Summary
Cholesterol depletion reversibly abolishes carbachol-evoked Ca2+ release from inositol (1,4,5)-trisphosphate (IP3)-sensitive stores, without affecting the distribution of IP3 receptors (IP3R) or endoplasmic reticulum, IP3 formation or responses to photolysis of caged IP3. Receptors that stimulate cAMP formation do not alone evoke Ca2+ signals, but they potentiate those evoked by carbachol. We show that these potentiated signals are entirely unaffected by cholesterol depletion and that, within individual cells, different IP3-sensitive Ca2+ stores are released by carbachol alone and by carbachol combined with receptors that stimulate cAMP formation. We suggest that muscarinic acetylcholine receptors in lipid rafts deliver IP3 at high concentration to associated IP3R, stimulating them to release Ca2+. Muscarinic receptors outside rafts are less closely associated with IP3R and provide insufficient local IP3 to activate IP3R directly. These IP3R, probably type 2 IP3R within a discrete Ca2+ store, are activated only when their sensitivity is increased by cAMP. Sensitization of IP3R by cAMP extends the effective range of signalling by phospholipase C, allowing muscarinic receptors that are otherwise ineffective to recruit additional IP3-sensitive Ca2+ stores.
Key words: Calcium signalling, Cholesterol, Cyclic AMP, IP3 receptor, Spatial organization
Introduction
Many cells respond to extracellular stimuli with an increase in intracellular free Ca2+ concentration, [Ca2+]i. The complex spatio-temporal organization of these Ca2+ signals provides the versatility that allows them selectively to regulate many cellular processes (Berridge et al., 2000). Inositol (1,4,5)-trisphosphate (IP3), by stimulating Ca2+ release from the endoplasmic reticulum (ER), provides a common link between receptors at the plasma membrane and cytoplasmic Ca2+ signals. Diverse stimuli, via their receptors, stimulate phospholipase C (PLC) and so formation of IP3, which binds to IP3 receptors (IP3R) causing Ca2+ to leak into the cytosol (Foskett et al., 2007; Taylor and Tovey, 2010).
Parathyroid hormone (PTH) stimulates adenylyl cyclase (AC) via G protein-coupled receptors (GPCR). The type 1 PTH receptor (PTHR1) has attracted most attention because it has essential roles in bone remodelling and plasma Ca2+ homeostasis, and it is a target of drugs used to treat osteoporosis (Mannstadt et al., 1999). PTHR1 can stimulate both AC and an increase in [Ca2+]i, but the relationships between these events are complex (reviewed in Taylor and Tovey, 2012). PTH invariably stimulates AC, but it can also stimulate PLC directly when the signalling proteins are expressed at high levels (Offermanns et al., 1996; Schwindinger et al., 1998; Taylor and Tovey, 2012) or when PTHR1 associates with the scaffold proteins, Na+-H+-exchanger regulatory factors (NHERF) (Wang et al., 2010). Cyclic AMP (cAMP), via exchange protein activated by cAMP (epac) and the monomeric G protein Rap, can also stimulate PLCε (Schmidt et al., 2001). We (Short and Taylor, 2000; Tovey et al., 2008; Tovey et al., 2003) and others (Schwindinger et al., 1998) find that in HEK 293 cells stably expressing physiological levels of PTHR1 (HEK-PR1 cells), PTH stimulates AC, but it does not alone evoke an increase in [Ca2+]i. However, PTH (or endogenous β2-adrenoceptors) potentiates the Ca2+ signals evoked by receptors that stimulate PLC (Kurian et al., 2009; Tovey et al., 2008). This effect of PTH is entirely mediated by cAMP, it requires activation of neither cAMP-dependent protein kinase (PKA) nor epacs, and it results from cAMP binding to IP3R directly or to a protein that tightly associates with IP3R (Tovey et al., 2008; Tovey et al., 2010). Although cAMP increases the sensitivity of all three IP3R subtypes to IP3 (Tovey et al., 2010), in intact HEK-PR1 cells a specific association of IP3R2 and AC6, an ‘AC-IP3R junction’, allows AC to deliver cAMP directly and at high concentration to IP3R (Fig. 1A) (Tovey et al., 2008).
Fig. 1.
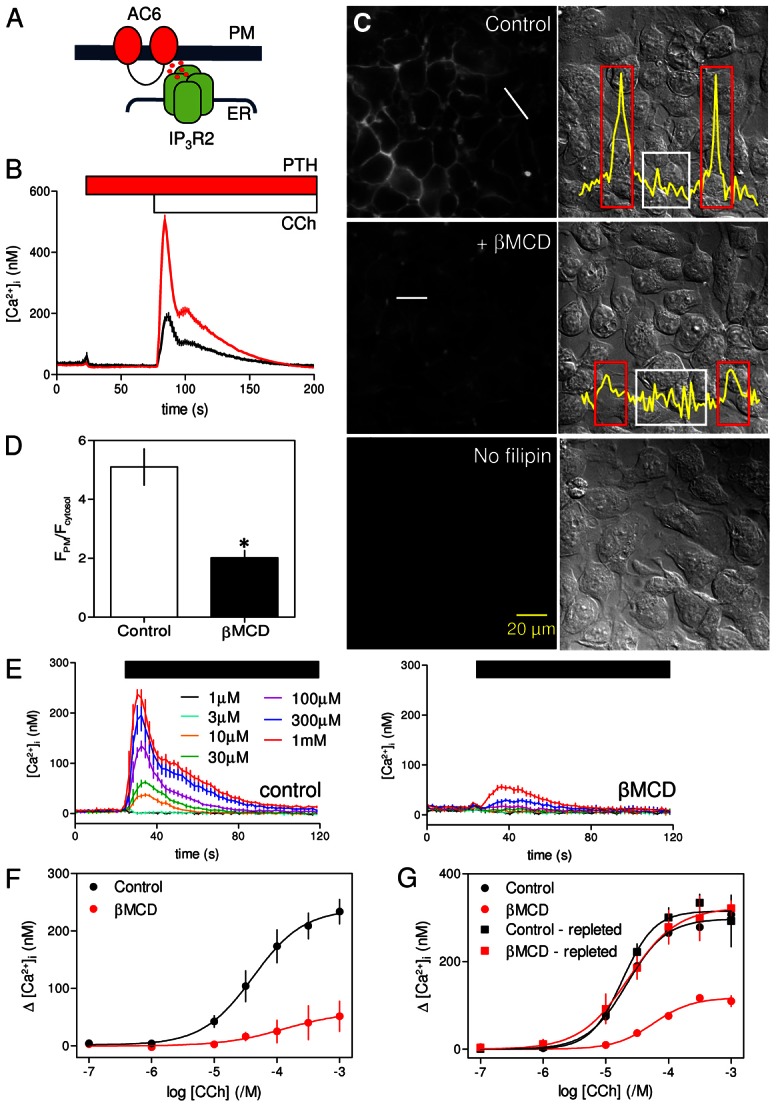
Cholesterol depletion reversibly inhibits CCh-evoked Ca2+ release. (A) Potentiation of CCh-evoked Ca2+ release by cAMP is mediated by a close association of IP3R2 and AC6 that delivers high concentrations of cAMP directly to IP3R (Tovey et al., 2008). PM, plasma membrane. (B) Populations of HEK-PR1 cells in Ca2+-free HBS were stimulated with a maximal concentration of CCh (1 mM) alone (black) or after addition of PTH (100 nM) (red). Results show means ± s.e.m. for 3–4 wells from a single experiment, each typical of at least three experiments. (C) Typical fields of cells showing filipin staining (left) or differential interference contrast images (right) for control cells, cells treated with βMCD (2%, 20°C), or unstained control cells. Images are typical of at least three experiments. Scale bar applies to all images. Free cholesterol levels in the plasma membrane were quantified by measuring fluorescence intensities along lines drawn across cells. Profiles, corresponding to white lines in panels, are shown in the overlaid traces. (D) From the fluorescence transects, regions of interest (ROI) corresponding to the plasma membrane (red boxes in C) or cytosol (white boxes) were identified and the average fluorescence intensities within each were calculated (FPM and Fcytosol, respectively). The histogram shows the FPM/Fcytosol ratios from control cells and cells treated with βMCD (2%, 20°C). Results are means ± s.e.m. from three experiments with 10 cells analysed in each field. *P<0.05. Similar analysis of the effects of treatment with βMCD at 37°C is shown in supplementary material Fig. S2. (E) Populations of HEK-PR1 cells in Ca2+-free HBS were stimulated with the indicated concentrations of CCh (left) and after pre-treatment with βMCD at 20°C (right). Results are means ± s.e.m. from a single experiment with three replicates for each condition. (F) Summary of results shows concentration-dependent effects of CCh on Ca2+ release with and without βMCD-treatment. (G) Concentration-dependent effects of CCh on Ca2+ signals under the conditions indicated. Cholesterol depletion with βMCD and repletion with βMCD∶cholesterol were performed at 37°C and before loading cells with fluo-4/AM. Results in F and G are means ± s.e.m. from three experiments, each with matched control and treated cells.
