Abstract
Atomic force microscopy (AFM) was used to directly measure the adhesion forces between three test proteins and low density polyethylene (LDPE) surfaces treated by glow discharge plasma to yield various levels of water wettability. The adhesion of proteins to the LDPE substrates showed a step dependence on the wettability of surfaces as measured by the water contact angle (θ). For LDPE surfaces with θ > ∼60–65°, stronger adhesion forces were observed for bovine serum albumin, fibrinogen and human FXII than for the surfaces with θ < 60°. Smaller adhesion forces were observed for FXII than for the other two proteins on all surfaces although trends were identical. Increasing the contact time from 0 to 50 s for each protein–surface combination increased the adhesion force regardless of surface wettability. Time varying adhesion data was fit to an exponential model and free energies of protein unfolding were calculated. This data, viewed in light of previously published studies, suggests a 2-step model of protein denaturation, an early stage on the order of seconds to minutes where the outer surface of the protein interacts with the substrate and a second stage involving movement of hydrophobic amino acids from the protein core to the protein/surface interface.
Impact statement
The work described in this manuscript shows a stark transition between protein adherent and protein non-adherent materials in the range of water contact angles 60–65°, consistent with known changes in protein adsorption and activity. Time-dependent changes in adhesion force were used to calculate unfolding energies relating to protein–surface interactions. This analysis provides justification for a 2-step model of protein denaturation on surfaces.
Keywords: AFM, Protein, Adhesion, Wettability
1. Introduction
Surface-induced thrombosis remains one of the main problems associated with the long term use of blood-contacting medical devices [1,2] and understanding the factors influencing thrombus formation is a key to the development and application of new biomaterials. It is well accepted that the protein adsorption is the first event following blood–material contact [3–5]. Protein adsorption is a nonspecific event and has been suggested to arise from solvent–protein interactions that provide an energetic basis to drive proteins from solution [6], solvent–surface interactions related to the adhesion of water to adsorbent surfaces [6], or as a result of one or more interactions between proteins and surfaces including van der Waal's interactions, electrostatic interactions, hydrogen bonding, and hydrophobic interactions [7–11].
Surface wettability (generally referred to as hydrophobicity/hydrophilicity) is one of the most important parameters affecting the biological response to an implanted material. Wettability affects protein adsorption, platelet adhesion/activation, blood coagulation and cell and bacterial adhesion [12–17]. However, observations regarding the effects of surface wettability on protein adhesion have not always been consistent. Generally hydrophobic surfaces are considered to be more protein-adsorbent than are hydrophilic surfaces because of the strong hydrophobic interactions occurring at these surfaces, in direct contrast to the repulsive solvation forces arising from strongly bound water at the hydrophilic surface [6,8,18]. The adhesion of proteins to a surface is a time-dependent process that can involve relatively large energy scales in addition to dynamic conformational changes and reorientation following contact with the surface [19–21]. Surface chemistry and wettability influence the time-dependent conformational changes in adsorbed proteins and mediate adsorption kinetics and binding strengths [22–24], as well as subsequent protein activity [25,26]. Several methods have been used to examine the conformation of proteins including antibody assays [27], circular dichroism [26,28], infrared spectroscopy [29], total internal reflection fluorescence [30], time-of-flight secondary ion mass spectrometry [31,32] and atomic force microscopy (AFM) [33,34]. AFM provides opportunities to examine not only the high resolution morphology, but also the interaction forces between protein and surface by either modifying AFM probes directly with the protein of interest [10,16,35] or by utilizing a protein-coated colloid [11]. AFM can also examine the interaction forces as the function of time by changing the time between initial contact and subsequent separation of the probe and surface [36–38].
The aim of the present work was to evaluate the effects of surface wettability on protein adhesion to polymeric biomaterial surfaces using AFM. A series of LDPE surfaces spanning a range of water wettability from hydrophilic to hydrophobic were obtained through glow-discharge plasma modification. Three different proteins were tested: bovine serum albumin (BSA, Fraction V, 69kDa), human fibrinogen (340 kDa) and human Factor XII (80 kDa). These three proteins are important participants in blood–material interactions including blood coagulation and thrombosis. Albumin is the most abundant protein in the circulatory system and it is believed that albumin adsorption would lead to passivation of a surface thereby slowing thrombus generation. Fibrinogen is a key structural glycoprotein involved in blood clotting by assembling to form a fibrin clot following thrombin activation [5]. Fibrinogen is also largely responsible for mediating platelet–surface interactions by serving as a ligand for the αIIbβ3 integrin receptor on the platelet membrane, while Factor XII is involved in contact activation of the intrinsic pathway of the blood coagulation cascade.
2. Materials and methods
2.1. General
Low-density polyethylene (Abiomed, Danvers, MA) was used as the base material for preparation of modified surfaces spanning a range of water wettability. Phosphate buffered saline (PBS) (150 mm NaCl, pH 7.4) was purchased as a powder from Sigma Chemicals and prepared using water from a Millipore Simplicity 185 System incorporating dual UV filters (185 and 254nm) to remove carbon contamination. Bovine serum albumin (BSA) was obtained from Sigma Chemical Co. (St. Louis, MO), human fibrinogen was from Calbiochem (La Jolla, CA), and Factor XII was purchased from Haematologic Technologies Inc. (Essex Junction, VT). All proteins were used as received.
2.2. Glow discharge plasma modification of LDPE substrates
A commercial glow discharge plasma cleaner (Harrick, Ithaca, NY) was used for modification of LDPE substrates. The chamber pressure was maintained at ∼200mTorr at a power of 100 W for time periods up to 150min. After plasma treatment, LDPE surfaces were either directly measured for surface wettability by water contact angle and subsequent AFM experiments or stored in a vacuum desiccator prior to use within 3 days. Sample wettabilities were measured immediately before use in AFM experiments.
