PREFACE
The family of protein deacetylases represented by yeast Sir2 has been the focus of intense investigation because of its longevity activity in yeast, worms and flies. Research in mammals has mainly focused on Sirt1, the closest homologue of Sir2. Emerging evidence from mouse models is yielding a sharper picture where Sirt1 is a potent protector from aging associated-pathologies, such as diabetes, liver steatosis, cardiovascular disease, neurodegeneration, osteoporosis and, importantly, various types of cancer.
INTRODUCTION
Over the past ten years, the aging research community has developed a growing interest in the mammalian sirtuin family (formed by paralogues Sirt1 to Sirt7), and more specifically in Sirt1, the closest homologue of yeast Sir21. This interest stems from pioneer reports linking Sir2 to lifespan regulation in lower organisms. In particular, genetic overexpression of Sir2 was shown to increase lifespan in yeast2, worms3 and flies4. This remarkable effect was further corroborated in studies using purported Sir2-chemical activators, most notably resveratrol, that extended lifespan in yeast5, worms and flies6 in a Sir2-dependent manner. Moreover, calorie restriction (CR), which is the most effective dietary intervention to extend lifespan in different organisms1, was shown to require Sir2, both in yeast7 and flies4, in order to produce lifespan extension. All these studies led to the appealing hypothesis that Sir2 is an evolutionary conserved longevity gene that mediates CR effects. If this was the case, then Sirt1 activation in mammals might improve aging without the necessity to reduce food intake. As discussed below, recent research in genetically-modified mice has demonstrated clear positive effects of Sirt1 on a variety of aspects related to mammalian health, including aging-associated diseases. Yet, current evidence does not support that Sirt1 can increase mammalian longevity.
Tidal changes
The story of Sirt1 has had its tidal changes, which should not be surprising when dealing with proteins that integrate and coordinate multiple stimuli and responses. Often, expectations run too high, and this is inevitably followed by counter expectations that go too low. In the case of Sirt1, the high expectations on its role in longevity were dimmed by studies that challenged the notion that S. cerevisiae Sir2 mediates CR-associated lifespan extension8,9 and, subsequently, by a report that failed to reproduce lifespan extension by resveratrol in D. melanogaster and C. elegans10. Regarding mammals, resveratrol was shown to have beneficial effects on health, including improved glucose tolerance and protection from fatty liver, but without extending lifespan11-13. However, accumulating evidence indicates that resveratrol is not a direct activator of Sirt114-16, but rather acts indirectly through AMPK17,18 (see Box1). Together, these conflictive results have casted a shadow on the relevance of Sirt1 in mammalian aging19.
Box1: Resveratrol and Sirt1.
Resveratrol has been widely used as a Sirt1 activator, yet a number of studies have concluded that resveratrol is not a direct activator of Sirt114-16. This does not invalidate the concept that resveratrol, through indirect mechanisms, can activate Sirt1 in vivo. In fact, earlier reports more than 10 years ago already indicated that resveratrol was an inhibitor of mitochondrial ATP synthase70 and this was corroborated by the crystallographic structure of ATP synthase complexed with resveratrol71. Inhibition of ATP synthase is predicted to increase AMP levels and therefore activate AMPK. In fact, resveratrol has been recently demonstrated to activate AMPK18, and, finally, it is now known that AMPK increases NAD+ levels and this, in turn, activates Sirt117. This chain of events now provides a rationale for how resveratrol, albeit indirectly, can activate Sirt1.
Another aspect that has generated debate is the relationship between Sirt1 and cancer, which, in turn, has its roots at the interplay between Sirt1 and p53. Early studies with in vitro cultured cells showed that Sirt1 is able to interact with and deacetylate the tumour suppressor protein p53, therefore inhibiting its transactivation potential20,21. In support of this, studies in Sirt1 knockout mice showed p53 hyperactivation, leading to increased thymocyte apoptosis22, although this could not be confirmed by other investigators23. Moreover, Sirt1 is upregulated in several types of human tumours (reviewed in24), which has further supported the idea that Sirt1 could be oncogenic. However, contrary to this, all the currently available data indicate that Sirt1 is a tumour suppressor in vivo25-28 and the possible consequences of Sirt1 on p53-mediated tumour suppression remain to be elucidated.