Lipid rafts are ordered regions of the plasma membrane in which the outer leaflet is enriched in cholesterol and sphingolipids (Simons and Toomre, 2000). Many signalling proteins, including GPCRs, G proteins, ACs, IP3Rs and PLCs, associate with lipid rafts and are thereby organized into signalling complexes (Isshiki and Anderson, 1999; Ostrom and Insel, 2004; Simons and Toomre, 2000). Disruption of lipid rafts by cholesterol depletion has been reported to inhibit IP3-evoked Ca2+ release (Nagata et al., 2007; Singleton and Bourguignon, 2004; Weerth et al., 2007) and to disrupt selective regulation of AC6 and AC8 by store-operated Ca2+ entry (Willoughby and Cooper, 2007). Evidence that delivery of cAMP from AC6 to IP3R2 mediates the effects of PTH on IP3-evoked Ca2+ signals in HEK-PR1 cells (Tovey et al., 2008) alongside reports that AC5/6 (Head et al., 2005; Ostrom et al., 2002; Willoughby and Cooper, 2007) and IP3R2 (Weerth et al., 2007) can associate with cholesterol-rich lipid rafts prompted us to examine the role of lipid rafts in signalling by PTH.
Results
Loss of cholesterol inhibits carbachol-evoked Ca2+ release, but not its potentiation by PTH
Carbachol (CCh), a stable analogue of acetylcholine that stimulates IP3 formation via endogenous M3 muscarinic receptors (M3R) (Luo et al., 2008), evoked Ca2+ release from the intracellular stores of HEK 293 cells (Short et al., 2000). This IP3-evoked Ca2+ release was potentiated by PTH in HEK-PR1 cells (Fig. 1B), by isoproterenol, which stimulates endogenous β2-adrenoceptors (supplementary material Fig. S1A) (Kurian et al., 2009), or by high concentrations of a membrane-permeant analogue of cAMP, 8-bromo-cAMP (8-Br-cAMP) (supplementary material Fig. S1B). Neither PTH nor isoproterenol alone stimulated Ca2+ release (Fig. 1B and supplementary material Fig. S1A). These results are consistent with earlier work suggesting that AC6-IP3R2 junctions allow cAMP to be delivered directly to IP3R at high concentrations to cause an increase in their sensitivity to IP3 (Tovey et al., 2008; Tovey et al., 2010). Subsequent experiments assess whether cholesterol, an essential component of lipid rafts (Simons and Ikonen, 1997), is required for this selective communication (Fig. 1A).
Filipin-staining established that most cholesterol was in the plasma membrane and that exposure to β-methylcyclodextrin (βMCD, 2% w/v, 2 hours at 20°C) caused loss of plasma membrane cholesterol without perturbing cell morphology (Fig. 1C,D). Similar results were obtained with brief exposure (10 minutes) to βMCD (2% w/v) at 37°C (supplementary material Fig. S2A–C). In most experiments, the more prolonged (30 minutes to 2 hours) treatment at 20°C was used because it was then easier to adjust incubation times to achieve effective cholesterol depletion without causing cellular damage. Pre-treatment of cells with βMCD at 20°C massively attenuated the Ca2+ release evoked by CCh in populations of HEK-PR1 cells (Fig. 1E). The peak increase in [Ca2+]i evoked by a maximal concentration of CCh (1 mM) was reduced by 80±11% from 234±22 nM to 51±26 nM (Fig. 1F). The sensitivity to CCh was not significantly affected: the pEC50 (−logEC50, where EC50 is the half-maximally effective concentration) was 4.38±0.14 and 3.88±1.07 for control and βMCD-treated cells, respectively (Fig. 1F). CCh-evoked Ca2+ release was similarly inhibited by brief treatment with βMCD (2% w/v, 10 minutes) at 37°C (supplementary material Fig. S2D). The effects of cholesterol depletion on CCh-evoked Ca2+-release were reversed by cholesterol repletion (Fig. 1G).
Although loss of cholesterol almost abolished responses to CCh, it had no significant effect on the Ca2+ signals evoked by subsequent addition of PTH (Fig. 2A–C). Neither the amplitude of the Ca2+ signal evoked by addition of a maximal concentration of PTH after CCh (229±28 nM and 187±30 nM, in control and βMCD-treated cells, respectively) nor the sensitivity to PTH (pEC50 = 7.25±0.21 and 7.11±0.17) was affected by loss of cholesterol (Fig. 2D). It is worth noting that in these and related experiments (Figs 2, 3; supplementary material Figs S1, S3B and S7), the Ca2+ signal evoked by addition of PTH directly reports the potentiated response because although CCh is present throughout, [Ca2+]i has returned to its basal level before PTH is added. The results with cell populations were confirmed in single cells, where a maximal concentration of CCh (1 mM) evoked a transient release of Ca2+ in 92±3% of cells, and subsequent addition of PTH (100 nM) caused further Ca2+ release in 97±2% of cells (Fig. 2E–H). Pre-treatment with βMCD significantly reduced both the number of cells responding to CCh alone (to 63±7%, Fig. 2G) and the amplitude of the peak Ca2+ signal (Fig. 2H). Even in those cells that responded to CCh, the amplitude of the Ca2+ signal was reduced from 350±52 nM to 191±7 nM. However, βMCD had no effect on the number of cells in which PTH potentiated CCh-evoked Ca2+ signals (98±1% of cells, Fig. 2G) or the amplitude of the peak response to PTH (229±8 nM and 278±27 nM in control and βMCD-treated cells, respectively). It is important to recall that PTH evokes Ca2+ signals only when cells are co-stimulated with CCh (Fig. 1B), yet after βMCD-treatment many cells (37%) failed to respond to CCh alone, but almost all cells (98%) responded to CCh with PTH (Fig. 2G). Many βMCD-treated cells therefore responded to PTH with CCh despite not responding to CCh alone.
Fig. 2.
