 | 0.6 |
| HAM-D factor VI | 2.4 ± 2.0 | 3.4 ± 1.9 | 0.7 |
| TAS-20 total | 57.4 ± 14.3 | 57 ± 9.8 | 0.04* |
| TAS-20 subscale I | 21.3 ± 7.4 | 22.6 ± 5.8 | 0.1 |
| TAS-20 subscale II | 15.1 ± 5.3 | 13.7 ± 3.9 | 0.09 |
| TAS-20 subscale III | 21.0 ± 5.8 | 20.4 ± 5.0 | 0.4 |
Notes: The comparative statistical analysis, between individuals aged 30–60 years and 61–80 years, is shown, taking into consideration the total scores and the subscales of the Hamilton for depression rating scale as well as the Toronto Alexithymia scale. All values are presented as mean ± standard deviation, except for the number of subjects. Statistical significance is represented by: *P ≤ 0.05.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
The linear regression model applied did not change the strength of the associations found in Pearson’s correlation.
Characteristics of the control group
Sixty-three nondepressed subjects were enrolled as a control group (45 males and 18 females; aged 59 ± 13.05 years). No significant differences in age (59.3 ± 11.6 years old among males versus 58.8 ± 16.6 years old among females, P = 0.06) and years of education (9.5 ± 4.2 years among males versus 9.2 ± 3.8 years among females, P = 0.6) were recorded. See Tables 5 and 6 for more details.
Table 5.
The baseline characteristics of the control group
| Number | 63 |
| Gender (female/male) | 18/45 |
| Age (years) | 59 ± 13.05 |
| Education (years) | 9.3 ± 5.1 |
| HAM-D total score | 3.6 ± 2.2 |
| HAM-D factor I | 1.2 ± 1.2 |
| HAM-D factor II | 0.03 ± 0.2 |
| HAM-D factor III | 0.9 ± 0.9 |
| HAM-D factor IV | 0 |
| HAM-D factor V | 0.8 ± 1 |
| HAM-D factor VI | 0.6 ± 0.9 |
| TAS-20 total score | 51 ± 13.1 |
| TAS-20 subscale I | 16 ± 6.4 |
| TAS-20 subscale II | 13 ± 3.7 |
| TAS-20 subscale III | 22 ± 5 |
Notes: The baseline characteristics of the control group (n = 63) are shown. All values are presented as mean ± standard deviation, except for number.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
Table 6.
The baseline characteristics and gender differences in the control group
| Female | Male | P-value | |
|---|---|---|---|
| Number (total = 63) | 18 | 45 | / |
| Age (years) | 58 ± 16.6 | 59.3 ± 11.6 | 0.06 |
| Education (years) | 9.2 ± 3.8 | 9.5 ± 4.2 | 0.6 |
| HAM-D total score | 4.5 ± 2.2 | 3.2 ± 2.1 | 0.7 |
| HAM-D factor I | 1.6 ± 1.2 | 1 ± 1.1 | 0.6 |
| HAM-D factor II | 0 | 0.04 ± 0.2 | <0.01** |
| HAM-D factor III | 1 ± 1 | 0.9 ± 0.9 | 0.5 |
| HAM-D factor IV | 0 | 0 | / |
| HAM-D factor V | 1 ± 1.4 | 0.7 ± 0.9 | <0.01** |
| HAM-D factor VI | 0.9 ± 1.2 | 0.5 ± 0.7 | <0.01** |
| TAS-20 total score | 54.7 ± 14 | 49.5 ± 12.6 | 0.5 |
| TAS-20 factor I | 18.5 ± 6.8 | 15 ± 6 | 0.4 |
| TAS-20 factor II | 13.9 ± 4.1 | 12.7 ± 3.5 | 0.3 |
| TAS-20 factor III | 22.3 ± 4.8 | 21.9 ± 5.1 | 0.8 |
Notes: The baseline characteristics of the control group (n = 63) are shown with regard to the gender. All values are presented as mean ± standard deviation, except for number. Statistical significance is represented by: **P < 0.01.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
Results of the HAM-D and the TAS-20 in the control group
With regard to the whole sample of the control group, the HAM-D total mean score was 3.6 ± 2.2 and the TAS-20 total mean score was 51 ± 13.1 (see Table 5). No statistically significant differences were found either in the HAM-D total scores (P = 0.7) or in the TAS-20 scores (P = 0.5) when comparing females and males. However, some statistically significant differences were found when considering the HAM-D factors (see Table 6 for more details).