2.3. Contact angle measurements
The water wettability of each LDPE sample was determined by sessile drop measurements of the advancing water contact angle (θ) using a Krüss contact angle goniometer. All measurements were made using PBS as a probe liquid. Advancing contact angles were measured by a minimum of eight independent measurements and are presented as mean ± standard deviation. The water adhesion tension (τ) was calculated by
| (1) |
where θ is the measured water contact angle and γ = 72.8 dyn/cm for water.
2.4. Protein modification of AFM probes
The three test proteins were covalently coupled to AFM probes having long-narrow Si3N4 triangular cantilevers (Veeco Instruments, Santa Barbara, CA, nominal k = 0.06 N/m). Probes were treated by glow discharge plasma at 100 W power for 30min and then incubated in a 1% (v/v) solution of aminopropyltriethoxysilane (Gelest Inc., PA) in ethanol for 1 h to provide reactive amine groups on the tip. After thoroughly rinsing with Millipore water, the probes were reacted with 10% gluteraldehyde in aqueous solution for 1 h. The probes were again rinsed with Millipore water to remove all glutaraldehyde from the solution after which the activated probes were incubated in protein solution (20 μg/ml) for 1 h. This attachment method has been shown to provide sufficient mobility and flexibility for proteins to rotate and orient themselves for binding [39,40]. The probes were rinsed with PBS after removal from protein solution and were stored in PBS at 4 °C until use within 2 days. Multiple probes (>3) were prepared at the same time to improve consistency between experiments.
2.5. Spring constant measurements
The spring constants of cantilevers (all taken from the same wafer) were determined using the thermal tuning method (Nanoscope V6.12r2) using a multimode AFM with a PicoForce attachment and Nanoscope IIIa control system (Veeco Instruments, Santa Barbara, CA). The average value of the spring constants was found to be 0.06 ± 0.01 N/m.
2.6. AFM measurements
All AFM experiments were performed using a Multimode AFM equipped with a Nanoscope IIIa controller system (Veeco Instruments, Santa Barbara, CA). The topography of the modified LDPE surfaces was visualized by tapping mode AFM imaging under ambient conditions using standard silicon probes (k∼20–75 N/m, NSC15, MikroMasch, Wilson-ville, OR). Average roughness (Rq) was analyzed by Nanoscope software (Version 5.12r3)
All force measurements were made under PBS at a vertical scan rate of 1 Hz with a z ramp size of 1 μm. The trigger mode was set at a relative deflection threshold of 100 nm so that the total loading force was ∼6.0 nN. Force data were collected using force volume imaging mode to obtain a 16 × 16 array of force curves over a scan area of 2 × 2 μm2 ensuring that no area was sampled multiple times. At least three different locations were examined for each sample with a specific wettability and multiple probes were used on each sample. To study the effects of contact time on adhesion forces, a delay in probe turnaround was implemented using the standard AFM software. The time needed for the tip to reach the desired loading force (∼6 nN) from the point of contacting the sample is ∼0.05 s and is not included in the contact time. Force measurements were performed with delay times ranging from 0–50 s at five random locations on each sample. Ten force curves were obtained at each location with a fixed contact time so that 50 measurements were made for each delay time.
The adhesion force was calculated from the distance between the zero deflection value (obtained from the noncontact portion of the force curve) to the point of maximum deflection during probe separation from the surface. A second value, termed the rupture distance, was measured as the piezo movement (during separation) between the point corresponding to zero cantilever deflection and the point where the probe underwent final complete separation from the sample. All AFM force data were extracted and analyzed offline with tools developed in Matlab™ (version 7.01, MathWorks Inc., MA).
2.7. Modeling of dynamic processes
Quantitative evaluation of the change in adhesion forces with contact time was modeled by a simple exponential of the form
| (2) |
where Fe is the adhesion force at t = 50 s (assumed to be equilibrium for sake of the model), F0 is an empirical coefficient related to the initial interaction force, t is the contact time, and ks is the rate constant determined by regression using the commercial software Microcal Origin 6.0. The rate constant was used to calculate an energy barrier for unfolding of the protein by using the Arrhenius equation
| (3) |
where T is the absolute temperature, Ea is the activation energy for protein unfolding, k is Boltzmans' constant and A(T) is a prefactor of 107 –109/s [41].
2.8. Data analysis
Statistical analysis of protein adhesion data was performed by ANOVA utilizing the commercial software program GraftPad Instat (version 3.06). p<0.05 was considered significant.
3. Results
3.1. Characteristics of plasma-treated LDPE surfaces
Plasma treatment was found to decrease the water contact angle of the LDPE surfaces (Table 1). The LDPE surface prior to plasma modification had an advancing water contact angle of 91 ± 2° and became more wettable following plasma treatment. Plasma treatment also led to minor changes in the topography of the PE surface. The topographic images show small islands distributed on the surface after plasma treatment (Fig. 1) and the dimensions of these island-like features generally decreased with plasma treatment time. Surface roughness of the substrates initially increased with time, from Rq = 4.6 ± 1.0 nm at 0 min to 12.4 ± 0.8nm at 60min, then decreased back to 5.5 ± 0.4 nm at 150min. All measurements were obtained using a scan area of 2 × 2 μm2 (Table 1).
Table 1. Characterization of LDPE surfaces after plasma treatment.
| Plasma treatment time (min) | 0 | 15 | 45 | 60 | 90 | 150 |
|---|---|---|---|---|---|---|
| Water contact angle (°) | 91±2 | 48±2 | 71±2 | 71±2 | 53±3 | 41±2 |
| Roughness (Rq) (nm) | 4.6±1.0 | 5.1±0.5 | 9.8±0.8 | 12.4±0.8 | 8.6±0.8 | 5.5±0.4 |
Fig. 1.
AFM topographic images of LDPE surfaces following plasma treatment. (a) 0, (b) 15, (c) 45, (d) 60, (e) 90, and (f)150 min. The mean water contact angle value of each surface is shown below the image. Scan size is 2 mm × 2 mm, z scale is 100 nm.