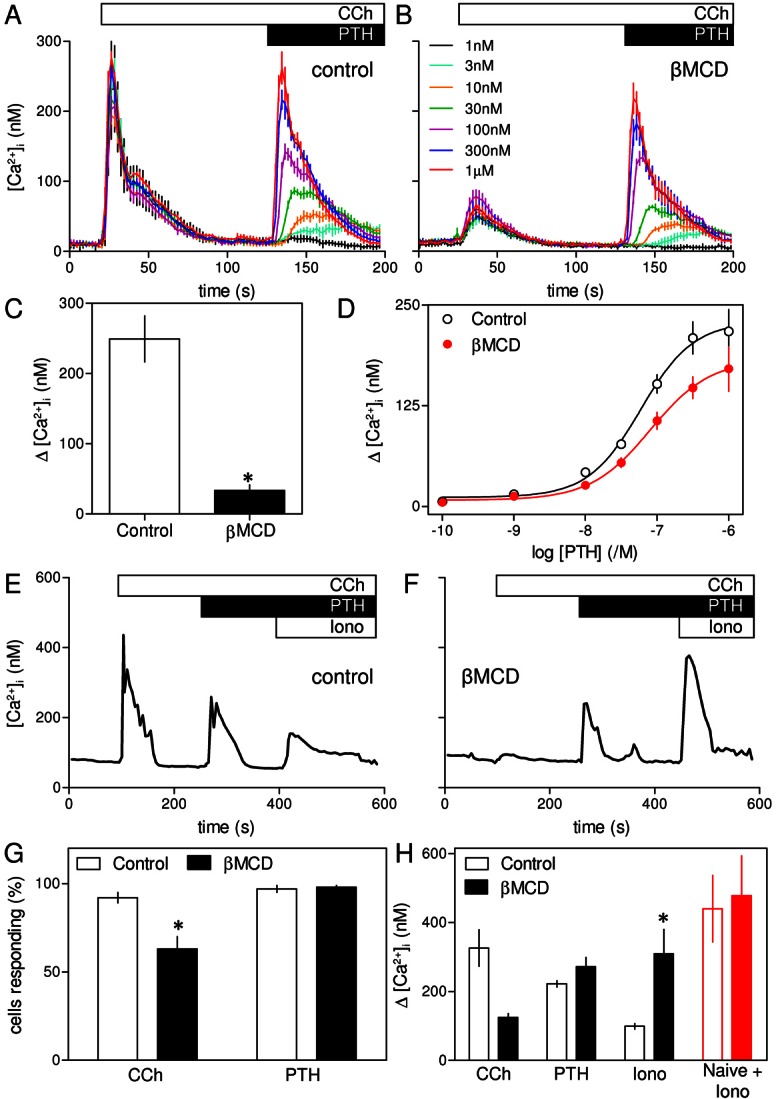
Potentiation of CCh-evoked Ca2+ release by PTH is unaffected by loss of cholesterol. (A) In Ca2+-free HBS, CCh (1 mM) evoked a transient release of Ca2+ from the intracellular stores of populations of HEK-PR1 cells. Addition of the indicated concentrations of PTH in the continued presence of CCh then evoked a further concentration-dependent Ca2+ release. (B) Similar experiments in cells pre-treated with βMCD (at 20°C) inhibited the initial response to CCh without significantly affecting the subsequent response to PTH. Results in A and B are means ± s.e.m. from a single experiment with three replicates for each condition. The code applies to both panels. (C,D) Summary of results (means ± s.e.m. from three experiments) shows peak increases in [Ca2+]i evoked by CCh (1 mM) (C) and the concentration-dependent effects of a subsequent addition of PTH on [Ca2+]i (D) in control and βMCD-treated cells. (E,F) Typical response of a single HEK-PR1 cell in Ca2+-free HBS to CCh (1 mM) followed by PTH (100 nM) and then ionomycin (Iono, 1 µM) treatment to release any Ca2+ remaining within intracellular stores. Results are shown for control cells (E) or after pre-treatment with βMCD (at 20°C) (F). (G) Summary of results shows the percentage of all cells that responded to CCh, or CCh with PTH. (H) Summary of results from experiments similar to those shown in E and F, showing the Ca2+ signals evoked by addition of CCh or of PTH after CCh. Ionomycin was added after stimulation with CCh and PTH to define the residual Ca2+ content of the intracellular stores. In parallel experiments, the initial Ca2+ content of the stores was defined by addition of ionomycin to cells that had not been stimulated (naive, red). Results in G and H are means ± s.e.m. from at least three coverslips, with >50 cells analyzed for each. *P<0.05.
Fig. 3.
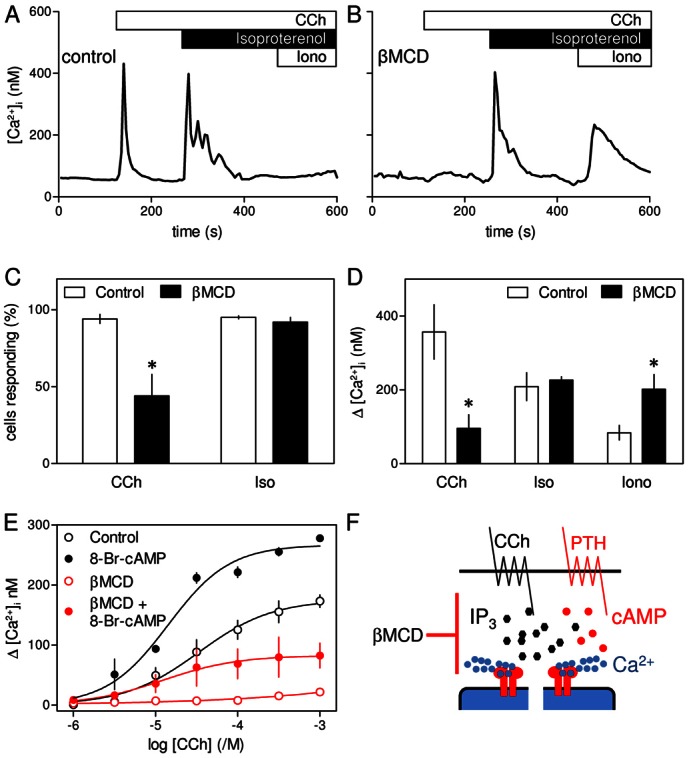
Cholesterol depletion attenuates CCh-evoked Ca2+ signals without affecting their potentiation by β-adrenoceptors. (A,B) Typical responses from a single HEK 293 cell with (B) or without (A) treatment with βMCD (at 20°C) and then stimulated in Ca2+-free HBS with CCh (1 mM), then isoproterenol (1 µM) and finally with ionomycin (Iono, 1 µM) to release any Ca2+ remaining within the intracellular stores. (C,D) Summary of results shows the percentage of cells responding to CCh alone, or to CCh with isoproterenol (C), and the amplitude of the peak increase in [Ca2+]i evoked by each stimulus (D) for control and βMCD-treated cells. Results are means ± s.e.m. from n≥3 coverslips, with >50 cells analyzed from each. *P<0.05. (E) Populations of HEK-PR1 cells were stimulated with the indicated concentrations of CCh alone or with 8-Br-cAMP (10 mM, 20 minutes) with or without βMCD treatment (at 20°C). Results are means ± s.e.m. from three experiments. (F) Depletion of cholesterol selectively inhibits Ca2+ release evoked by CCh alone without affecting Ca2+ release by CCh with PTH (or isoproterenol).
Addition of ionomycin, a Ca2+ ionophore, to cells in Ca2+-free HBS was used to define the amount of Ca2+ within intracellular stores (Fig. 2E,F). The results demonstrate that loss of cholesterol had no effect on the initial Ca2+ content of the stores (Fig. 2H, red), but their residual Ca2+ content after stimulation with CCh and PTH was significantly greater in βMCD-treated cells (Fig. 2H). We conclude that cholesterol depletion has no direct effect on the Ca2+ content of the intracellular stores, but it attenuates the Ca2+ signals evoked by CCh alone, without affecting those evoked by CCh with PTH.
Potentiation of CCh-evoked Ca2+ release by β2-adrenoceptors is unaffected by loss of cholesterol
Endogenous β2-adrenoceptors in HEK 293 cells stimulate AC and thereby potentiate CCh-evoked Ca2+ release (supplementary material Fig. S1A). The response to β2-adrenoceptors is larger in the parental HEK 293 cells; we therefore used these cells to examine responses to isoproterenol, a selective agonist of β-adrenoceptors. In populations of these cells, βMCD almost abolished the Ca2+ signals evoked by CCh (supplementary material Fig. S3A) without affecting the amplitude or sensitivity of the subsequent responses to isoproterenol (supplementary material Fig. S3B). In measurements of single cells, CCh (1 mM) caused a transient increase in [Ca2+]i in 94±3% of cells, and subsequent addition of isoproterenol (1 µM) caused further Ca2+ release from 95±1% of cells (Fig. 3A–C). As with HEK-PR1 cells, pre-treatment with βMCD reduced the number of cells responding to CCh (Fig. 3C), and in those cells that responded the amplitude of the increase in [Ca2+]i was significantly reduced (Fig. 3D). However, the Ca2+ signals evoked by addition of isoproterenol after CCh were unaffected by loss of cholesterol: 92±3% of cells responded and the amplitude of the increase in [Ca2+]i was similar with (209±38 nM) or without βMCD-treatment (227±9 nM) (Fig. 3C,D). Just as with PTH, therefore, many cells in which βMCD-treatment abolished responses to CCh alone responded normally to stimulation with CCh and isoproterenol. Furthermore, the residual Ca2+ content of the intracellular stores after stimulation with CCh and isoproterenol was significantly larger in cells that had been depleted of cholesterol (Fig. 3D).
