Abstract
Connections of primary (V1) and secondary (V2) visual areas were revealed in macaque monkeys ranging in age from 2 to 16 weeks by injecting small amounts of cholera toxin subunit B (CTB). Cortex was flattened and cut parallel to the surface to reveal injection sites, patterns of labeled cells, and patterns of cytochrome oxidase (CO) staining. Projections from the lateral geniculate nucleus and pulvinar to V1 were present at 4 weeks of age, as were pulvinar projections to thin and thick CO stripes in V2. Injections into V1 in 4- and 8-week-old monkeys labeled neurons in V2, V3, middle temporal area (MT), and dorsolateral area (DL)/V4. Within V1 and V2, labeled neurons were densely distributed around the injection sites, but formed patches at distances away from injection sites. Injections into V2 labeled neurons in V1, V3, DL/V4, and MT of monkeys 2-, 4-, and 8-weeks of age. Injections in thin stripes of V2 preferentially labeled neurons in other V2 thin stripes and neurons in the CO blob regions of V1. A likely thick stripe injection in V2 at 4 weeks of age labeled neurons around blobs. Most labeled neurons in V1 were in superficial cortical layers after V2 injections, and in deep layers of other areas. Although these features of adult V1 and V2 connectivity were in place as early as 2 postnatal weeks, labeled cells in V1 and V2 became more restricted to preferred CO compartments after 2 weeks of age.
Indexing Terms: V1, V2, pulvinar, lateral geniculate nucleus, macaque monkey
The connections of V1 and V2 in adult macaque monkeys have been extensively characterized (for reviews, see Felleman and Van Essen, 1991; Casagrande and Kaas, 1994; Sincich et al., 2010; Markov et al., 2011). Only a few studies have explored connections of these areas during early development (Barone et al., 1995; Coogan and Van Essen, 1996; Batardière et al., 2002); however, these studies did not relate connection patterns to anatomical markers of the modular organizations of V1 and V2. Previous studies of the visual system have provided evidence that connections are sometimes exuberant early in development and mature patterns of connections arise from subsequent pruning of these imprecise connections, usually as a result of sensory experience (Innocenti et al., 1977; Wiesel, 1982). The results of other studies suggest that many adult patterns of connections are in place prior to visual experience (Dehay and Kennedy, 1988; Coogan and Van Essen, 1996; Horton and Hocking, 1996). Determining how and when adult-like patterns of afferent projections emerge within and to V1 and V2 would promote our understanding of when adultlike visual functions may begin to emerge.
Physiological and anatomical studies indicate that there are at least three distinct cortical processing streams emerging from V1 and V2 (Roe and Ts'o, 1995; Sincich and Horton 2002, 2005; Casagrande and Xu, 2003). Bands or stripes of tissue crossing the width of V2 can be identified in brain sections processed for cytochrome oxidase (CO) or myelin, and three processing streams have been related to the three different CO band types (Livingstone and Hubel, 1983, 1984; Tootell et al., 1983; Olavarria and Van Essen, 1997; Sincich and Horton, 2002; Kaskan et al., 2008; Lim et al., 2009). CO-dark thick stripes have been associated with neurons sensitive to image disparities between two eyes (information used in stereopsis), thin stripes with neurons selective for color, and pale stripes with neurons selective for aspects of shape analysis (Tootell et al., 1983; Livingstone and Hubel, 1984a, 1987; DeYoe and Van Essen, 1985; Shipp and Zeki, 1985; Hubel and Livingstone, 1987; Tootell and Hamilton, 1989).
Each stripe type preferentially receives different V1 afferents and projects to various cortical targets. For instance, cortex associated with the CO-dense blobs of V1 preferentially projects to V2 thin stripes (Livingstone and Hubel, 1983, 1984; Sincich and Horton, 2002, 2005; Sincich et al., 2007), whereas thick and pale stripes receive projections from interblob regions around CO blobs of V1 (Xiao and Felleman, 2004; Sincich et al., 2010). Horizontal connections in V2 have been described as widely distributed (Livingstone and Hubel, 1984; Rockland, 1985; Amir et al., 1993; Levitt et al., 1994; Malach et al., 1994; Roe and Ts'o, 1995). Like stripe types tend to have the most connections with one another, especially at greater distances (Livingstone and Hubel, 1984; Levitt et al., 1994), although there are also connections with other stripe types (Levitt et al., 1994; Rockland, 1985). Additionally, thick stripes have connections with MT, whereas thin and pale stripes have connections with V4 (Livingstone and Hubel, 1983, 1984; DeYoe and Van Essen, 1985; Shipp and Zeki, 1985; Hubel and Livingstone, 1987), demonstrating the emergence of the two cortical processing streams (Ungerleider and Mishkin, 1982; Goodale and Westwood, 2004). Subcortically, the visual pulvinar projects to CO-rich stripes of V2, but not pale stripes (Livingstone and Hubel, 1982; Levitt et al., 1995).
Previous reports have suggested that adult-like connections are present before birth, with patchy patterns of label formed after V1 and V2 injections (Coogan and Van Essen, 1996). However, the same studies and others also suggest that a substantial amount of “reorganization” occurs between neonatal monkeys and adults (Barone et al., 1995; Batardière et al., 2002). The results of recent electrophysiological studies have suggested, as well, that there may be some anatomical refinement of V1 and V2 connections over the first few weeks after birth. Although response latencies for neurons in V1 and V2 appear to be adult-like as early as 2 weeks after birth (Zhang et al., 2008), the receptive field (RF) center/surround characteristics within V1 and V2 change between 2 and 8 weeks in that center, and surround sizes are initially larger than those found in adults and then decrease over time (Zhang et al., 2005; Zheng et al., 2007).
The observed differences in RF center/surround properties are hypothesized to have anatomical correlates. Models of the anatomical sources of RF centers and surrounds propose that feed-forward projections contribute to RF center characteristics (Bauer et al., 1999), horizontal/intrinsic projections account for near surround suppressive characteristics (Gilbert et al., 1996), and far surround suppressive characteristics are a result of feedback projections (Angelucci and Bullier 2003; Angelucci and Bressloff, 2006). Therefore, the observed decreases in RF center and surround sizes of subjects between 2 and 8 weeks of age may be associated with the reorganization of the feed-forward, as well as intrinsic and feedback connections in V1 and V2 soon after birth.
In the present study, we examined connections of V1 and V2 of monkeys aged 2, 4, 8, and 16 weeks. These same animals were undergoing an electrophysiological analysis of receptive field properties of neurons in the contralateral V1 and V2. We found that subcortical and cortical connection patterns were largely adult-like as early as 2 weeks of age, with a large proportion of afferent projections to V2 thin stripes arising from V1 blobs, and preferential connections between like stripes within V2; however, these connections may undergo some pruning and refinement between 2 and 8 weeks of age.
Materials and Methods
We placed small injections of an anatomical tracer, cholera toxin subunit B (CTB) into V1 and V2 of the right hemisphere of anesthetized infant macaque monkeys (Macaca mulatta) at 2, 4, 8, and 16 weeks of age. After injections, microelectrode recording experiments were carried out in the left hemisphere for the following 2–4 days (Zheng et al., 2007; Maruko et al., 2008). At the end of recording sessions, the monkeys were deeply anesthetized and perfused with fixative, and the brains were subsequently processed to reveal architecture and anatomical connections. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Houston, and adhered to National Institutes of Health guidelines. A total of 10 macaque monkeys weighing between 480 and 600 g and ranging in age from 2 to 16 weeks were used in this study (Table 1).
Table 1. Summary of Cases.
| Case no. | Age (wk) | Sex | Injection location | Figures |
|---|---|---|---|---|
| MV1b2 | 2 | F | V1/V2 border | 13 |
| MV2n2 | 2 | M | V2 thin stripe | 3, 5, 7 |
| MV1b4 | 4 | M | V1/V2 border | 12 |
| MV2b2 | 2 | M | V1/V2 border | 11 |
| MV2b4 | 4 | F | V1/V2 border and somewhere in V2 | None |
| MV1c6 | 16 | M | V1 | None |
| MV2n4 | 4 | M | V2 thin stripe | 2, 3, 6, 15 |
| MV2k4 | 4 | M | V2 thick stripe | 6, 16 |
| MV1c4 | 4 | M | V1 | 10, 14 |
| MV2n8 | 8 | M | V2 thin stripe | 4, 5, 9, 15 |
Tracer injections
Monkeys were initially anesthetized with an intramuscular injection of ketamine hydrochloride, (15-20 mg/kg) and acepromazine maleate, (0.15-0.2 mg/kg). All subsequent surgical procedures were carried out with additional propofol anesthesia (4–6 mg/kg/hr), administered intravenously as needed to maintain a surgical level of anesthesia. A tracheotomy was performed in order to facilitate artificial breathing, and the head was secured in a stereotaxic instrument. Heart rates, temperatures, CO2 levels, and electroencephalograms (EEGs) were monitored throughout all procedures. A portion of V1 and V2 was exposed with a small craniotomy (approximately 7 × 5 mm) and a durotomy over the right lunate sulcus of each monkey. Using a glass pipette, glued over the tip of a Hamilton syringe, pressure injections of 0.05 μl of 1% CTB (Sigma-Aldrich, St. Louis, MO) were placed into the caudal lip of the lunate sulcus near the V1-V2 border. Injections were placed 0.8-1.3 mm deep, in the middle layers of cortex.
Following the injections, the dura was replaced and the exposed region was covered with a clear contact lens. A thin cap of dental cement was used to seal the opening of the skull and the scalp was sutured closed. Following the surgical procedures on the right hemisphere of each monkey, a small region of visual cortex of the left hemisphere was exposed for microelectrode recording experiments as described elsewhere (Zheng et al., 2007; Zhang et al., 2008).
Histology
Physiological experiments lasted 2–4 days, after which animals were given a lethal dose of sodium pentobarbital (100 mg/kg) and perfused transcardially with phosphate-buffered saline (PBS) and then with 2% paraformaldehyde, in PBS. The brains were then removed. Both the right and left cortical hemispheres were carefully separated from underlying brain structures. Most of the visual cortex from the right hemisphere was separated from more anterior cortex and manually flattened as described previously (Stepniewska et al., 2005). The left hemisphere was left intact for further processing of electrophysiological electrode tract data. Both hemispheres and thalamus were then placed in 30% sucrose in PBS for 24–48 hours. The flattened right hemisphere was cut on a freezing microtome parallel to the cortical surface at a thickness of 40 or 50 μm. The thalamus was cut coronally into 40- or 50-μm sections. Every third section of cortex was processed for cytochrome oxidase (CO; Wong-Riley, 1979b), to reveal the V1–V2 border and the CO-dense stripes of V2, and another series of every third section was processed for CTB by using an antibody and the histological procedures described in Bruce and Grovfova (1992). See Table 2 for the CTB antibody source. The CTB antibody failed to stain cells in tissue from animals without CTB injections. A third series was processed for myelin, or set aside. Every third section of the thalamus was processed for CO to help determine lateral geniculate nucleus (LGN) and pulvinar borders. A second series of every third sections was processed for CTB, and the third series was set aside.
Table 2. Antibody Used in This Study.
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Cholera toxin subunit B (CTB) | Purified CTB isolated from Vibrio cholerae | List Biological Laboratories (Campbell, CA), goat polyclonal #703 | 1:5,000 |
Data analysis
Neurons labeled for CTB in both cortex and thalamus were plotted under high resolution by using a Leitz microscope coupled to a Neurolucida system (MicroBrightField, Colchester, VT). Plotted sections were aligned with adjacent CO sections, by using blood vessels and other landmarks as references. Adobe (San Jose, CA) Photoshop and Illustrator were used in order to relate connection patterns with architectonic borders. During alignment, sections were adjusted for differential section shrinkage by using scale and rotate functions in Adobe Illustrator. Photographs of tissue sections were adjusted for luminance and contrast, but were otherwise unaltered.
The connection patterns from the different age groups were compared for the numbers and locations of labeled cells. Borders between subcortical visual nuclei and visual cortical areas were identified in brain sections processed for CO, or were estimated from previous established relationships to other borders and cortical features. V1 and V2 were easily identified by using CO-stained preparations due to the characteristic blob–interblob CO staining pattern and CO-dense middle cortical layers (Horton and Hubel, 1981; Horton, 1984; Livingstone and Hubel, 1984; Hendrickson, 1985; Levitt et al., 1994; Malach et al., 1994; Olavarria and Van Essen, 1997) and the alternating CO dense and pale stripe pattern within V2 (Livingstone and Hubel, 1983, 1984; Tootell et al., 1983; Horton, 1984; Hendrickson, 1985; Olavarria and Van Essen, 1997).
