Abstract
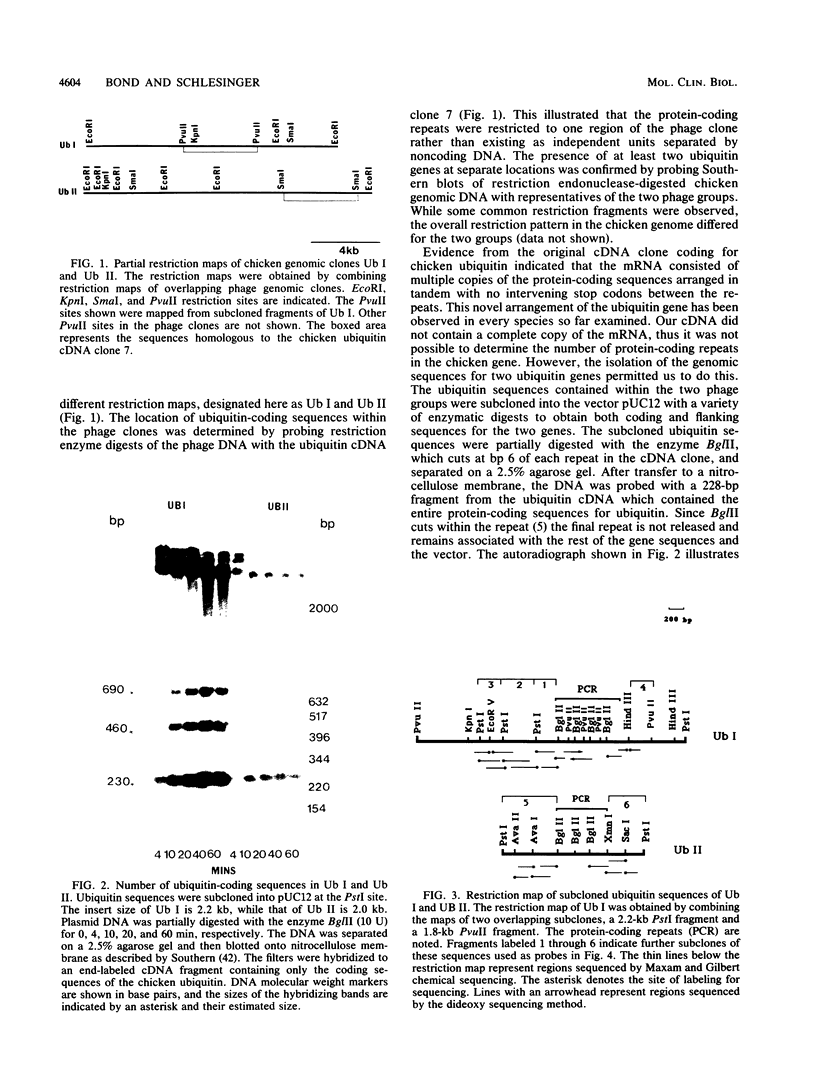
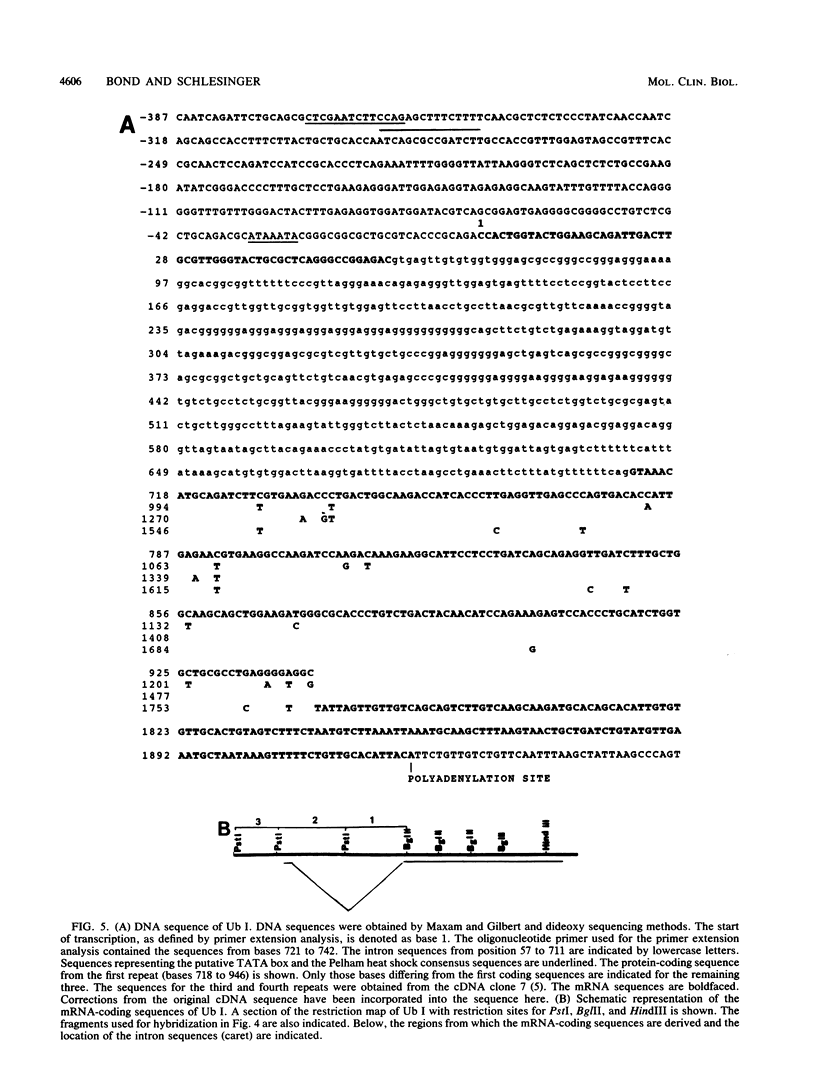
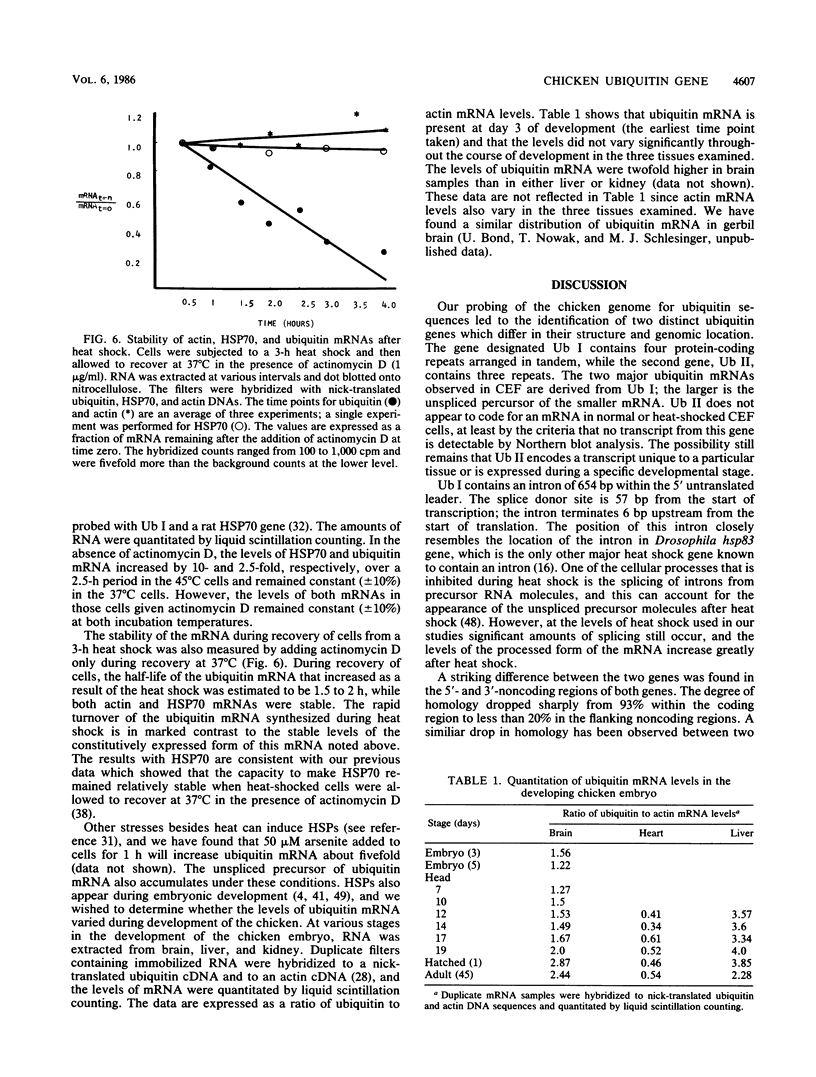
A chicken genomic library was screened to obtain genomic clones for ubiquitin genes. Two genes that differ in their genomic location and organization were identified. One gene, designated Ub I, contains four copies of the protein-coding sequence arranged in tandem, while the second gene, Ub II, contains three. The origin of the two major mRNAs that are induced after heat shock in chicken embryo fibroblasts was determined by generating DNA probes from the 5'-and 3'-noncoding regions of the two genes. Both mRNAs are transcribed from Ub I, the larger being the unspliced precursor of the smaller. A 674-base-pair intron was located within the 5'-noncoding region of Ub I. The second gene, Ub II, does not appear to code for an RNA species in normal or heat-shocked chicken embryo fibroblasts. The expression of ubiquitin mRNA during heat shock and recovery was examined. Addition of actinomycin D before heat shock completely abolished the response of ubiquitin mRNA to the stress. Analysis of the stability of the mRNA during recovery revealed that the mRNA accumulated during the heat shock is rapidly degraded with a half-life of approximately 1.5 h, suggesting a specialized but transient role for ubiquitin during heat shock.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Ballal N. R., Goldknopf I. L., Busch H. Protein A24 lyase activity in nucleoli of thioacetamide-treated rat liver releases histone 2A and ubiquitin from conjugated protein A24. Biochemistry. 1981 Mar 3;20(5):1100–1104. doi: 10.1021/bi00508a009. [DOI] [PubMed] [Google Scholar]
