Abstract
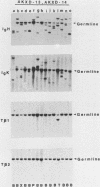
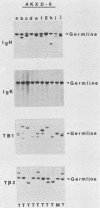
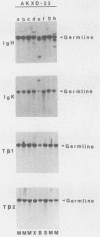
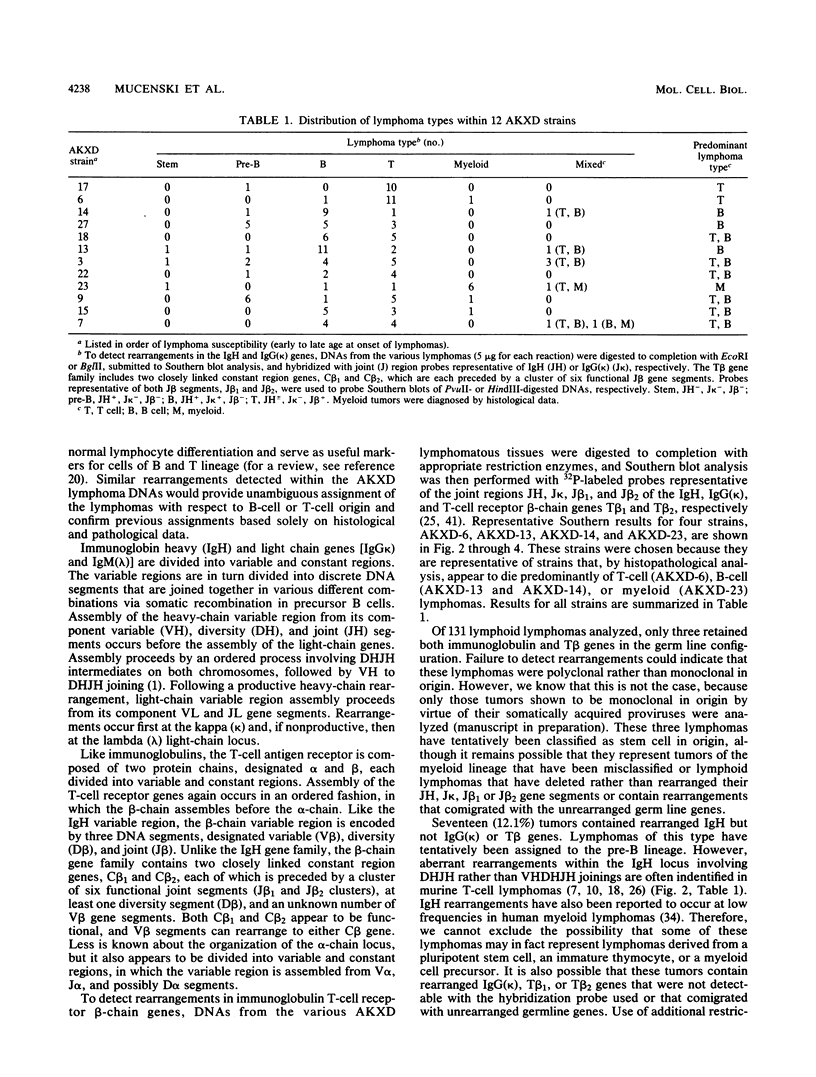
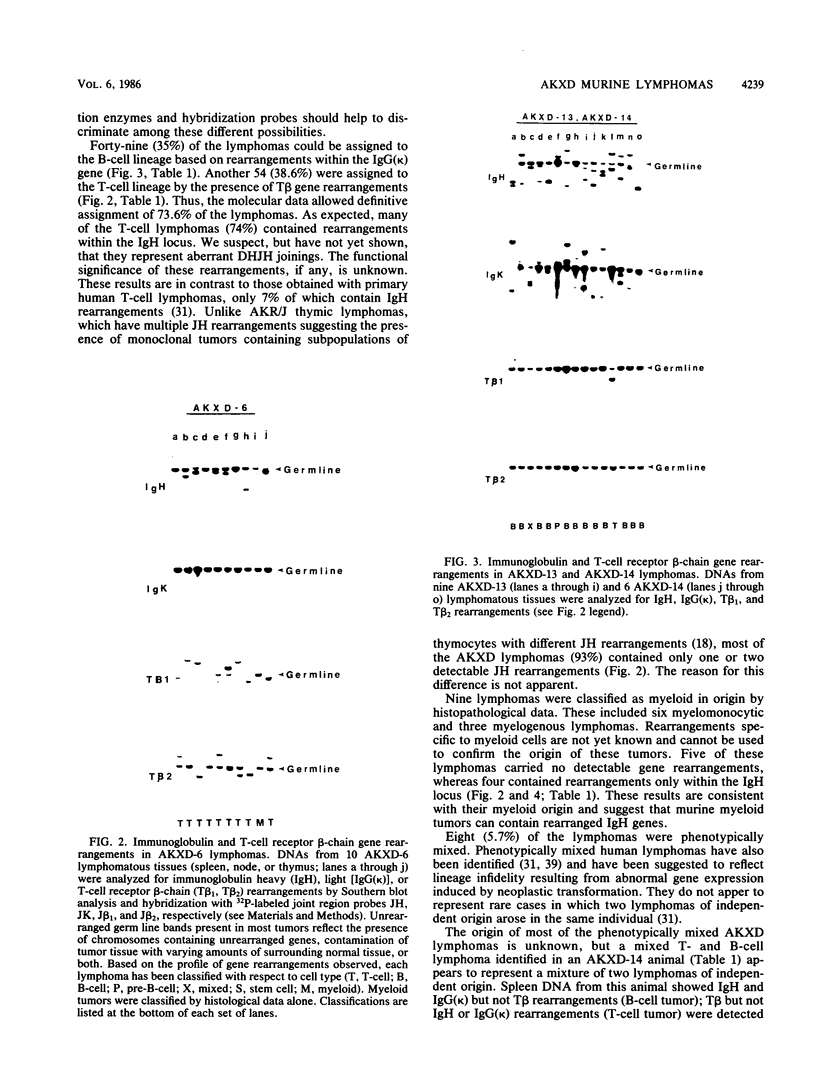
We analyzed the lymphoma susceptibility of 13 AKXD recombinant inbred mouse strains derived from AKR/J, a highly lymphomatous strain, and DBA/2J, a weakly lymphomatous strain. Of the 13 strains used, 12 showed a high incidence of lymphoma development. However, the average age at onset of lymphoma varied considerably among the different AKXD strains, suggesting that they have segregated several loci that affect lymphoma susceptibility. A relatively unambiguous classification of lymphomas was made possible by using histopathology in addition to detailed molecular characterization of rearrangements in immunoglobulin heavy and kappa light genes and in T-cell receptor beta-chain genes. Among the 12 highly lymphomatous strains, only 2 were identified that, like the parental AKR/J strain, died primarily of T-cell lymphomas. Three strains died primarily of B-cell lymphomas, and one strain primarily of myeloid lymphomas. Six strains were susceptible to both T-cell and B-cell lymphomas. Thus, these strains have segregated genes that affect both lymphoma susceptibility and lymphoma type and should prove to be useful models for studying the molecular genetic basis of murine lymphomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel J. M., Bedigian H. G. Expression of murine leukemia viruses in B-cell lymphomas of CWD/Agl mice. J Virol. 1984 Nov;52(2):691–694. doi: 10.1128/jvi.52.2.691-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedigian H. G., Johnson D. A., Jenkins N. A., Copeland N. G., Evans R. Spontaneous and induced leukemias of myeloid origin in recombinant inbred BXH mice. J Virol. 1984 Sep;51(3):586–594. doi: 10.1128/jvi.51.3.586-594.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lilly F. Suppression of spontaneous lymphoma by previously undiscovered dominant genes in crosses of high- and low-incidence mouse strains. Virology. 