Abstract
Blood vessel networks provide nutrients and gaseous exchange that are essential for functions. Pancreatic islet capillaries deliver oxygen to endocrine cells while transporting hormones to organs and peripheral locations throughout the body. We have developed a zebrafish diabetes model in which adult islets can be followed in vivo during beta cell regeneration while calibrating changes in beta cell mass and fasting blood glucose levels. After genetic ablation, beta cells are initially dysfunctional or dying, and blood glucose levels increase fourfold. During a 2-week period, hyperglycemia eventually normalizes as beta cell mass regenerates. We show that mCherry-fluorescent, insulin-positive beta cells re-emerge in close contact with the vascular endothelium. Alterations in the dense vascular network of zebrafish islets were visualized by the expression of green fluorescent protein (GFP) in endothelial cells derived from the Fli transcription factor promoter. The rapid destruction and regeneration of beta cell mass was evaluated in the same animal over time, providing a functional model for investigating the interactions of islet cell types with vascular cells as well as the consequences of hyperglycemia on other tissues. Regenerating adult zebrafish can be utilized as vertebrate, metabolically active models for generating new insights into treatments for type 2 diabetes.
Introduction
Pancreatic beta cells control metabolic homeostasis by secreting insulin into islet capillaries after sensing increases in blood glucose. Sustained hyperglycemia in adults due to increased demand on beta cells is a metabolic disease that leads to multiple end organ failure, including functional complications of the eye, heart, kidney, liver, muscle, and vasculature. The irreplaceable loss of beta cell function in human diabetes has driven research into methods that can be used in generating new islets in situ from existing progenitors, differentiating beta cells, or improving transplantation of exogenous islets. At least two human cadavers should be rapidly processed to provide enough islets for transplantation into a single type 1 diabetic patient, and alternative supplies of islets are necessary. The variable nature of human islet preparations, influenced by factors such as donor health, time from death, and exposure to ischemia, limits the use of islets for transplantation and research. During transport, continued degradation of the severed islet vasculature and the resulting hypoxia undermine islet integrity and function.1 Islet vascularization controls the expansion of beta cell mass in response to increased insulin demand, a condition that has been linked to glucose intolerance and type 2 diabetes.2 Understanding the role of islet vasculature in vivo and the supporting factors needed to sustain beta cell function will enhance our ability to produce medical interventions for a growing diabetes epidemic.
While some expansion of beta cell mass is seen in very young mammals, this ability is lost in aging adults.3 In disease states such as diabetes, beta cells become larger, compensating by producing more insulin to control blood glucose. Beta cells eventually cease functioning, and insulin injections are required. Unlike mammals, adult zebrafish can regenerate beta cells after several methods of destruction,4 and blood glucose levels return to normal without intervention. The cell types and signaling mechanisms responsible for the recovery of beta cells in adults are under investigation. Since beta cell regeneration relies on nutritional factors delivered by the vasculature as well as non-nutritional metabolic cues that rely on the systemic circulation, we sought to evaluate changes in islet vasculature in concert with beta cell mass during regeneration in adult zebrafish. Methods to visualize changes in mammalian beta cell mass have relied on fluorescent tags to image pancreatic tumors in situ.5 In addition, islets transplanted onto the retina of immunodeficient mice have been used to measure islet re-vascularization and normalization of blood glucose after streptozotocin treatment.6 However, the adult zebrafish model is advantageous in that the conditional genetic ablation of endogenous beta cells followed by the return of beta cell mass can be directly imaged in the same animal over time without surgical resection, providing a reproducible physiological model. We describe the interaction of regenerating beta cells and the vasculature of adult islets. Ablation of endogenous pancreatic beta cells is followed by the return of beta cell mass. Transparent transgenic adults expressing both mCherry in pancreatic beta cells and green fluorescent protein (GFP) in vascular cells have distinct morphologies that were evaluated at a discrete time points during a 2-week regeneration period. These unique changes in beta cell mass and their associations with islet endothelial cells during regeneration in 1-year-old zebrafish were accompanied by a return to glucose homeostasis without further intervention.
The adult zebrafish pancreas shares important similarities with its human counterpart. The endocrine pancreas contains multiple large islets (Fig. 1) incorporating specialized endocrine cells7 that can secrete characteristic hormones in response to metabolic signals present in the blood. Islets are embedded in a dense capillary network. Mammalian islet capillaries have been described as being more compact and fenestrated than the surrounding exocrine tissue, providing rapid responses to changes in blood glucose.8 Structural support for islets is produced by the basement membrane of endothelial cells and smooth muscle cells called pericytes, promoting beta cell proliferation and insulin expression.9 Vascular pericytes are supporting cells of the islet vasculature that serve to regulate capillary blood flow and permeability, influencing changes in beta cell mass.10 During early development, islet endothelial cells are required for pancreas specification; while at later stages, the vasculature restricts and coordinates growth that does not appear to depend on perfusion.11 Beta cells secrete both pro- and anti-angiogenic factors that have been implicated in hyperglycemia-induced vasculopathy in adults.12 Angiogenic factors, including vascular endothelial growth factor (VEGF) are secreted from beta cells, maintaining a dense and fenestrated capillary network; while VEGF receptors are differentially expressed in islets versus acinar endothelium.13 Understanding the cross-talk between angiogenic instructions exchanged between endothelial cells and beta cells during pancreas development, pathological conditions,14 or regeneration can lead to novel therapies for diabetes.
