Abstract
We delimited sequences necessary for in vivo expression of the Drosophila melanogaster dopa decarboxylase gene Ddc. The expression of in vitro-altered genes was assayed following germ line integration via P-element vectors. Sequences between -209 and -24 were necessary for normally regulated expression, although genes lacking these sequences could be expressed at 10 to 50% of wild-type levels at specific developmental times. These genes showed components of normal developmental expression, which suggests that they retain some regulatory elements. All Ddc genes lacking the normal immediate 5'-flanking sequences were grossly deficient in larval central nervous system expression. Thus, this upstream region must contain at least one element necessary for this expression. A mutated Ddc gene without a normal TATA boxlike sequence used the normal RNA start points, indicating that this sequences is not required for start point specificity.
Full text
PDF

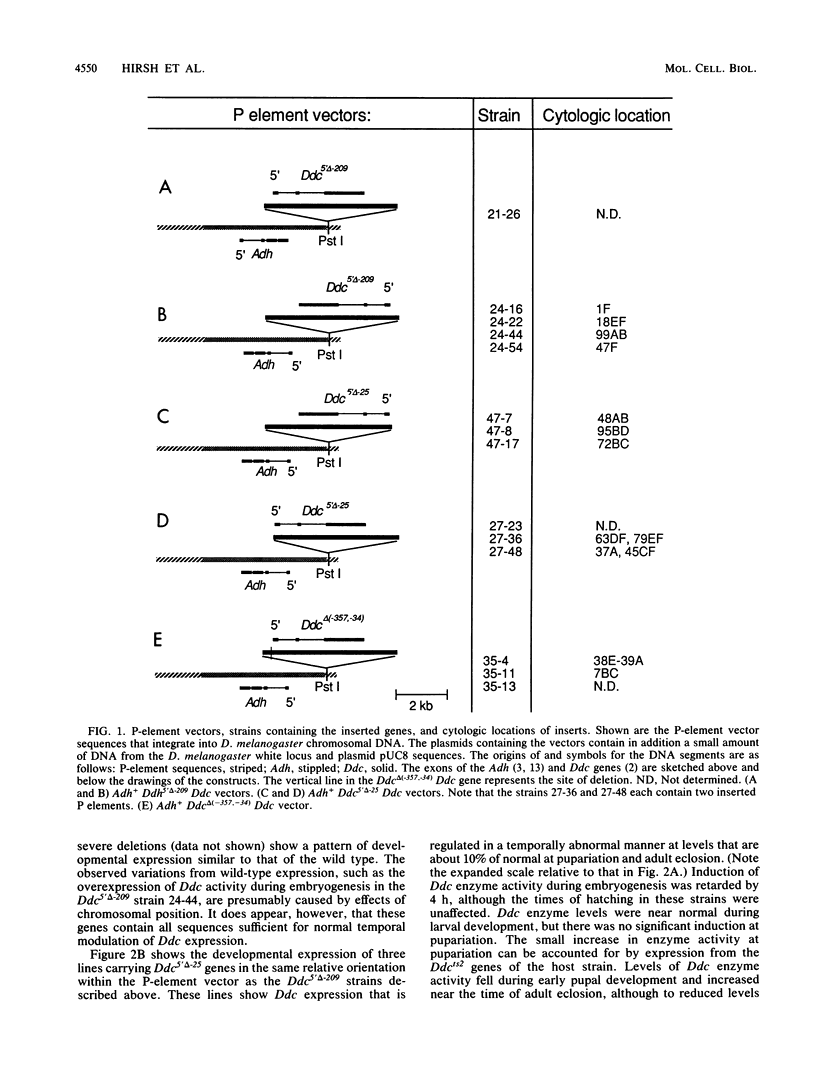
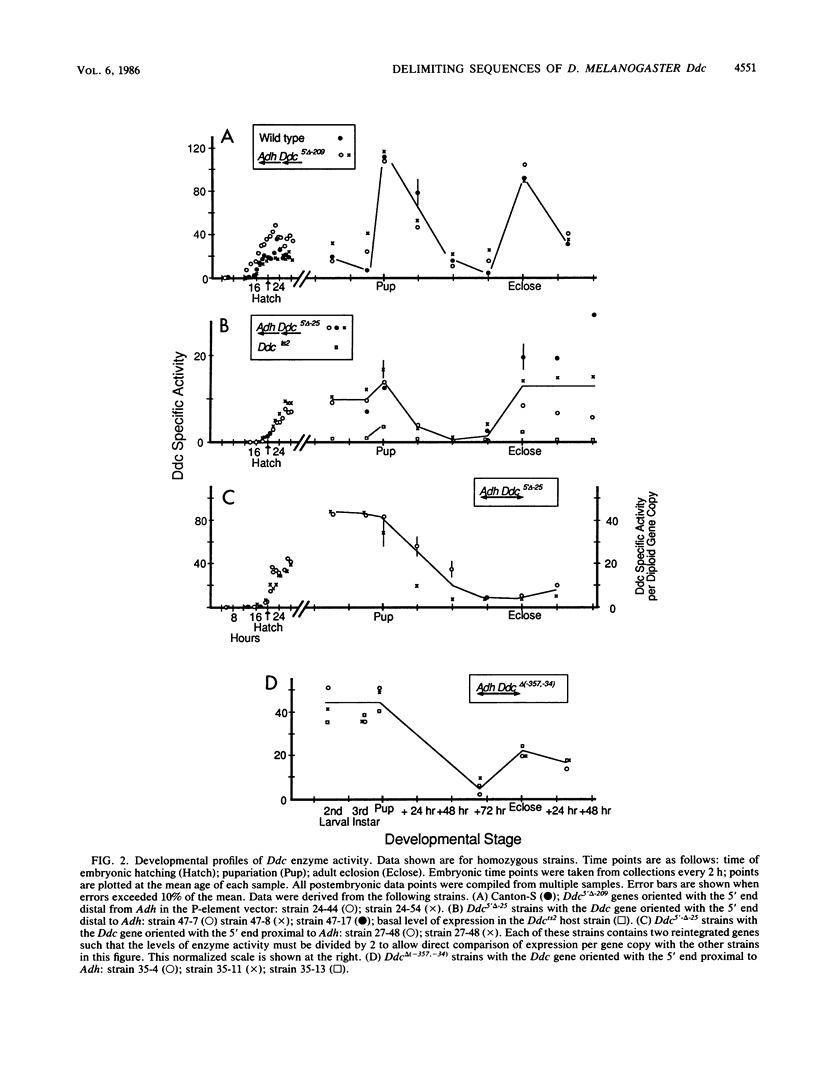

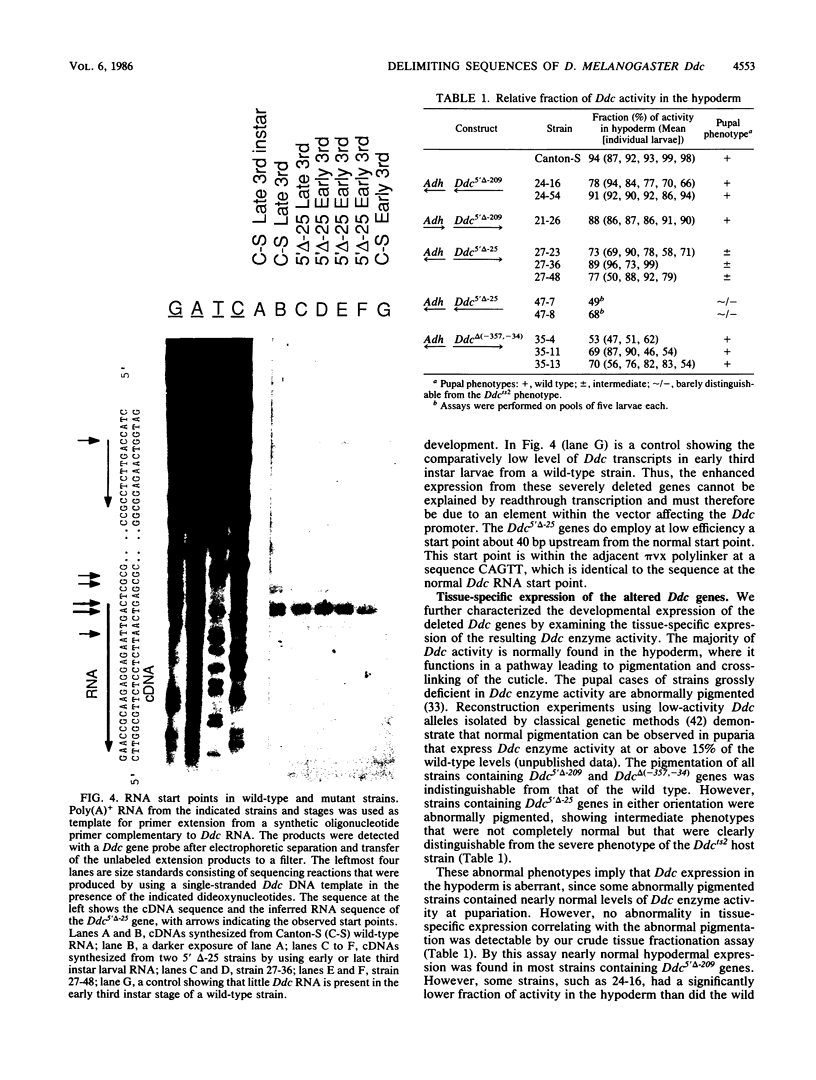
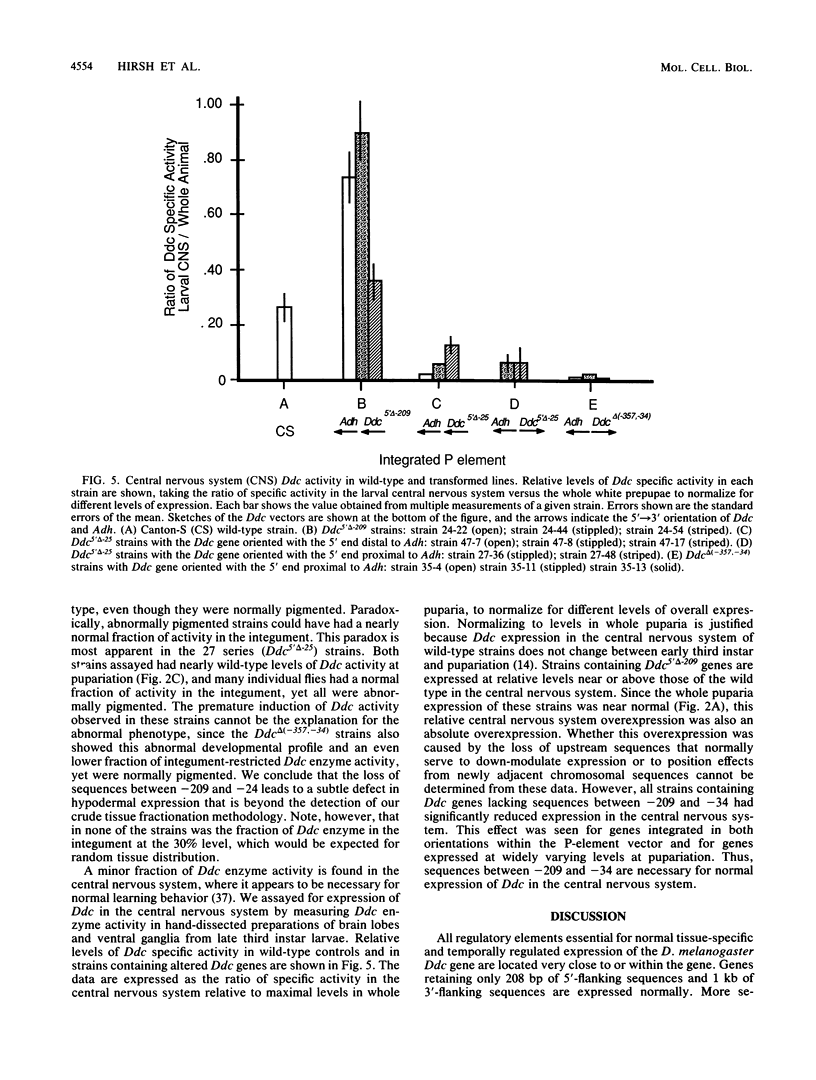