The above controversies highlight the importance of genetically-modified mouse models to address Sirt1 effects in the context of the organism. In this Progress, we discuss recent studies using genetic mouse models of Sirt1, which have shed new light into the field. For more comprehensive reviews on Sirt1, the reader is referred to recent reviews29,30.
Sirt1 and metabolism
Sirt1 effects in metabolism are the best-characterized ones. A considerable amount of recent literature based on a variety of genetically-modified mouse models has solidly demonstrated a protective role for Sirt1 against pathologies associated to high dietary fat intake (HFD, high fat diet), such as glucose intolerance and liver steatosis (also known as fatty liver) (Table 1).
Table 1.
Sirt1 mouse models and their effects on cancer and metabolism
Phenotypes in green colour are those that imply a beneficial effect of Sirt1, on the contrary, those in red imply a detrimental effect of Sirt1.
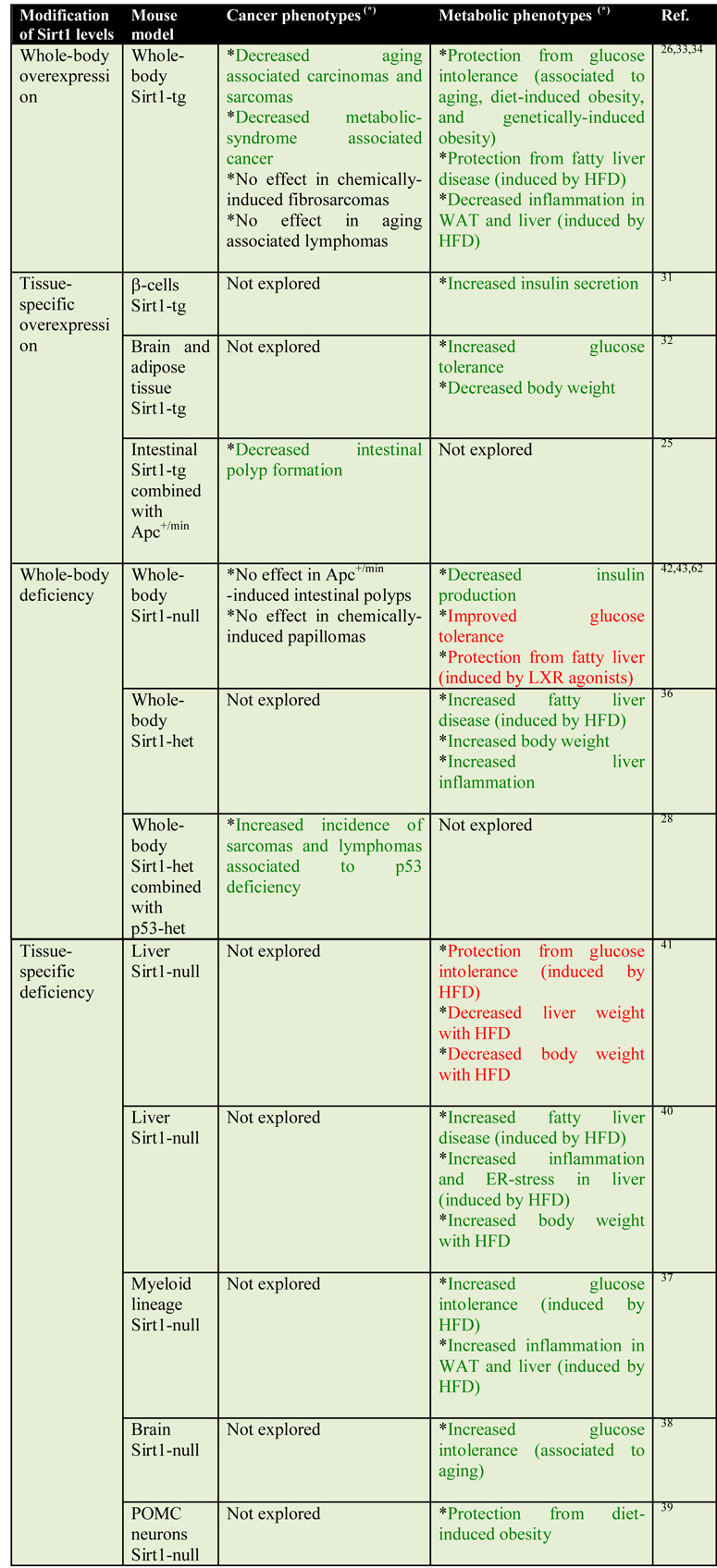
The first mouse models that linked Sirt1 to improved glucose tolerance were studies in which Sirt1 overexpression was restricted, in one case, to pancreatic β-cells31 and, in the other one, to brain and adipose tissue32. The effect of Sirt1 on metabolism has been further explored in mice with whole-body transgenic expression of moderately increased levels of Sirt1 (2-3 x-fold) under its own endogenous transcriptional regulation33,34. Importantly, these Sirt1 transgenic mice were protected from diabetes associated to diet-induced obesity33,34 or to genetically-induced obesity34. The impact of moderate systemic Sirt1 overexpression on protection from high dietary fat goes beyond glucose tolerance. In fact, whole body Sirt1 transgenic mice are remarkably protected from developing liver steatosis33. Consistent with this, mice deficient in Dbc1, a negative regulator of Sirt1, are