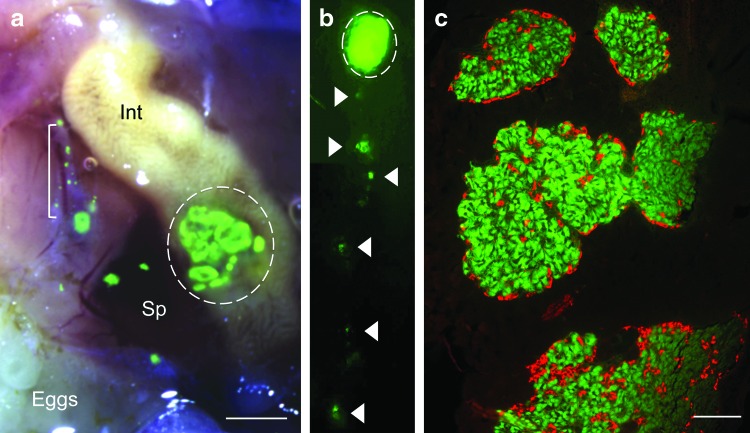
FIG. 1.
Multiple islets are organized within the endocrine pancreas of adult zebrafish and express endocrine hormones. (a) Right lateral bright field view of a dissected INSGFP adult female zebrafish with superimposed fluorescent image. Green fluorescent protein (GFP) expression is seen in multiple large islets located in the endocrine pancreas (scale bar=400 μm). Sp: spleen, Int: intestine, L: liver, dashed line: GFP+ islets surrounded by translucent exocrine tissue: bracket. (b) Whole mount fluorescence image of the adult zebrafish endocrine pancreas, right lateral view. Dashed line: multiple large islets. Arrowheads: isolated islets and individual beta cells extending caudally along the intestine. (c) Five micrometer paraffin section of multiple large islets (scale bar=40 μm). Immunohistochemistry was performed without antigen retrieval. Green: endogenous GFP expressed in beta cells within multiple large islets, red: anti-glucagon antibody in alpha cells. Color images available online at www.liebertpub.com/zeb
Transgenic Tg (Fli1:eGFP)y1 zebrafish used in this study label the endothelial lineage from angioblasts during early development15 and during normal or defective pancreas development.16 The Fli transcription factor is a highly conserved member of the Ets-domain family of transcriptional activators and repressors. Using Fli as an endothelial cell marker in developing zebrafish, distinct requirements for VEGF-A in specifying axial or intersegmental vessels were discovered.17 Fli is expressed early in zebrafish angioblasts, endothelial cells, and human lymphocytes,18 generating signals that promote maturation and migration, even in the absence of oxygen or nutrient delivery.19 However, many of these observations have been made at a time when organogenesis is incomplete and systemic influences are immature. To evaluate the re-growth of beta cells after conditional ablation in contact with islet vascular endothelium in adult zebrafish, we employed the transparent casper strain,20 where changes in beta cell mass were followed throughout regeneration in situ and analyzed at discrete time points for vascular density and blood glucose levels. We observed clumps of insulin-positive beta cells around remodeling islet vasculature as blood glucose levels returned to normal. Functional beta cells increasingly occupied the area between blood vessels, rebuilding islets within 2 weeks.
Materials and Methods
Zebrafish husbandry and transgenics
Zebrafish were maintained with IACUC approval in an Aquatic Habitats 8-rack, continuous flow system with a 14 h on/10 h off light/dark cycle. Water quality was maintained daily at 800 μS conductivity, 27°C–28°C, and pH 7.6–7.8. Fish were fed twice daily: AM-shrimp containing Zoe Marine vitamins and PM-Zeigler Zebrafish Adult Diet dry food. AB Tg (1.0ins:eGFP)sc1 zebrafish referred to as INSGFP21 are currently available through the ZIRC stock center (http://zfin.org). Casper20 embryos (gift of R.M. White) were injected with the conditional DNA construct Tg(T2Kins:nfsB-mCherry)jh4 (generous gift of M.J.Parsons22) using tol2-mediated recombination,23 generating transparent beta cell conditional knock out zebrafish. Casper Insulin Nitroreductase mCherry (INC) F2 heterozygotes were bred with Tg(Fli1:eGFP)y1 casper zebrafish (generous gift of B.M. Weinstein) to yield the 1-year-old compound heterozygous INCFli adult males or 6 day (unfed) INCFli larvae that were used in this study.