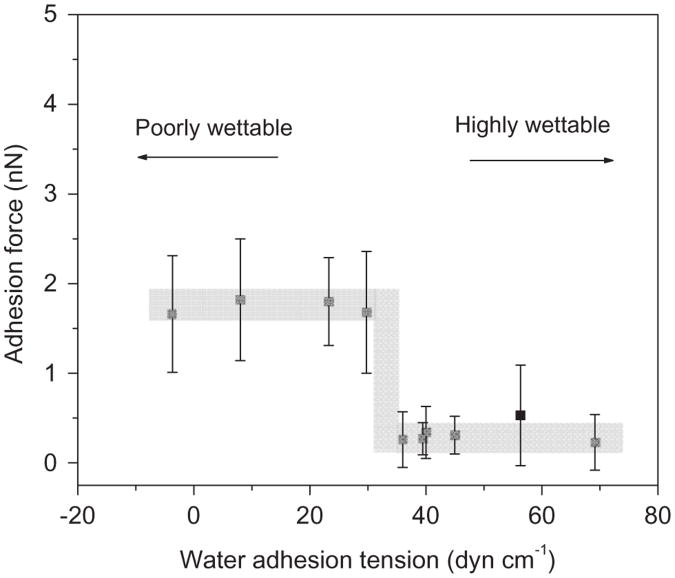
3.2. Adhesion forces for unmodified Si3N4 tip to LDPE surfaces
A series of control experiments were conducted using unmodified Si3N4 tips and the treated LDPE surfaces under PBS solution. Larger adhesion forces were consistently observed on the less wettable (more hydrophobic) surfaces than on the more wettable (hydrophilic) surfaces. Furthermore, adhesion forces were remarkably similar between the bare Si3N4 tip and all LDPE surfaces having τ≤30.8 dyn/cm (corresponding to water contact angle θ≥65°) with the average adhesion force being 1.7 ± 0.2nN (Fig. 2). Similarly, the adhesion forces between Si3N4 tips and the wettable LDPE surfaces with τ≥ 36.4 dyn/cm (θ≤60°) were also quite similar with values of just 0.3 ± 0.1nN. Thus, there appears to be a step dependence in the probe–surface adhesion forces at surface wettability values in the range of water contact angles ∼60–65°.
Fig. 2.
Average adhesion forces for Si3N4 probes to LDPE surfaces having different water adhesion tension values. Shaded area is drawn to aid the eye.
3.3. Adhesion forces between protein-coated probes and LDPE surfaces
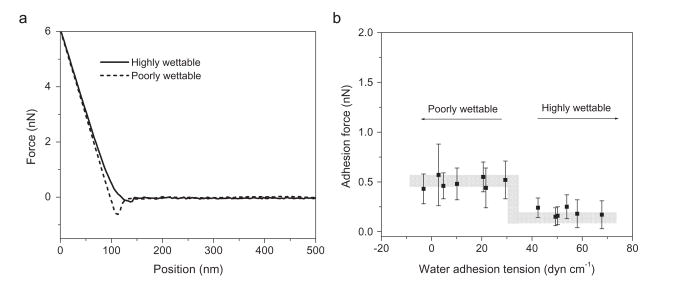
When BSA was covalently immobilized on the probe tip, the typical saw-tooth shaped retraction force curves often seen with proteins were observed, indicating multiple interactions between the protein and LDPE during the tip separation from the surfaces (Fig. 3a). However, these two curves show a striking difference in the adhesive forces between the protein-modified probes and the different substrates, with the wettable surfaces having a maximum adhesive force of just 0.3 nN while the maximum adhesion force on the poorly wettable surface was 2.0 nN. This difference between the wettable and poorly wettable substrates can be seen more clearly in Fig. 3b, illustrating the mean adhesive forces for all the different substrates against the BSA-modified probe. There is a pronounced step in the BSA/LDPE interactions, similar to that seen between the bare tip and the sample, indicating a difference between samples with contact angles ≤ 55° (τ≥ 41.8 dyn/cm) and surfaces with contact angles ≥62° (τ ≤ 34.2 dyn/cm) (Fig. 3b). Overall, the mean adhesive force for the protein-probe against the wettable surfaces was 0.4 ± 0.2 nN while for the poorly wettable surface the adhesive force value was 2.2 ± 0.4 nN. The separation curves also show longer rupture distances when the probe is removed from the poorly wettable surface, suggesting that the protein is more adherent to the less wettable surface and is being stretched during separation.
Fig. 3.
(a) Representative separation force curves for BSA coated probes and LDPE surfaces, showing larger adhesion forces for BSA and poorly wettable surfaces than BSA and wettable surfaces. (b) Average adhesion forces of BSA coated probes to LDPE surfaces with different water adhesion tension values. Shaded area is drawn to aid the eye.
Similar observations were seen for probes coupled with human fibrinogen when measured against the treated LDPE surfaces in PBS (Fig. 4). The interactions between the fibrinogen probes and modified LDPE surfaces again exhibited a step-like response to wettability (Fig. 4b) where surfaces with water contact angles ≥63° (τ≤33.0 dyn/cm) had average adhesion forces of 1.9 ± 0.2 nN and substrates with water contact angle ≤ 55° (τ ≤ 41.8 dyn/cm) had adhesion forces of 0.6 ± 0.2 nN. Fibrinogen also produced longer rupture distances compared to BSA (up to 400–500 nm) on the poorly wettable surfaces (Fig. 4a), presumably because fibrinogen is a much larger molecule having a rod-like shape ∼46 nm in length [42] that can be stretched to a greater extent than can albumin, which possesses a globular shape having dimensions ∼9 × 5.5 × 5.5 nm [43].
Fig. 4.
(a) Representative separation force curves for Human Fibrinogen coated probes with LDPE surfaces, showing larger adhesion forces to non-wettable surfaces than to wettable surfaces. (b) Average adhesion forces for Human Fibrinogen coated tips to LDPE surfaces with different water adhesion tension values. Shaded area is drawn to aid the eye.
Human FXII was the third protein studied and the trends for this protein were consistent with the other two, although the actual magnitudes of the forces were decreased in both cases (Fig. 5). Again a step exists in the region of water contact angle = 60°, with substrates having water contact angle ≥66° (τ≤29.6 dyn/cm) having adhesive forces of 0.5 ± 0.1 nN and substrates with water contact angle ≤54° (τ ≥ 42.8 dyn/cm) having average adhesion values of 0.2 ± 0.1 nN.