Correlations between the HAM-D and the TAS-20 in the control group
Taking into consideration the whole sample of the control group, Pearson’s correlation coefficient between the HAM-D total mean score and the TAS-20 total mean score was 0.1 (P = 0.1); in other words, no correlation was recorded.
Hence, as was done for the patients, the following possible correlations were also researched in the control group: (a) the HAM-D factors and the TAS-20 total score; (b) the TAS-20 subscales and the HAM-D total score; (c) the TAS-20 subscale and the HAM-D factors. A positive correlation between the HAM-D factor I (anxiety/somatization) and the TAS-20 total score was found (r = 0.2; P = 0.04). Furthermore, analyzing the TAS-20 subscales and its possible correlations with the HAM-D total score, the TAS-20 subscale I (difficulty in identifying feelings) was found to be positively correlated with the HAM-D total score (r = 0.2; P = 0.03). See Table 7 for more details.
Table 7.
The correlations between depression and the tendency towards alexithymia in the control group
| Pearson’s coefficient (r) | P-value | |
|---|---|---|
| HAM-D total/TAS-20 total | 0.1 | 0.1 |
| HAM-D total/TAS-20 total female | 0.1 | 0.6 |
| HAM-D total/TAS-20 total male | 0.15 | 0.3 |
| HAM-D factors/TAS-20 total | ||
| HAM-D factor I/TAS-20 total | 0.2 | 0.04* |
| HAM-D factor II/TAS-20 total | 0.1 | 0.2 |
| HAM-D factor III/TAS-20 total | 0.06 | 0.6 |
| HAM-D factor IV/TAS-20 total | / | / |
| HAM-D factor V/TAS-20 total | −0.06 | 0.5 |
| HAM-D factor VI/TAS-20 total | 0.09 | 0.4 |
| TAS-20 subscales/HAM-D total | ||
| TAS subscale I and HAM-D total | 0.2 | 0.03* |
| TAS subscale II and HAM-D total | 0.06 | 0.6 |
| TAS subscale III and HAM-D total | 0.09 | 0.4 |
Notes: The correlations between the results of the Hamilton depression rating scale and of the Toronto Alexithymia scale, taking into consideration the total scores and the subscales of both instruments of assessment, are shown. Statistical significance is represented by: *P ≤ 0.05.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
In addition, an investigation of the possible correlations between the HAM-D factors and the TAS-20 subscales revealed a positive correlation between the HAM-D factor I and the TAS-20 subscale I (r = 0.3; P = 0.003).
Moreover, even the sample of the control group was divided into two groups: subjects aged 30–60 years and subjects aged 61–80 years. The correlation between the HAM-D factor I and the TAS-20 subscale I that was found in the whole control group, as reported above, was confirmed in the former group (aged 30–60 years) (r = 0.49; P = 0.01) but was not evidenced in the latter (aged 61–80 years). No differences in the tendency towards alexithymia were found when comparing the two groups (P = 0.5) (see Table 8).
Table 8.
The comparison between the subjects in the control group aged 30–60 years and those aged 61–80 years
| Age 30–60 years | Age 61–80 years | P-value | |
|---|---|---|---|
| Total number (female/male) | 25 (8/17) | 38 (10/28) | / |
| Age (years) | 46 ± 10.3 | 67.4 ± 5.44 | <0.001*** |
| Education (years) | 9.8 ± 3 | 8.6 ± 1 | <0.001*** |
| HAM-D total | 3.9 ± 2.2 | 3.4 ± 2.1 | 0.8 |
| HAM-D factor I | 1.3 ± 1.4 | 1.2 ± 1.1 | 0.2 |
| HAM-D factor II | 0.04 ± 0.2 | 0.03 ± 0.2 | 0.9 |
| HAM-D factor III | 1.1 ± 1 | 0.8 ± 0.9 | 0.5 |
| HAM-D factor IV | 0 | 0 | / |
| HAM-D factor V | 0.7 ± 1 | 0.9 ± 1.1 | 0.6 |
| HAM-D factor VI | 0.8 ± 1 | 0.5 ± 0.8 | 0.2 |
| TAS-20 total | 51.8 ± 14.1 | 50.5 ± 12.6 | 0.5 |
| TAS-20 subscale I | 16.6 ± 6.7 | 15.6 ± 6.2 | 0.6 |
| TAS-20 subscale II | 12.5 ± 4.3 | 13.4 ± 3.3 | 0.1 |
| TAS-20 subscale III | 22.7 ± 5 | 21.5 ± 5 | 1 |
Notes: The comparative statistical analysis, between individuals aged 30–60 years and 61–80 years, is shown, taking into consideration the total scores and the subscales of the Hamilton for depression rating scale as well as the Toronto Alexithymia scale. All values are presented as mean ± standard deviation, except for total number. Statistical significance is represented by: ***P < 0.001.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
The linear regression model applied did not change the strength of the associations found in Pearson’s correlation.
