Abstract
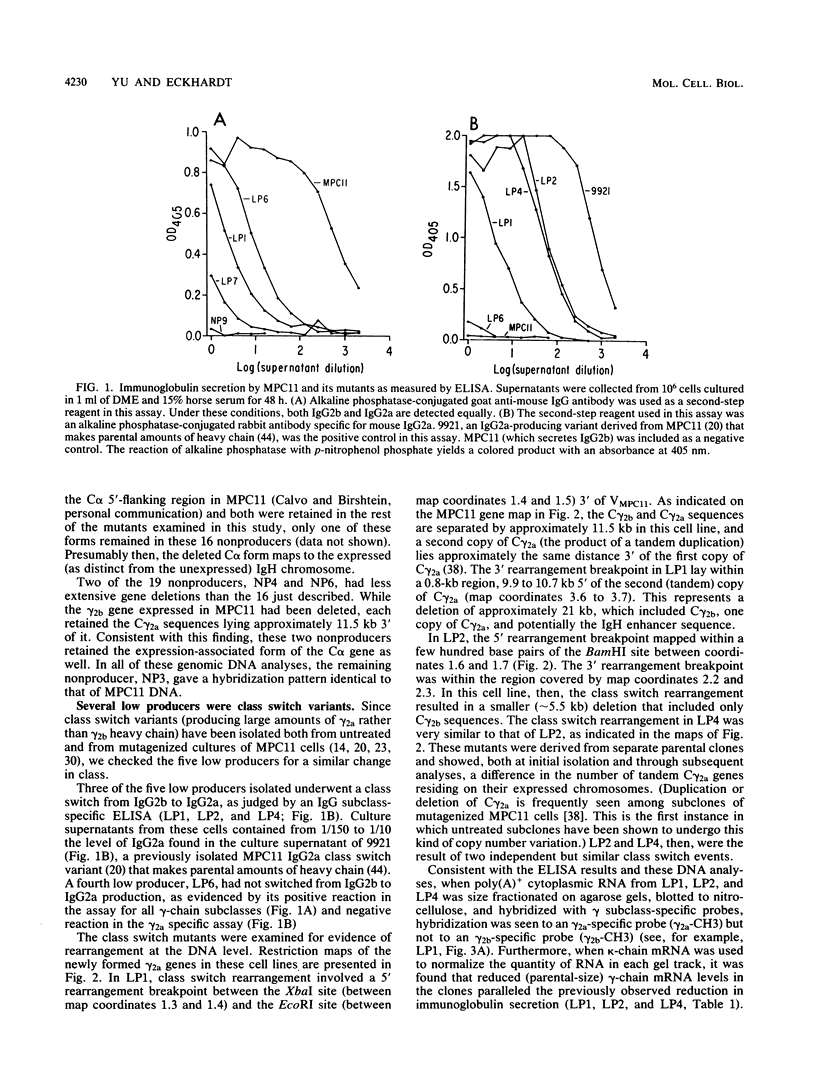
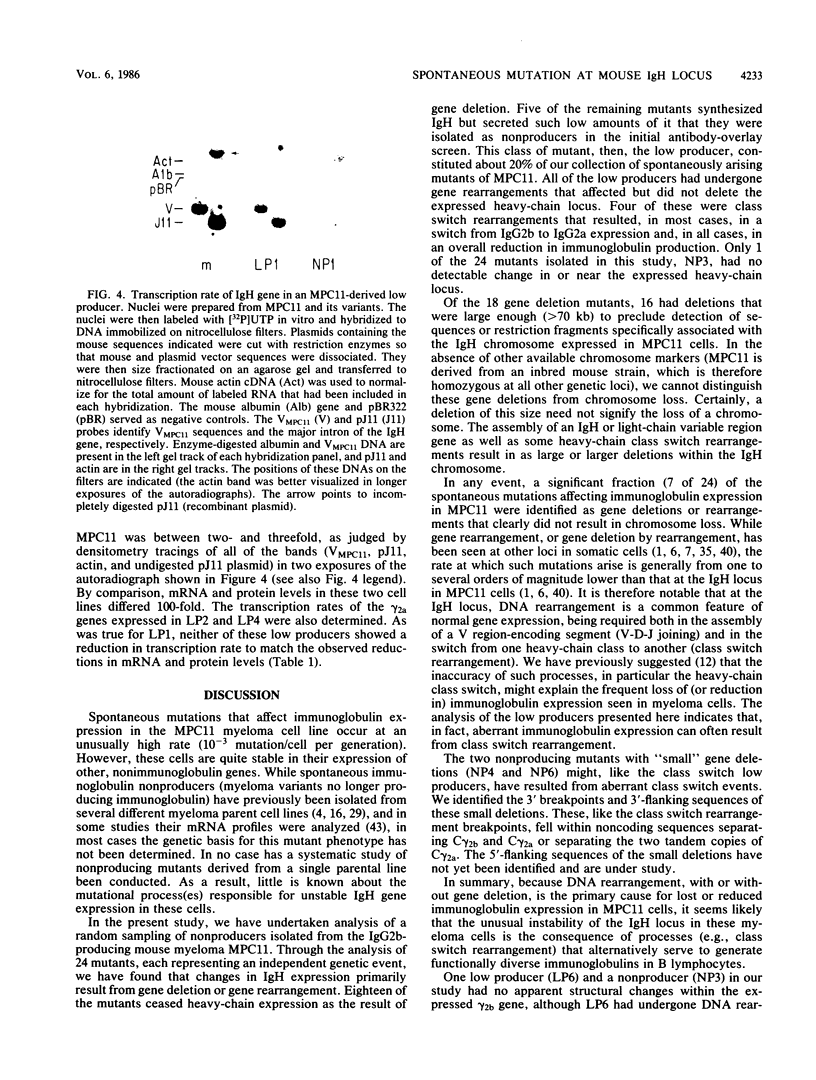
The spontaneous mutation rate of immunoglobulin genes expressed in myeloma cells is well above that of other genes expressed in these or in other cell types. The nature of such mutations in one myeloma cell line, MPC11, was explored at the molecular level. Included in this study were MPC11 variants representing 24 independent and spontaneous mutations affecting immunoglobulin secretion. Of the mutants studied, 19 had ceased immunoglobulin heavy chain (IgH) production (nonproducers), and 5 produced from as little as 1/1,000 to as much as 1/10 the amount of immunoglobulin produced by MPC11 (low producers). Only one of the MPC11 mutants (a nonproducer) showed no evidence of DNA rearrangement in or near the expressed IgH gene. The formerly expressed gamma 2b gene had been deleted in 18 of the 19 nonproducers. All of the low producers had undergone DNA rearrangement in or near the expressed IgH gene, and three of them produced immunoglobulin of a new heavy chain class. The cause for reduced heavy-chain synthesis in the low producers is not yet known. However, in several of these mutants, the defect appeared to be posttranscriptional. In these cell lines, steady-state IgH mRNA levels were much lower than in the parent cell line, while the heavy-chain gene transcription rate remained unchanged.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Nageotte R., Chambraud B., Rougeon F. Mouse immunoglobulin genes: a bacterial plasmid containing the entire coding sequence for a pre-gamma 2a heavy chain. Nucleic Acids Res. 1980 Mar 25;8(6):1231–1241. doi: 10.1093/nar/8.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. E., Letovanec D. High-frequency nonrandom mutational event at the adenine phosphoribosyltransferase (aprt) locus of sib-selected CHO variants heterozygous for aprt. Somatic Cell Genet. 1982 Jan;8(1):51–66. doi: 10.1007/BF01538650. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt L. A., Birshtein B. K. Independent immunoglobulin class-switch events occurring in a single myeloma cell line. Mol Cell Biol. 1985 Apr;5(4):856–868. doi: 10.1128/mcb.5.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt L. A., Tilley S. A., Lang R. B., Marcu K. B., Birshtein B. K. DNA rearrangements in MPC-11 immunoglobulin heavy chain class-switch variants. Proc Natl Acad Sci U S A. 1982 May;79(9):3006–3010. doi: 10.1073/pnas.79.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francus T., Dharmgrongartama B., Campbell R., Scharff M. D., Birshtein B. K. IgG2a-producing variants of an IgG2b-producing mouse myeloma cell line. J Exp Med. 1978 Jun 1;147(6):1535–1550. doi: 10.1084/jem.147.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Klein S., Gerster T., Picard D., Radbruch A., Schaffner W. Evidence for transient requirement of the IgH enhancer. Nucleic Acids Res. 1985 Dec 20;13(24):8901–8912. doi: 10.1093/nar/13.24.