Abstract
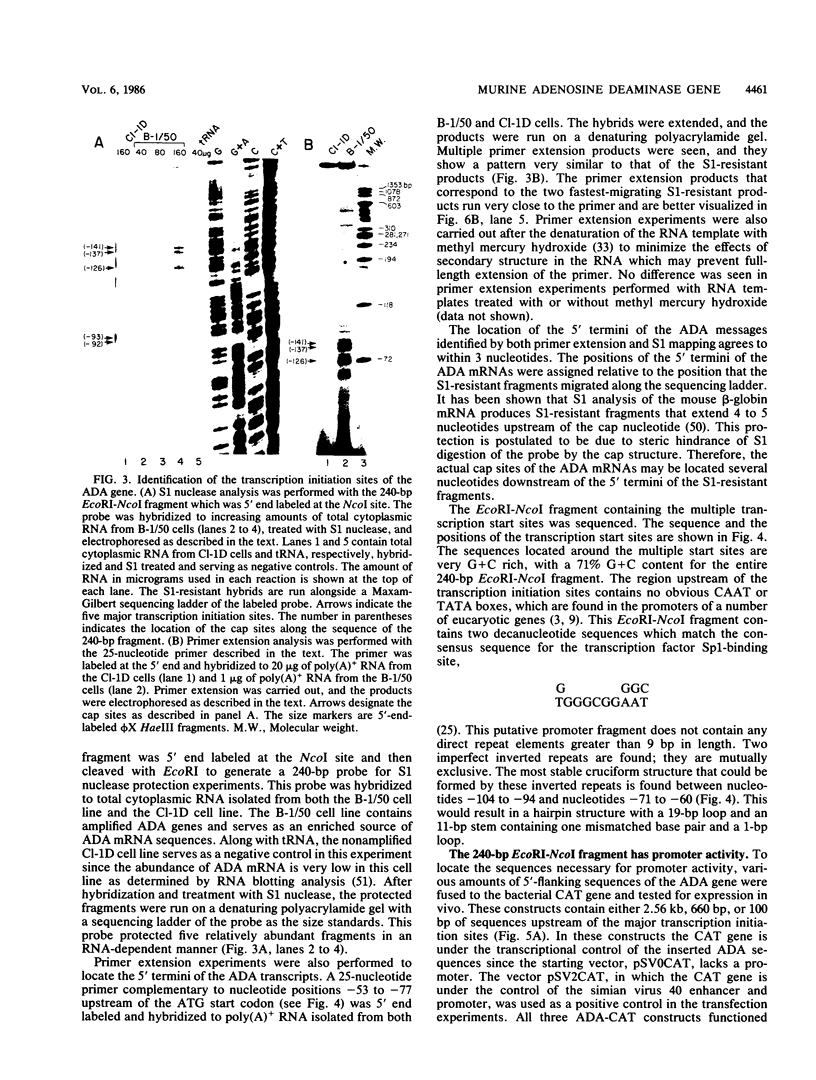
A genomic library was prepared with DNA from a genetically enriched mouse cell line in which amplified copies of the adenosine deaminase (ADA) gene account for over 5% of the genome. Overlapping cosmid clones encompassing the entire ADA structural gene were isolated from this genomic library and used for subsequent structural and functional analyses. Nuclease protection and primer extension analyses served to identify the location of multiple transcription initiation sites at the 5' end of the structural gene. Promoter activity was found by functional analyses to reside within a 240-base-pair fragment which contains the transcription initiation sites. Sequences upstream of the transcription initiation sites are very G + C rich (77%) and include a 22 nucleotide stretch of deoxyguanylate residues and two potential Sp1 transcription factor-binding sites. Comparison of the mouse and human ADA gene promoters revealed the presence of several regions that are highly conserved with regard to both sequence content and location and may represent genetic elements which are involved in ADA gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. W. The effects of an induced adenosine deaminase deficiency on T-cell differentiation in the rat. Cell Immunol. 1985 Oct 15;95(2):297–310. doi: 10.1016/0008-8749(85)90317-x. [DOI] [PubMed] [Google Scholar]
- Barton R., Martiniuk F., Hirschhorn R., Goldschneider I. Inverse relationship between adenosine deaminase and purine nucleoside phosphorylase in rat lymphocyte populations. Cell Immunol. 1980 Jan;49(1):208–214. doi: 10.1016/0008-8749(80)90071-4. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bresnick E. Deoxythymidine kinase in regenerating rat liver. Methods Enzymol. 1978;51:360–365. doi: 10.1016/s0076-6879(78)51048-3. [DOI] [PubMed] [Google Scholar]
- Bullock D. W., Woo S. L., O'Malley B. W. Uteroglobin messenger RNA: translation in vitro. Biol Reprod. 1976 Nov;15(4):435–443. doi: 10.1095/biolreprod15.4.435. [DOI] [PubMed] [Google Scholar]
- Cheng S. M., Schildkraut C. L. A family of moderately repetitive sequences in mouse DNA. Nucleic Acids Res. 1980 Sep 25;8(18):4075–4090. doi: 10.1093/nar/8.18.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Shewach D. S., Kelley W. N., Argos P., Markham A. F., Orkin S. H. Human adenosine deaminase. cDNA and complete primary amino acid sequence. J Biol Chem. 1984 Oct 10;259(19):12101–12106. [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Emerson B. M., Lewis C. D., Felsenfeld G. Interaction of specific nuclear factors with the nuclease-hypersensitive region of the chicken adult beta-globin gene: nature of the binding domain. Cell. 1985 May;41(1):21–30. doi: 10.1016/0092-8674(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Evans T., Schon E., Gora-Maslak G., Patterson J., Efstratiadis A. S1-hypersensitive sites in eukaryotic promoter regions. Nucleic Acids Res. 1984 Nov 12;12(21):8043–8058. doi: 10.1093/nar/12.21.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Ginder G. D., Wood W. I., Felsenfeld G. Isolation and characterization of recombinant clones containing the chicken adult beta-globin gene. J Biol Chem. 1979 Sep 10;254(17):8099–8102. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Martiniuk F., Rosen F. S. Adenosine deaminase activity in normal tissues and tissues from a child with severe combined immunodeficiency and adenosine deaminase deficiency. Clin Immunol Immunopathol. 1978 Mar;9(3):287–292. doi: 10.1016/0090-1229(78)90100-9. [DOI] [PubMed] [Google Scholar]
- Ingolia D. E., Yeung C. Y., Orengo I. F., Harrison M. L., Frayne E. G., Rudolph F. B., Kellems R. E. Purification and characterization of adenosine deaminase from a genetically enriched mouse cell line. J Biol Chem. 1985 Oct 25;260(24):13261–13267. [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizaki H., Habu S., Ohsaka F., Sakurada T. Purine nucleoside metabolizing enzyme activities in mouse thymocytes at different stages of differentiation and maturation. Cell Immunol. 1983 Dec;82(2):343–351. doi: 10.1016/0008-8749(83)90168-5. [DOI] [PubMed] [Google Scholar]