Potentiation of CCh-evoked Ca2+ signals by PTH and β2-adrenoceptors depends on their ability to deliver cAMP locally at high concentrations to associated IP3R (Fig. 1A) (Tovey et al., 2008), but the effects are mimicked by a uniformly high concentration of 8-Br-cAMP (supplementary material Fig. S1B). As with PTH and isoproterenol, under conditions where βMCD almost abolished responses to CCh alone, 8-Br-cAMP still potentiated CCh-evoked increases in [Ca2+]i (Fig. 3E). These results demonstrate that whether cAMP is delivered focally or globally, it effectively allows CCh to evoke Ca2+ release under conditions where CCh alone is ineffective.
We conclude that loss of cholesterol massively attenuates Ca2+ signals evoked by CCh without affecting the potentiated signals evoked by CCh in combination with 8-Br-cAMP or stimulation of AC by endogenous β2-adrenoceptors or heterologously expressed PTH receptors (Fig. 3F). There is, therefore, a surprising independence of the Ca2+ release evoked by IP3 in response to CCh alone or CCh with cAMP that does not require spatially organized cAMP signals.
Cholesterol depletion affects neither CCh-evoked IP3 formation nor the sensitivity of IP3 receptors
Maximal activation of endogenous M3R in HEK cells generates insufficient IP3 to directly release all IP3-sensitive Ca2+ stores (Tovey et al., 2008) (Figs 2, 3). This is consistent with the EC50 for CCh-evoked Ca2+-release and the KD of CCh binding to M3R (Burford et al., 1995) being similar. The lack of ‘spare receptors’ is an experimental advantage in that it allows use of saturating concentrations of CCh to evoke submaximal Ca2+ signals similar to those evoked by physiological stimuli, but it requires sensitive methods to resolve CCh-stimulated IP3 formation. We used a FRET-based IP3-biosensor to allow real-time recording of IP3 levels in CCh-stimulated HEK-PR1 cells (see Materials and Methods and supplementary material Fig. S4). Results shown in Fig. 4A show that CCh (20 µM) evoked a rapid and sustained increase in cytosolic IP3 concentration that was unaffected by subsequent addition of PTH (100 nM), but increased further when the CCh concentration was increased (1 mM). This confirms that PTH does not affect the intracellular concentration of IP3 (Short and Taylor, 2000). Treatment with βMCD had no effect on the IP3 signals evoked by CCh alone or CCh with PTH (Fig. 4A,B).
Fig. 4.
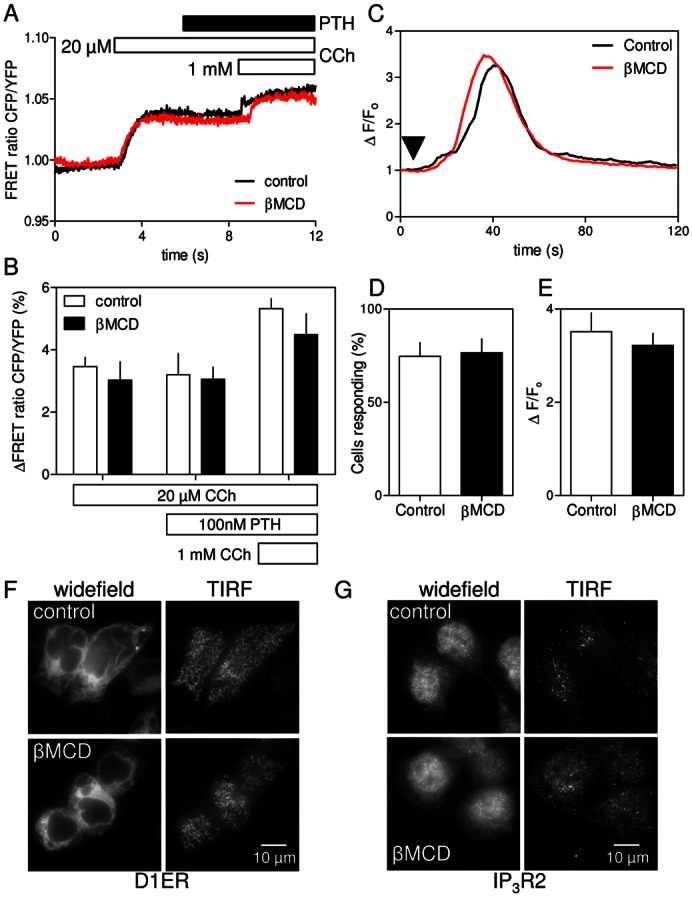
Cholesterol depletion has no effect on IP3 formation, IP3 receptor sensitivity or ER morphology. (A) Normalized changes in the CFP/YFP FRET ratio of an IP3 sensor (see Materials and Methods and supplementary material Fig. S4) in HEK-PR1 cells stimulated with CCh (20 µM), then PTH (100 nM) and finally a maximal concentration of CCh (1 mM). Traces show changes in FRET ratio for single cells, each representative of six experiments, with or without βMCD treatment. (B) Summary (means ± s.e.m.) shows cumulative changes in FRET ratio for 15 control cells and 11 βMCD-treated cells from six independent experiments. (C) Ca2+ release evoked by photolysis of ciIP3 (arrow) in HEK-PR1 cells with and without pre-treatment with βMCD (2% w/v, 20°C). Each trace shows the average change in fluorescence (ΔF/F0) for ≥20 cells from a single field. Examples of responses from individual cells are shown in supplementary material Fig. S5. (D,E) Summary of results shows the fraction of cells responding to iIP3 (D) and average amplitude (ΔF/F0) of the Ca2+ transient in cells that responded (E). Results are means ± s.e.m. for n≥3 coverslips, with 5 fields per coverslip (∼150 cells/coverslip). (F,G) Distribution of the ER marker, D1ER (F) and IP3R2 (G) in control and βMCD-treated HEK-PR1cells. Images show the distributions in widefield and in the region immediately beneath the plasma membrane using TIRFM. Images are representative of at least three experiments. Supplementary material Fig. S6 shows similar analyses of IP3R1 and IP3R3.
We used photolysis of ciIP3 (caged iIP3) non-disruptively loaded into cells as ciIP3/PM [cell-permeant caged iIP3, D-2,3-O-isopropylidene-6-O-(2-nitro-4,5-dimethoxy)benzyl-myo-inositol 1,4,5-trisphosphate-hexakis(propionoxymethyl)ester] (Dakin and Li, 2007) to assess the effects of βMCD on the Ca2+ signals evoked by activation of IP3R directly. Photolysis of ciIP3 in HEK-PR1 cells caused transient increases in [Ca2+]i (Fig. 4C). The amplitudes of these Ca2+ signals varied between cells (supplementary material Fig. S5), perhaps reflecting differences in loading and de-esterification of ciIP3/PM. However, in a large sample (n>400 cells), the response to iIP3 was unaffected by treatment with βMCD (Fig. 4C–E). Neither the fraction of cells responding to photolysis of ciIP3 (75±7% and 77±7% for control and βMCD-treated cells, respectively) nor the average amplitude of the increase in fluo-4 fluorescence (ΔF/F0, 3.52±0.40 and 3.22±0.26) was affected by βMCD.
Treatment with βMCD caused HEK-PR1 cells to become slightly more rounded (Fig. 1C; Fig. 4F), but there was no obvious redistribution of the ER and it remained associated with the plasma membrane (Fig. 4F). Immunostaining for each of the three IP3R subtypes confirmed that their distributions, whether assessed by widefield microscopy or total internal reflection fluorescence microscopy (TIRFM), were also unaffected by cholesterol depletion (Fig. 4G and supplementary material Fig. S6).
We conclude that selective inhibition of CCh-evoked Ca2+ release by cholesterol depletion occurs under conditions where CCh-evoked increases in cytosolic IP3 are unaffected (Fig. 4B), intracellular Ca2+ stores are intact (Fig. 2H), IP3R respond normally to IP3 (Fig. 4C–E) and there are no obvious changes in the distribution of the ER (Fig. 4F) or IP3Rs (Fig. 4G; supplementary material Fig. S6). This suggests that for cells stimulated with CCh alone cholesterol depletion disrupts effective delivery of IP3 to IP3R, yet it has no effect on IP3 delivery to IP3R that respond in the presence of either cAMP or 8-Br-cAMP.