Fig. 5.
(a) Representative separation force curves for Human Factor XII coated probes with LDPE surfaces, showing larger adhesion forces for poorly wettable surface than wettable surfaces. (b) Average adhesion forces of HFXII coated tips to PE surfaces with different water adhesion tension values. Shaded area is drawn to aid the eye.
3.4. ANOVA analysis
Preliminary examination of the data suggested differences in the protein–surface adhesive forces for substrates having contact angles above or below ∼60–65°. ANOVA was performed for each of the protein–substrate combinations in order to test the accuracy of this observation. Similar results were obtained for each test protein; these are summarized in Table 2. Poorly wettable surfaces were always found to have statistically larger adhesive forces than highly wettable surfaces. This statistical analysis confirms the preliminary observation of a step change in adhesive forces at or around an advancing water contact angle of ∼60° for each of the proteins studied.
Table 2. ANOVA analysis of adhesion forces for BSA, fibrinogen, HFXII against modified LDPE surfaces.
| BSA |
|
Highly wettable | τ | Poorly wettable |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
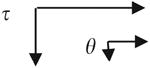
|
67.8 | 61.3 | 50.9 | 50.2 | 46.3 | 44.5 | 41.7 | 34.1 | 32.4 | 19.3 | 8.0 | 4.4 | |
| 21.3 | 32.7 | 45.6 | 46.4 | 50.5 | 52.3 | 55.0 | 62.1 | 63.6 | 74.6 | 83.7 | 86.5 | ||
| 67.8 | 21.3 | NS | NS | NS | NS | *** | NS | *** | *** | *** | *** | *** | |
| 61.3 | 32.7 | NS | NS | NS | *** | NS | *** | *** | *** | *** | *** | ||
| 50.9 | 45.6 | NS | NS | *** | NS | *** | *** | ** | *** | *** | |||
| 50.2 | 46.4 | NS | *** | NS | *** | *** | *** | *** | *** | ||||
| 46.3 | 50.5 | ** | NS | *** | *** | ** | *** | *** | |||||
| 44.5 | 52.3 | *** | *** | *** | *** | *** | *** | ||||||
| 41.7 | 55.0 | *** | *** | *** | *** | *** | |||||||
|
| |||||||||||||
| 34.1 | 62.1 | NS | NS | NS | NS | ||||||||
| 32.4 | 63.6 | NS | NS | *** | |||||||||
| 19.3 | 74.6 | NS | NS | ||||||||||
| 8.0 | 83.7 | NS | |||||||||||
| 4.4 | 86.5 | ||||||||||||
|
| |||||||||||||
| Fibrinogen |
|
Highly wettable | τ | Poorly wettable |
|
||||||||
|
| |||||||||||||
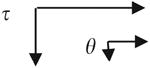
|
67.2 | 61.1 | 56.2 | 50.0 | 39.4 | 33.3 | 24.8 | 21.9 | 12.3 | 3.43 | 0.76 | -16.1 | |
| 22.7 | 33.0 | 39.5 | 46.6 | 57.2 | 62.8 | 70.1 | 72.5 | 80.3 | 87.3 | 89.4 | 102.8 | ||
|
| |||||||||||||
| 67.2 | 22.7 | *** | NS | NS | NS | *** | *** | *** | *** | *** | *** | *** | |
| 61.1 | 33.0 | NS | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| 56.2 | 39.5 | NS | *** | *** | ** | *** | *** | ** | *** | *** | |||
| 50.0 | 46.6 | NS | *** | *** | *** | *** | *** | *** | *** | ||||
| 39.4 | 57.2 | *** | *** | *** | *** | ** | *** | *** | |||||
|
| |||||||||||||
| 33.3 | 62.8 | NS | *** | *** | NS | NS | NS | ||||||
| 24.8 | 70.1 | NS | NS | NS | NS | NS | |||||||
| 21.9 | 72.5 | NS | NS | NS | NS | ||||||||
| 12.3 | 80.3 | *** | NS | NS | |||||||||
| 3.43 | 87.3 | NS | NS | ||||||||||
| 0.76 | 89.4 | NS | |||||||||||
| −16.1 | 102.8 | ||||||||||||
|
| |||||||||||||
| HFXII |
|
Highly wettable | τ | Poorly wettable |
|
||||||||
|
| |||||||||||||
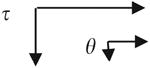
|
67.8 | 58.0 | 53.8 | 49.3 | 42.4 | 29.4 | 21.6 | 20.6 | 10.1 | 4.7 | 2.8 | -3.2 | |
| 21.3 | 37.2 | 42.3 | 47.3 | 54.4 | 66.2 | 72.7 | 73.6 | 82.0 | 86.3 | 87.8 | 92.5 | ||
|
| |||||||||||||
| 67.8 | 21.3 | NS | NS | NS | NS | *** | *** | *** | *** | *** | *** | *** | |
| 58.0 | 37.2 | NS | NS | NS | *** | *** | *** | *** | *** | *** | *** | ||
| 53.8 | 42.3 | NS | NS | *** | ** | *** | *** | ** | *** | *** | |||
| 49.3 | 47.3 | NS | *** | *** | *** | *** | *** | *** | *** | ||||
| 42.4 | 54.4 | *** | *** | *** | *** | ** | *** | *** | |||||
|
| |||||||||||||
| 29.4 | 66.2 | NS | NS | NS | NS | NS | NS | ||||||
| 21.6 | 72.7 | NS | NS | NS | * | NS | |||||||
| 20.6 | 73.6 | NS | NS | NS | NS | ||||||||
| 10.1 | 82.0 | NS | NS | NS | |||||||||
| 4.7 | 86.3 | NS | NS | ||||||||||
| 2.8 | 87.8 | ** | |||||||||||
| −3.2 | 92.5 | ||||||||||||
NS = not-significant,
= Significant (p<0.001),
= Significant (p<0.01),
= Significant (p<0.05).