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Beall C. J., Hirsh J. High levels of intron-containing RNAs are associated with expression of the Drosophila DOPA decarboxylase gene. Mol Cell Biol. 1984 Sep;4(9):1669–1674. doi: 10.1128/mcb.4.9.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Rice D., Grosschedl R., Baltimore D. Two regulatory elements for immunoglobulin kappa light chain gene expression. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7041–7045. doi: 10.1073/pnas.81.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Treisman R., Mellon P., Chao M., Axel R., Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984 Aug;38(1):251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- Chen W., Struhl K. Yeast mRNA initiation sites are determined primarily by specific sequences, not by the distance from the TATA element. EMBO J. 1985 Dec 1;4(12):3273–3280. doi: 10.1002/j.1460-2075.1985.tb04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Cloning exons of mapping of transcription: characterization of the Drosophila melanogaster alcohol dehydrogenase gene. Nucleic Acids Res. 1983 Jul 25;11(14):4735–4752. doi: 10.1093/nar/11.14.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Davidson N. Isolation and characterization of the dopa decarboxylase gene of Drosophila melanogaster. Mol Cell Biol. 1981 Jun;1(6):475–485. doi: 10.1128/mcb.1.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kraminsky G. P., Clark W. C., Estelle M. A., Gietz R. D., Sage B. A., O'Connor J. D., Hodgetts R. B. Induction of translatable mRNA for dopa decarboxylase in Drosophila: an early response to ecdysterone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4175–4179. doi: 10.1073/pnas.77.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Tempel B. L. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983 May 5;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Gibbs P. D., Timmons P. M. Developmental control of transduced dopa decarboxylase genes in D. melanogaster. Mol Gen Genet. 1985;198(3):393–403. doi: 10.1007/BF00332929. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Wright T. R. Developmental relationship between dopa decarboxylase, dopamine acetyltransferase, and ecdysone in Drosophila. Dev Biol. 1980 Dec;80(2):379–387. doi: 10.1016/0012-1606(80)90412-1. [DOI] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Okuno S., Fujisawa H. Accurate assay of dopa decarboxylase by preventing nonenzymatic decarboxylation of dopa. Anal Biochem. 1983 Mar;129(2):412–415. doi: 10.1016/0003-2697(83)90570-5. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Richards G., Cassab A., Bourouis M., Jarry B., Dissous C. The normal developmental regulation of a cloned sgs3 'glue' gene chromosomally integrated in Drosophila melanogaster by P element transformation. EMBO J. 1983;2(12):2137–2142. doi: 10.1002/j.1460-2075.1983.tb01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Livingstone M. S., Quinn W. G. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Green M. R., Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. doi: 10.1073/pnas.80.24.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallés A. M., White K. Development of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin. J Neurosci. 1986 May;6(5):1482–1491. doi: 10.1523/JNEUROSCI.06-05-01482.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vigue C., Sofer W. Adh-n5: a temperature-sensitive mutant at the Adh locus in Drosophila. Biochem Genet. 1974 May;11(5):387–396. doi: 10.1007/BF00486412. [DOI] [PubMed] [Google Scholar]
- Wright S., Rosenthal A., Flavell R., Grosveld F. DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells. Cell. 1984 Aug;38(1):265–273. doi: 10.1016/0092-8674(84)90548-8. [DOI] [PubMed] [Google Scholar]