In some CO preparations V3 can be identified by a banding-like pattern of CO staining yet the bands in V3 are much wider and less obvious than those observed in V2 (Lyon and Kaas, 2002). For the most part we identified V3 based on measurements and the location of V3 within the lunate sulcus observed in Van Essen et al. (1986), Burkhalter et al. (1986), and Lyon and Kaas (2002). In the present material cortex between the anterior lip of the lunate sulcus and the posterior edge of the superior temporal sulcus (STS) was designated as DL/V4, similar to Stepniewska et al. (2005). We were unable to differentiate visual areas within the STS and therefore considered all visual areas within this region as the MT complex (Kaas and Morel, 1993).
In the primary visual cortex (V1), the borders of CO blobs with interblobs were estimated by visual inspection after adjusting for contrast in Adobe Photoshop (Fig. 1). Blob borders were also estimated by using filtering software from Image J and Adobe Photoshop. First images were imported into Image J and adjusted by using the auto local threshold plug-in; images were then smoothed and imported into Adobe Photoshop where they were thresholded and filtered to remove noise. Finally, edges were detected by using the stylize filter (Fig. 1). All CO images were processed by using the same parameters in all of the above-mentioned steps. We determined numbers of labeled neurons within blob and non-blob compartments of V1 only from regions with good CO staining where we were able to determine blob borders. Percent total area within blobs in the sampled regions was determined by using Image J software. The computer filtering often resulted in multiple separate blobs merging into a single large blob (e.g., Fig. 1E). This frequently resulted in a greater percent area dedicated to blobs and, therefore, a greater number of cells within those blob borders. A similar problem was previously reported by Purves and La Mantia (1993).
Figure 1.
Filtering figure showing the two methods used for determining blob borders. A: Photomicrograph of CO blob patterns in V1 of an 8-week-old macaque monkey adjusted for contrast. Two procedures were used to determine blob borders. In the first method, images were imported into Image J, adjusted by using the auto local threshold plug-in, and smoothed. B: Then images were imported into Adobe Photoshop where the threshold levels were adjusted and images were noise filtered. D: Finally, edges were determined by using the stylize filter. In the second method, images were imported into Adobe Photoshop, where they were adjusted for contrast (A); C: after this borders were determined with visual inspection. E: A comparison of blob borders for these two methods. Scale bar = 0.5 mm in A.
Accordingly, we provide subjective and computer-based filtering estimates. As in previous studies (e.g., Olavarria and Van Essen, 1997), stripe types were classified as CO dark and CO light. The CO dark stripes were further divided into thick and thin types based on a difference in width. As the shapes of the functionally distinct stripe types can vary within individuals and especially across species (Krubitzer and Kaas, 1990), occasional misidentifications of functional types of stripes may occur. In one case (MV2n2: Fig. 2D,E), there were two thin stripes next to one another. Multiple thin stripes separated by only pale stripes versus two pale stripes and a thick stripe have been observed previously by Olavarria and Van Essen (1997). Numbers of labeled cells within estimated stripe borders were determined by using Adobe Illustrator. The average widths for the different stripe types were based on our subjective borders and were measured using Image J.
Figure 2.
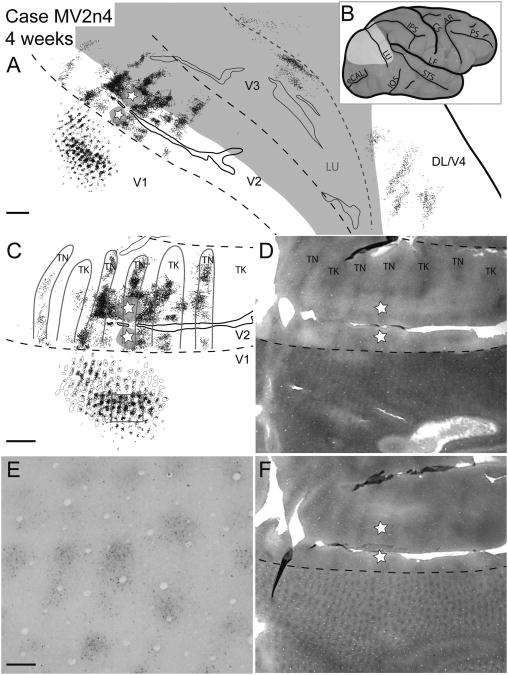
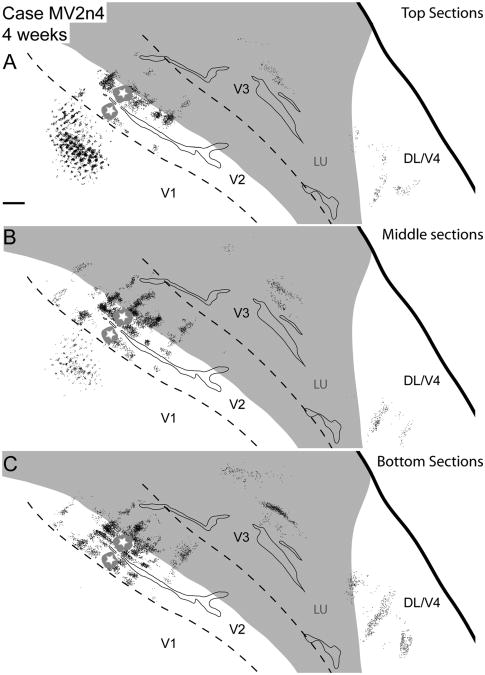
Case MV2n4. Reconstruction of a V2 thin stripe injection and labeled neurons in a 2-week-old macaque. A: Reconstruction of labeled cells throughout the brain of a 2-week-old macaque after a CTB injection into a thin stripe in V2. The star and gray halo represent the injection site location and spread, respectively. In this and subsequent figures, black dots represent retrogradely labeled CTB cells. Gray shaded areas represent unfolded sulci and dashed black lines represent borders between visual areas determined with cytochrome oxidase and local cortical landmarks. B: A lateral view of the macaque brain before artificial flattening. The highlighted area corresponds to the area of the brain shown in A. C: A magnified view of CTB label along the V1/V2 border overlaid with stripe type and blob borders of cytochrome oxidase staining based on E and F. D: Cytochrome oxidase photomicrograph showing the stripe type pattern in V2. TN and TK correspond to thin and thick stripes, respectively. E: A photomicrograph of a portion of V1 (boxed area from C) of a single CTB section with retrogradely labeled cells. F: Cytochrome oxidase photomicrograph demonstrating the blob pattern in V1. For abbreviations, see list. Scale bar = 2 mm in A and C (applies to C,D,F); 0.25 mm in E. CAL is the calcarine sulcus, IPS the intraparietal sulcus, STS the superior temporal sulcus, and IOS the inferior occipital sulcus. V1 is the primary visual area, V2 is the secondary visual area, V3 is the third visual area, DL/V4 is the dorsolateral/fourth visual area. TN, TK are thin and thick stripes respectively.
Results
Cortical connections were analyzed after V1 or V2 injections in 2-, 4-, and 8-week-old macaque monkeys, and one V1 injection in a 16-week-old monkey using the anatomical tracer CTB to determine any age-dependent changes. In most cases, examined cortical tissue was from the V1, V2, V3, dorsomedial area (DM), dorsolateral area (DL), and middle temporal area (MT). In other cases a subset of these areas was examined. In all cases, the border between V1 and V2 was determined by using CO staining. Other borders were estimated based on their expected location relative to V1 and V2 and the location of cortical fissures. V1 is easily recognized in CO preparations as a region with alternating CO-dense blobs and CO-weak interblobs (see Fig. 2F for an example), whereas V2 is apparent as a result of its distinctive CO-dense and CO-weak banding pattern (see Fig. 2D for an example). We also analyzed thalamocortical connections in some monkeys.
Because our most extensive results were on cortical connections, especially V2, we start with a description of the cortical connections of V2, followed by V1, and then subcortical connections of V1 and V2. Cortical connections revealed by V2 injections were obtained from four cases. Within these cases, three injection sites involved thin CO stripes, and one involved a thick CO stripe.
V1 projections to V2
Adult-like patterns of connections between V1 and V2 (Federer et al., 2009 ; Livingstone and Hubel, 1987a; Sincich et al., 2010) were present as early as 2 weeks of age (Fig. 3). For instance, injections into thin stripes preferentially labeled blob regions within V1 (Figs. 2–5), whereas injections into a thick stripe within V2 (Fig. 6) labeled cells mainly within interblobs, with some spread into the outer limits of blobs in V1. None of the injections were centered on a pale stripe of V2.
Figure 3.
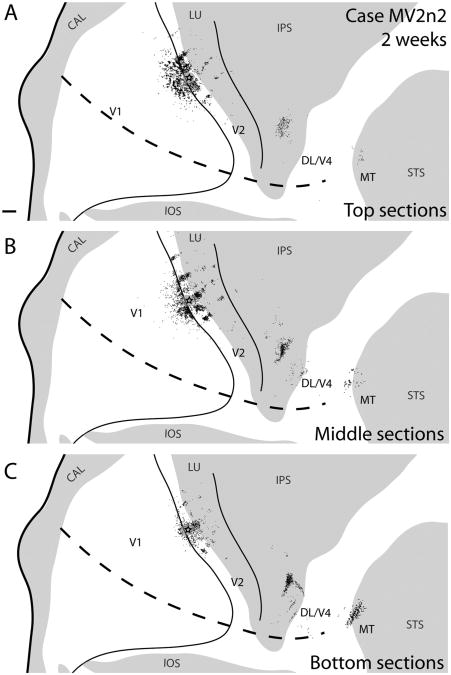
Case MV2n2. Reconstruction of a V2 thin stripe injection and labeled neurons in a 2-week-old macaque. A: Reconstruction of labeled cells throughout the brain of a 2-week-old macaque after a CTB injection into a thin stripe in V2. The star and gray halo represent the injection site location and spread, respectively. Solid black lines represent borders between visual areas determined with cytochrome oxidase; dashed black line presents the estimated horizontal meridian. B: A lateral view of the macaque brain before artificial flattening. The highlighted area corresponds to the area of the brain shown in A. C: A magnified view of CTB label along the V1/V2 border overlaid with CO stripe and blob borders determined by using images D and F. D: Cytochrome oxidase photomicrograph showing the stripe type pattern in V2. E: A photomicrograph of a portion of V1 (boxed area from C) of a single CTB section with retrogradely labeled cells. F: Cytochrome oxidase photomicrograph demonstrating the blob pattern in V1. Tn, thin stripes; Tk, thick stripes. For other abbreviations, see list. Scale bar = 2 mm in A,C,D,F; 0.25 mm in E.
Figure 5.
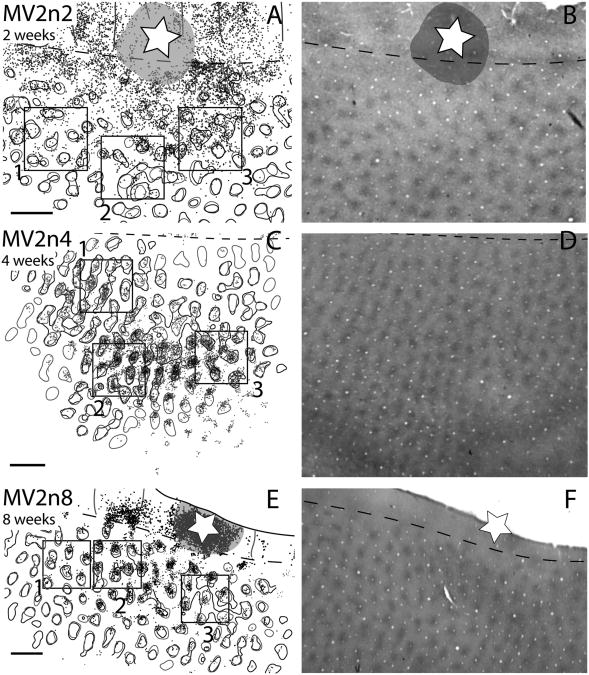
V1 blob and interblob cell locations after injections in thin stripes for 2-, 4-, and 8-week-old macaques. A,C,E: Close-up views of V1 from Figures 2, 3, and 4. B,D,E: The corresponding photomicrographs of V1 blob patterns expressed with cytochrome oxidase staining for A, C, and E, respectively. Gray circles indicate the blob borders determined with visual inspection, and black circles represent blob borders determined with computer filtering. Scale bar = 1 mm in A (applies to A,B), C (applies to C,D), and E (applies to E,F).