Blood glucose monitoring and metronidazole treatment
Fasted blood glucose was measured after cold-water anesthesia24,25 at four different time points in a terminal assay using the Abbott AlphaTRAK monitor and blood glucose strips set at code 7. This veterinary monitor is commonly used in metabolic rodent research and functions identically to the Abbott Freestyle monitor for humans.4 The inserted glucose oxidase-coated strips (Abbott #32006-6) have not been optimized by the company to determine the ratios of glucose in plasma vs. red blood cells for zebrafish. However, we acquired similar baseline readings for fasted adult male zebrafish irrespective of whether we used Abbott's strips and Freestyle monitor (57.4 mg/dL,4) or AlphaTRAK blood glucose test strips and the AlphaTRAK monitor set at code 7 (65 mg/dL, Fig. 3a). After 90 s of anesthesia in 14°C system water, gill movements became slow and regular. The fish were transferred to a paper towel with a spoon, patted dry, and the heads were severed at the caudal edge of the operculum. The strip was then placed directly in the severed heart and held there to measure blood glucose. Sixteen to 20 adult, age-matched males 8 months–1 year old were imaged after an overnight fast on days 1, 3, 7 and/or 14 in each of three independent studies. The average weight of male fish was 0.42±0.15 g, n=48 (total of three independent studies). At least four fish were imaged at each time point and were then sacrificed for fasting blood glucose measurements. Each fish was transferred into a 125 mL glass jar holding 100 mL of 0.2 μm filtered system water containing 12 mM metronidazole (Met; Sigma) or vehicle. After 18 h in a 28°C incubator where the light/dark cycle was maintained, fish were transferred individually into 1 L tanks and returned to the aquatic system. Each fish was fed shrimp and monitored for feeding and swimming behavior.
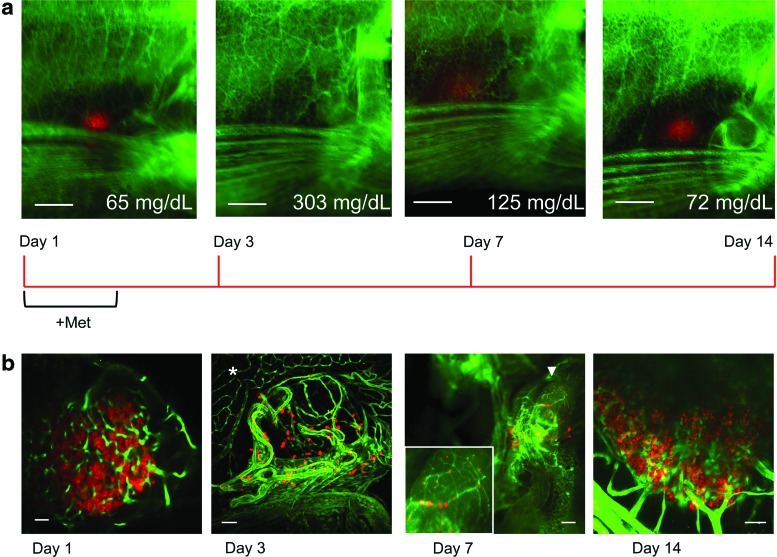
FIG. 3.
Regeneration in living adults, associated changes in blood glucose, and alterations in islet morphology. (a) Images of an affected INCFli adult male casper during regeneration days 1, 3, 7, and 14. Scale bars=0.5 mm. Red: pancreatic beta cells, green: vasculature. The average blood glucose of animals sacrificed at each time point is indicated (mg/dL). A regeneration timeline is depicted below the images. (b) Single multiphoton confocal images of fixed gut sections from affected individuals (1, 3, and 14 day). Scale bar=10 μm. *: vasculature in exocrine pancreas at day 3. Whole mount, Leica MZFlIII microscope image of green vasculature and red beta cells in affected adult on dissection at day 7. Scale bar=100 μm. Arrowhead: multiple large islets. Inset: magnification of multiple large islets. Color images available online at www.liebertpub.com/zeb
Imaging
Microscopy of anesthetized zebrafish was accomplished by placing the adult with the right side facing the 1.0×lens in a shallow, black tray containing tricaine.26 Images were taken at the same settings for all fish using a Leica MZFl III microscope and mCherry or GFP 2 filters (Chroma). A VariSpec color wheel (Caliper Life Sciences) was used to capture color images. Openlab (Improvision) software driving a Hamamatsu ORCA camera generated images that were processed in Adobe Photoshop on Apple computers. Multiphoton laser scanning microscopy was performed on an Olympus FV1000 Imaging System using a Chameleon Vision II single box Ti:Saph fsec laser with 960-nm pulses (Coherent, Inc.) and a 25×water objective after mounting in agarose. Stacks of frame-averaged sections were collected digitally and processed using open source software (FiJi/ImageJ 1.47h, http:/imagej.nih.gov.ij) or Imaris software.
Histology
After sacrifice, the intestine, pancreas, spleen, gall bladder, and liver were excised as a single piece and placed in buffered 4% paraformaldehyde at 4°C overnight. Larvae were fixed at room temperature for 4 h, placed in 55°C histogel (Thermo Scientific), cooled, and transferred to a six-compartment biopsy cassette (Lab Storage Systems #503-2) for paraffin processing. Five-micrometer sections were cut, de-paraffinized, and hybridized with insulin (Dako anti-guinea pig; 1:500) or glucagon (Dako anti-rabbit; 1:500) antibodies. The Alexafluor (Invitrogen) secondary antibodies (555 nm or 488 nm) were used at concentrations of 1:5000. Slides were imaged on a Zeiss Axioplan 2 microscope using a Retiga Exi (Qimaging) camera. For confocal imaging, larvae or adult gut sections were fixed for 2 h, mounted using low-melt agarose in 6 cm dishes, overlaid with phosphate buffered saline, and imaged immediately.