1982 Apr 15;118(1):76–85. doi: 10.1016/0042-6822(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Bedigian H. G., Thomas C. Y., Jenkins N. A. DNAs of two molecularly cloned endogenous ecotropic proviruses are poorly infectious in DNA transfection assays. J Virol. 1984 Feb;49(2):437–444. doi: 10.1128/jvi.49.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A., Hobart M., Hengartner H., Rabbitts T. H. An immunoglobulin heavy-chain gene is altered in two T-cell clones. Nature. 1980 Aug 28;286(5776):897–899. doi: 10.1038/286897a0. [DOI] [PubMed] [Google Scholar]
- Graham M., Adams J. M., Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. 1985 Apr 25-May 1Nature. 314(6013):740–743. doi: 10.1038/314740a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Yetter R. A., Morse H. C., 3rd A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J Exp Med. 1983 Jul 1;158(1):16–24. doi: 10.1084/jem.158.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig J. S., Glimcher L. H., Kranz D. M., Clayton L. K., Greenstein J. L., Saito H., Maxam A. M., Burakoff S. J., Eisen H. N., Tonegawa S. Expression of the T-cell-specific gamma gene is unnecessary in T cells recognizing class II MHC determinants. Nature. 1985 Sep 5;317(6032):68–70. doi: 10.1038/317068a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Free and integrated recombinant murine leukemia virus DNAs appear in preleukemic thymuses of AKR/J mice. J Virol. 1984 Apr;50(1):155–162. doi: 10.1128/jvi.50.1.155-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Perlmutter A. P., Gilbert W. Monoclonal AKR/J thymic leukemias contain multiple JH immunoglobulin gene rearrangements. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7433–7436. doi: 10.1073/pnas.80.24.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Ishimoto A., Adachi A., Sakai K., Matsuyama M. Long terminal repeat of Friend-MCF virus contains the sequence responsible for erythroid leukemia. Virology. 1985 Feb;141(1):30–42. doi: 10.1016/0042-6822(85)90180-1. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Frith C. H. Immunomorphologic classification of spontaneous lymphoid cell neoplasms occurring in female BALB/c mice. J Natl Cancer Inst. 1983 Jan;70(1):169–179. [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Boelens W., van Wezenbeek P., Cuypers T., Maandag E. R., Selten G., Berns A. Generation of AKR mink cell focus-forming viruses: a conserved single-copy xenotrope-like provirus provides recombinant long terminal repeat sequences. J Virol. 1984 May;50(2):432–438. doi: 10.1128/jvi.50.2.432-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovigatti U., Mirro J., Kitchingman G., Dahl G., Ochs J., Murphy S., Stass S. Heavy chain immunoglobulin gene rearrangement in acute nonlymphocytic leukemia. Blood. 1984 May;63(5):1023–1027. [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Genes affecting mink cell focus-inducing (MCF) murine leukemia virus infection and spontaneous lymphoma in AKR F1 hybrids. J Exp Med. 1983 Aug 1;158(2):353–364. doi: 10.1084/jem.158.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Potter M., Mushinski J. F., Lavu S., Reddy E. P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984 Nov 30;226(4678):1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- Silver J., Kozak C. Common proviral integration region on mouse chromosome 7 in lymphomas and myelogenous leukemias induced by Friend murine leukemia virus. J Virol. 1986 Feb;57(2):526–533. doi: 10.1128/jvi.57.2.526-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. J., Curtis J. E., Messner H. A., Senn J. S., Furthmayr H., McCulloch E. A. Lineage infidelity in acute leukemia. Blood. 1983 Jun;61(6):1138–1145. [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Quint W., Cuypers T., Radaszkiewicz T., Schoenmakers H., de Goede R., Melief C. Ecotropic and mink cell focus-forming murine leukemia viruses integrate in mouse T, B, and non-T/non-B cell lymphoma DNA. J Virol. 1986 Mar;57(3):1037–1047. doi: 10.1128/jvi.57.3.1037-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., de Goede R. E., Schoenmakers H., Radaszkiewicz T., Melief C. J. Ecotropic and dualtropic mink cell focus-inducing murine leukemia viruses can induce a wide spectrum of H-2 controlled lymphoma types. Virology. 1984 Oct 30;138(2):198–211. doi: 10.1016/0042-6822(84)90345-3. [DOI] [PubMed] [Google Scholar]