Depressed patients versus the control group
The comparative statistical analysis between the depressed patients and the subjects in the control group did not show statistically significant differences in the demographical characteristics. Obviously, the differences between the HAM-D total scores and the HAM-D factors were highly significant. However, no statistically significant differences were reported when comparing the TAS-20 total scores (P = 0.8) and the TAS-20 subscales, except for the TAS-20 subscale II (P = 0.03). See Table 9 for more details regarding the comparative statistical analysis between the depressed patients and the subjects in the control group.
Table 9.
The comparison between depressed patients and control subjects
| Depressed patients | Controls | P-value | |
|---|---|---|---|
| Total number | 75 | 63 | / |
| Age (years) | 54.8 ± 15.3 | 59 ± 13.05 | 0.2 |
| Education (years) | 9.1 ± 4.8 | 9.3 ± 5.1 | 0.6 |
| HAM-D total | 25.8 ± 8.2 | 3.6 ± 2.2 | <0.001*** |
| HAM-D factor I | 8 ± 3.2 | 1.2 ± 1.2 | <0.001*** |
| HAM-D factor II | 1.1 ± 1.4 | 0.03 ± 0.2 | <0.001*** |
| HAM-D factor III | 6 ± 3.5 | 0.9 ± 0.9 | <0.001*** |
| HAM-D factor IV | 2 ± 1.5 | 0 | / |
| HAM-D factor V | 6.3 ± 2.8 | 0.8 ± 1 | <0.001*** |
| HAM-D factor VI | 3 ± 2 | 0.6 ± 0.9 | <0.001*** |
| TAS-20 total | 57.3 ± 1.8 | 51 ± 13.1 | 0.8 |
| TAS-20 subscale I | 21.8 ± 6.9 | 16 ± 6.4 | 0.5 |
| TAS-20 subscale II | 14.6 ± 4.8 | 13 ± 3.7 | 0.03* |
| TAS-20 subscale III | 20.8 ± 5.5 | 22 ± 5 | 0.4 |
Notes: The comparative statistical analysis, between the depressed patients and the subjects in the control group, is shown, taking into consideration the total scores and the subscales of the Hamilton for depression rating scale as well as the Toronto Alexithymia scale. All values are presented as mean ± standard deviation, except for total number. Statistical significance is represented by: *P ≤ 0.05, ***P < 0.001.
Abbreviations: HAM-D, Hamilton rating scale for depression; TAS-20, 20-item Toronto Alexithymia scale.
Discussion and conclusion
From the statistical analysis of our sample, a positive correlation emerged between the HAM-D total score and the TAS-20 total score, and this was confirmed only in females. This data is consistent with literature: as a matter of fact, several studies have demonstrated a certain correlation between alexithymia and depression, and have also put forward the hypothesis that alexithymia might not be a stable personality construct, but a state-dependent trait which also relates to the severity of the depressive state.13,14 A correlation between the HAM-D factor V and all the TAS-20 subscales was found in the 30–60 year group; however, the data regarding a higher tendency towards alexithymia in young (30–60 years old) and better educated subjects is inconsistent with literature which reports exactly the opposite.17 The previously reported correlation between the TAS-20 subscale I (difficulty in identifying feelings) and the affective symptoms8 was confirmed in this study, not only among the depressed subjects, but also among the nondepressed ones; this data would suggest that the strong link between this subscale and the affective symptoms is not exclusively related to the presence of a clinically evident depressive state, at least among younger people (as is evident from the analysis of the group of people aged 30–60 years). Indeed, a more interesting data which, to the best of our knowledge, is still unpublished, is the positive correlation between the HAM-D factor V (psychomotor retardation) and the TAS-20 subscale III (externally oriented thinking and lack of introspective capacities) found among depressed patients. Practically, the more the patients tended to be poorly introspective, the more they presented psychomotor retardation, and vice versa. This correlation was confirmed among young and female patients.