Results
Beta cells and other endocrine cells are organized in principal islets surrounded by exocrine tissue in adult zebrafish (Fig. 1). The translucent exocrine pancreas can be observed surrounding individual islets in Figure 1a (bracket) and encompasses the islets (dashed line), though it is difficult to identify with light microscopy.27 Digestive enzymes produced by the exocrine pancreas are delivered in response to hormonal signals to collecting ducts that empty into a main pancreatic duct attached to the intestine. In contrast, islet hormones are secreted into capillaries that eventually flow into the portal vein, regulating diverse metabolic processes.28 GFP in beta cells was expressed as a function of the zebrafish insulin promoter in AB Tg(1.0ins:eGFP)sc1 transgenics (INSGFP). We observed GFP only in beta cells that were either organized in large multiple islets (Fig. 1a, dashed line) or in smaller, caudal islets or individual beta cells (Fig. 1a, bracket). Fluorescence within the entire endocrine pancreas is shown in Figure 1b. A 0.4 mm volume of clustered, large multiple islets located anteriorly is followed by a trail of small groups of islets or individual beta cells extending caudally along the intestine. A compact segment of the intestinal loop29 and three overlying liver lobes30 along with the attached pancreas and spleen were excised, allowing precise comparison of gut anatomy and protein expression after paraffin embedding (Fig. 1c). A 5 μm paraffin section was de- paraffinized, re-hydrated, and treated with anti-glucagon antibody. Endogenous beta cell-specific GFP fluorescence was retained during paraffin processing unless antigen retrieval was used. Endocrine alpha cells within the islets expressed glucagon (red), indicating that zebrafish islets share morphological characteristics with human islets, having a randomly distributed alpha and beta cell phenotype.31 The disadvantages of using INSGFP adults for studying adult regeneration are twofold. Pigmentation in the AB strain obscures in vivo fluorescence. In addition, off-target effects from injected beta cell toxins4,32 cause damage to the liver and kidneys. To improve the metabolic zebrafish beta cell regeneration system, we transferred a conditional toxigene into the unpigmented, casper strain that combines nacre and roy mutations.20 Nacre lacks melanocytes and roy is a spontaneous mutant having uniformly black eyes, translucent skin, a few melanocytes, and no iridophores. The toxigene (Insulin:NtrmCherry [INC]) contains nfsB, a gene expressing bacterial nitroreductase (Ntr) which is fused to the mCherry fluorescent protein that is expressed by the zebrafish insulin promoter only in beta cells.22 Delivery of the prodrug Met to zebrafish water destroys beta cells but causes no known off-target effects. INC male, heterozygous F2 caspers were bred with Tg(Fli1:eGFP)y1 casper zebrafish (Fli) to generate the beta cell/endothelial cell-labeled INCFli transgenic zebrafish (Fig. 2).
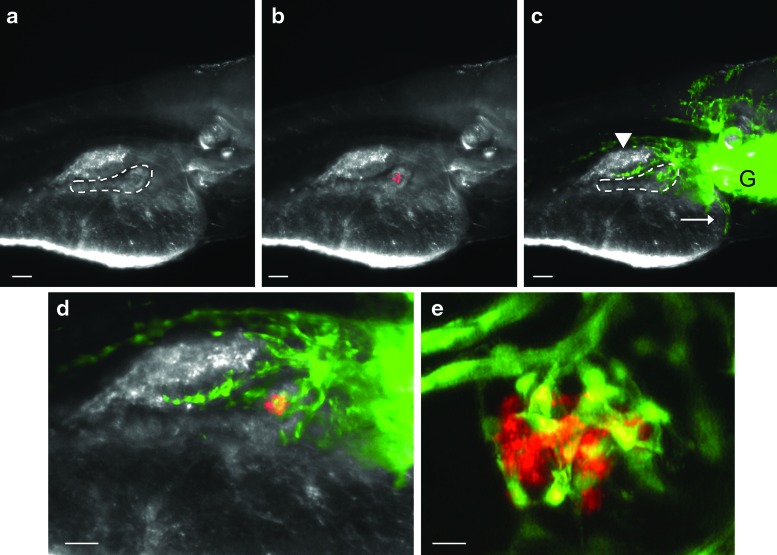
FIG. 2.