CCh mobilizes Ca2+ from different stores in the presence of PTH
The ability of CCh with PTH to evoke Ca2+ release when responses to CCh alone are almost abolished by cholesterol depletion is unexpected because PTH increases [Ca2+]i only when paired with a stimulus, like CCh, that stimulates IP3 production (Fig. 1B) (Tovey et al., 2008). How might loss of cholesterol disrupt delivery of IP3 to IP3R when CCh alone is the stimulus without affecting IP3 delivery when CCh is combined with activation of receptors that stimulate AC? The effect cannot simply result from sensitization of IP3R by cAMP counteracting inhibition of M3R coupling to PLC because there was no detectable inhibition of IP3 production (Fig. 4A), and inhibition of PLC with U73122 similarly inhibited responses to CCh alone or CCh with PTH (supplementary material Fig. S7). In most of our experiments, where cells were first stimulated with CCh before addition of PTH, there was more Ca2+ in the intracellular stores at the time of PTH addition for cells treated with βMCD (Fig. 2H; Fig. 3D). We had therefore to consider whether the lack of effect of βMCD on responses to CCh with PTH might result from a fortuitous combination of a larger intracellular Ca2+ pool together with PTH, via cAMP, increasing the sensitivity of IP3R (Tovey et al., 2008). That explanation is unlikely because responses to every concentration of PTH were insensitive to βMCD (Fig. 2D). We nevertheless assessed the proposal directly using a protocol that allowed the CCh-sensitive Ca2+ stores to be depleted before treatment with βMCD and then stimulation with CCh and PTH.
Cells were first stimulated with CCh in nominally Ca2+-free HBS to deplete the stores that respond to CCh alone. They were then rapidly depleted of cholesterol by incubation with βMCD (2% w/v, 10 minutes, 37°C, supplementary material Fig. S2) and then the effects of PTH with CCh on [Ca2+]i were assessed. The results demonstrate that prior stimulation with CCh or βMCD massively attenuated the Ca2+ signals evoked by subsequent addition of CCh, and combining the treatments almost abolished the response to CCh (Fig. 5A, black). However, the increase in [Ca2+]i evoked by CCh with PTH was entirely unaffected by any of the treatments. Prior depletion of the CCh-sensitive Ca2+ stores, cholesterol depletion, or both together had no effect on the ability of PTH to potentiate the increase in [Ca2+]i evoked by CCh (Fig. 5A, blue). These results demonstrate that the Ca2+ stores released by CCh alone and those released by CCh combined with PTH are functionally independent (Fig. 5B). We conclude that M3R communicate with different IP3R in different Ca2+ stores when cells are activated by CCh alone or by CCh with stimuli that increase cAMP. Cholesterol depletion distinguishes these two pathways, and although cAMP is delivered focally to IP3R when its production is stimulated by PTH (Tovey et al., 2008) globally applied 8-Br-cAMP has the same effect (Fig. 3E and supplementary material Fig. S1B). The two pathways from M3R to IP3R are not, therefore, dependent on local delivery of cAMP, they must differ in how they deliver IP3 to IP3R.
Fig. 5.
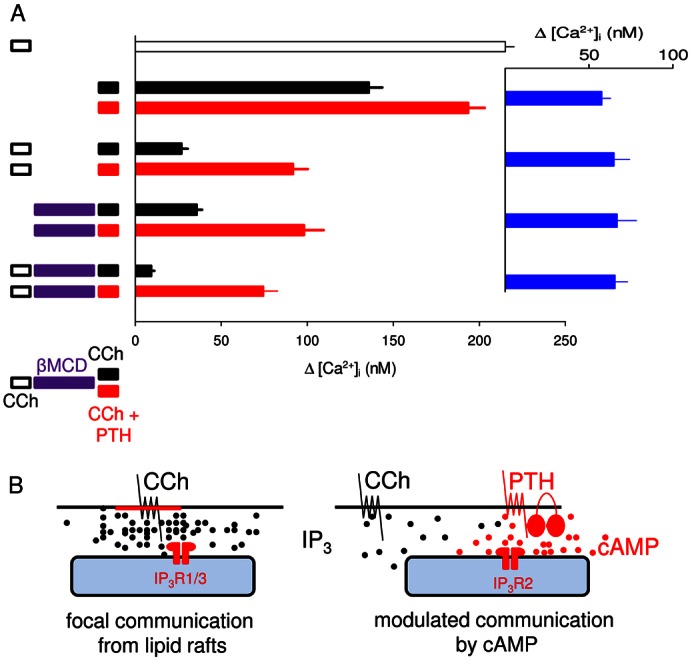
CCh and CCh with PTH stimulate Ca2+ release from functionally distinct stores. (A) Cells were stimulated with CCh (1 mM, 2 minutes), βMCD (2% w/v, 10 minutes, 37°C) and then for 2 minutes with CCh alone (1 mM) or combined with PTH (100 nM) as indicated. All treatments were carried out in nominally Ca2+-free HBS. Results show the peak increase in [Ca2+]i (means ± s.e.m. from eight experiments) evoked by the final stimulus. Inset (blue) shows the difference between paired observations for cells stimulated with CCh alone or CCh with PTH. (B) Focal and modulated communication between M3R and IP3R. Different Ca2+ stores respond to CCh alone (left) or CCh with PTH (right). We suggest that activation of M3R within lipid rafts delivers a saturating concentration of IP3 to closely associated IP3R (probably IP3R1 or IP3R3) allowing their robust ‘all-or-nothing’ activation. Graded responses to increasing concentrations of CCh result from recruitment of these digital junctions, rather than graded activity within each junction. Because each junction is either inactive or maximally activated, sensitization of IP3R within them by cAMP or 8-Br-cAMP has no effect on IP3-evoked Ca2+ release. M3R outside lipid rafts are less intimately associated with IP3R. Stimulation of these M3R provides insufficient IP3 to activate the more distant IP3R directly. cAMP focally directed from AC6 to IP3R2 in response to PTH (Tovey et al., 2008), or 8-Br-cAMP delivered globally, both increase IP3R sensitivity and thereby bring these IP3R within the sensitivity range of the IP3 provided by more distant M3R. Modulation of IP3R by cAMP extends the effective signalling range of PLC signalling pathways, allowing M3R that were ineffective in the absence of cAMP to trigger Ca2+ release from a distinct store in the presence of cAMP.
Discussion
Functionally distinct IP3-sensitive Ca2+ stores respond independently to CCh alone or to CCh delivered with stimuli, like PTH, that activate AC (Fig. 5B). Cholesterol depletion almost abolishes responses to CCh (Fig. 1) without affecting Ca2+ signals evoked by CCh with PTH (Fig. 2), isoproterenol or 8-Br-cAMP (Fig. 3). Responses to PTH with CCh are unchanged despite there being more Ca2+ within the intracellular stores after cholesterol depletion (Fig. 2), and they are unaffected by depletion of the Ca2+ stores that respond to CCh alone (Fig. 5A). These results establish that even when M3R are maximally activated, a population of M3R stimulates Ca2+ release via IP3R from a store that is unaffected by cAMP or 8-Br-cAMP. Because cAMP increases the sensitivity of all IP3R subtypes to IP3 (Tovey et al., 2010), we conclude that the IP3 concentration to which these CCh-regulated IP3Rs are locally exposed must be sufficient to cause their maximal activation. Evidence that βMCD massively attenuates CCh-evoked Ca2+ signals (Figs 1–3, Fig. 5A; supplementary material Figs S2, S3 and S7) without affecting IP3 formation (Fig. 4) is consistent with such a spatially organized delivery of IP3 to IP3R that is disrupted by cholesterol depletion (Fig. 5B, left). Responses to CCh alone are attenuated by loss of IP3R1, but not of IP3R2 (Tovey et al., 2008), suggesting that these locally activated IP3R are likely to be IP3R1 (or IP3R3). Depleting cells, probably the plasma membrane (Fig. 1C), of cholesterol, disrupts communication between these M3R and IP3R (Fig. 1). Lipid rafts are enriched in M3R, Gαq and PLCβ, they associate with IP3R, and in many cells disrupting lipid rafts inhibits M3R-evoked Ca2+ signals (Gosens et al., 2007; Lockwich et al., 2000). We propose that M3R within lipid rafts locally deliver saturating concentrations of IP3 to closely associated IP3R (Fig. 5B, left). The concentration-dependent effects of CCh must then come largely from all-or-nothing recruitment of these IP3R junctions rather than from graded recruitment of IP3R within a junction. The graded CCh signal is transduced into a digital recruitment of IP3R junctions. Cholesterol depletion disrupts lipid rafts and thereby the interactions between M3R and IP3R that facilitate focal delivery of IP3, so that diffusion and degradation of IP3 rapidly reduce its concentration to below the threshold for IP3R activation, thereby abolishing the CCh-evoked Ca2+ signals (Fig. 5B, left).