Literature on this data is scarce due to the fact that studies have focused their attention on the opposite topic, or rather, on the high levels of introspection in depressed patients, thus explaining some typical aspects of MD, such as the focus on their (patients) negative aspects and the tendency towards ruminations.18,19 The correlation between psychomotor retardation and externally oriented thinking offers interesting food for thought. Psychomotor retardation is an important diagnostic and prognostic feature of MD:20 in fact, it is part of the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders Text Revision 4th edition (DSM-IV-TR),21 and it has been associated with greater lifetime severity of depression.22 The manifestations of psychomotor retardation, including slow speech, decreased movement, and impaired cognitive functions, are frequent among patients with melancholic and psychotic depression23 and, for some authors, these may be seen as indicators of bipolarity.22 In a study published in 2011, it was demonstrated that patients with high levels of psychomotor retardation exhibited an early age at onset, long duration of illness, high frequency of episodes, many lifetime suicide attempts and a positive family history of psychiatric disorders.22 The cognitive impairment related to psychomotor retardation could explain the association with impaired introspective capacities found in this study. Naturally, good introspective capacities need a good cognitive functioning, and it has been demonstrated that introspection relates to the quantity of gray matter in the brain.24 As to introspection, another fundamental aspect, related to it and worthwhile considering in the psychiatric field, is “insight”, more generally intended as the ability to recognize one’s own illness. In fact, despite the tremendous impact of MD in the patients’ lives, only a small number of patients seek the help of a doctor.25 Taking MD into consideration, in particular severe depression (as in the case of our sample), it is not unusual to find patients with impaired insight into awareness of their illness, into attribution of symptoms, and into the need for treatment.26 Indeed, it is easy to imagine how difficult it would be for an alexithymic patient to become aware of his/her disease condition, to correctly perceive and quantify his/her symptoms (or even the residual abilities), and to seek help. With regard to psychomotor retardation, according to cognitive theories, this could be related to the feelings of incapacity perceived by the patient: feelings of hopelessness and powerlessness, metaphorically conceptualized as “motor incapacity”, would affect the periphery, thus producing symptoms of lack of energy and psychomotor retardation.27 On the other hand, it is known that alexithymic patients are likely to present somatic depressive symptoms.28 Hence, it could be assumed that a patient with an externally oriented thinking and, therefore, with poor resources of introspection, might run into a distorted perception of his/her own ability to function, thus causing a psychomotor “fattening”. This condition confirms the feeling of incapacity and, consequently, as emerged in this study, a vicious circle is formed whereby a feeble introspection and psychomotor retardation positively correlate.
The females and younger patients seem to be more vulnerable to this psychopathological manifestation. Indeed, previous studies have evidenced that the TAS-20 subscale III seems to be a stable trait of the alexithymic construct without being influenced by changes in mood.13 One might venture the hypothesis (to be confirmed) that this specific personological construct might favour the manifestation of specific depressive symptoms. The hypotheses raised in this study require further confirmation due to the lack of some data (such as: the level of patients’ insight, the prevalence of the feelings of incapacity among patients with psychomotor retardation) which could be interesting to acquire in a future study. Furthermore, we have not reported follow-up data on the possible changes of the alexithymia levels after recovery from depression. Prospective controlled studies are needed. On the other hand, it is well known that the premorbid characteristics of patients play an important role in promoting the manifestation of certain subtypes of depression.29,30 With this in mind, the alexithymic construct is worthy of further investigation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Farges F, Corcos M, Speranza M, et al. Alexithymie et toxicomanie [Alexithymia, depression and drug addiction] Encephale. 2004;30:201–211. doi: 10.1016/s0013-7006(04)95431-0. French. [DOI] [PubMed] [Google Scholar]
- 2.Leweke F, Leichsenring F, Kruse J, Hermes S. Is alexithymia associated with specific mental disorders? Psychopathology. 2012;45:22–28. doi: 10.1159/000325170. [DOI] [PubMed] [Google Scholar]