The pancreatic vasculature at larval day 6. (a) Bright field image of the right side of a living Insulin Nitroreductase mCherry (INCFli) zebrafish (scale bars in a–c=30 μm). The dashed border defines the pancreas where islet endocrine cells are developing in a single principal islet surrounded by exocrine tissue. (b) Endogenous mCherry fluorescence in transgenic INCFli islets is restricted to beta cells. (c) Endogenous GFP expressed by the Fli promoter within the trunk vasculature. Arrowhead: swim bladder, arrow: heart, G: gills, dashed line: pancreas. (d) Merged red and green fluorescence defines the endocrine pancreas containing nascent blood vessels. Scale bar=60 μm. (e) Multiphoton confocal image of living casper larva. Scale bar=10 μm. Color images available online at www.liebertpub.com/zeb
We evaluated living, 6 day-old casper INCFli heterozygous larvae for red and green fluorescence before them being placed in the aquatic system (Fig. 2). At this stage of development, larval yolk had diminished and the pancreas could be identified as a single principal islet surrounded by refractile exocrine tissue under a dissecting microscope (Fig. 2a, dashed line). Red fluorescence due to mCherry expression was observed only in pancreatic beta cells (Fig. 2b). Secondary islets33 expressing mCherry were not apparent in most larvae at this stage. Green fluorescence was present in the vasculature of the gill, heart, swim bladder, and ventral extensions of the trunk (Fig. 2c, dashed line). A few endothelial cells were identified in distinct patterns within the developing pancreas (Fig. 2d). To increase resolution of the nascent islet vasculature, multiphoton confocal imaging analysis (Fig. 2e) verified that cells marked with Fli were present in the principal islet, representing an environment in which active angiogenic and vasculogenic modeling was occurring. In support of previous studies,34 we did not observe blood flow through islet capillaries and concluded that systemic insulin action was not occurring at this stage. Although there may be important factors relevant for beta cell regeneration in embryos and larvae, it is not clear that the same signals would be relevant in the adult.
To understand re-growth of adult islets and their functionally mature islet capillaries, we followed GFP-labeled vascular cells and mCherry-labeled beta cells in the pancreas of living, regenerating adult casper zebrafish and recorded terminal blood glucose concentrations at four regeneration time points (Fig. 3a). A timeline is included to indicate the treatment regimen. INCFli adults were evaluated before treatment (day 1) and after 3, 7, or 14 days of regeneration within the endocrine pancreas. Placement of zebrafish in 12 mM Met at day 1 resulted in beta cell ablation through the conversion of Ntr into a compound that forms a cytotoxic metabolite only in beta cells.35,36 Two to 3 fish from each of three independent studies were either unaffected by Met treatment or died by day 3 (15%). This increased toxicity of Met treatment in caspers compared with the previously studied EK strain4 may be due to differences in drug susceptibility or genetic background. By treatment day 3, GFP- positive vasculature was visible in the trunk and fins, while only a few remaining red fluorescent beta cells could be observed beneath the unpigmented skin and liver after ablation (Fig. 3a, day 3). Average fasted blood glucose levels were recorded from affected fish after cold-water anesthesia and could be functionally linked to the re-appearance of beta cells. Values from a baseline of 65±5 mg/dL (n=9) to 303±24 mg/dL (n=11) at day 3 were followed by a return to near normal levels by day 14 (72±7 mg/dL [n=11]). Hyperglycemia had fallen significantly by day 7, when blood glucose values were recorded as 125±21 mg/dL (n=11), and mCherry-positive beta cells were clearly visible in living caspers (Fig. 3, day 7). We were able to rapidly identify regenerating beta cells in affected individuals and to determine the time required to achieve glucose homeostasis in INCFli adults using this medium throughput, standardized protocol.
Gut sections from affected adults at each time point were briefly fixed and mounted in agarose for multiphoton confocal (days 1, 3 or 14) or microscopic (day 7) analysis (Fig. 3b). On day 1, a representative whole mount section in which the overlying liver had been removed indicated groups of mCherry-positive beta cells clustered throughout a volume of 200 μm within the endocrine pancreas. GFP-labeled islet capillaries invested the islets before Met treatment. By day 3, when blood glucose was recorded at maximum levels, a few remaining mCherry-positive beta cells or new beta cells appeared to line an expanded GFP-positive islet capillary bed. The surrounding exocrine capillaries had a distinct morphology (day 3, *). Affected adults sacrificed at day 7 portrayed variable phenotypes that may be correlated to variability in blood glucose levels recorded at this regeneration time point. In Figure 3b, a representative 7 day pancreas is indicated with an arrowhead and magnified in the inset. mCherry-positive beta cells are located along the GFP-labeled vasculature. By day 14, the endocrine pancreas regenerated within the region that had been initially defined by multiple large islets in control, animals and the vasculature surrounded and invested these mCherry-positive clusters.