A second population of M3R is unaffected by cholesterol depletion and less intimately associated with IP3R. Activation of these M3R provides insufficient IP3 to activate IP3R directly, but these IP3R respond when they are co-stimulated with cAMP (Fig. 5B, right). Previous work showed that loss of IP3R2 attenuated Ca2+ signals evoked by CCh and PTH without affecting responses to CCh alone (Tovey et al., 2008). This suggests that IP3R2 populate the Ca2+ store from which CCh evokes Ca2+ release only in the presence of cAMP (Fig. 5B, right).
We showed earlier that activation of M3R and P2Y receptors in single HEK cells evokes Ca2+ release from functionally distinct IP3-sensitive stores (Short et al., 2000), suggesting that IP3 can be locally delivered to IP3R. HEK cells are well-suited to addressing this feature because even maximally activated M3R generate insufficient IP3 to release Ca2+ from all IP3-sensitive stores. Signalling from M3R to IP3R therefore remains focal even when M3R are fully activated. In most cells with larger numbers of receptors, maximal activation is likely to generate high concentrations of IP3 that flood the cytosol and obscure spatial organization. HEK cells provide the experimental convenience of working with all M3R activated while retaining physiologically relevant spatial organization. This experimental opportunity allowed us to demonstrate that M3R in lipid rafts deliver IP3 at high local concentration to associated IP3R (Fig. 5B, left). Such digital communication is robust and analogous to the digital signalling we proposed for cAMP delivery from AC6 to IP3R2 (Fig. 1A) (Tovey et al., 2008). M3R outside lipid rafts are less intimately associated with IP3R and their activation fails to deliver enough IP3 to activate IP3R directly. Cyclic AMP, by sensitizing IP3R (Tovey et al., 2008; Tovey et al., 2010), extends the range over which these M3R can signal, allowing M3R that are otherwise ineffective to recruit additional IP3-sensitive Ca2+ stores. We conclude that M3R can signal directly to IP3R via digital junctions or more loosely via IP3 diffusing over longer distances. The latter is ineffective unless cAMP tunes IP3R sensitivity, effectively extending the range of action of IP3 produced in response to these otherwise ineffective M3R.
Materials and Methods
Materials
Cell culture media, Lipofectamine 2000, Alexa-488 conjugated secondary antibodies, fluo-4/AM, fura-2/AM and Ca2+ standard solutions for calibration of fura-2 fluorescence signals to [Ca2+]i were from Life Technologies (Paisley, UK). Ionomycin and 8-bromo-cAMP (8-Br-cAMP) were from Merck Bioscience (Nottingham, UK). 1,2-bis(o-aminophenoxy)ethane- N,N,N′,N′-tetraacetic acid (BAPTA) was from Molekula (Gillingham, UK). U73122 was from Tocris (Bristol, UK). Foetal bovine serum, carbamylcholine chloride (CCh), poly-l-lysine, β-methylcyclodextrin (βMCD), filipin and isoproterenol were from Sigma–Aldrich (Poole, UK). G418 was from ForMedium (Hunstanton, UK). Cholesterol (plant-derived) was from Avanti Polar Lipids Inc. (Alabaster, USA). A peptide comprising residues 1–34 of human PTH (hereafter described as PTH) was from Bachem (St. Helens, UK). Cell-permeant caged iIP3 (ciIP3/PM) was from Sichem (Bremen, Germany). Inositol 1,4,5-trisphosphate (IP3) was from Alexis Biochemicals (Nottingham, UK) and 3H-IP3 (681Gbq/mmol) was from Perkin Elmer (Cambridge, UK). Cell culture plastics and 96-well assay plates were from Greiner (Stonehouse, UK). Imaging dishes (35-mm diameter with a 7-mm glass insert) were from MatTek Corporation (Ashland, USA) or PAA laboratories (Yeovil, UK). VECTASHIELD mounting reagent was from Vector Laboratories (Peterborough, UK). A plasmid encoding a Ca2+-indicator protein targeted to the ER lumen, D1ER, was a gift from R. Tsien (San Diego, USA) (Palmer et al., 2004). It was used to identify ER rather than to measure free [Ca2+]. An affinity-purified polyclonal antibody against the C-terminal of IP3R1 has been described (Cardy et al., 1997). D. Yule (University of Rochester, USA) provided affinity-purified antibodies to the N- (residues 320–338) and C-terminals (residues 2686–2702) of IP3R2 (Betzenhauser et al., 2009; Betzenhauser et al., 2008). A monoclonal antibody against IP3R3 was from BD Transduction Laboratories (Oxford, UK). A polyclonal anti-GFP antibody was from Abcam (Cambridge, UK).
Cell culture and transfection
HEK 293 and HEK 293 cells stably transfected with the human type 1 PTH receptor (HEK-PR1 cells) (Short and Taylor, 2000) were cultured at 37°C in Dulbecco’s modified Eagle’s medium/Ham’s F12 with GlutaMAX™-1, supplemented with foetal bovine serum (10%) and 800 µg/ml G418 (HEK-PR1 cells only) in a humidified atmosphere containing 95% air and 5% CO2. Medium was replaced every third day, and cells were passaged when they reached about 80% confluence. For experiments with cell populations, cells were seeded into 96-well plates (∼2×104 cells/well). For single-cell analyses, cells were seeded (1.2×105 cells/well) onto either 35-mm imaging dishes with a 7-mm glass insert or 22-mm round glass coverslips, each pre-coated with 0.01% poly-l-lysine. Cells were grown for a further 2–3 days before experiments or transfection. The latter used Lipofectamine 2000 according to the manufacturer’s instructions with 1 µg DNA/well for cells in 35-mm imaging dishes.
Measurement of [Ca2+]i
Almost confluent cultures of HEK 293 or HEK-PR1 cells in 96-well plates were washed, loaded with fluo-4/AM (2 µM), and [Ca2+]i was measured in populations of cells using a FlexStation fluorescence plate-reader (MDS Analytical Devices, Wokingham, UK) (Tovey et al., 2008). All experiments were performed at 20°C in HBS, nominally Ca2+-free HBS or Ca2+-free HBS. HBS had the following composition: 135 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 11.6 mM HEPES, 11.5 mM glucose, pH 7.3; CaCl2 was omitted from nominally Ca2+-free HBS; Ca2+-free HBS was supplemented with 10 mM BAPTA. Single-cell analyses of [Ca2+]i in HEK 293/HEK-PR1 cells loaded with fura-2/AM (2 µM) were performed as previously reported (Tovey et al., 2003). Fluorescence ratios were calibrated, after correction for background fluorescence, to [Ca2+]i by reference to Ca2+-calibration solutions.
To allow photolysis of ciIP3, HEK-PR1 cells were first incubated at 20°C for 45 minutes in HBS containing ciIP3/PM (1 µM) (Dakin and Li, 2007; Smith and Parker, 2009) and then for 45 minutes with HBS containing fluo-4/AM (2 µM) and ciIP3/PM (1 µM). After at least 60 minutes (during which any treatments used to deplete cholesterol were applied), cells were used for single-cell imaging. Imaging dishes were mounted on the stage of an Olympus IX81 inverted fluorescence microscope (60× TIRF objective, numerical aperture 1.45). Excitation light was provided by a 488-nm diode-pumped solid-state laser (Olympus Digital Laser Systems, Milton Keynes, UK) and emitted fluorescence (500–550 nm) was captured with an eMCCD camera (Andor ixon 897, Andor, Belfast, UK). Two high-intensity flashes (∼1 millisecond, 3000 µF, 300 V, ∼170 J) from a JML-C2 xenon flash-lamp (Rapp OptoElectronic, Hamburg, Germany) equipped with a 395-nm barrier filter were used to photolyse ciIP3. Photolysis of ciIP3 releases a form of IP3 (iIP3) in which the 2- and 3-hydroxyl groups are protected by an isopropylidene group. This does not prevent it from activating IP3R, but iIP3 is more resistant than IP3 to degradation by endogenous enzymes (Dakin and Li, 2007). For these measurements of [Ca2+]i using a non-ratiometric indicator (fluo-4), responses are reported as F/F0, where F0 is the average fluorescence intensity recorded from a defined region of interest (ROI) in the 5 seconds immediately before flash photolysis, and F is the fluorescence intensity from the same region after the flash.