also protected from fatty liver35 and, conversely, Sirt1 heterozygous mice are prone to develop this disease36. Mice with tissue-specific deletion of Sirt1 have further refined the picture and have indicated that Sirt1 exerts its protective metabolic effects through its actions on multiple tissues. Namely, myeloid lineage-specific deletion of Sirt1 resulted in insulin resistance37; brain-specific Sirt1 deletion increased aging-associated glucose intolerance38; Sirt1 deletion in the pro-opiomelanocortin (POMC) neurons, that control food intake and energy expenditure, resulted in leptin resistance in these neurons and increased susceptibility to diet-induced obesity39; and, finally, liver-specific deletion of Sirt1 protected mice from steatosis40. However, regarding the latter, it should be mentioned that other investigators have reported the opposite phenotype using the same genetic model of Sirt1 deficiency in the liver41; the basis for these conflictive results are unclear. A separate note must be made of the few metabolic studies using whole-body Sirt1-null mice because of their paradoxical results. In particular, Sirt1-null presented higher glucose tolerance42 and protection from fatty liver induced by LXR agonists43, which is in sharp contrast with all above-described evidence indicating that Sirt1 protects from glucose intolerance and fatty liver. However, interpretation of the results obtained with whole-body Sirt1-null mice is highly complex given that the majority of these mice die in the perinatal period and the survivors have developmental defects22,44 that may obscure the role of Sirt1 in a normal physiological context. In summary, as listed in Table 1, most studies using a variety of mouse models have concluded that Sirt1 is a robust metabolic protector acting at several systems, including not only the liver, but also the brain and the immune system.