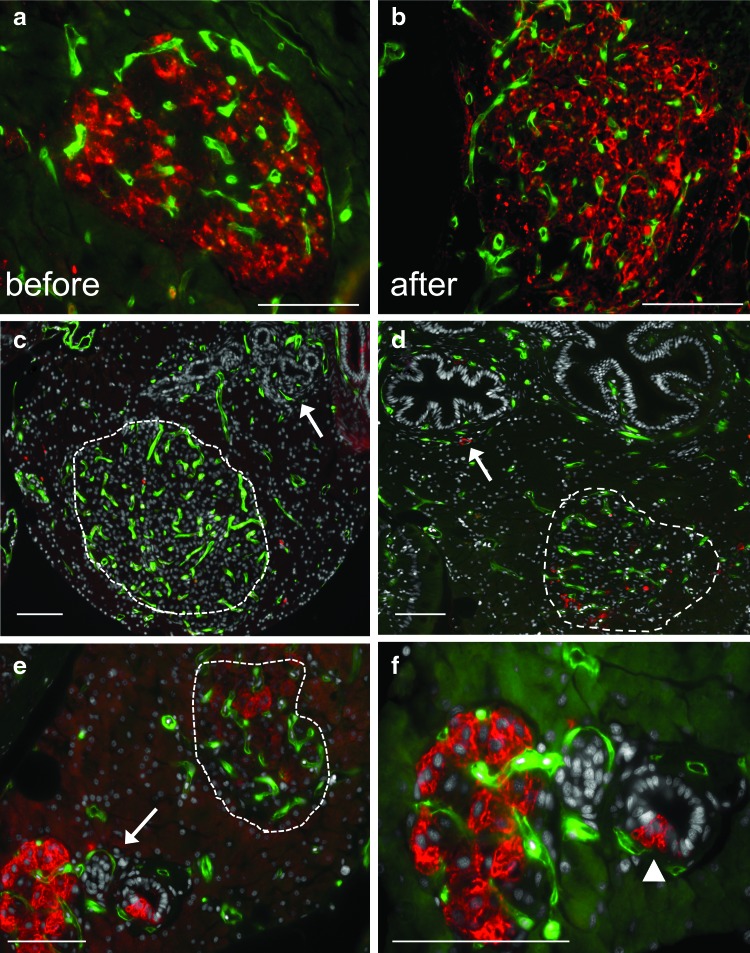
Changes in the morphology of islets were analyzed using paraffin sections prepared from each regeneration time point (Fig. 4). Endogenous GFP expression marked blood vessel morphology, and either endogenous mCherry expression or an insulin antibody was used to identify beta cells using immunohistochemistry without antigen retrieval. Untreated islets contained a dense, GFP-positive vasculature that was closely aligned with mCherry-positive beta cells (Fig. 4a). After a 14 day regeneration period, islet mass had been restored and contained large numbers of insulin-expressing beta cells and condensed, GFP-positive endothelial cells (Fig. 4b). These before and after images portray the rapid regeneration properties of many adult zebrafish organs. To assess intermediate stages of regeneration, we analyzed sections acquired from affected adults 3 days after the addition of Met (Fig. 4c, d). Sparse insulin staining was present in islets and beta cells near the exterior of what appeared to be small pancreatic ducts (Fig. 4c). These cells were previously described during pancreas regeneration.3,37 We were still able to define islet vasculature (dashed line), as it was more compact than vasculature in surrounding exocrine tissue. However, there were very few insulin-positive beta cells present at day 3 (Fig. 4d). Extra-islet blood vessels (arrow) surrounded exocrine ducts at day 3 where insulin-positive cells were found. A few positive cells were infrequently seen at the islet periphery but not in the center of the islet. It was unclear whether every differentiated beta cell had first died or had only ceased producing insulin.4 By day 7, mCherry expression in islets had increased (Fig. 4e, f). Changes in both vascular and beta cells were apparent. Large islets (dashed line) containing a few insulin-positive beta cells as well as smaller islets with robust mCherry staining were present. Islets were frequently observed budding from pancreatic ducts in the vicinity of labeled capillaries (Fig. 4f, arrowhead). mCherry-positive beta cells and GFP-positive capillaries were imaged with a 63×objective in Figure 4f. Large islet capillaries invested, regenerating islets at a time when blood glucose levels were returning to normal.
FIG. 4.
Changes in islet vasculature during beta cell regeneration. Five-micrometer paraffin sections. Representative sections from each time point are shown. Scale bars=20 μm (a–f) endogenous green fluorescence in vasculature. Cell nuclei (stained with Hoechst) are shown in gray (sections c–f). Beta cells are marked with mCherry red fluorescence (a, e, f) or insulin antibodies (b, c, d). (a) Day 1 control (before Met treatment). (b) A regenerated pancreas from an affected INCFli adult sacrificed at day 14 (after). (c, d) Independent, affected male casper transgenics sacrificed at day 3. (c) Arrow points to pancreatic ducts. An islet containing labeled vasculature is circled. (d) A different affected adult sacrificed at day 3 with labeled beta cells in an islet (dashed circle). Arrow points to a beta cell near labeled vasculature surrounding main pancreatic duct. (e) Affected INCFli adult sacrificed at day 7. Arrow points to region magnified in (f) Dashed circle: islet. (f) Regeneration day 7. Magnification of region in (e). Arrowhead: beta cells emerging from small pancreatic duct. Color images available online at www.liebertpub.com/zeb
Discussion
Zebrafish embryos have been used as inexpensive, tractable models for discovering new signaling pathways that affect beta cell mass during development.38,39 In contrast, we have engineered regenerating zebrafish to interrogate beta cell morphology and the effects of hyperglycemia on islet vasculature in living adults. Insulin action is initiated on secretion of insulin from beta cells into islet capillaries, maintaining metabolic homeostasis after a meal by removing glucose from the blood. To probe the supporting role of the vasculature beta cell growth in adults, we developed a transparent adult zebrafish regeneration model to observe islet vasculature in conditionally ablated beta cells during a return to normoglycemia. We verified that the vasculature in developing zebrafish was not fully formed by larval day 6. Developing islets require endothelial cell growth factors (including VEGF) for proper specification and differentiation40 despite their lack of a fully functional vasculature. Although arterial-venous pathways are in place at this stage,34 it is known that both nutritional and non-nutritional signals are required for normal islet and vascular function.14 Therefore, we assayed morphological interactions of islet endothelial cells with beta cells in the adult zebrafish pancreas during the onset and resolution of hyperglycemia. These experiments set the stage for additional studies, where end organs affected by high blood glucose can be evaluated in regenerating adult zebrafish using both morphological and metabolic analyses. Although we have not evaluated what happens when hyperglycemia is sustained through multiple applications of Met, a single dose induced a reproducible return to normoglycemia in INCFli adult zebrafish by day 14. Other metabolic measurements made from zebrafish blood are being used to define the functional status of adult zebrafish,41 providing rapid assays that can help determine the relevance of morphological changes with regard to physiological function. Differences in biology between mammals and zebrafish can be exploited to better understand how to promote regeneration, as in the case of spinal cord injury42 or blood stem cells.43