Identification of endoplasmic reticulum and IP3 receptors
Total internal reflection fluorescence microscopy (TIRFM) using an Olympus IX81 inverted microscope with a triple-line TIRF combiner and a 150× TIRF objective (numerical aperture 1.45) was used to visualize the ER in HEK-PR1 cells expressing D1ER (Palmer et al., 2004). Fluorescence of EYFP was excited using a 488-nm diode-pumped solid-state laser, with emitted fluorescence (500–550 nm) captured using an eMCCD camera (Andor ixon 897). Widefield images of D1ER were obtained by excitation at 504 nm, with emission collected at 530–570 nm.
Endogenous IP3R were immunostained as described (Tovey et al., 2001), with all steps performed at 20°C in phosphate-buffered saline (PBS) unless stated otherwise. Cells on 35-mm imaging dishes were washed, fixed with paraformaldehyde (4% w/v in PBS) for 30 minutes, washed again and permeabilized with Triton X-100 (0.2% v/v in PBS, 10 minutes). Cells were then incubated in blocking medium (3% w/v BSA in PBS) for 1 hour, then with primary antibody (1∶100 in blocking medium) overnight at 4°C, washed (3×10 minutes), incubated with Alexa-Fluor-488 conjugated secondary antibody for 1 hour (1∶1000 in blocking medium) and washed (3×10 minutes). Immunofluorescence was imaged as described for D1ER. PBS had the following composition: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4.
Measurement of IP3
Intracellular IP3 was measured using a FRET-based IP3-sensor derived from the IP3-binding core (IBC) of IP3R1 tagged at its N- and C-termini with ECFP and EYFP, respectively, each attached by a short linker (supplementary material Fig. S4A). The sequence encoding residues 224–604 of IP3R1 was PCR-amplified from a full-length rat IP3R1(S1+) sequence (GenBank: GQ233032.1) using primers 1 and 2. The sequences of all primers are provided in supplementary material Table S1. The fragment was cloned as a BamHI/XhoI fragment into the pENTR1 vector (Gateway) to generate the construct pENTR1-IBC. The open reading frame (ORF) of ECFP was PCR-amplified from the pECFP-ER vector (Clontech) using primers 3 and 4 and cloned as a BamHI/MluI fragment into pENTR1-IBC to generate the construct pENTR1-ECFP-IBC. The ORF of EYFP was PCR-amplified from the pC1-EYFP vector (Clontech) using primers 3 and 5, and cloned as an EcoRI/XhoI fragment into pENTR1-ECFP-IBC to generate the construct pENTR1-ECFP-IBC-EYFP. The latter was inserted into the expression vector pcDNA3.2 (Gateway) to generate the IP3-sensor expression plasmid. Properties of the IP3-sensor are shown in supplementary material Fig. S4.
Cells on 35-mm imaging dishes were transfected with the plasmid encoding the IP3-sensor (1 µg/dish) using Lipofectamine 2000, and used 2 days later. An Olympus IX81 inverted microscope equipped with a 60× TIRF objective (numerical aperture 1.45) and a 440/520 nm dual band-pass dichroic mirror was used to record fluorescence using widefield excitation at 427 nm and simultaneous collection of CFP (455–485 nm) and YFP (520–550 nm) emissions using an Olympus U-SIP split imaging TV port fitted with a 505-nm dichroic mirror (supplementary material Fig. S4E). Split images were obtained at 1-second intervals using an eMCDD camera (Andor ixon 897). CFP and YFP emissions were background corrected and a normalized CFP/YFP ratio was calculated for each cell. IP3 binding causes an increase in CFP/YFP ratio, indicative of decreased FRET (supplementary material Fig. S4D,E).
3H-IP3 binding and western blotting of the IP3-sensor
HEK cells transfected with the IP3-sensor in 6-well plates were washed, scraped into PBS containing protease inhibitors (1 tablet/10 ml, Roche Diagnostics, Mannheim, Germany) and centrifuged (650 g, 2 minutes). The pellet was lysed by two freeze–thaw cycles in liquid nitrogen, sonication (1 minute) and passage through a syringe needle. After centrifugation (12,000 g, 5 minutes), the supernatant was used for western blotting or 3H-IP3 binding. For blotting, proteins (50 µg) were loaded onto NuPAGE 4–12% Bis Tris gels (Life Technologies) and blotted with an anti-GFP antibody (1∶1000). Equilibrium-competition binding assays were performed as described (Rossi et al., 2009). Briefly, incubations (500 µl) at 4°C were performed in 50 mM Tris, 1 mM EDTA, pH 8.3 with 3H-IP3 (0.75 nM) and cell supernatant (150 µg protein). Reactions were terminated after 5 minutes by addition of poly(ethylene glycol) 8000 (500 µl, 30% w/v) and γ-globulin (30 µl, 25 mg/ml) and centrifugation (20,000 g, 5 minutes). Radioactivity was determined by liquid scintillation counting. Non-specific binding was determined by addition of 1 µM IP3.
Cholesterol depletion, repletion and measurement
After loading with a Ca2+ indicator (and, where appropriate, ciIP3), cells were depleted of intracellular cholesterol by incubation with βMCD (2% w/v, ∼15 mM) for either 10 minutes at 37°C or for up to 2 hours at 20°C (Rodal et al., 1999; Sampson et al., 2004). After washing, cells were used for experiments. Cholesterol was restored as a βMCD∶cholesterol complex (10∶1, 0.26% w/v (∼2 mM) βMCD:200 µM cholesterol) added to cells for 1 hour at 37°C. Briefly, a 100 mM solution of cholesterol was prepared in methanol∶chloroform (2∶1) and complexes of βMCD∶cholesterol were formed by drop-wise addition of cholesterol to a continuously stirred (∼2 hours) solution of 0.26% w/v βMCD in HBS maintained at 80°C.
Free cholesterol was measured using filipin, a fluorescent antibiotic that binds to the free 3β-hydroxyl of cholesterol (McCabe and Berthiaume, 2001). Cells were fixed with paraformaldehyde at 20°C (4% w/v, 30 minutes), washed three times with PBS, and then with PBS containing glycine (1.5 mg/ml, 10 minutes) to terminate fixation. Cells were stained with filipin (50 µg/ml) in PBS containing foetal bovine serum (10%) for 2 hours at 20°C, washed with PBS (3×10 minutes), and mounted (VECTASHIELD). Filipin staining was visualized using an Olympus IX81 inverted fluorescence microscope with excitation at 380 nm and emission at 460–550 nm. Identical microscope and eMCCD camera settings were used to capture each image. The method used to quantify filipin staining is described in the legend to Fig. 1C.
Analysis
Concentration–effect relationships for each experiment were individually fitted to Hill equations using non-linear curve-fitting (GraphPad Prism, version 5) and the results obtained from each (pEC50, Hill coefficient h, maximal response) were pooled for statistical analysis and presentation. Student’s t-test was used for statistical analyses.
Supplementary Material
Acknowledgments
We thank Dr Ana Rossi for production and initial characterization of the IP3-sensor, and Roger Tsien and David Yule for gifts of the D1ER expression plasmid and antibodies to IP3R2, respectively.
Footnotes
Author contributions
S.C.T. completed all experimental work. Both authors devised experiments, analyzed data and wrote the manuscript.