Figure 6.
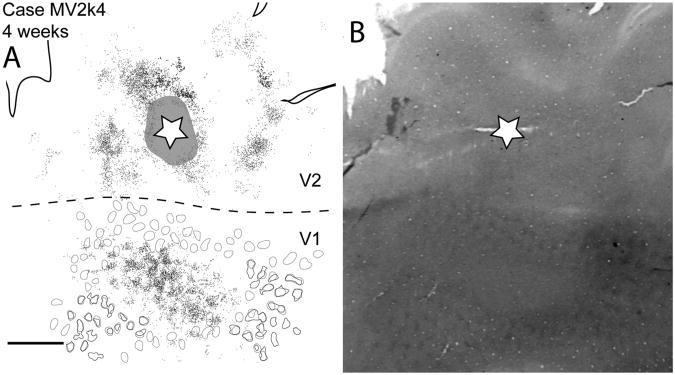
Case MV2k4. A: Reconstruction of a V2 CTB injection and labeled neurons in a 4-week-old macaque monkey most likely in a thick CO stripe. Gray circles in V1 represent blob borders determined by visual inspection. Black circles represent blob borders determined by computer filtering by Image J and Photoshop. B: Photomicrograph of a cytochrome oxidase stained section used for determining blob, and V2/V2 border. For abbreviations, see list. Scale bar = 2 mm in A (applies to A,B).
Feed-forward connections are thought to be involved with RF center properties (Bauer et al., 1999). Neurons labeled in V1 represent feed-forward connections from V1 to V2. In the present cases, most of the V2 injections were along the V1/V2 border and the resulting retrogradely labeled neurons in V1 were located just caudal to the injection sites near the V1/V2 border, as expected for the mirrored retinotopy of V1 and V2 and known connection patterns of V2 with V1 in adult monkeys (Stepniewska and Kaas, 1996; Sincich et al., 2010). Injections in the thin stripes of V2 revealed thin stripe connections with V1 at postnatal ages 2, 4, and 8 weeks (Figs. 2–4). In adults, cells within blobs tend to project to thin stripes (Livingstone and Hubel, 1984; Sincich and Horton, 2002, 2005; Sincich et al., 2007). In our three cases, clusters of retrogradely labeled cells in V1 were largely within CO blobs. In addition, the labeled cells appeared to become more restricted to blobs as the monkeys matured from 2 to 8 weeks of age, suggesting that some interblob neurons with inappropriate connections with thin stripes lost those connections over the first few weeks of postnatal life.
Figure 4.
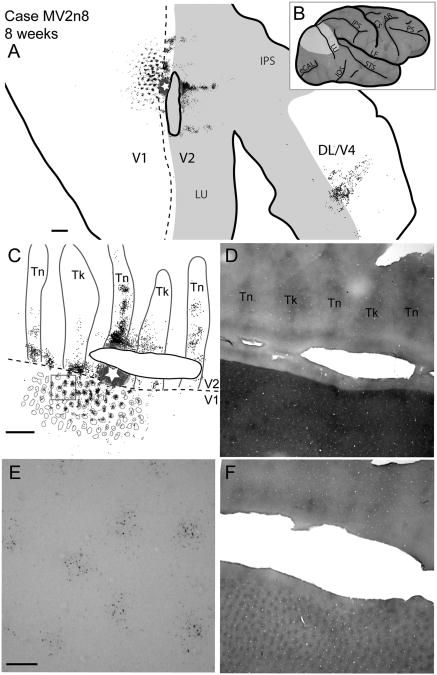
Case MV2n8. Reconstruction of a CTB injection into a V2 thin stripe and resultant label of an 8-week-old macaque. A: A reconstruction of all cortical sections with retrogradely labeled CTB cells. B: A lateral view of an iconic macaque brain. The light gray area represents the area shown in A. C: A magnified view of CTB label along the V1/V2 border overlaid with CO stripe and blob borders determined by using images D and F. D: A photomicrograph of a CO section used to determine the V1, V2 border, as well as the stripe types within V2. E: A photomicrograph of a portion of V1 (boxed area from C) of a single CTB section with retrogradely labeled cells. F: A photomicrograph of the CO section used to determine CO blob locations. For abbreviations, see list. Scale bar = 2 mm in A, C (applies to C,D,F); 0.25 mm in E.
To further evaluate the apparent reduction of projections from interblob neurons to thin stripes during postnatal maturation, we determined the projections of labeled neurons within and outside of V1 blobs at the three developmental ages. Blob borders were outlined either by visual inspection of CO-stained sections or by a computer thresholding procedure (see Materials and Methods and Fig. 1). By either measure of blob borders, the projection of labeled neurons within blobs increased over postnatal maturation (Fig. 5). However, the borders produced by the two methods varied somewhat (Table 3 and Fig. 1). At 2 weeks of age, 36.6–41.2% (subjective vs. computer) of labeled neurons were within blobs for our sampled tissue. By 4 weeks, this number grew to 69.3– 75.8% (subjective vs. computer), and 74.9–79.4% (subjective vs. computer) at 8 weeks. We also measured percent area dedicated to blobs used in the above calculations. For all ages, the subjective borders resulted in 25– 32% of the surface area of V1 being within blobs, whereas these values were slightly higher, 28–39%, for computer filtering values. Similar values were previously reported for blob and interblob territories in adult and infant macaques (Purves and La Mantia, 1993; Sincich et al., 2007).
Table 3. A. Summary of the Percent Number of Cells Within Blobs of V1 After V2 Thin Stripe Injections With Raw Data Included.
| % No. of cells in blobs for a given area1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Box 1 | Box 2 | Box 3 | Total | |||||||
|
|
|
|
|
|||||||
| Case | Age (wk) | Hand | Filter | Hand | Filter | Hand | Filter | Hand | Filter | |
| MV2n8 | 8 | 72.7 (141/194) | 68.6 (133/194) | 70.2 (471/671) | 73.6 (494/671) | 82.7 (387/468) | 92.3 (432/468) | 74.9 (999/1,333) | 79.4 (1,059/1,333) | |
| MV2n4 | 4 | 78.58 (389/495) | 93.13 (461/495) | 71.32 (1,042/1461) | 71.87 (1,050/1461) | 63.27 (782/1,236) | 73.38 (907/1,236) | 69.32 (2,213/3,192) | 75.75 (2,418/3,192) | |
| MV2n2 | 2 | 40.1 (93/232) | 32.3 (75/232) | 48.2 (151/313) | 55 (172/313) | 29.1 (172/592) | 37.5 (222/592) | 36.6 (416/1,137) | 41.2 (469/1137) | |
|
| ||||||||||
| 1Raw cell counts are in parentheses. | ||||||||||
| B. Summary of Percent Area of Space Defined as Blobs in V1 | ||||||||||
|
| ||||||||||
| % Area of space of blobs in a 1.5 mm2 space | ||||||||||
|
|
||||||||||
| Box 1 | Box 2 | Box 3 | Total | |||||||
|
|
|
|
|
|||||||
| Case | Age (wk) | Hand | Filter | Hand | Filter | Hand | Filter | Hand | Filter | |
|
| ||||||||||
| MV2n8 | 8 | 21.9 | 24.3 | 27 | 34.8 | 26.3 | 58.7 | 25.1 | 39.3 | |
| MV2n4 | 4 | 31.9 | 46.5 | 38.9 | 35.3 | 26.5 | 33.9 | 32.4 | 38.6 | |
| MV2n2 | 2 | 28.6 | 20.9 | 24.9 | 35.2 | 23.3 | 27.8 | 25.6 | 28 | |
Thus, the loss of connections of thin stripes from inter-blob V1 neurons does not appear to result from any change in defining blob boundaries. We also feel that the result was not simply due to the possibility that injections were less well confined to thin stripes at early ages. Whereas the injection site in the 2-week-old monkey was somewhat larger than in the 4- and 8-week-old monkeys, the extent of the labeled region in V1 was only 10% larger in the 2-week-old monkey than in the 8-week-old monkey, suggesting little differences in the effectiveness of thin stripe injections across cases.
In a different 4-week-old monkey, an injection was placed in what appeared to be a thick stripe (Fig. 6). This is not completely certain as both stripes in V2 and blobs in V1 were unevenly stained for CO in this case. However, near the edge of the labeled region in V1 of this case, blobs were apparent, and labeled neurons were located outside of blobs (see top left portion of V1 in Fig. 6). In addition, where labeled neurons were more closely distributed, they formed a matrix around holes, the sizes and distribution of blobs, providing additional evidence that the labeled neurons were in the interblob territory.
After injections in V2, the majority of labeled cells in V1 were found in the most superficial sections for all cases (Figs. 7–9). Because we cut our tissue tangentially to the cortical surface, it was difficult to precisely determine the laminar locations of labeled neurons. However, layer 4 of V1 (IVC of Brodmann, 1909) can easily be identified in tangential sections by dark CO staining and a lack of the blob and interblob pattern (e.g., Fig. 4D for an example). We used this characteristic to determine whether labeled cells were above or below layer IVC in V1. At 2 weeks of age, labeled cells in V1 were both above and below layer IVC.
Figure 7.
Case MV2n2. A–C: Reconstructions of a V2 thin stripe injection and labeled neurons in a 2-week-old macaque at various depths of flattened cortex. The superficial section reconstruction is composed of the two most superficial CTB sections, the intermediate sections reconstruction is composed of the three intermediate CTB sections, and the deep sections reconstruction is composed of the two deepest CTB sections in the series of all cortical sections. For abbreviations, see list. Scale bar = 2 mm in A (applies to A–C).
Figure 9.
MV2n8. Reconstruction of a V2 thin stripe CTB injection and labeled neurons in an 8-week-old macaque at various depth planes. A: The two most superficial sections with CTB label. B: The two intermediate sections with CTB label. C: The two deepest sections with CTB label. For abbreviations, see list. Scale bar = 2 mm in A (applies to A–C).
However, the majority of cells in V1 were found superficial to the IVC (Fig. 7A; 61%; 2,914/4,752 cells) and deeper sections, near (Fig. 7B; 35%; 1,642/4,752 cells) or below the IVC (Fig. 7B,C; 4%; 196/4,752 cells), had few labeled cells. In the 4-week-old cases for both thin (Fig. 8) and thick stripe injections (not shown), no cells were found within V1 at the level of or deeper than layer IVC. A similar pattern was also observed for the 8-week-old monkey following a thin stripe injection (Fig. 9). In adults, a small proportion of V2 projecting neurons in V1 is located in layers 5 and 6 (for review, see Callaway, 2003; Casagrande and Xu, 2003), and some are even in midlayer IV (IVC) (Yabuta and Callaway, 1998).
Figure 8.
Case MV2n4. A–C: Reconstructions of a V2 thin stripe injection and labeled neurons in a 4-week-old macaque at various depths of flattened cortex. The superficial section reconstruction is composed of the two most superficial CTB sections, the intermediate sections reconstruction is composed of the three intermediate CTB sections, and the deep sections reconstruction is composed of the two deepest CTB sections in the series of all cortical sections. For abbreviations, see list. Scale bar = 2 mm in A (applies to A–C).
Intrinsic connections of V2
The distribution of labeled cells around injection sites in V2 of 2-, 4-, and 8-week-old monkeys were most dense immediately adjacent to the injection site, and became progressively less dense at greater distances, where they formed a patchy, band-like pattern (Figs. 2–4). These more distant patches of labeled cells were focused on CO stripe types that matched that of the injection site, especially at longer projection distances. Similar patchy patterns of labeled cells in V2 have been reported in previous studies of intrinsic cortical connections of V2 in adult macaques (Livingstone and Hubel, 1984; Rockland, 1985; Levitt et al., 1994). After injections in V2, labeled cells within V2 were located throughout the full depth of tissue sections from top to bottom, with most labeled cells within the middle sections for all cases studied (Figs. 7–9). The horizontal distribution of labeled cells in V2 covered a greater extent of cortical space than the distribution in V1. This was apparent along the same axis as the V1/V2 border, as well as the width of V2.