Adult zebrafish recover from damage to pancreatic beta cells without insulin therapy, rapidly normalizing blood glucose levels using as yet to be defined regenerative signals. We would expect that chronic elevation of blood glucose would have negative long-term effects on beta cell proliferation in the zebrafish as it does for mice.44 The failure in humans of an adaptive response between blood vessels and beta cells in the islet contributes to diseases such as type 2 diabetes. During growth, pregnancy, or insulin resistance, hyperglycemia exacerbates beta cell proliferation. Adult rodent models of type 2 diabetes have been shown to lack the regenerative plasticity of the adult zebrafish pancreas, instead resorting to beta cell compensation until beta cell exhaustion and hyperglycemia lead to cell death.28 Injectable agents causing beta cell toxicity, including streptozotocin, caused a permanent loss of intraislet vasculature, limiting beta cell regeneration and permanently impairing glucose tolerance in neonatal rats.45 Changes in glycemic control in adult zebrafish are correlated with changes in beta cell mass, suggesting an increase in insulin secretion from beta cells into the functional islet circulation. Although blood glucose levels are linked to beta cell proliferation in vivo, chronic changes in beta cell glucose metabolism have recently been shown to positively regulate basal and compensatory growth.46 After genetic ablation, surviving beta cells proliferated in young mice due to circulating high glucose levels, causing elevated glucokinase activity, KATP channel closure, and subsequent membrane depolarization in beta cells. Therefore, there may be a temporal requirement for hyperglycemia to precede beta cell regeneration, followed by a return to normoglycemia once beta cell mass has been restored.
The limited regeneration of beta cells found in young adult mice is controlled by systemic as well as local factors.47 Reciprocal signaling between beta cells and islet capillaries relies on VEGFA activity as well as other paracrine signals, promoting a dynamic cross-talk during development and disease.14 Although islet capillaries coordinate islet growth and are known to provide vascular niches for survival and maintenance of beta cell function, they also serve to restrict outgrowth and morphogenesis depending on localized VEGFA expression and modulation of Delta-Notch signaling.11 Our results provide a basis for discovering mechanisms that coordinate islet vascular remodeling with increases in beta cell mass in adults. We found that islet vascular morphology dramatically changed 3 days after beta cell ablation had been initiated. A looser, lower-density scaffold of GFP-labeled capillaries was visible within hyperglycemic adult zebrafish where few differentiated beta cells were present. By comparison, whole mount imaging of vascular-marked, anti-CD31 (PECAM)-hybridized diabetic mouse islets indicated differences in intra-islet capillary length and volume from normoglycemic mice.48 In addition, beta cells engineered to chronically over-express VEGF in adult mice induced hyperglycemia, impaired insulin secretion, and decreased beta cell mass.49 Since we observed a few mCherry as well as insulin-positive beta cells attached to the islet vasculature at this stage, these sites may represent a vascular niche in regenerating zebrafish. By day 14 of zebrafish regeneration, clusters of beta cells had either recovered and/or were formed from precursors via neogenesis within a tightly woven mesh of islet capillaries. The levels of expression of VEGFA and other angiogenic signaling molecules in the observed beta cells at different stages of regeneration could be used to understand the mechanisms required to potentiate endogenous expansion of beta cell mass, revealing important inducers of beta cell neogenesis and/or function. A further analysis of cell-type-specific expression of VEGFs and VEGF receptors, as well as angiogenic signaling molecules will be necessary to unravel the signaling between vascular and beta cells during regeneration. It may also be possible to interfere with this signaling using small molecules that selectively affect islet/trunk vasculature without excessive damage to other organs.50,51
Acknowledgments
Funding source: JDRF # 1-2005-1177 to L.G. Moss. The authors would like to thank Chris Newgard for reviewing their work and providing support; Jacob Wood, Brian Thompson, Wei Wei Ye, and David Lloyd for taking care of the fish; and Ken Poss for understanding the value of regeneration.