3.5. Effect of contact time on adhesion forces of proteins to LDPE surfaces
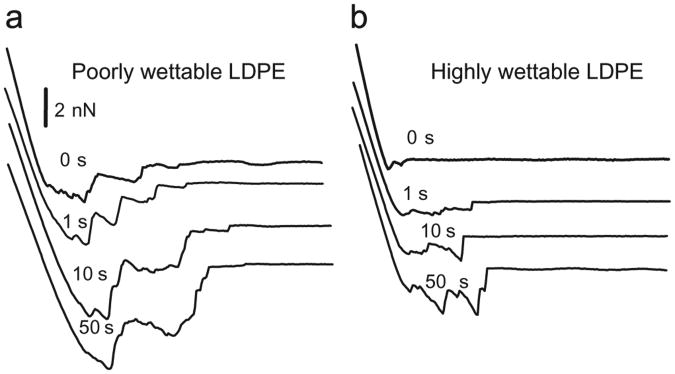
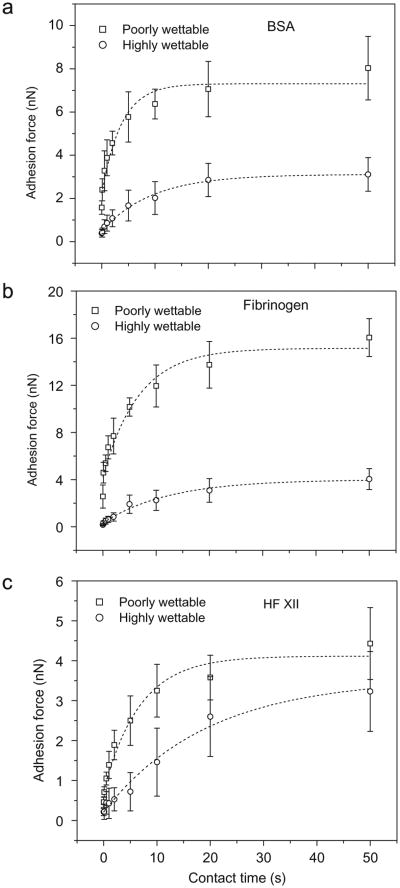
The time-dependence of protein–surface adhesion was investigated by adding a delay of up to 50 s between initial protein–surface contact and subsequent separation. In all cases, the adhesion forces between proteins and substrates were found to increase with increasing protein–surface contact time. Fig. 6 illustrates a representative series of separation force curves between BSA tips and a poorly wettable LDPE surface (Fig. 6a) and a wettable LDPE surface (Fig. 6b). Similar results were observed for both fibrinogen and FXII. Fig. 7 summarizes the average adhesion forces between each protein on LDPE surfaces at different contact times, again differentiating between the wettable and poorly wettable surfaces based on the transition observed in the previous section. Forces increased rapidly through a contact time range of 1–20 s, with only a slight further increase seen at 50 s for both wettable and poorly-wettable substrates.
Fig. 6.
Representative retraction curves for BSA probes and (a) poorly wettable LDPE (water contact angle = 83.7°) or (b) highly wettable LDPE (water contact angle = 50.5°) surfaces at increasing contact times.
Fig. 7.
Mean values of adhesion forces between protein-coated probes and LDPE surfaces with contact time, (a) BSA, (b) Fibrinogen, (c) HF XII. Curves illustrate fit of the exponential described in Eq. (2) to the experimental data.
An exponential model for the increase in adhesion force yielded fits to the data with R2 ≥ 0.95 (Fig. 7). The fits yielded rate constants of 0.15–0.27 s−1 for the non-wettable surfaces and 0.05–0.10s−1 for the wettable surfaces (Table 3). Application of these rate constants into the Arrhenius equation utilizing temperature-dependent pre-factors of 107–109 [41] yields energies of unfolding of 17.4–22.6 kT for the non-wettable surfaces and 18.4–23.7 kT for the wettable surfaces.
Table 3. Fitting parameters for exponential model and unfolding energies (Ea).
| Proteins | Non-wettable | Wettable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Fe (nN) | F0 (nN) | ks (1/s) | R2 | Ea (kT) | Fe (nN) | F0 (nN) | ks (1/s) | R2 | Ea (kT) | |
| BSA | 7.3±0.4 | 5.0±0.5 | 0.27±0.08 | 0.95 | 17.4–22.0 | 3.1±0.1 | 2.6±0.1 | 0.10±0.01 | 0.99 | 18.4–23.0 |
| Fibrinogen | 15.1±0.9 | 10.9±0.9 | 0.15±0.04 | 0.96 | 18.0–22.6 | 4.0±0.2 | 3.7±0.2 | 0.08±0.01 | 0.99 | 18.6–23.2 |
| HFXII | 4.1±0.2 | 3.4±0.3 | 0.15±0.03 | 0.97 | 18.0–22.6 | 3.6±0.3 | 3.3±0.3 | 0.05±0.01 | 0.98 | 19.1–23.7 |
4. Discussion
The three proteins that were tested in this study are all considered to be important in blood–material interactions. Albumin and fibrinogen behaved similarly with respect to adhesion although their size and roles in blood–material interactions are strikingly different; albumin is an 80 kDa protein considered a “passivating” protein while fibrinogen is a 340 kDa protein widely believed to be a prime mediator of surface thrombosis. Similar trends were also observed for FXII, a protein responsible for contact activation of the blood coagulation cascade although the absolute values of the adhesion forces were found to be substantially smaller. Statistical analysis of the adhesion force measurements demonstrate that proteins were more strongly adherent onto the poorly wettable surfaces than to the wettable surfaces, which is consistent with the observations of other investigators [18,25,44,45].