For the three cases with thin stripe injections, the numbers of labeled cells within different stripes were determined based on the borders we could visualize in CO-stained sections. In the 2- and 4-week-old monkeys, approximately half of all labeled cells were located within thin stripes (50% for 2 weeks, and 49% for 4 weeks), whereas 75% of the labeled cells were within thin stripes for our 8-week-old monkey (Table 4). Close to the injection site, labeled cells were within all stripe types; however, at greater distances from the injection, the majority of labeled cells were found in like stripe types (Figs. 3A,C,E, 2A,C,E, 4A,C,E). Within stripes, there was a patchy distribution of retrogradely labeled cells, which could reflect functional domains within stripe types (e.g., Roe and Ts'o, 1995). This patchy pattern within stripes was apparent as early as 2 weeks of age (Fig. 8). The number of dark CO stripes with retrogradely labeled cells decreased over time, with a span of seven to eight dark CO stripes containing labeled cells at 2 weeks, but only five dark CO stripes containing labeled cells by 8 weeks (Figs. 3E, 2E, 4E). The furthest distance along the length of V2 for labeled cells was 15 mm in the 2-week-old, 13.1 mm in the 4-week-old, and 13.4 mm for the 8-week-old monkeys. This is a decrease in the span of labeled cells of around 10.6% between 2 and 8 weeks of age and does not take cortical growth into consideration, which would expand, not decrease, the distribution.
Table 4. Percent Number of Cells in V2 Stripe Types With Raw Data Included.
| % No. of cells in a given V2 stripe of all V2 stripe cells1 | ||||
|---|---|---|---|---|
|
|
||||
| Case | Age (wk) | Thin | Thick | Pale |
| MV2n8 | 8 | 74.8 (5,035/6,735) | 12.4 (832/6,735) | 12.8 (868/6,735) |
| MV2n4 | 4 | 49.2 (8,542/17,352) | 16.8 (2,917/17,352) | 34 (5,893/17,352) |
| MV2n2 | 2 | 50.1 (2,599/5,185) | 30.9 (1,601/5,185) | 19 (985/5,185) |
Raw cell counts are in parentheses.
We also measured the average width of the different stripe types at the different ages and found that, at 2 weeks, the average width of thin stripes was 0.98 mm and thick stripes were 1.26 mm, by 4 weeks these numbers increased to 1.2 and 1.67 mm for thin and thick stripes, respectively, and finally by 8 weeks the average thin stripe widths were around 1.29 mm, and thick stripe widths were 1.85 mm. The total length of a complete thin, pale, thick, pale cycle of stripes at 2 weeks averaged 3.6 mm; by 4 weeks this increased to 4.7 mm (an increase of 31% from week 2), and by 8 weeks the length was 5.5 mm. Previous reports have indicated that the length of the stripe cycle in adults is approximately 6 mm (Ts'o et al., 2009). The distance across a full stripe cycle for our 2-week-old monkey (3.6 mm) is consistent with a previous estimate of the stripe cycle length for 2-week-old macaques (3.5 mm/cycle: Horton and Hocking, 1996).
CO staining in V2 was weak for our thick stripe injections and the tissue located at distances greater than that shown in the figure was damaged (Fig. 6). However, it does appear that the injection for this case did label clusters of cells at variable distances that would match dark CO bands and avoid pale stripes given our measurement data collected from other 4-week-old cases in this study.
Connections with other cortical areas
We analyzed afferent connections from visual areas rostral to V2 in three cases by using thin stripe injections. In adult macaques, V2 is known to have connections with DL/V4, DM, V3, MT, medial superior temporal area (MST), and the fundal superior temporal area (FST) (Rockland and Pandya, 1981; Maunsell and Van Essen, 1983; DeYoe and Van Essen, 1985; Kennedy and Bullier, 1985; Ungerleider and Desimone, 1986; Zeki and Shipp, 1989; Stepniewska and Kaas, 1996; Felleman et al., 1997; Gattass et al., 1997) and specifically V2 thin stripes are known to receive projections from DL/V4 (DeYoe and Van Essen, 1985; Shipp and Zeki, 1985, 1989,1995; DeYoe et al., 1994). We were unable to determine the precise borders of MT, MST, and FST in any of our cases, and therefore we combined MT, MST, and FST into an MT complex. As early as 2 weeks of age, connections between V3, DM, DL/V4, and the MT complex were present after V2 thin stripe injections (Figs. 2–4).
For the most part, V2 thin stripe injections resulted in multiple loci of labeled cells that formed a band-like pattern within DL/V4 (Figs. 1A, 3A), similar to the band-like patches of label in adults observed in Stepniewska and Kaas (1996). These bands run in a mediolateral direction, and encompass a much smaller area than the labeled cells within V2. In one case, labeled cells in V3 were clustered into two short bands parallel and adjacent to the proposed V3/DM border (Fig. 2). The locations of the labeled cells are consistent with the proposed retinotopies of V3 (Gattass et al., 1988) and DM in macaques (Stepniewska and Kaas, 1996; Lyon and Kaas, 2002), or V3 and DM adjoined along a representation of the vertical meridian of the lower visual quadrant. The two bands may correspond to the locations of two injection sites within a V2 thin stripe (Fig. 2) being in one visual area (either V3 or DM), or they could correspond to topographic connections in both V3 and DM across the vertical meridian. Patches of labeled cells in other areas that were placed in DL/V4 in our figures were close to the expected outer border of V3, and may have included V3 but were more lateral than expected for topographically matched locations for V3 (Fig. 3). Much like the patches of labeled cells within DL/V4, the extent of cortex occupied by these labeled cells after V2 injections is much smaller than the distributions within V2.
Labeled cells in the MT complex were present as early as 2 weeks of age (Fig. 3), but we did not examine this region of cortex in monkeys of later ages. The patch of labeled cells within the MT complex of our 2-week-old monkey was located on the outermost lip of the STS and could correspond to MTc/V4t (Kaas and Morel, 1993; Desimone and Ungerleider, 1986). Another possibility is that this cluster of cells was within the most rostral part within DL/V4. Previously, DeYoe and colleagues (1994) placed an injection in a similar location close to the superior temporal sulcus within DL/V4 of an adult macaque and observed retrogradely labeled cells within thin CO stripes of V2.
The numbers of labeled cells within the DL/V4 complex in tissue sections of different depths varied with the majority of retrogradely labeled cells within the middle and deep sections (Figs. 7–9). At 2 weeks of age, 16% (279/1,740 cells) of the labeled cells were within the top sections, 39% (679/1,740 cells) in the middle, and 45% (782/1,740 cells) in the bottom sections. By 4 weeks of age 18% (248/1,359 cells) were within the top, 19% (253/1359 cells) within the middle, and 63% (858/1,359 cells) within the bottom sections. At 8 weeks of age 22% (187/839 cells) of the labeled cells were found in the top sections, 34% (286/839 cells) were within the middle sections, and 44% (366/839 cells) were in the bottom sections. Whereas the percentages across the depths varied somewhat between cases, the deeper sections had nearly half or more of the labeled cells overall, as expected for feedback connections (Rockland and Pandya, 1979; Maunsell and Van Essen, 1983).
V1 injections
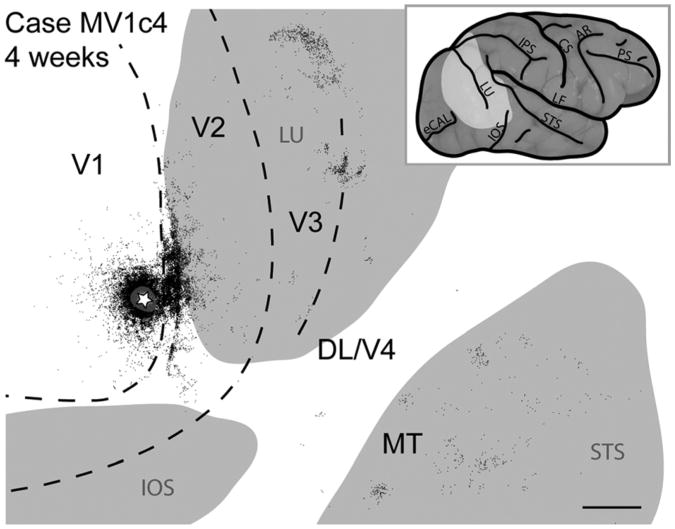
V1 connections were studied in two monkeys, one 4-week-old monkey (MKV1c4: Fig. 10), and one 16-week-old monkey (not shown). In both cases retrogradely labeled cells were observed in V1, V2, V3, and MT, with only a few cells labeled in DL/V4. Within V1, labeled cells were homogeneously distributed around the injection site but formed patches at greater distances (Fig. 10), as reported in adult monkeys (Rockland and Lund, 1983). However, these patches did not completely correspond to CO blobs or interblobs. Within V2 of the 4-week-old monkey, retrogradely labeled cells were densely distributed along the V1/V2 border adjacent to the injection site (Fig. 10). The distribution of labeled neurons in V2 was broader along the V1/V2 border than the injection site in V1, or even the distribution of labeled cells within V1, and thus covered more of the representation of the visual hemifield.
Figure 10.
Case MV1c4. Reconstruction of a CTB injection into V1 and labeled neurons of a 4-week-old macaque monkey. Gray regions represent the lunate sulcus (LU), the inferior occipital sulcus (IOS), and the superior temporal sulcus (STS). For other abbreviations, see list. Scale bar = 5 mm.
Labeled cells in V2 formed a somewhat patchy pattern, especially as they became less densely distributed in more rostral, medial, and lateral directions from the injection site (Fig. 10). When the distribution of labeled cells in this case was compared with CO stripe types in V2, the pattern of patches appeared to align preferentially with CO-dark stripes. Cells were also found along the outer border of V3, and multiple patches of labeled cells were within the superior temporal sulcus (including the expected location of MT and possibly other MT complex areas). Patches of labeled cells marked the rostral border of V3, where the vertical meridian is represented, and some of the neurons of the large medial patch could be in DM/V3A (Lyon and Kaas, 2002) (Fig. 10).
The injection in the 16-week-old monkey labeled relatively few cells outside of V1, but labeled cells were located in V2 and V3. As the V1 injection was slightly displaced from the V1/V2 border, the labeled cells in V2 were displaced from the V1/V2 border, and the labeled cells in V3 were displaced from the outer border of V3 in topographically matched locations relative to the injection site.
V1/V2 border injections
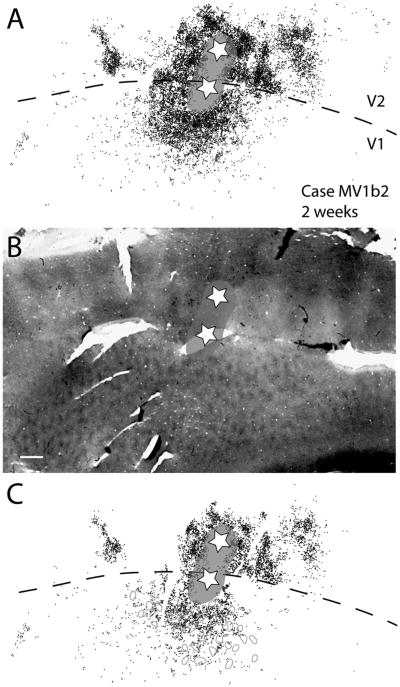
There were four cases with injection sites located along the V1/V2 border. Two of these cases were 2-week-old macaques and the other two cases were 4-week-old macaques. Labeled cells were found in V1, V2, V3, DM, and the MT complex and possibly DL/V4 as early as 2 weeks of age (Figs. 11, 12). Within V1, labeled cells were uniformly distributed along the V1/V2 border but, at greater distances, more caudal to the V1/V2 border, labeled cells were in a somewhat patchy arrangement (Figs. 11–13). These patches do not appear to be associated with blobs or interblobs (Figs. 11, 12). Within V2, labeled cells were not preferentially located within any given stripe type and the majority of cells were within 2 mm of the V1/V2 border (Figs. 11–13). Case MV1b2 (Fig. 13) has two injection sites located on either side of the V1/V2 border, with the cores of the injections fused at the V1/V2 border. Consequently, we categorized this case as a border injection.
Figure 11.