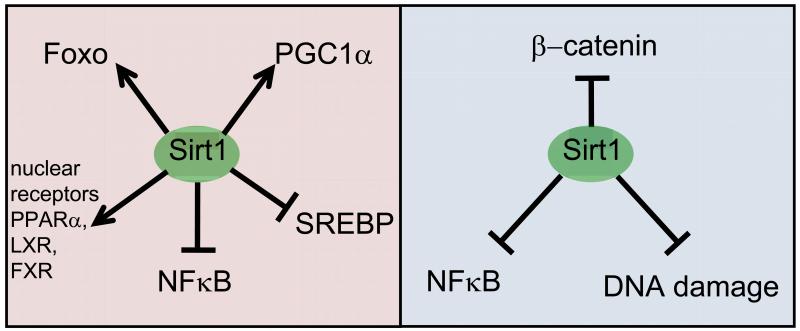
A recurrent mechanistic finding in the above-described mouse models linking Sirt1 to protection from diabetes and fatty liver is a reduced inflammatory response associated to lower levels of NFκB activity33,35-37,40 (Figure 1). This fully supports in mice the earlier finding in cultured cells that Sirt1 binds, deacetylates and inhibits NFκB45. Another recurrent mechanism is the deacetylation and activation of key transcription factors involved in lipid metabolism, such as Foxo134 and the nuclear receptors PPARα40, LXR43, and FXR46, as well as, their general co-activator PGC1α33,40,47 (Figure 1). Numerous additional mechanisms implicating Sirt1 in metabolism have been also reported, but their detailed discussion goes beyond the scope of this Progress article. Briefly, these additional mechanisms include increased insulin production by repression of Ucp231,42; deacetylation and inhibition of SREBP33,40,48,49; repression of the Pparg promoter50; activation of the Sirt6 promoter51 which, in turn, may contribute to protect from metabolic damage as demonstrated in Sirt6-overexpressing mice52; and deacetylation and activation of Lkb153,54.
Figure 1. Summary of the main mechanisms through which Sirt1 protects from metabolic damage and cancer.
The left panel shows the main mechanisms by which Sirt1, through direct deacetylation, protects from metabolic damage. Other mechanisms are mentioned in the text. The right panel shows the three mechanisms reported so far supporting cancer protection by Sirt1 in mice.
Diabetes and fatty liver are diseases that often occur concurrently, and in association with other pathologies, notably including cardiovascular disease. This group of associated pathologies is referred to as the metabolic syndrome55. In this regard, it is worth mentioning that there is also evidence from Sirt1-mouse models indicating that Sirt1 can improve cardiovascular function56-59. The metabolic syndrome is characteristic of aged individuals chronically exposed to high caloric intake and low physical activity, a lifestyle of increasing prevalence that explains the high incidence of metabolic syndrome-associated diseases worldwide55. Based on the above-mentioned data in genetically-modified mice, Sirt1 activation is an attractive target for pharmaceutical interventions aimed to delay or ameliorate the pathologies associated to the metabolic syndrome.
Sirt1 and cancer
The analysis of cancer in mice with genetically-modified Sirt1 levels has consistently supported the concept that Sirt1 possesses strong tumour suppressive activity (Table 1). In particular, increased or decreased Sirt1 expression results, respectively, in delayed or accelerated sarcoma and lymphoma development in a p53-heterozygous background27,28. Interestingly, both studies obtained supporting in vitro evidence indicating that this tumour suppressive activity reflected the ability of Sirt1 to preserve genomic integrity in the face of p53 deficiency27,28. Upon DNA damage, chromatin-bound Sirt1 relocates from repeated elements to the actual DNA damaged sites, presumably repressing transcription27 (Figure 1).
Following on the reported functions of Sirt1 in protection from DNA damage and in protection from fatty liver, whole-body Sirt1 transgenic mice were subjected to a novel carcinogenic treatment that models metabolic syndrome-associated liver cancer. Feeding mice with a high fat diet (HFD) dramatically increases the incidence of liver carcinomas in mice previously treated with a hepatic carcinogen (diethylnitrosamine, DEN) which on its own has poor carcinogenic activity26,60. Importantly, Sirt1 transgenic mice were remarkably protected from metabolic syndrome-driven liver carcinogenesis26. This protection was due to the combined effects of Sirt1, first, in reducing DNA damage by the hepatic mutagen and, then, in preventing inflammation and fatty liver by HFD26 (Figure 1). Dietary obesity leads to liver inflammation by enhancing the expression of IL-6 and TNFα, well known targets of NFκB, and this inflammation promotes tumorigenesis60. Sirt1 transgenic mice show decreased NFκB activation and, concomitantly, decreased inflammation in the liver resulting in the observed protection from liver tumorigenesis26,33. Of note, human liver cancers present decreased levels of Sirt1 compared to normal liver28. It remains to be elucidated whether Sirt1 would protect from inflammation-associated tumorigenesis in general or just from carcinogenesis associated to dietary-induced inflammation.