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Sablitzky F., Radbruch A. Deletion of the IgH enhancer does not reduce immunoglobulin heavy chain production of a hybridoma IgD class switch variant. EMBO J. 1984 Nov;3(11):2473–2476. doi: 10.1002/j.1460-2075.1984.tb02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskimies S., Birshtein B. K. Primary and secondary variants in immunoglobulin heavy chain production. Nature. 1976 Dec 2;264(5585):480–482. doi: 10.1038/264480a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Potash M. J., Lehrach H., Shulman M. J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1(5):555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang B., Radbruch A., Rajewsky K. Isolation of myeloma variants with predefined variant surface immunoglobulin by cell sorting. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3901–3905. doi: 10.1073/pnas.75.8.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk R. J., Morrison S. L., Milcarek C. Heavy-chain mutants derived from gamma 2b mouse myeloma: characterization of heavy-chain messenger ribonucleic acid, proteins, and secretion in delection mutants and messenger ribonucleic acid in gamma2a mutant progeny. Biochemistry. 1981 Apr 14;20(8):2330–2339. doi: 10.1021/bi00511a040. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W. High-frequency mutation at the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells due to deletion of the gene. Proc Natl Acad Sci U S A. 1983 Feb;80(3):810–814. doi: 10.1073/pnas.80.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Yang J. Q., Eckhardt L. A., Harris L. J., Birshtein B. K., Marcu K. B. Products of a reciprocal chromosome translocation involving the c-myc gene in a murine plasmacytoma. Proc Natl Acad Sci U S A. 1984 Feb;81(3):829–833. doi: 10.1073/pnas.81.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley S. A., Birshtein B. K. Unequal sister chromatid exchange. A mechanism affecting Ig gene arrangement and expression. J Exp Med. 1985 Aug 1;162(2):675–694. doi: 10.1084/jem.162.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Wabl M. R., Burrows P. D. Expression of immunoglobulin heavy chain at a high level in the absence of a proposed immunoglobulin enhancer element in cis. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2452–2455. doi: 10.1073/pnas.81.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach M., Ishay-Michaeli R., Givol D., Laskov R. Analysis of immunoglobulin mRNA in murine myeloma cell variants defective in the synthesis of the light or heavy polypeptide chains. J Immunol. 1982 Feb;128(2):684–690. [PubMed] [Google Scholar]
- Zaller D. M., Eckhardt L. A. Deletion of a B-cell-specific enhancer affects transfected, but not endogenous, immunoglobulin heavy-chain gene expression. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5088–5092. doi: 10.1073/pnas.82.15.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]