Case MV2b2. A: Reconstruction of a V1/V2 border injection and labeled neurons in a 2-week-old macaque. B: Lateral view of an intact macaque brain with the highlighted region indicating the region of brain shown in A. C: Close-up view of the injection site, and labeled cells along the V1/V2 border. Gray circles represent the location of cytochrome oxidase blobs. D: Photomicrograph of a cytochrome oxidase section showing the location of blobs and interblobs. For abbreviations, see list. Scale bar = 2 mm in A; 1 mm in C (applies to C,D).
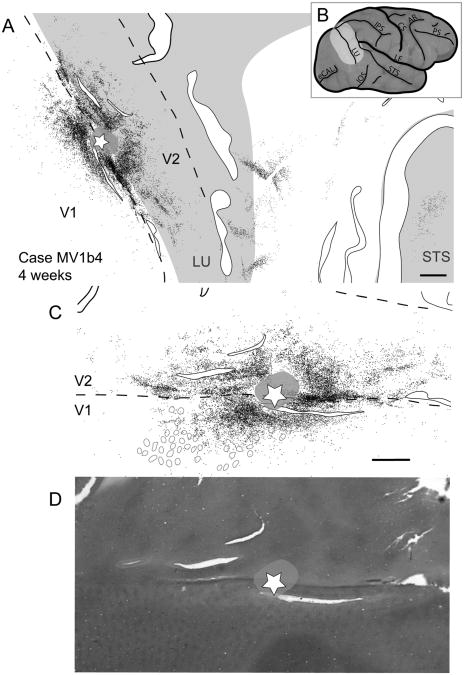
Figure 12.
Case MV1b4. A: Reconstruction of a V1/V2 border injection and labeled neurons in a 4-week-old macaque monkey. Solid black lined white spaces represent tears in the tissue. B: Lateral view of a macaque brain with the estimated tissue shown in A outlined on the surface of the folded brain in light gray. Gray circles indicate the location of detectable blobs in the vicinity of labeled cells. C: Close-up view of labeled cells along the V1/V2 border from A. D: Cytochrome oxidase section showing the V1/V2 border as well as blob and interblob locations. For abbreviations, see list. Scale bar = 2 mm in A; 1 mm in C (applies to C,D).
Figure 13.
Case MV1b2. A: Reconstruction of retrogradely labeled cells after two closely spaced CTB injections along the V1/V2 border in a 2-week-old macaque. The two stars represent the injection locations. One injection site is more in V1, whereas the second injection site is more in V2 (the stripe type is uncertain). The gray halo represents the tracer spread, which continues from one injection site to the other across the V1/V2 border. This reconstruction was made by merging three CTB sections together. B: Photomicrograph of a cytochrome oxidase stain with the injection site locations and tracer spread superimposed on top. C: A single middle CBT section from the three CTB sections shown in A. Close to the injection site in V1, the label is quite dense; however, further away from the injection site the label is somewhat patchy. For abbreviations, see list. Scale bar = 1 mm in B (applies to A–C).
However, the patchy stripe-like label within V2 reflected features of a V2 stripe injection. This stripe-like pattern of connections was not observed in our other border injections, possibly because they did not extend far enough into V2 (Figs. 11, 12). Labeled cells within V2 span a larger mediolateral distance than those in V1 (Figs. 12, 13), suggesting that labeled intrinsic and V1–V2 connections span a greater proportion of V2 than V1. Multiple patches of retrogradely labeled cells were found between the rostral border of V2 and the superior temporal sulcus, possibly indicating the locations of the vertical meridian representations within visual areas V3, DM/V3A, and DL/V4.
Thalamocortical connections
We examined the thalamus for neurons labeled by injections in visual cortex in four cases. Three involved V2 injections in a thick stripe and two in thin stripes, whereas the fourth case involved a V1 injection (Table 1). To determine the locations of retrogradely labeled cell bodies, we aligned the labeled cells in CTB sections with matching parts of adjacent CO-stained sections. Labeled cells were found within the pulvinar complex and the dorsal lateral geniculate nucleus (LGNd). For our analysis, we divided the pulvinar complex into three traditional divisions, the medial pulvinar (PM), the lateral pulvinar (PL), and the inferior pulvinar (PI). Although each of these divisions can be divided further, these three divisions can be reliably identified in CO preparations (Stepniewska and Kaas, 1997).
The medial pulvinar is located lateral to the superior colliculus, and dorsal to the brachium of the superior colliculus (BrSC). PM stains moderately for CO, and contains few fiber bundles running through it relative to PL. PL lies along the most lateral border of the pulvinar complex, lateral to both PM and PI, but also dorsal to PI, stains moderately for CO, and is striated in appearance due to fiber bundles. PI is ventral to PM, and medial and ventral to PL. The brachium of the superior colliculus (BrSC), for the most part, creates a border between PI and PL; however, some aspects of PI are present dorsal to the BrSC (Gutierrez et al., 1995; Stepniewska and Kaas, 1997; Adams et al., 2000; Jones, 2007).
Injections of CTB into V1 of a 4-week-old monkey resulted in retrogradely labeled cells within the parvocellular layers of the LGNd, as well as the lateral pulvinar (Fig. 14). The injection site was centered 1.5 mm from the V1/V2 border within the representation of the central 1°-2° of vision of the lower visual quadrant (Gattass et al., 1981, 1987) and is relatively close to central vision (see Fig. 10A for injection site). Retrogradely labeled cells were found within a middle portion of PL just dorsal to the brachium of the superior colliculus (Fig. 14D). This general region of the pulvinar is known to project to V1 and V2 (Kennedy and Bullier, 1985; Adams et al., 2000). Labeled cells were also found dorsally in the parvocellular layers of the LGNd (Fig. 14B). Possibly because of the small size of the injection, and the narrowing of the column of projection neurons as it reaches the magnocellular layers (Kaas et al., 1972), we did not observe labeled cells within the magnocellular layers of the LGNd.
Figure 14.
Thalamocortical connections of a CTB injection into V1 of a 4-week-old macaque monkey. A,C: Photomicrographs of coronally cut cytochrome oxidase sections used to determine the location of CTB-labeled cells (black dots). B,D: Location of CTB-labeled cells within the dorsal lateral geniculate nucleus (LGNd: top) and pulvinar complex (bottom). The magnocellular layers of the LGNd are outlined using thin gray lines within the drawing of the LGN in the top right panel. Solid black lines represent borders between nuclei of the thalamus, and dashed lines represent borders between the medial, lateral, and inferior pulvinar. For abbreviations, see list. Scale bar = 1 mm in A (applies to A–D).
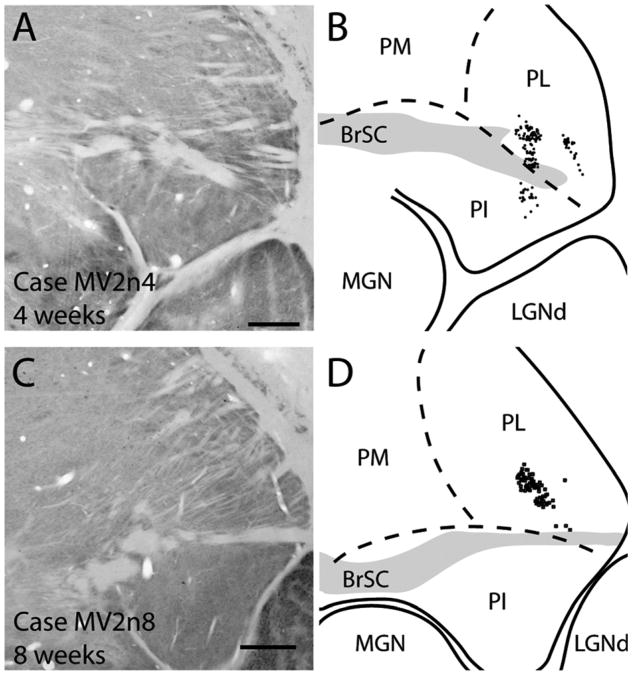
Thalamocortical projections to thin stripes of V2 were observed in both 4- and 8-week-old monkeys (Fig. 15). Both cases resulted in retrogradely labeled cells mostly in the ventral half of the lateral pulvinar, in a similar region of the pulvinar as that for our V1 injection. In the 4-week-old monkey (Fig. 15C), two patches of label were present within PL, possibly reflecting the two closely spaced injection sites for this case (Fig. 2). The pattern of label seemed to form a V-like shape similar to the pattern of label described by Kennedy and Bullier (1985) after V2 injections. Additionally, retrogradely labeled cells were present within the lateral third of the inferior pulvinar. In our 8-week-old monkey, the thin stripe injection (Fig. 15C,D) labeled a small patch of cells in the lateroventral portion of the lateral pulvinar just above the brachium of the superior colliculus (not shown) in a location similar to the medial patch of cells within the lateral pulvinar after a thin stripe injection in a 4-week-old monkey (Fig. 15A,B).
Figure 15.
Thalamocortical connections of V2 thin stripe injections in 4- (A,B) and 8-week-old (C,D) macaque monkeys. A,C: Photomicrographs of coronally cut cytochrome oxidase section used to determine borders within the thalamus. B,D: Locations of CTB labeled cells within the pulvinar complex. For abbreviations, see list. Scale bar = 1 mm in A (applies to A,B) and C (applies to C,D).
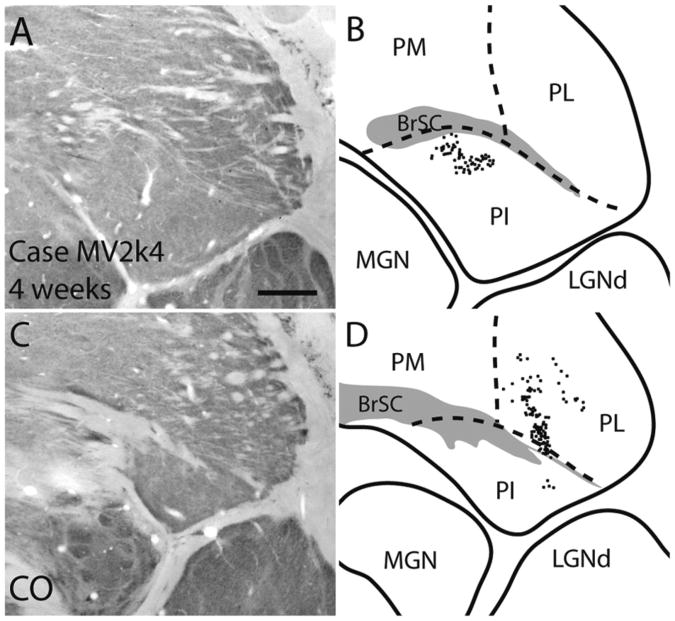
Our injection in case MV2k4, a 4-week-old monkey, appeared to be in a thick stripe based on darker CO staining around the injection site (Fig. 6) and the pattern of labeled cells within interblobs within V1. Additionally, previous studies have reported that the pulvinar complex projects only to CO dark stripes (Levitt et al., 1995). Retrogradely labeled cells were in the lateral and inferior pulvinar. Labeled cells in PL were in two patches, one laterally within PL, and another denser patch of cells ventromedially within PL. Two patches of labeled cells were also found in PI, one located dorsally within central PI (Fig. 16B), and another small patch located laterally within PI (Fig. 16D). No labeled cells were within the LGN.
Figure 16.
Thalamocortical connections of a V2 thick stripe injection in a 4-week-old macaque monkey. A,B: Photomicrographs of coronal cytochrome oxidase sections used to determine the location of retrogradely labeled CTB cells within the pulvinar complex. C,D: Reconstructions of labeled cells within the pulvinar complex at two different locations. D is in a more rostral section than B. For abbreviations, see list. Scale bar = 1 mm in A (applies to A–D).
In summary, all injections resulted in labeled cells within the lateral pulvinar, and injections in V2 also labeled neurons in the inferior pulvinar. The thick stripe injection in V2 resulted in labeled cells within both the lateral and medial aspects of the inferior pulvinar, whereas thin stripe injections resulting in labeled cells only within the lateral aspect of the inferior pulvinar. V1 injections also labeled cells within the LGNd.
Discussion
We studied the connections of V1 and V2 in 2-, 4-, 8-, and 16-week-old macaque monkeys. The results indicate that much of an adult-like pattern of V1 and V2 connections is present as early as 2 weeks of age, but there is also a refinement of connections from 2 to 8 weeks of age so those originating from appropriate CO modules in V1 and V2 are preserved over those from other CO domains. This refinement period parallels the maturation of receptive field properties of V2 neurons that occurs over the same time (Zhang et al., 2005, 2008; Zheng et al., 2007; Maruko et al., 2008). Our results are discussed below in relation to previous anatomical and physiological findings in adult and developing macaque monkeys.