On another line of research, Sirt1 overexpression in enterocytes protected from intestinal tumours in the Apc+/min model25. This observation led to discovery that Sirt1 deacetylates and inhibits β-catenin in the intestine of these mice25 (Figure 1). In further support of this, subsequent studies showed that Sirt1 suppresses the growth of colon tumour xenografts assays using human colon cancer lines61. Of note, recent studies using Sirt1-null mice revealed no difference in tumour development when combined with the Apc+/min mutation62. It is formally possible that Sirt1 overexpression could protect from intestinal tumorigenesis, while in the absence of Sirt1, compensatory mechanisms could supply the missing activity of Sirt1. As briefly alluded above, caution must be taken when interpreting the phenotypes observed in whole-body Sirt1-null strains due to their developmental defects22,44.
Finally, regarding aging-associated spontaneous cancer, whole-body Sirt1 transgenic mice displayed a global decrease in cancer incidence26. This protection, however, was restricted to carcinomas and sarcomas, while lymphoma development was not affected by Sirt1 dosage. This differential effect is currently not well understood and it contrasts with the previously mentioned Sirt1-dependent protection from p53-deficient lymphomas. The aging-associated lymphomas developed in a p53-functional context are conceivably less aggressive and less genetically unstable than the lymphomas developed in the absence of p53. Hence, the ability of Sirt1 to preserve genomic stability could be less relevant in the context of aging-associated lymphomas. In addition to aging-associated lymphomas, Sirt1 was also found to be neutral regarding chemically-induced fibrosarcomas26. Apart from these two cancer models in which Sirt1 levels do not seem to affect tumorigenesis, the general finding in the above-summarized studies is that Sirt1 protects from cancer through a number of mechanisms, including protection from DNA damage, protection from diet-induced inflammation, and inhibition of the oncogenic activity of β-catenin (Figure 1). At present, there are no reports of mouse models of cancer in which Sirt1 plays an oncogenic role (Table 1).
Sirt1 and aging
The effect of Sirt1 on mammalian aging is a long-sought result in the field. Importantly, mice moderately overexpressing Sirt1 (3x-fold) under its own transcriptional regulatory elements do not live longer under standard diet conditions, but they show a healthier aging26. In particular, aged Sirt1 transgenic mice showed improved glucose homeostasis, better preservation of bone mineralization, reduced incidence of sarcomas and carcinomas, and reduced levels in molecular markers of aging, such as p16Ink4a or DNA damage26. Moreover, recent studies in mice have also demonstrated a protective role for Sirt1 against Alzheime’s disease and amyotrophic lateral sclerosis63,64. The emerging picture is one in which Sirt1, through its well-established protective activities against diet-induced metabolic damage, genomic instability, and cancer, improves some aspects of aging and delays or ameliorates a number of aging-associated diseases such as metabolic syndrome, Alzheimer or some types of cancer. In summary, although current evidence does not allow to categorize Sirt1 as a “longevity” gene, it clearly is a beneficial gene for aging and aging-associated diseases.