- Kohler P. O., Bridson W. E., Hammond J. M., Weintraub B., Kirschner M. A., Van Thiel D. H. Clonal lines of human choriocarcinoma cells in culture. Acta Endocrinol Suppl (Copenh) 1971;153:137–153. doi: 10.1530/acta.0.068s137. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Larsen A., Weintraub H. An altered DNA conformation detected by S1 nuclease occurs at specific regions in active chick globin chromatin. Cell. 1982 Jun;29(2):609–622. doi: 10.1016/0092-8674(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C. Developmental changes of adenosine deaminase, xanthine oxidase, and uricase in mouse tissues. Dev Biol. 1973 Apr;31(2):227–233. doi: 10.1016/0012-1606(73)90259-5. [DOI] [PubMed] [Google Scholar]
- Lindenmaier W., Hauser H., de Wilke I. G., Schütz G. Gene shuttling: moving of cloned DNA into and out of eukaryotic cells. Nucleic Acids Res. 1982 Feb 25;10(4):1243–1256. doi: 10.1093/nar/10.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKeon C., Schmidt A., de Crombrugghe B. A sequence conserved in both the chicken and mouse alpha 2(I) collagen promoter contains sites sensitive to S1 nuclease. J Biol Chem. 1984 May 25;259(10):6636–6640. [PubMed] [Google Scholar]
- Melton D. W., McEwan C., McKie A. B., Reid A. M. Expression of the mouse HPRT gene: deletional analysis of the promoter region of an X-chromosome linked housekeeping gene. Cell. 1986 Jan 31;44(2):319–328. doi: 10.1016/0092-8674(86)90766-x. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Miwa S., Fujii H., Matsumoto N., Nakatsuji T., Oda S., Asano H., Asano S. A case of red-cell adenosine deaminase overproduction associated with hereditary hemolytic anemia found in Japan. Am J Hematol. 1978;5(2):107–115. doi: 10.1002/ajh.2830050205. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., LaBella L. A., Buss M., Daddona P. E. Immunohistochemistry of adenosine deaminase: implications for adenosine neurotransmission. Science. 1984 Apr 13;224(4645):166–168. doi: 10.1126/science.6142530. [DOI] [PubMed] [Google Scholar]
- PONTEN J., JENSEN F., KOPROWSKI H. Morphological and virological investigation of human tissue cultures transformed with SV40. J Cell Comp Physiol. 1963 Apr;61:145–163. doi: 10.1002/jcp.1030610206. [DOI] [PubMed] [Google Scholar]
- Parkman R., Gelfand E. W., Rosen F. S., Sanderson A., Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975 Apr 3;292(14):714–719. doi: 10.1056/NEJM197504032921402. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Paglia D. E., Tartaglia A. P., Gilsanz F. Hereditary hemolytic anemia with increased red cell adenosine deaminase (45- to 70-fold) and decreased adenosine triphosphate. Science. 1977 Feb 25;195(4280):783–785. doi: 10.1126/science.836588. [DOI] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weyden M. B., Kelley W. N. Human adenosine deaminase. Distribution and properties. J Biol Chem. 1976 Sep 25;251(18):5448–5456. [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C. Y., Frayne E. G., Al-Ubaidi M. R., Hook A. G., Ingolia D. E., Wright D. A., Kellems R. E. Amplification and molecular cloning of murine adenosine deaminase gene sequences. J Biol Chem. 1983 Dec 25;258(24):15179–15185. [PubMed] [Google Scholar]
- Yeung C. Y., Ingolia D. E., Bobonis C., Dunbar B. S., Riser M. E., Siciliano M. J., Kellems R. E. Selective overproduction of adenosine deaminase in cultured mouse cells. J Biol Chem. 1983 Jul 10;258(13):8338–8345. [PubMed] [Google Scholar]
- Yeung C. Y., Ingolia D. E., Roth D. B., Shoemaker C., Al-Ubaidi M. R., Yen J. Y., Ching C., Bobonis C., Kaufman R. J., Kellems R. E. Identification of functional murine adenosine deaminase cDNA clones by complementation in Escherichia coli. J Biol Chem. 1985 Aug 25;260(18):10299–10307. [PubMed] [Google Scholar]