Our present findings indicate that many of the connections of V1 and V2 that have been described in adult macaques are already present in early postnatal macaques. This is not too surprising, as some features develop well before birth, and macaques have functional vision at birth. For instance, retinal projections to the lateral geniculate nucleus (LGN) and superior colliculus (SC) emerge and segregate to form LGN layers prenatally (Rakic, 1976; Miessirel et al., 1997) and LGN projections to V1 develop and segregate into ocular dominant layers prenatally (Rakic, 1976). Our injections into V1 labeled neurons in the LGN at 2 weeks of age, and in the pulvinar at 4 weeks of age.
Our results also indicate that many features of the cortical connection patterns of V1 and V2 are also present in early postnatal monkeys.
Intrinsic connections of V1
Our injections in V1 were limited in number, but neurons were labeled around the injection sites in V1 at 4 and 16 weeks of age, where they formed a somewhat patchy pattern in the close vicinity of the injection core at 4 weeks. It is not known when the more extensive intrinsic connection pattern of V1 of adult macaques (Rockland and Lund, 1983; Livingstone and Hubel, 1984) emerges, but this pattern does not appear to be fully developed in our cases. However, our postinjection survival times were necessarily short. Using a diffusible fluorescent tracer in postmortem, perfused brains (diI), Coogan and Van Essen (1996) reported that prenatal macaques have a patchy distribution of intrinsic connections in V1 as early as 3 weeks before birth, but results were limited and difficult to compare with the adult patterns. In the present study the patches of label found within V1 did not correlate with blob and interblob regions. In adults, the patchy patterns could reflect a concentration of connections to those between nearby neurons of similar preferences for stimulus orientation (Malach et al., 1993), between neurons between blobs, and between neurons in interblob regions (Livingstone and Hubel, 1984).
V1 projections to V2
V1 projections to V2 were revealed by injections of CTB into V2. For injections in V2 thin stripes, only 36.6– 41.2% of the cells were within CO blobs at 2 weeks of age, but by 8 weeks of age 74.9–79.4% were found within blobs, with the greatest change in percentage of cells within blobs occurring between 2 and 4 weeks of age. The proportions observed by 8 weeks of age are similar to those observed in adult macaques (Sincich et al., 2007). This suggests that projections of V1 neurons to V2 are initially nonselective or only weakly selective, and that neurons in non-blob portions of V1 lose their projections to V2 thin stripes by 8 weeks of age.
Our injection that appeared to be within a thick stripe labeled cells predominantly within interstripe regions and regions around blobs of V1, which is similar to the distribution observed after thick stripe injections in adult macaques by Sincich et al. (2010). The surface area we measured as blob space comprised 25–39% of the total V1 space. Other reports measuring V1 space dedicated to blobs range between 20 and 33% (Purves and LaMantia 1993; Farias et al., 1997; Solomon, 2002; Sincich et al., 2007).
Intrinsic horizontal connections of V2
The organization of intrinsic connections of V2 in adult macaques has been variously described. For the most part, intrinsic connections of V2 in adults tend to form aggregate clusters in preferred sites (Wong-Riley, 1979a; Matsubara et al., 1985; Rockland, 1985; Livingstone and Hubel, 1984; Hubel and Livingstone, 1987; Amir et al., 1993, Malach et al., 1994 ). Hubel and Livingstone (1984) reported that CO dense stripes (both thick and thin) are interconnected, but have few connections with pale stripes. However, Rockland (1985) found no clear relationship of intrinsic connections and CO stripes in V2. Levitt et al. (1994) described dense connections between CO dark stripes, but found that pale stripes were equally connected to both pale and CO dense stripes. Coogan and Van Essen (1996) established that by embryonic day E130, horizontal connections within V2 form a patchy distribution, and by E145 an adult pattern of connections is present; however, they did not assess these connections with respect to CO staining patterns of stripe types.
In the present study, we found that an adult-like patchy pattern of connections is present at 2 weeks of age, and these patches of connections tend to be confined to like-stripe types the further away they are from the injection site. Stepniewska and Kaas (1996) reported that intrinsic connection of V2 extended 5 mm in either direction of the injection site in adult macaques. This distance is much smaller than what we observed for all our age groups in the current study, suggesting that there is likely a reduction in the extent of horizontal connections between 2 weeks of age and adulthood. However, the size, number, and lateral extent of horizontal connections are affected by the size of tracer injections (Lund et al., 1993; Malach et al., 1997; Tanigawa et al., 2005). Indeed, the injection site size for our 2-week-old macaque was larger than that of the 8-week-old macaque. Additionally, we did observe some evidence of cortical surface expansion within V2 between 2 and 8 weeks of age with an increase in stripe cycle sizes of 52%, which could further result in discrepancies between the size and extent of our injection sites for the different ages. Regardless, we can be sure that our injections are mainly located within single stripes as the labeling pattern is confined to like-stripe types only at greater distances for all cases.
When analyzing the connections based on architectonic modules within V2, we see a decrease in the number of like stripes with retrogradely labeled cells (from eight to five) as the monkeys aged. This could reflect a developmental loss of longer connections or, perhaps, a sampling bias in our full set of cases based on differences in injection size, or even the injection site location within the stripe type. Nevertheless, our injection sites were centered within a single stripe, and the difference between 2 and 8 weeks is considerable, suggesting that the extent of intrinsic horizontal connections decreases during development.
Cortical feedback connections to V1 and V2
Previous studies (Barone et al., 1995; Batardière et al., 2002) involving retrograde tracer injections into V1 revealed substantial feedback connections from areas V2, V4, and MT as early as embryonic day 130, after transport times that were much longer than those of the present study (9–15 days). We also observed labeled neurons in extrastriate visual areas including V2, V3, V4, and MT, after V1 and V2 injections with only 3 days of transport time in 4-week-old monkeys, although more neurons might have been labeled with longer transport times. Barone et al. (1995) showed that connections from V2 to V1 are present as early as E115 but that the laminar pattern was less restricted than that found in the adult. We also found that projections from V1 to V2 become more restricted to the superficial layers between 2 and 8 weeks of age, a pattern of label similar to that found in adults.
Feedback connections of V2 have been extensively investigated in adult posimians (Collins et al., 2001), New World Monkeys (Kaas and Lin, 1977; Rockland and Knutson 2000), and Old World macaque monkeys (DeYoe and Van Essen, 1985; Shipp and Zeki 1985; Stepniewska and Kaas 1996). The consensus is that V2 receives feedback projections mainly from V3, DL/V4, MT and DM/V3A. More recently, it was shown that if very sparse and possibly variable connections are considered, V2 may have feedback connections from as many as 25 cortical areas (Markov et al., 2011). The present study demonstrated that feedback connections from V3 and DL/V4 are present as early as 2 weeks of age. Additionally, the connections from these higher order areas back to V2 may be organized in segregated modular streams of visual processing much like those observed between V1 and V2 (Shipp and Zeki, 1985; DeYoe et al., 1994).
Multiple studies have examined connections between V2 and V4 in both adults and during development (DeYoe and Van Essen, 1985; DeYoe et al., 1994; Shipp and Zeki 1985; Barone et al., 1995). Some of these studies have suggested the presence of segregated modular compartments within DL/V4 (Van Essen et al., 1984; Shipp and Zeki 1985) or rostral and caudal subdivisions (Stepniewska and Kaas, 1996). More recently, intrinsic optical imaging has been used to identify functional domains for color, luminance, and orientation within DL/V4 (Tanigawa et al., 2010). Additionally, these domains appear to contain internal, repeated topographic organizations similar to those found in V2 stripes (Roe and Ts'o, 1995). In the present study, we found two or more patches of labeled cells within the DL/V4 region in 2-, 4-, and 8-week-old monkeys (Figs. 2–4). These patches could reflect different functional domains or divisions within DL/V4. Similar “patches” within DL/V4 have been observed in adult macaques after V2 injections (Stepniewska and Kaas, 1996).
V2 connections with DL/V4 likely emerge before birth in macaques. Barone et al. (1994) reported that injections in V4 in prenatal monkeys at E129 resulted in patches of retrogradely labeled cells in V2 that coincided with acetylcholinesterase (AChE)-enriched bands. AChE stripes may become thin and thick CO stripes (Barone et al., 1994), which project to V4 (Shipp and Zeki, 1985; DeYoe et al., 1994), indicating that adult-like patterns of feedforward connections are present prior to birth. We found similar adult-like patterns of V2–DL/V4 connections at 2 weeks of age.
V1-V2 border injections
Injections along the V1/V2 border have rarely been mentioned with respect to ipsilateral connection patterns in either adult or developing monkeys. Most injections along the V1/V2 border are usually incorporated into studies of callosal connections, and such connections have been implicated in midline stereopsis and perceptual fusion of the two hemifields (Choudhury et al., 1965; Berlucchi and Rizzolatti, 1967; Blakemore et al., 1983; Land et al., 1983; Livingstone and Hubel, 1984). In primates, callosal connections are primarily restricted to the rostral side of the V1/V2 border prior to birth (Dehay and Kennedy, 1988; Chalupa et al., 1989). Close to the V1/V2 border, callosal connections are distributed within a largely continuous band that becomes patchy and forms stripe-like patterns more rostrally into V2. These bands extending away from the V1/V2 border have been associated with CO-dense stripes (Olavarria and Abel, 1996). Additionally, callosal connections from the V1/V2 border region are also found along other vertical meridian locations of cortex such as along the anterior bank of the lunate sulcus, and within the posterior banks of the superior temporal sulcus (Kennedy et al., 1986; Olavarria and Abel, 1996).
Consistent with these finding on callosal connections, the ipsilateral connections of the V1/V2 border in the present cases show retrogradely labeled cells evenly distributed along the V1/V2 border that form patches more rostrally within V2. However, these patches usually did not correspond to CO stripes. Additionally, labeled cells were found along the anterior bank of the lunate sulcus, possibly representing the vertical meridian border on the rostral end of V3, as well as cells within the superior temporal sulcus that could represent the vertical meridian border of MT as early as 2 weeks of age.
Thalamic projections to V1 and V2
V1 is known to receive inputs from the LGN and the pulvinar complex in adult macaque monkeys (Gutierrez and Cusick, 1997; Adams et al., 2000). Indeed, we observed this pattern of connections in the present study in infants. However, unlike Gutierrez and Cusick (1997) and Adams et al. (2000), we did not observe retrogradely labeled cells within the inferior pulvinar after V1 injections at 4 or 16 weeks of age. This could be because the pulvinar projects to layers 3 and 4 of V2, and mainly to layer 1 of V1, and the thinner projections to V1 were less effectively labeled and would require longer transport times. Pulvinar projections to V2 thin stripes originated from the inferior and lateral pulvinar. Within the inferior pulvinar, labeled cells were found only within the most lateral portion, possibly within the region of central lateral inferior pulvinar, PICL, of Stepniewska and Kaas (1997). Pulvinar projections to V2 thick stripes originated from the lateral pulvinar and two locations within the inferior pulvinar that could include medial inferior pulvinar, PIM, or central medial inferior pulvinar, PICM, of Stepniewska and Kaas (1997). Previously PIM or PICM have been described to project to dorsal stream visual areas such as MT, MTc, DM, and the rostral region of DL/V4 (Kaas and Lyon, 2007). Thick stripes in V2 are also associated with the dorsal visual stream, and they project to MT (Shipp and Zeki, 1985; DeYoe and Van Essen, 1985). Thus, the projections we observed could be a further indicator of the thick stripe involvement of dorsal stream processing.
Comparisons with electrophysiological and perceptual studies
Visual capacities of newborn primates are limited. Acuity is poor and contrast sensitivity is much lower in infants than adults for the first few months after birth (for reviews, see Teller et al., 1978; Boothe et al., 1985; Blakemore, 1990; Chino et al., 2004; Kiorpes and Movshon, 2004; Zheng et al., 2007). Immaturities of the retina and, to a greater extent, the visual brain, largely set a limit on perceptual development. Contrary to earlier observations (e.g., Blakemore and Vital-Durand, 1981; Blakemore, 1990), the responses of individual V1 neurons are qualitatively adult-like as early as 6–14 days after birth and are well tuned to stimulus orientation, spatial and temporal frequencies, size, contrast, and disparity by 4 weeks of age. This is roughly equivalent to 4 months of age in humans (Chino et al., 1997; Kumagami et al., 2000; Endo et al., 2000; Zhang et al., 2005, 2008; Maruko et al., 2008; see Table 1 of Zheng et al., 2007; also see Kiorpes and Movshon, 2003).