- Andersen M. W., Goldknopf I. L., Busch H. Protein A24 lyase is an isopeptidase. FEBS Lett. 1981 Sep 28;132(2):210–214. doi: 10.1016/0014-5793(81)81162-3. [DOI] [PubMed] [Google Scholar]
- Atidia J., Kulka R. G. Formation of conjugates by 125I-labelled ubiquitin microinjected into cultured hepatoma cells. FEBS Lett. 1982 Jun 1;142(1):72–76. doi: 10.1016/0014-5793(82)80222-6. [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Dworkin-Rastl E., Shrutkowski A., Dworkin M. B. Multiple ubiquitin mRNAs during Xenopus laevis development contain tandem repeats of the 76 amino acid coding sequence. Cell. 1984 Dec;39(2 Pt 1):321–325. doi: 10.1016/0092-8674(84)90010-2. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Gavilanes J. G., Gonzalez de Buitrago G., Perez-Castells R., Rodriguez R. Isolation, characterization, and amino acid sequence of a ubiquitin-like protein from insect eggs. J Biol Chem. 1982 Sep 10;257(17):10267–10270. [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. W., Lis J. T. Localization of the hsp83 transcript within a 3292 nucleotide sequence from the 63B heat shock locus of D. melanogaster. Nucleic Acids Res. 1983 Oct 25;11(20):7011–7030. doi: 10.1093/nar/11.20.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Eytan E., Ciechanover A., Haas A. L. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J Biol Chem. 1982 Dec 10;257(23):13964–13970. [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Selective arrangement of ubiquitinated and D1 protein-containing nucleosomes within the Drosophila genome. Cell. 1982 Feb;28(2):375–385. doi: 10.1016/0092-8674(82)90355-5. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lund P. K., Moats-Staats B. M., Simmons J. G., Hoyt E., D'Ercole A. J., Martin F., Van Wyk J. J. Nucleotide sequence analysis of a cDNA encoding human ubiquitin reveals that ubiquitin is synthesized as a precursor. J Biol Chem. 1985 Jun 25;260(12):7609–7613. [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Varshavsky A. The yeast ubiquitin gene: head-to-tail repeats encoding a polyubiquitin precursor protein. Nature. 1984 Dec 13;312(5995):663–666. doi: 10.1038/312663a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- Sirotkin K., Davidson N. Developmentally regulated transcription from Drosophila melanogaster chromosomal site 67B. Dev Biol. 1982 Jan;89(1):196–210. doi: 10.1016/0012-1606(82)90307-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St John T., Gallatin W. M., Siegelman M., Smith H. T., Fried V. A., Weissman I. L. Expression cloning of a lymphocyte homing receptor cDNA: ubiquitin is the reactive species. Science. 1986 Feb 21;231(4740):845–850. doi: 10.1126/science.3003914. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2B can be modified by the attachment of ubiquitin. Nucleic Acids Res. 1980 Oct 24;8(20):4671–4680. doi: 10.1093/nar/8.20.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Pedersen M. S., Wind A., Berglund L. E., Marcker K. A., Vuust J. The human ubiquitin multigene family: some genes contain multiple directly repeated ubiquitin coding sequences. EMBO J. 1985 Mar;4(3):755–759. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]