Funding
Supported by the Wellcome Trust [grant number 085295] and Biotechnology and Biological Sciences Research Council [grant number BB/H009736/1]. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.126144/-/DC1
References
- Berridge M. J., Lipp P., Bootman M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Betzenhauser M. J., Wagner L. E. 2nd., Iwai M., Michikawa T., Mikoshiba K., Yule D. I. (2008). ATP modulation of Ca2+ release by type-2 and type-3 InsP3R: Differing ATP sensitivities and molecular determinants of action. J. Biol. Chem. 283, 21579–21587 10.1074/jbc.M801680200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzenhauser M. J., Fike J. L., Wagner L. E., 2nd, Yule D. I. (2009). Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J. Biol. Chem. 284, 25116–25125 10.1074/jbc.M109.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford N. T., Tobin A. B., Nahorski S. R. (1995). Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 274, 134–142 [PubMed] [Google Scholar]
- Cardy T. J. A., Traynor D., Taylor C. W. (1997). Differential regulation of types-1 and -3 inositol trisphosphate receptors by cytosolic Ca2+. Biochem. J. 328, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin K., Li W. H. (2007). Cell membrane permeable esters of D-myo-inositol 1,4,5-trisphosphate. Cell Calcium 42, 291–301 10.1016/j.ceca.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Foskett J. K., White C., Cheung K. H., Mak D. O. (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R., Stelmack G. L., Dueck G., Mutawe M. M., Hinton M., McNeill K. D., Paulson A., Dakshinamurti S., Gerthoffer W. T., Thliveris J. A. et al. (2007). Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am. J. Physiol. 293, L1406–L1418 10.1152/ajplung.00312.2007 [DOI] [PubMed] [Google Scholar]
- Head B. P., Patel H. H., Roth D. M., Lai N. C., Niesman I. R., Farquhar M. G., Insel P. A. (2005). G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J. Biol. Chem. 280, 31036–31044 10.1074/jbc.M502540200 [DOI] [PubMed] [Google Scholar]
- Isshiki M., Anderson R. G. W. (1999). Calcium signal transduction from caveolae. Cell Calcium 26, 201–208 10.1054/ceca.1999.0073 [DOI] [PubMed] [Google Scholar]
- Kurian N., Hall C. J., Wilkinson G. F., Sullivan M., Tobin A. B., Willars G. B. (2009). Full and partial agonists of muscarinic M3 receptors reveal single and oscillatory Ca2+ responses by β2-adrenoceptors. J. Pharmacol. Exp. Ther. 330, 502–512 10.1124/jpet.109.153619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwich T. P., Liu X., Singh B. B., Jadlowiec J., Weiland S., Ambudkar I. S. (2000). Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 275, 11934–11942 10.1074/jbc.275.16.11934 [DOI] [PubMed] [Google Scholar]
- López Sanjurjo C. I., Tovey S. C., Prole D. L., Taylor C. W. (2013). Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 126, 289–300 10.1242/jcs.116103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Busillo J. M., Benovic J. L. (2008). M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol. Pharmacol. 74, 338–347 10.1124/mol.107.044750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannstadt M., Jüppner H., Gardella T. J. (1999). Receptors for PTH and PTHrP: their biological importance and functional properties. Am. J. Physiol. 277, F665–F675 [DOI] [PubMed] [Google Scholar]
- McCabe J. B., Berthiaume L. G. (2001). N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol. Biol. Cell 12, 3601–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata J., Guerra M. T., Shugrue C. A., Gomes D. A., Nagata N., Nathanson M. H. (2007). Lipid rafts establish calcium waves in hepatocytes. Gastroenterology 133, 256–267 10.1053/j.gastro.2007.03.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S., Iida-Klein A., Segre G. V., Simon M. I. (1996). G α q family members couple parathyroid hormone (PTH)/PTH-related peptide and calcitonin receptors to phospholipase C in COS-7 cells. Mol. Endocrinol. 10, 566–574 10.1210/me.10.5.566 [DOI] [PubMed] [Google Scholar]
- Ostrom R. S., Insel P. A. (2004). The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br. J. Pharmacol. 143, 235–245 10.1038/sj.bjp.0705930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom R. S., Liu X., Head B. P., Gregorian C., Seasholtz T. M., Insel P. A. (2002). Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol. Pharmacol. 62, 983–992 10.1124/mol.62.5.983 [DOI] [PubMed] [Google Scholar]
- Palmer A. E., Jin C., Reed J. C., Tsien R. Y. (2004). Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA 101, 17404–17409 10.1073/pnas.0408030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S. K., Skretting G., Garred O., Vilhardt F., van Deurs B., Sandvig K. (1999). Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. M., Riley A. M., Tovey S. C., Rahman T., Dellis O., Taylor E. J. A., Veresov V. G., Potter B. V. L., Taylor C. W. (2009). Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 5, 631–639 10.1038/nchembio.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson L. J., Hayabuchi Y., Standen N. B., Dart C. (2004). Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ. Res. 95, 1012–1018 10.1161/01.RES.0000148634.47095.ab [DOI] [PubMed] [Google Scholar]
- Schmidt M., Evellin S., Weernink P. A. O., vom Dorp F., Rehmann H., Lomasney J. W., Jakobs K. H. (2001). A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 3, 1020–1024 10.1038/ncb1101-1020 [DOI] [PubMed] [Google Scholar]
- Schwindinger W. F., Fredericks J., Watkins L., Robinson H., Bathon J. M., Pines M., Suva L. J., Levine M. A. (1998). Coupling of the PTH/PTHrP receptor to multiple G-proteins. Endocrine 8, 201–209 10.1385/ENDO:8:2:201 [DOI] [PubMed] [Google Scholar]
- Short A. D., Taylor C. W. (2000). Parathyroid hormone controls the size of the intracellular Ca2+ stores available to receptors linked to inositol trisphosphate formation. J. Biol. Chem. 275, 1807–1813 10.1074/jbc.275.3.1807 [DOI] [PubMed] [Google Scholar]
- Short A. D., Winston G. P., Taylor C. W. (2000). Different receptors use inositol trisphosphate to mobilize Ca2+ from different intracellular pools. Biochem. J. 351, 683–686 10.1042/0264-6021:3510683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Ikonen E. (1997). Functional rafts in cell membranes. Nature 387, 569–572 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- Simons K., Toomre D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 10.1038/35036052 [DOI] [PubMed] [Google Scholar]
- Singleton P. A., Bourguignon L. Y. (2004). CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp. Cell Res. 295, 102–118 10.1016/j.yexcr.2003.12.025 [DOI] [PubMed] [Google Scholar]
- Smith I. F., Parker I. (2009). Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA 106, 6404–6409 10.1073/pnas.0810799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Tovey S. C. (2010). IP3 receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2, a004010 10.1101/cshperspect.a004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Tovey S. C. (2012). From parathyroid hormone to cytosolic Ca2+ signals. Biochem. Soc. Trans. 40, 147–152 10.1042/BST20110615 [DOI] [PubMed] [Google Scholar]
- Tovey S. C., de Smet P., Lipp P., Thomas D., Young K. W., Missiaen L., De Smedt H., Parys J. B., Berridge M. J., Thuring J. et al. (2001). Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J. Cell Sci. 114, 3979–3989 [DOI] [PubMed] [Google Scholar]
- Tovey S. C., Goraya T. A., Taylor C. W. (2003). Parathyroid hormone increases the sensitivity of inositol trisphosphate receptors by a mechanism that is independent of cyclic AMP. Br. J. Pharmacol. 138, 81–90 10.1038/sj.bjp.0705011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey S. C., Dedos S. G., Taylor E. J. A., Church J. E., Taylor C. W. (2008). Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J. Cell Biol. 183, 297–311 10.1083/jcb.200803172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey S. C., Dedos S. G., Rahman T., Taylor E. J. A., Pantazaka E., Taylor C. W. (2010). Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J. Biol. Chem. 285, 12979–12989 10.1074/jbc.M109.096016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Ardura J. A., Romero G., Yang Y., Hall R. A., Friedman P. A. (2010). Na/H exchanger regulatory factors control parathyroid hormone receptor signaling by facilitating differential activation of Gα protein subunits. J. Biol. Chem. 285, 26976–26986 10.1074/jbc.M110.147785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerth S. H., Holtzclaw L. A., Russell J. T. (2007). Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells. Cell Calcium 41, 155–167 10.1016/j.ceca.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Willoughby D., Cooper D. M. F. (2007). Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87, 965–1010 10.1152/physrev.00049.2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