All of the proteins studied exhibited a step increase in adhesion force as the contact angles of the surface increased above θ∼60° (τ < 36.4 dyn/cm). The step occurred within a narrow range of wettabilities, apparently over the range of contact angles of ∼60–65° (Figs. 3b, 4b, 5b). These data suggest that this value of water wettability might then be viewed as a criterion for distinguishing a surface as either “protein adherent” or “protein non-adherent”. This is supported by other studies, including Yoon et al. who measured the hydrophobic (attractive) and hydrophilic (repulsive) forces on different wettable silica surfaces using AFM and suggested that hydrophobic forces were not supported on surfaces with θ <62.4° [12,46], and Berg et al. [47] who suggested a water contact angle limit of 65° for the observation of long range hydrophobic attractive forces on surfaces. A similar step change in protein adhesion force with wettability was observed in a previous study [16], where the step in adhesion force was observed between protein-modified AFM probes and self-assembled monolayer (SAM) surfaces and also during SAM/SAM interactions. In this current study, we extend these types of measurements to polymeric biomaterial surfaces which have more relevance to a clinical environment and present a challenge for force measurements due to additional nonspecific forces arising from an increase in the area of probe–surface contact due to compression of the polymer. The presence of the step increase in these adhesion forces suggests that the effect of surface wettability on protein adhesion for these three proteins is actually quite straightforward and that subtle changes in wettability will not be a useful tool in affecting protein adhesion to surfaces unless that change yields a transition across this θ = 60–65° region.
The constant adhesion forces observed across all of the wettable and all of the non-wettable surfaces also suggest that the small changes in surface roughness between these samples are relatively unimportant in protein adhesion. The plasma treatment produced LDPE surfaces with different roughness values (up to 3 × different Rq values) when contact angles were greater than 65° (Table 1 and Fig. 1). The fact that protein adhesion forces remained largely constant on these surfaces suggests that the nanometer scale topography of LDPE does not influence protein adhesion. Cai et al. [48] also reported that the surface roughness had little effect on protein adsorption and cell proliferation on titanium materials with roughness values in the range of 2–21 nm.
Increasing the protein–surface contact time consistently increased the adhesion forces for all three proteins on both the wettable and the poorly wettable surfaces (Fig. 7). This observation is suggestive of time-dependent physiochemical changes in proteins confined near the surface, consistent with adsorption-induced conformational changes. As contact time increases the protein undergoes conformational changes, presumably to move hydrophobic amino acids from the interior core of the protein to the surface where they can interact with the substrate. Similar effects of contact time on protein adhesion were also seen in other studies. Mondon [38] observed the adhesion force between a protein-modified AFM tip and titanium surfaces increased with interaction time, reaching a maximum adhesion force within ∼2s. Hemmerle et al. [49] observed multiple consecutive ruptures during tip retraction when a fibrinogen-coated AFM tip interacted with a silica surface, and found that the mean number and strength of these ruptures increased steadily with interaction time. They suggested that the extent of bonding increased with retention time resulting in increased adhesion forces. Conformational changes in proteins following adsorption was also directly seen in a previous study by our group where the heights of individual fibrinogen molecules were observed to undergo changes following adsorption to muscovite mica (θ< 10°) and to highly ordered pyrolitic graphite (θ∼110°) [33].
The Santore group has addressed fibrinogen adsorption and conformation changes utilizing total internal reflection fluorescence (TIRF) in a series of studies [41,50–52]. The results suggest that fibrinogen undergoes changes following adsorption that are consistent with an increase in molecular footprint and that cannot be explained by a simple transition from end-on to side-on adsorption. Santore used a similar Arrhenius calculation to yield an activation energy of 23–28 kT on hydrophobic surfaces. We have previously used direct measurements of conformational changes by atomic force microscopy and obtained a rate constant of 4.7 × 10−4 s−1, with a corresponding activation energy of unfolding of ∼37kT for fibrinogen on a hydrophobic surfaces [33], although we had used a slightly different prefactor in the Arrhenius analysis. Recalculating the previous data using the same rate constant but with a prefactor of 107–109 yields activation energies of 24–28 kT, similar to the range of values found previously by Santore and consistent with what we have now obtained in this current study.
It is somewhat surprising that similar rate constants are seen at these very early time points. Studies by Santore as well as our previous study suggested that it takes as much as hours for fibrinogen to reach a final conformational state. In this study, we are limited to a maximum contact time to just 50 s, but even at that relatively short time point we see very good fit to the exponential form as well as a range of activation energies that overlap the ranges from these previous studies. The rate constants are much higher in this study, however it should be noted that in this current study an applied force is being applied to the protein by compression with the AFM probe. Xu et al. [36] have shown that adhesion force increases dramatically with loading force even for two relatively incompressible substrates. This suggests that the applied loading force may increase the denaturation of the protein during protein–surface contact.
The observation of significant changes in adhesion force and presumably protein structure at early time points is consistent with our previous study using AFM imaging of individual proteins in which we found that when the curves illustrating domain height as a function of time were extrapolated back to t = 0, the heights were less than 50% of what is expected for the native fibrinogen structure. We speculated in that study that this might arise from a two-step spreading model, with the first step being very rapid (on the order of seconds to minutes) and involving rearrangement of protein surface amino acids and the second step taking much longer and involving rearrangement of the internal amino acids.
Such a process is consistent with the repeatability observed in this study. Each probe was used multiple times yet results remained consistent over the lifetime of the probe. There are two potential explanations for this observation. First, that the interaction forces measured over this less than 1 min time scale arise from rearrangement of functional groups at the outer protein surface. The proteins are likely to contact the surface slightly differently on each approach so that this surface process continues to occur even after repeated contacts. The second alternative is that the protein undergoes refolding back to the native or near-native structure after separation but prior to the next contact. However, at this time we have no reason to suspect one of these explanations over the other.