A related issue is whether Sirt1 mediates the effects of calorie restriction (CR) on aging. Only one report has addressed this directly using Sirt1-null mice under CR65. As mentioned above, most Sirt1-null mice die in the perinatal period and those that survive have developmental defects22,44, including a shortened lifespan (median lifespan of approximately 1 year)65. Interestingly, CR started between 5-7 months of age did not have an impact on the longevity of Sirt1-null mice65. Although suggestive, the abnormally short lifespan of Sirt1-null mice makes it difficult to interpret their lack of response to CR. Additionally, some of the beneficial effects of Sirt1 on metabolism are also characteristically produced by CR, such as improved insulin sensitivity. Finally, a number of studies have reported a variety of molecular and phenotypic effects of CR that are mediated by Sirt1 in mice38,65-69. Overall, there is growing suggestive evidence indicating that Sirt1 participates in CR.
Conclusions and future perspectives
Recent work with genetically-modified mice has firmly established Sirt1 as a protector of metabolic syndrome and as a tumour suppressor in a wide range of cancers. Moreover, emerging evidence implicate Sirt1 in protection from cardiovascular disease56-59 and neurodegeneration63,64. The fact that Sirt1 impinges on such a variety of aging-associated diseases suggests that it could be a longevity gene, although direct evidence for this is still lacking in mammals. The only available study of longevity in a mouse model with systemic Sirt1 overexpression (3x-fold) reported improved health during aging, but normal longevity26. In this regard, it would be interesting to test whether higher levels of Sirt1 overexpression or contemporaneous overexpression of several sirtuins could extend longevity.
Regarding metabolism, it would be of critical importance to know if, once diabetes has developed, Sirt1 activation could be used as a treatment. To this end, and until potent Sirt1 activators are developed, Sirt1 inducible knock-in mice could be used to explore this possibility. If these activators showed a benefit in diabetic patients, and given the cancer protection activity of Sirt1, it is tempting to speculate that they could diminish cancer incidence concomittantly. Precedence for this can be found in the anti-diabetic drug metformin that decreases cancer incidence both in mice63,64 and humans63,64.
With respect to cancer, genetic studies published so far support a protective role for Sirt1 in many types of cancer. However, this is not general and there are examples of mouse cancer types where Sirt1 does not play a role (Table 1). Besides, numerous studies based on in vitro cultured cancer cell lines have suggested that Sirt1 has oncogenic activities24. Although this has not been confirmed yet in a more physiological setting, it remains possible that future studies could reveal oncogenic activities of Sirt1 in vivo. Finally, a key pending question is whether Sirt1 activators have therapeutic activity on cancer.
REFERENCES
- 1.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 4.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 6.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 7.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 8.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabrizio P, et al. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Pearson KJ, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeberlein M, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 15.Beher D, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 16.Pacholec M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber K. A mid-life crisis for aging theory. Nat Biotechnol. 2008;26:371–374. doi: 10.1038/nbt0408-371. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng HL, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamel C, Abrol M, Jardine K, He X, McBurney MW. SirT1 fails to affect p53-mediated biological functions. Aging Cell. 2006;5:81–88. doi: 10.1111/j.1474-9726.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 24.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firestein R, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herranz Sirt1 improves healthy ageing and protects from metabolic syndrome - associated cancer. Nature Communications. 2010;1:3 doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberdoerffer P, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RH, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moynihan KA, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 33.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks AS, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escande C, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120:545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu F, et al. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology. 2010;151:2504–2514. doi: 10.1210/en.2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schug TT, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010 doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramadori G, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen D, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordone L, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 44.McBurney MW, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung F, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemper JK, et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 48.Walker AK, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponugoti B, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010 doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picard F, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanfi Y, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9:162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 53.Hou X, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 56.Alcendor RR, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 57.Zhang QJ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein S, et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein S, et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kabra N, et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem. 2009;284:18210–18217. doi: 10.1074/jbc.M109.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 63.Kim D, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donmez G, Wang D, Cohen DE, Guarente L. Sirt1 supresses β-amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Boily G, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 67.Qin W, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 68.Satoh A, et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kume S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]