The average peak firing rate of V1 neurons doubles between 2 and 4 weeks of age and approaches near adult levels by 8 weeks of age (Zheng et al., 2007). If brief stationary gratings are turned on and off over the RF centers of V1 neurons (step stimuli), the transient onset discharges in 2-week-old infant monkeys are as responsive and as reliable as those in adults (Zhang et al., 2008). Whereas the sustained component of the discharge is relatively immature during the first 4 postnatal weeks, it becomes adult-like by 8 weeks of age. The contrast sensitivity of V1 neurons also reaches near adult levels by 8 weeks (Zheng et al., 2007; Zhang et al., 2008). The RF center-surround interactions in the majority of V1 neurons are likewise qualitatively adult-like as early as 2 weeks of age although considerable immaturities persist during the first 4 weeks of a monkey's life. By 8 weeks of age, however, the RF center-surround interactions of V1 neurons are indistinguishable from those of adults (Zhang et al., 2005). The adult-like spatiotemporal filter properties of V1 neurons in infant monkeys dovetail nicely with the behavioral performances of human neonates and infants for stimulus orientation discrimination (Atkinson et al., 1988; Slater et al., 1991) and the detection of simple targets (e.g., Matsuzawa and Shimojo, 1997).
With respect to V2 development, the basic spatiotemporal filter properties of V2 neurons (Zheng et al., 2007; see also Fig. 7 in Maruko et al., 2008), their responsiveness (e.g., peak firing rate and contrast sensitivity; Zheng et al., 2007), and reliability of neuronal firing (Zhang et al., 2008) become largely adult-like as early as 8 weeks of age. However, it was also found that some response properties of V2 neurons functionally develop at a slower rate than V1 neurons (Zhang et al., 2005; Zheng et al., 2007). This delayed development in V2 neurons is most notable in the RF center-surround interactions assessed with high-contrast sinusoidal gratings (Zhang et al., 2005). At 2 weeks of age, many V2 neurons do not show measurable suppressive surrounds, but by 8 weeks of age, the center-surround interactions are largely adultlike, except in a small proportion of V2 units.
Taken together, previous physiological studies in V1 and V2 show that, although the basic response properties of individual neurons are present as early as 6–14 days after birth and neurons' responsiveness is low, substantial changes in RF properties and responsiveness have taken place between 2 and 4 weeks of age in V1 and V2.
Interestingly, the overall maturation of RF properties is delayed in V2 relative to V1 until 8 weeks of age. These physiological data are consistent with the maturation of the connections of V1 and V2 described in this study. For example, following injections of tracer into thin CO stripes at 2 weeks of age, only 36.6–41.2% of the labeled cells were found within blobs for our sampled tissues. By 4 weeks of age this number grew to 69.3–75.8%, and 74.9– 79.4% of the labeled cells were within blobs at 8 weeks (Fig. 5). Models describing the underlying anatomical features suggest that feed-forward projections contribute to RF center characteristics (Bauer et al., 1999).
Thus the reductions in the extent of convergence of feed-forward projections from V1 to V2 from 2- to 4-week-old monkeys may account for the reduction in RF center sizes over these same ages. Assuming that horizontal/intrinsic projections account for RF surround suppressive characteristics (Gilbert et al., 1996), and far RF surround suppressive characteristics reflect feedback connections from higher visual areas (Angelucci and Bullier 2003; Angelucci and Bressloff, 2006), developmental changes in the pattern and amount of convergence of connections within V2 is expected and was observed. Our findings on the maturation of the intrinsic connections and feedback connections in V2 are generally consistent with the development of center/surround RF organization of V2 neurons.
In summary, we conclude that the many adult-like connectional features of V1 and V2 are present in the macaque monkey as early as 2 weeks of age or earlier, but that intrinsic connections in V2, as well as connections of V1 to V2 are further refined by selective loss of connections from birth to around 4–8 weeks of postnatal age. Our results are consistent with the previous studies of cats and ferrets, in which modular patterns of connections were shown to emerge as a result of regressive forces (Callaway and Katz, 1990; Luhmann et al., 1990; Katz and Callaway, 1992; Katz and Shatz, 1996; Galuske and Singer, 1996; Durack and Katz, 1996; Ruthazer and Stryker, 1996) involving the pruning of exuberant connections and the formation of new horizontal connections by branching axons. Axon pruning, the progressive loss of inappropriate connections, is one of the classically regressive events of brain development (Cowan et al., 1984).
Acknowledgments
Grant sponsor: the National Eye Institute; Grant numbers: RO1-EY-008128 (to Y.M.C), RO1 EY-02686 (to J.H.K), T32-EY-007135 (to P.M.K), and CORE grants P30 EY-007751, and P30 EY-008126.
Abbreviations
- CAL
Calcarine sulcus
- CO
Cytochrome oxidase
- CTB
Cholera toxin subunit B
- DL
Dorsolateral area
- DM
Dorsomedial area
- IOS
Inferior occipital sulcus
- LU
Lunate sulcus
- MT
Middle temporal area
- STS
Superior temporal sulcus
- V1
Primary visual area
- V2
Secondary visual area
- V3
Third visual area
- V4
Fourth visual area
Literature Cited
- Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Amir Y, Harel M, Malach R. Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortex. J Comp Neurol. 1993;334:19–46. doi: 10.1002/cne.903340103. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- Angelucci, A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 2003;97:141–54. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Anker S, Evans C, Hall R, Pimm-Smith E. Visual acuity testing of young children with the Cambridge Crowding Cards at 3 and 6 m. Acta Ophthalmol (Copenh) 1988;66:505–508. doi: 10.1111/j.1755-3768.1988.tb04371.x. [DOI] [PubMed] [Google Scholar]
- Barone P, Dehay M, Berland M, Kennedy H. Developmental changes in the distribution of acetylcholinesterase in the extrastriate cortex of monkey. Dev Brain Res. 1994;77:290–294. doi: 10.1016/0165-3806(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Barone P, Dehay C, Berland M, Bullier J, Kennedy H. Developmental remodeling of primate visual cortical pathways. Cereb Cortex. 1995;5:22–38. doi: 10.1093/cercor/5.1.22. [DOI] [PubMed] [Google Scholar]
- Batardière A, Barone P, Knoblauch K, Giroud P, Berland M, Dumas AM, Kennedy H. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb Cortex. 2002;12:453–465. doi: 10.1093/cercor/12.5.453. [DOI] [PubMed] [Google Scholar]
- Bauer U, Scholz M, Levitt JB, Obermayer K, Lund JS. A model for the depth-dependence of receptive field size and contrast sensitivity of cells in layer 4C of macaque striate cortex. Vision Res. 1999;39:613–629. doi: 10.1016/s0042-6989(98)00172-2. [DOI] [PubMed] [Google Scholar]
- Blakemore C. Maturation of mechanisms for efficient spatial vision. In: Blakemore C, editor. Vision: coding and efficiency. Cambridge: Cambridge University Press; 1990. pp. 254–256. [Google Scholar]
- Blakemore C, Vital-Durand F. Postnatal development of the monkey's visual system. Ciba Found Symp. 1981;86:152–171. doi: 10.1002/9780470720684.ch7. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Diao Y, Pu M, Wang Y, Xiao Y. Possible functions of the interhemispheric connections between visual cortical areas in the cat. J Physiol. 1983;337:334–349. doi: 10.1113/jphysiol.1983.sp014627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationlehre der Grosshirnrinde in ihren Prinzipien Dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Barth; 1909. [Google Scholar]
- Bruce K, Grofova I. Notes on a light and electron microscopic double-labeling method combining anterograde tracing with Phaseolus vulgaris leucoagglutinin and retrograde tracing with cholera toxin subunit B. J Neurosci Methods. 1992;45:23–33. doi: 10.1016/0165-0270(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Felleman DJ, Newsome WT, Van Essen DC. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res. 1986;26:63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Cell types and local circuits in the primary visual cortex of the macaque monkey. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2003. pp. 680–691. [Google Scholar]
- Callaway EM, Katz LC. Emergence of refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990;10:1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Cerebral cortex. Vol. 10. New York: Plenum; 1994. pp. 201–259. [Google Scholar]
- Casagrande VA, Xu X. Parallel visual pathways: a comparative perspective. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2003. pp. 494–505. [Google Scholar]
- Chalupa LM, Killackey HP, Snider CJ, Lia B. Callosal projection neurons in area 17 of fetal rhesus monkey. Brain Res Dev Brain Res. 1989;46:303–308. doi: 10.1016/0165-3806(89)90294-0. [DOI] [PubMed] [Google Scholar]
- Chino YM, Smith EL, 3rd, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci. 1997;17:296–307. doi: 10.1523/JNEUROSCI.17-01-00296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino YM, Bi H, Zhang B. Normal and abnormal development of the neuronal response properties in primate visual cortex. In: Kaas JH, Collins CE, editors. The primate visual system. Boca Raton, FL: CRC; 2004. pp. 81–108. [Google Scholar]
- Choudhury BP, Whitteridge D, Wilson ME. The function of the callosal connections of the visual cortex. Q J Exp Physiol. 1965;50:214–219. doi: 10.1113/expphysiol.1965.sp001783. [DOI] [PubMed] [Google Scholar]
- Collins CE, Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in prosimian primate (Galago garnetti) J Comp Neurol. 2001;431:155–167. [PubMed] [Google Scholar]
- Coogan TA, Van Essen DC. Development of connections within and between areas V1 and V2 of macaque monkeys. J Comp Neurol. 1996;372:327–342. doi: 10.1002/(SICI)1096-9861(19960826)372:3<327::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Fawcette VW, O'Leary DDM, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Absence of interhemispheric connections of area 17 during development in the monkey. Nature. 1988;331:348–350. doi: 10.1038/331348a0. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Van Essen DC. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Felleman DJ, Van Essen DC, McClendon E. Multiple processing streams in occipitotemporal visual cortex. Nature. 1994;371:151–154. doi: 10.1038/371151a0. [DOI] [PubMed] [Google Scholar]
- Durack JC, Katz LC. Development of horizontal projections in layer 2/3 of ferret visual cortex. Cereb Cortex. 1996;6:178–183. doi: 10.1093/cercor/6.2.178. [DOI] [PubMed] [Google Scholar]
- Endo M, Kaas JH, Jain N, Smith EL, 3rd, Chino Y. Binocular cross-orientation suppression in the primary visual cortex (V1) of infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2000;41:4022–4031. [PubMed] [Google Scholar]
- Farias MF, Gattass R, Pinon MC, Ungerleider LG. Tangential distribution of cytochrome oxidase-rich blobs in the primary visual cortex of macaque monkeys. J Comp Neuol. 1997;386:217–228. doi: 10.1002/(sici)1096-9861(19970922)386:2<217::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Federer F, Ichida J, Jeffs J, Schiessl I, McLoughlin N, Angelucci A. Four projection streams from primate V1 to the cytochrome oxidase stripes of V2. J Neurosci. 2009;29:15455–15471. doi: 10.1523/JNEUROSCI.1648-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Burkhalter A, Van Essen DC. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J Comp Neurol. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Singer W. The origin and topography of long-range intrinsic projections in cat visual cortex: a developmental study. Cereb Cortex. 1996;6:417–430. doi: 10.1093/cercor/6.3.417. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. J Comp Neurol. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Rosa MG. Visual topography of V1 in the cebus monkey. J Comp Neurol. 1987;259:529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Gross CG. Topographic organization and extent of V3 and V4 of the macaque. J Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. Cortical projections of area V2 in the macaque. Cereb Cortex. 1997;7:110–129. doi: 10.1093/cercor/7.2.110. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Das A, Ito M, Kapadia M, Westheimer GA. Spatial integration and cortical dynamics. Proc Natl Acad Sci U S A. 1996;93:615–622. doi: 10.1073/pnas.93.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Westwood, DA An evolving new of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Cusick CG. Area V1 in macaque monkeys project to multiple histochemically defined subdivisions of the inferior pulvinar complex. Brain Res. 1997;765:349–365. doi: 10.1016/s0006-8993(97)00696-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J Comp Neurol. 1995;363:545–562. doi: 10.1002/cne.903630404. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal and lateral geniculate nucleus and visual cortex of Old World and New World primates. J Comp Neurol. 1978;182:123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Horton JC. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B. 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J Neurosci. 1996;16:1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkeys. Nature. 1981;292:762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987;7:3378–415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Fiore L, Caminiti R. Exuberant projections into the corpus callosum from the visual cortex of newborn cats. Neursci Lett. 1977;4:237–242. doi: 10.1016/0304-3940(77)90185-9. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Kaas JH, Lin R. Cortical projections of area 18 in owl monkeys. Vision Res. 1977;17:739–741. doi: 10.1016/s0042-6989(77)80013-8. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal ventral subdivisions of FST. J Neurosci. 1993;13:534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol. 1972;6:253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- Kaskan PM, Lu HD, Dillenburger BC, Kaas JH, Roe AW. The organization of orientation-selective luminance-change and binocular-preference domains in the second (V2) and third (V3) visual areas of new world owl monkeys as revealed by intrinsic signal optical imaging. Cereb Cortex. 2008;19:1394–1407. doi: 10.1093/cercor/bhn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Callaway EM. Development of local circuits in mammalian visual cortex. Annu Rev Neurosci. 1992;15:31–56. doi: 10.1146/annurev.ne.15.030192.000335. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J Neurosci. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H, Dehay C, Bullier J. Organization of the callosal connections of visual areas V1 and V2 in the macaque monkey. J Comp Neurol. 1986;247:398–415. doi: 10.1002/cne.902470309. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge, MA: MIT Press; 2003. pp. 159–173. [Google Scholar]
- Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Vis Neurosci. 2004;21:851–859. doi: 10.1017/S0952523804216054. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Kumagami T, Zhang B, Smith EL, 3rd, Chino YM. Effect of onset age of strabismus on the binocular response of neurons in the monkey visual cortex. Invest Ophthalmol Vis Sci. 2000;41:948–954. [PubMed] [Google Scholar]
- Land EH, Hubel DH, Livingstone MS, Perry SH, Burns MM. Colour-generating interactions across the corpus callosum. Nature. 1983;303:616–618. doi: 10.1038/303616a0. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Yoshioka T, Lund JS. Intrinsic cortical connections in macaque visual area V2: evidence for interaction between different functional streams. J Comp Neurol. 1994;342:551–570. doi: 10.1002/cne.903420405. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Yoshioka T, Lund JS. Connections between the pulvinar complex and cytochrome oxidase-defined compartments in visual area V2 of macaque monkey. Exp Brain Res. 1995;104:419–430. doi: 10.1007/BF00231977. [DOI] [PubMed] [Google Scholar]
- Lim H, Wang Y, Xiao Y, Hu M, Felleman DJ. Organization of hue selectivity in macaque V2 thin stripes. J Neurophysiol. 2009;102:2603–2615. doi: 10.1152/jn.91255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Thalamic input to CO rich regions of monkey visual cortex. Proc Natl Acad Sci U S A. 1982;79:6098–6101. doi: 10.1073/pnas.79.19.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Specificity of cortico-cortical connections in monkey visual system. Nature. 1983;304:531–534. doi: 10.1038/304531a0. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Connections between layer 4B of area 17 and the thick cytochrome oxidase stripes of area 18 in the squirrel monkey. J Neurosci. 1987a;7:3371–3377. doi: 10.1523/JNEUROSCI.07-11-03371.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987b;7:3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Singer W, Martinez-Millan L. Horizontal interactions in cat striate cortex: 1. Anatomical substrate and postnatal development. Eur J Neurosci. 1990;2:344–357. doi: 10.1111/j.1460-9568.1990.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Lund JS, Yoshioka T, Levitt JB. Comparisons of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb Cortex. 1993;3:148–162. doi: 10.1093/cercor/3.2.148. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 2002;33:453–461. doi: 10.1016/s0896-6273(02)00580-9. [DOI] [PubMed] [Google Scholar]
- Malach R, Amir Y, Harel M, Grinvald A. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci U S A. 1993;90:10469–10473. doi: 10.1073/pnas.90.22.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Tootell RBH, Malonek D. Relationship between orientation domains, cytochrome oxidase stripes, and intrinsic horizontal connections in squirrel monkey V2. Cereb Cortex. 1994;4:151–165. doi: 10.1093/cercor/4.2.151. [DOI] [PubMed] [Google Scholar]
- Malach R, Schirman TD, Harel M, Tootell RB, Malonek D. Organization of intrinsic connections in owl monkey MT. Cereb Cortex. 1997;7:386–393. doi: 10.1093/cercor/7.4.386. [DOI] [PubMed] [Google Scholar]
- Markov NT, Misery P, Falchier A, Lamy C, Vezoli J, Quilodran R, Gariel MA, Giroud P, Ercsey-Ravasz M, Pilaz LJ, Huisoud C, Barone P, Dehay C, Toroczkai Z, Van Essen DC, Kennedy H, Knoblauch K. Weight consistency specifies regularities of macaque cortical networks. Cereb Cortex. 2011;21:1254–1272. doi: 10.1093/cercor/bhq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruko I, Zhang B, Tao X, Tong J, Smith EL, 3rd, Chino YM. Postnatal development of disparity sensitivity in visual area 2 (v2) of macaque monkeys. J Neurophysiol. 2008;100:2486–2495. doi: 10.1152/jn.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara J, Cynader M, Swindale NV, Stryker MP. Intrinsic projections within the visual cortex: evidence for orientation specific local connections. Proc Natl Acad Sci U S A. 1985;82:935–939. doi: 10.1073/pnas.82.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa M, Shimojo S. Infant's fast saccades in gap paradigm and development of visual attention. Infant Behav Dev. 1997;20:449–455. [Google Scholar]
- Maunsell JH, Van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissirel C, Wilker KC, Chalupa LM, Rakic P. Early divergence of magnocellular and parvocellular functional subsystems in the embryonic primate visual system. Proc Natl Acad Sci U S A. 1997;94:5900–5905. doi: 10.1073/pnas.94.11.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L, Hawken MJ, Cavanaugh JR. Functional maturation of the macaque's lateral geniculate nucleus. J Neurosci. 2005;10:2712–2722. doi: 10.1523/JNEUROSCI.2356-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olavaria JF, Abel PL. The distribution of callosal connections correlates with the pattern of cytochrome oxidase stripes in visual area V2 of macaque monkeys. Cereb Cortex. 1996;6:631–639. doi: 10.1093/cercor/6.4.631. [DOI] [PubMed] [Google Scholar]
- Olavarria JF, Van Essen DC. The global pattern of cytochrome oxidase stripes in visual area V2of the macaque monkey. Cereb Cortex. 1997;7:395–404. doi: 10.1093/cercor/7.5.395. [DOI] [PubMed] [Google Scholar]
- Purves D, LaMantia A. Development of blobs in the visual cortex of macaques. J Comp Neurol. 1993;334:169–175. doi: 10.1002/cne.903340202. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rockland KS. A reticular pattern of intrinsic connections in primate area V2 (area 18) J Comp Neurol. 1985;235:467–478. doi: 10.1002/cne.902350405. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Lund JS. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983;216:303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Knutson T. Feedback connections from area MT of the squirrel monkey to areas V1 and V2. J Comp Neurol. 2000;425:345–368. [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Cortical connections of the occipital lobe in the rhesus monkey: interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Res. 1981;212:249–270. doi: 10.1016/0006-8993(81)90461-3. [DOI] [PubMed] [Google Scholar]
- Roe AW, Ts'o DY. Visual topography in primate V2: multiple representations across functional stripes. J Neurosci. 1995;8:3689–3715. doi: 10.1523/JNEUROSCI.15-05-03689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Stryker MP. The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci. 1996;16:7253–7269. doi: 10.1523/JNEUROSCI.16-22-07253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur J Neurosci. 1989;1:33–54. doi: 10.1111/j.1460-9568.1989.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation and convergence of specialized pathways in macaque monkey visual cortex. J Anat. 1995;187:547–562. [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Horton J. Divided by cytochrome oxidase: a map of the projections from V1 to V2 in macaques. Science. 2002;295:1734–1737. doi: 10.1126/science.1067902. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton J. Input to V2 thin stripes arises from V1 cytochrome oxidase patches. J Neurosci. 2005;25:10087–10093. doi: 10.1523/JNEUROSCI.3313-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Jocson CM, Horton J. Neurons in V1 patch columns project to V2 thin stripes. Cereb Cortex. 2007;17:935–941. doi: 10.1093/cercor/bhl004. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Jocson CM, Horton J. V1 interpatch projections to V2 thick stripes and pale stripes. J Neurosci. 2010;30:6963–6974. doi: 10.1523/JNEUROSCI.5506-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A, Mattock A, Brown E, Bremmer JG. Form perception at birth: Cohen and Younger (1984) revisited. J Exp Child Psychol. 1991;51:395–406. doi: 10.1016/0022-0965(91)90084-6. [DOI] [PubMed] [Google Scholar]
- Solomon GS. Striate cortex in dichromatic and trichromatic marmosets: neurochemical compartmentalization and geniculate input. J Comp Neurol. 2002;450:366–381. doi: 10.1002/cne.10327. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in macaque monkeys. J Comp Neurol. 1996;371:129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas JH. Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis Neurosci. 1997;14:1043–1060. doi: 10.1017/s0952523800011767. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex. 2005;15:809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Wang Q, Fujita I. Organization of horizontal axons in the inferior temporal cortex and primary visual cortex of the macaque monkey. Cereb Cortex. 2005;15:1887–1899. doi: 10.1093/cercor/bhi067. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Haidong LD, Roe AW. Functional organization for color and orientation in macaque V4. Nat Neurosci. 2010;13:1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY, Regal DM, Videen TO, Pulos E. Development of visual acuity in infant monkeys (Macaca nemestrina) during early postnatal weeks. Vision Res. 1978;18:561–566. doi: 10.1016/0042-6989(78)90203-1. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hamilton SL. Functional anatomy of the second visual area (V2) in macaque. J Neurosci. 1989;9:2620–2644. doi: 10.1523/JNEUROSCI.09-08-02620.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Silverman MS, De Valois RL, Jacobs GH. Functional organization of the second cortical visual area in primates. Science. 1983;220:737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Ts'o DY, Zarella M, Burkitt G. Whither the hypercolumn? J Physiol. 2009;587:2791–2805. doi: 10.1113/jphysiol.2009.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of the visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual streams. In: Ingle DG, Goodale MA, Mansfield RJQ, editors. Analysis of visual behavior. Cambridge: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JHR, Bixby JL. The projections from striate cortex (V1) to areas V2 and V3 in macaque monkey: asymmetries, areal boundaries and patchy connections. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Columnar cortical-cortical interconnections within the visual system of squirrel and macaque monkeys. Brain Res. 1979a;162:201–217. doi: 10.1016/0006-8993(79)90284-1. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979b;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Felleman DJ. Projections from primary visual cortex to cytochrome oxidase thin stripes and interstripes of macaque visual area 2. Proc Natl Acad Sci U S A. 2004;101:7147–7151. doi: 10.1073/pnas.0402052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta NH, Callaway EM. Functional streams and local connections of layer 4C neurons in primary visual cortex of the macaque monkey. J Neurosci. 1998;18:9489–9499. doi: 10.1523/JNEUROSCI.18-22-09489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Shipp S. Modular connections between areas V2 and V4 of macaque monkey visual cortex. Eur J Neurosci. 1989;1:494–506. doi: 10.1111/j.1460-9568.1989.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng J, Watanabe I, Maruko I, Bi H, Smith EL, 3rd, Chino Y. Delayed maturation of receptive field center/surround mechanisms in V2. Proc Natl Acad Sci U S A. 2005;102:5862–5867. doi: 10.1073/pnas.0501815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Smith EL, 3rd, Chino YM. Postnatal development of onset transient responses in macaque V1 and V2 neurons. J Neurophysiol. 2008;100:1476–1487. doi: 10.1152/jn.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Zhang B, Bi H, Maruko I, Watanabe I, Nakatsuka C, Smith EL, 3rd, Chino YM. Development of temporal response properties and contrast sensitivity of V1 and V2 neurons in macaque monkeys. J Neurophysiol. 2007;97:3906–3916. doi: 10.1152/jn.01320.2006. [DOI] [PubMed] [Google Scholar]