5. Conclusions
The interaction forces between protein-modified atomic force microscope probes and glow discharge plasma-modified LDPE surfaces were measured. The surface wettability was shown to be an important factor in protein adhesion to biomaterial surfaces. Bovine serum albumin, fibrinogen and FXII all exhibited similar behavior on the test materials, showing a step dependence in adhesion force as water contact angles transitioned across the region of ∼60–65°. The remarkable similarities in adhesion force across the full range of the wettable surfaces and the full range of the non-wettable surfaces suggest that there may be little that can be done to change protein adhesion to surfaces short of changing the wettability across this transitional water contact angle region. Protein adhesion forces were found to increase with contact time on all surfaces, consistent with surface-induced conformational changes in the proteins. Calculated energies of unfolding were consistent with previous studies measured by different techniques, although slightly smaller, presumably because the protein had not reached the final denatured state. Remarkably, the protein adhesion forces showed similar trends over time, suggesting that the protein either can refold after separation or that these early unfolding processes are largely independent of the original state of the protein.
Acknowledgments
The authors would like to acknowledge Dr. Bruce Logan for assistance with measurements of cantilever spring constants. The authors gratefully acknowledge financial support for this work provided by the National Institutes of Health (RO1 HL69965), the Dorothy Foehr Huck and J. Lloyd Huck Institutes of the Life Sciences and by a grant from the Pennsylvania Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- 1.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 2.Castner DG, Ratner BD. Biomedical surface science: foundations to frontiers. Surface Sci. 2002;500(1–3):28–60. [Google Scholar]
- 3.Horbett TA. Principles underlying the role of adsorbed plasma-proteins in blood interactions with foreign materials. Cardiovasc Pathol. 1993;2(3):S137–48. [Google Scholar]
- 4.Montdargent B, Letourneur D. Toward new biomaterials. Infection Control Hosp Epidemiol. 2000;21(6):404–10. doi: 10.1086/501782. [DOI] [PubMed] [Google Scholar]
- 5.Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127(22):8168–73. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 6.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials. 2006;27(34):5801–12. doi: 10.1016/j.biomaterials.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Heynes CANW. Globular proteins at solid/liquid interfaces. Colloids Surfaces B—Biointerfaces. 1994;2:517–66. [Google Scholar]
- 8.Israelachvili J, Wennerstrom H. Role of hydration and water structure in biological and colloidal interactions. Nature. 1996;379(6562):219–25. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- 9.Sit PS, Marchant RE. Surface-dependent differences in fibrin assembly visualized by atomic force microscopy. Surface Sci. 2001;491(3):421–32. [Google Scholar]
- 10.Kidoaki S, Matsuda T. Mechanistic aspects of protein/material interactions probed by atomic force microscopy. Colloids Surfaces B—Biointerfaces. 2002;23(2–3):153–63. [Google Scholar]
- 11.Xu LC, Logan BE. Interaction forces between colloids and protein-coated surfaces measured using an atomic force microscope. Environ Sci Technol. 2005;39(10):3592–600. doi: 10.1021/es048377i. [DOI] [PubMed] [Google Scholar]
- 12.Vogler EA. Water and the acute biological response to surfaces. J Biomater Sci—Polym Ed. 1999;10(10):1015–45. doi: 10.1163/156856299x00667. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee HB. Platelet adhesion onto wettability gradient surfaces in the absence and presence of plasma proteins. J Biomed Mater Res. 1998;41(2):304–11. doi: 10.1002/(sici)1097-4636(199808)41:2<304::aid-jbm16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Choee JH, Lee SJ, Lee YM, Rhee JM, Lee HB, Khang G. Proliferation rate of fibroblast cells on polyethylene surfaces with wettability gradient. J Appl Polym Sci. 2004;92(1):599–606. [Google Scholar]
- 15.Faucheux N, Schweiss R, Lutzow K, Werner C, Groth T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials. 2004;25(14):2721–30. doi: 10.1016/j.biomaterials.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 16.Sethuraman A, Han M, Kane RS, Belfort G. Effect of surface wettability on the adhesion of proteins. Langmuir. 2004;20(18):7779–88. doi: 10.1021/la049454q. [DOI] [PubMed] [Google Scholar]
- 17.Vogler EA, Graper JC, Harper GR, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade. 1. Procoagulant surface energy and chemistry. J Biomed Mater Res. 1995;29:1005–16. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]
- 18.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A survey of structure–property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17(18):5605–20. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 19.Tan JS, Martic PA. Protein adsorption and conformational change on small polymer particles. J Colloid Interface Sci. 1990;136(2):415–31. [Google Scholar]
- 20.Lee SJ, Park K. Protein-interaction with surfaces—separation distance-dependent interaction energies. J Vac Sci Technol a—Vac Surfaces Films. 1994;12(5):2949–55. [Google Scholar]
- 21.Buijs J, Hlady V. Adsorption kinetics, conformation, and mobility of the growth hormone and lysozyme on solid surfaces, studied with TIRF. J Colloid Interface Sci. 1997;190(1):171–81. doi: 10.1006/jcis.1997.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont-Gillain CC, Fauroux CMJ, Gardner DCJ, Leggett GJ. Use of AFM to probe the adsorption strength and time-dependent changes of albumin on self-assembled monolayers. J Biomed Mater Res Part A. 2003;67A(2):548–58. doi: 10.1002/jbm.a.10092. [DOI] [PubMed] [Google Scholar]
- 23.Fang F, Satulovsky J, Szleifer I. Kinetics of protein adsorption and desorption on surfaces with grafted polymers. Biophys J. 2005;89(3):1516–33. doi: 10.1529/biophysj.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agashe M, Raut V, Stuart SJ, Latour RA. Molecular simulation to characterize the adsorption behavior of a fibrinogen gamma-chain fragment. Langmuir. 2005;21(3):1103–17. doi: 10.1021/la0478346. [DOI] [PubMed] [Google Scholar]