- 3.Onur E, Alkın T, Sheridan MJ, Wise TN. Alexithymia and Emotional Intelligence in Patients with Panic Disorder, Generalized Anxiety Disorder and Major Depressive Disorder. Psychiatr Q. 2012 doi: 10.1007/s11126-012-9246-y. [In press] [DOI] [PubMed] [Google Scholar]
- 4.Honkalampi K, Hintikka J, Tanskanen A, Lehtonen J, Viinamäki H. Depression is strongly associated with alexithymia in the general population. J Psychosom Res. 2000;48:99–104. doi: 10.1016/s0022-3999(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 5.Bamonti PM, Heisel MJ, Topciu RA, Franus N, Talbot NL, Duberstein PR. Association of alexithymia and depression symptom severity in adults aged 50 years and older. Am J Geriatr Psychiatry. 2010;18:51–56. doi: 10.1097/JGP.0b013e3181bd1bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogrodniczuk JS, Piper WE, Joyce AS. Alexithymia as a predictor of residual symptoms in depressed patients who respond to short-term psychotherapy. Am J Psychother. 2004;58:150–161. doi: 10.1176/appi.psychotherapy.2004.58.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Resarch. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet A, Bréjard V, Pasquier A, Pedinielli JL. Affectivité et alexithymie: deux dimensions explicatives des relations entre symptômes dépressifs et anxieux [Affectivity and alexithymia: two dimensions explicative of the relationship between anxiety and depressive symptoms] Encephale. 2012;38:187–193. doi: 10.1016/j.encep.2011.03.006. French. [DOI] [PubMed] [Google Scholar]
- 9.Duddu V, Isaac MK, Chaturvedi SK. Alexithymia in somatoform and depressive disorders. J Psychosom Res. 2003;54:435–438. doi: 10.1016/s0022-3999(02)00440-3. [DOI] [PubMed] [Google Scholar]
- 10.Son SH, Jo H, Rim HD, et al. A Comparative Study on Alexithymia in Depressive, Somatoform, Anxiety, and Psychotic Disorders among Koreans. Psychiatry Investig. 2012;9:325–331. doi: 10.4306/pi.2012.9.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honkalampi K, Saarinen P, Hintikka J, Virtanen V, Viinamäki H. Factors associated with alexithymia in patients suffering from depression. Psychother Psychosom. 1999;68:270–275. doi: 10.1159/000012343. [DOI] [PubMed] [Google Scholar]
- 12.Wise TN, Mann LS, Randell P. The stability of alexithymia in depressed patients. Psychopathology. 1995;28:173–176. doi: 10.1159/000284919. [DOI] [PubMed] [Google Scholar]
- 13.Saarijärvi S, Salminen JK, Toikka TB. Alexithymia and depression: a 1-year follow-up study in outpatients with major depression. J Psychosom Res. 2001;51:729–733. doi: 10.1016/s0022-3999(01)00257-4. [DOI] [PubMed] [Google Scholar]
- 14.Honkalampi K, Hintikka J, Laukkanen E, Lehtonen J, Viinamäki H. Alexithymia and depression: a prospective study of patients with major depressive disorder. Psychosomatics. 2001;42:229–234. doi: 10.1176/appi.psy.42.3.229. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiat. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor GJ, Bagby RM, Parker JDA. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge Univ. Press; 1997. [Google Scholar]
- 17.Mattilaa AK, Salminenc JK, Nummia T, Joukamaaa M. Age is strongly associated with alexithymia in the general population. Journal of Psychosomatic Research. 2006;61:629–635. doi: 10.1016/j.jpsychores.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Grimm S, Ernst J, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: effects of self-focus and analytic thinking. J Abnorm Psychol. 2001;110:353–357. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- 20.Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders Text Revision (DSM IV-TR) 4th ed. Washington, DC: 2000. [Google Scholar]
- 22.Calugi S, Cassano GB, Litta A, et al. Does psychomotor retardation define a clinically relevant phenotype of unipolar depression? J Affect Disord. 2011;129:296–300. doi: 10.1016/j.jad.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329:1541–1543. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bebbington PE, Brugha TS, Meltzer H, et al. Neurotic disorders and the receipt of psychiatric treatment. Psychol Med. 2000;30:1369–1376. doi: 10.1017/s0033291799002974. [DOI] [PubMed] [Google Scholar]
- 26.Yen CF, Chen CC, Lee Y, Tang TC, Ko CH, Yen JY. Insight and correlates among outpatients with depressive disorders. Compr Psychiatry. 2005;46:384–389. doi: 10.1016/j.comppsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Lindeman LM, Abramson LY. The mental simulation of motor incapacity in depression. Journal of Cognitive Psychotherapy. 2008;22:228–249. [Google Scholar]
- 28.Sayar K, Kirmayer LJ, Taillefer SS. Predictors of somatic symptoms in depressive disorder. Gen Hosp Psychiatry. 2003;25:108–114. doi: 10.1016/s0163-8343(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 29.Iacovides A, Fountoulakis KN, Fotiou F, Fokas K, Nimatoudis I, Kaprinis G. Relation of personality disorders to subtypes of major depression according both to DSM-IV and ICD-10. Can J Psychiatry. 2002;47:196–197. [PubMed] [Google Scholar]
- 30.Corruble E. Personality and vulnerability to severe depression. Encephale. 2009;35(Suppl 7):S282–S285. doi: 10.1016/S0013-7006(09)73487-6. French. [DOI] [PubMed] [Google Scholar]