- 25.Wu YG, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J Biomed Mater Res Part A. 2005;74A(4):722–38. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 26.Hylton DM, Shalaby SW, Latour RA. Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J Biomed Mater Res Part A. 2005;73A(3):349–58. doi: 10.1002/jbm.a.30295. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg ME. Investigating protein conformation, dynamics and folding with monoclonal-antibodies. Trends Biochem Sci. 1991;16(10):358–62. doi: 10.1016/0968-0004(91)90148-o. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield NJ. Applications of circular dichroism in protein and peptide analysis. Trac-Trends Anal Chem. 1999;18(4):236–44. [Google Scholar]
- 29.Chittur KK. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials. 1998;19(4–5):357–69. doi: 10.1016/s0142-9612(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 30.Bos MA, Kleijn JM. Determination of the orientation distribution of adsorbed fluorophores using Tirf. 1. Theory. Biophys J. 1995;68(6):2566–72. doi: 10.1016/S0006-3495(95)80439-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lhoest J-BED, van den Bosch de Aguilar P, Bertrand P. Fibronectin adsorption, conformation, and orientation on polystyrene substrates studied by radiolabeling, XPS, and ToF SIMS. J Biomed Mater Res. 1998;41(1):95–103. doi: 10.1002/(sici)1097-4636(199807)41:1<95::aid-jbm12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Lhoest JBMSW, Tidwell CD, Castner DG. Characterization of adsorbed protein films by time of flight secondary ion mass spectrometry. J Biomed Mater Res. 2001;57(3):432–40. doi: 10.1002/1097-4636(20011205)57:3<432::aid-jbm1186>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Agnihotri A, Siedlecki CA. Time-dependent conformational changes in fibrinogen measured by atomic force microscopy. Langmuir. 2004;20(20):8846–52. doi: 10.1021/la049239+. [DOI] [PubMed] [Google Scholar]
- 34.Sit PS, Marchant RE. Surface-dependent conformations of human fibrinogen observed by atomic force microscopy under aqueous conditions. Thrombosis Haemostasis. 1999;82:1053–60. [PubMed] [Google Scholar]
- 35.Kidoaki S, Matsuda T. Adhesion forces of the blood plasma proteins on self-assembled monolayer surfaces of alkanethiolates with different functional groups measured by an atomic force microscope. Langmuir. 1999;15(22):7639–46. [Google Scholar]
- 36.Xu LC, Vadillo-Rodriguez V, Logan BE. Residence time, loading force, pH, and ionic strength affect adhesion forces between colloids and biopolymer-coated surfaces. Langmuir. 2005;21(16):7491–500. doi: 10.1021/la0509091. [DOI] [PubMed] [Google Scholar]
- 37.Xu LC, Logan BE. Interaction forces measured using AFM between colloids and surfaces coated with both dextran and protein. Langmuir. 2006;22(10):4720–7. doi: 10.1021/la053443v. [DOI] [PubMed] [Google Scholar]
- 38.Mondon M, Berger S, Ziegler C. Scanning-force techniques to monitor time-dependent changes in topography and adhesion force of proteins on surfaces. Anal Bioanal Chem. 2003;375(7):849–55. doi: 10.1007/s00216-003-1751-2. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury PB, Luckham PF. Probing recognition process between an antibody and an antigen using atomic force microscopy. Colloids Surfaces A—Physicochem Eng Aspects. 1998;143(1):53–7. [Google Scholar]
- 40.Agnihotri A, Siedlecki CA. Adhesion mode atomic force microscopy study of dual component protein films. Ultramicroscopy. 2005;102(4):257–68. doi: 10.1016/j.ultramic.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Santore MM, Wertz CF. Protein spreading kinetics at liquid–solid interfaces via an adsorption probe method. Langmuir. 2005;21(22):10172–8. doi: 10.1021/la051059s. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Kollman JM, Pandi L, Doolittle RF. Crystal structure of native chicken fibrinogen at 2.7 angstrom resolution. Biochemistry. 2001;40(42):12515–23. doi: 10.1021/bi011394p. [DOI] [PubMed] [Google Scholar]
- 43.Rezwan K, Meier LP, Rezwan M, Voros J, Textor M, Gauckler LJ. Bovine serum albumin adsorption onto colloidal Al2O3 particles: a new model based on zeta potential and UV–vis measurements. Langmuir. 2004;20(23):10055–61. doi: 10.1021/la048459k. [DOI] [PubMed] [Google Scholar]
- 44.Sethuraman A, Vedantham G, Imoto T, Przybycien T, Belfort G. Protein unfolding at interfaces: slow dynamics of alpha-helix to beta-sheet transition. Proteins—Struct Funct Bioinformatics. 2004;56(4):669–78. doi: 10.1002/prot.20183. [DOI] [PubMed] [Google Scholar]
- 45.Sigal GB, Mrksich M, Whitesides GM. Effect of surface wettability on the adsorption of proteins and detergents. J Am Chem Soc. 1998;120(14):3464–73. [Google Scholar]
- 46.Yoon RH, Flinn DH, Rabinovich YI. Hydrophobic interactions between dissimilar surfaces. J Colloid Interface Sci. 1997;185(2):363–70. doi: 10.1006/jcis.1996.4583. [DOI] [PubMed] [Google Scholar]
- 47.Berg JM, Eriksson LGT, Claesson PM, Borve KGN. 3-Component Langmuir–Blodgett-films with a controllable degree of polarity. Langmuir. 1994;10(4):1225–34. [Google Scholar]
- 48.Cai KY, Bossert J, Jandt KD. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surfaces B—Biointerfaces. 2006;49(2):136–44. doi: 10.1016/j.colsurfb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Hemmerle J, Altmann SM, Maaloum M, Horber JKH, Heinrich L, Voegel JC, et al. Direct observation of the anchoring process during the adsorption of fibrinogen on a solid surface by force-spectroscopy mode atomic force microscopy. Proc Natl Acad Sci USA. 1999;96(12):6705–10. doi: 10.1073/pnas.96.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wertz CF, Santore MM. Adsorption and relaxation kinetics of albumin and fibrinogen on hydrophobic surfaces: single-species and competitive behavior. Langmuir. 1999;15(26):8884–94. [Google Scholar]
- 51.Wertz CF, Santore MM. Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single-species and competitive behavior. Langmuir. 2001;17(10):3006–16. [Google Scholar]
- 52.Wertz CF, Santore MM. Fibrinogen adsorption on hydrophilic and hydrophobic surfaces: geometrical and energetic aspects of interfacial relaxations. Langmuir. 2002;18(3):706–15. [Google Scholar]