Disclosure Statement
No competing financial interests exist.
References
- 1.Brissova M. Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57:2269–2271. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S. Perspective: postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 3.Rankin MM. Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss JB. Koustubhan P. Greenman M. Parsons MJ. Walter I. Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes. 2009;58:1844–1851. doi: 10.2337/db08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvet M. Hoffman RM. In vivo imaging of pancreatic cancer with fluorescent proteins in mouse models. Methods Mol Biol. 2012;872:51–67. doi: 10.1007/978-1-61779-797-2_4. [DOI] [PubMed] [Google Scholar]
- 6.Olerud J. Johansson M. Lawler J. Welsh N. Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes. 2008;57:1870–1877. doi: 10.2337/db07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S. Li C. Yuan G. Xie F. Anatomical and histological observation on the pancreas in adult zebrafish. Pancreas. 2007;34:120–125. doi: 10.1097/01.mpa.0000246661.23128.8c. [DOI] [PubMed] [Google Scholar]
- 8.Henderson JR. Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347–356. doi: 10.1113/expphysiol.1985.sp002920. [DOI] [PubMed] [Google Scholar]
- 9.Lammert E. Cleaver O. Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 10.Richards OC. Raines SM. Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343–363. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magenheim J. Ilovich O. Lazarus A. Klochendler A. Ziv O. Werman R, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderari S. Chougnet C. Clemessy M. Kempf H. Corvol P. Larger E. Angiopoietin 2 alters pancreatic vascularization in diabetic conditions. PLoS One. 2012;7:e29438. doi: 10.1371/journal.pone.0029438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammert E. Gu G. McLaughlin M. Brown D. Brekken R. Murtaugh LC, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 14.Cleaver O. Dor Y. Vascular instruction of pancreas development. Development. 2012;139:2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore AV. Monzo K. Cha YR. Pan W. Weinstein BM. Vascular development in the zebrafish. Cold Spring Harb Perspect Med. 2012;2:a006684. doi: 10.1101/cshperspect.a006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field HA. Dong PD. Beis D. Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 17.Nasevicius A. Larson J. Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pusztaszeri MP. Seelentag W. Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 19.Pham VN. Lawson ND. Mugford JW. Dye L. Castranova D. Lo B, et al. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White RM. Sessa A. Burke C. Bowman T. LeBlanc J. Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.diIorio PJ. Moss JB. Sbrogna JL. Karlstrom RO. Moss LG. Sonic hedgehog is required early in pancreatic islet development. Dev Biol. 2002;244:75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- 22.Pisharath H. Rhee JM. Swanson MA. Leach SD. Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- 24.AVMA Guidelines for the Euthanasia of Animals. www.avma.org/issues/animal_welfare/euthanasia_guidelines/species…aquatics…methods.asp www.avma.org/issues/animal_welfare/euthanasia_guidelines/species…aquatics…methods.asp
- 25.Eames SC. Philipson LH. Prince VE. Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7:205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerfield M. 4th. Eugene: University of Oregon Press; 2000. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) [Google Scholar]
- 27.Yee NS. Lorent K. Pack M. Exocrine pancreas development in zebrafish. Dev Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Muoio DM. Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 29.Wallace KN. Akhter S. Smith EM. Lorent K. Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Kan NG. Junghans D. Izpisua Belmonte JC. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 2009;23:3516–3525. doi: 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim A. Miller K. Jo J. Kilimnik G. Wojcik P. Hara M. Islet architecture: a comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen AS. Sarras MP., Jr. Intine RV. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010;18:532–542. doi: 10.1111/j.1524-475X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons MJ. Pisharath H. Yusuff S. Moore JC. Siekmann AF. Lawson N, et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isogai S. Lawson ND. Torrealday S. Horiguchi M. Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 35.Pisharath H. Validation of nitroreductase, a prodrug-activating enzyme, mediated cell death in embryonic zebrafish (Danio rerio) Comp Med. 2007;57:241–246. [PubMed] [Google Scholar]
- 36.Curado S. Anderson RM. Jungblut B. Mumm J. Schroeter E. Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 37.Inada A. Nienaber C. Katsuta H. Fujitani Y. Levine J. Morita R, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson O. Adams BA. Yoo D. Ellis GC. Gut P. Anderson RM, et al. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell Metab. 2012;15:885–894. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rovira M. Huang W. Yusuff S. Shim JS. Ferrante AA. Liu JO, et al. Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. Proc Natl Acad Sci U S A. 2011;108:19264–19269. doi: 10.1073/pnas.1113081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brissova M. Shostak A. Shiota M. Wiebe PO. Poffenberger G. Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor—a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 41.Pedroso GL. Hammes TO. Escobar TD. Fracasso LB. Forgiarini LF. da Silveira TR. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp. 2012;63:e3865. doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldshmit Y. Sztal TE. Jusuf PR. Hall TE. Nguyen-Chi M. Currie PD. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci. 2012;32:7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durand EM. Zon LI. Stem cells: the blood balance. Nature. 2010;468:644–645. doi: 10.1038/468644a. [DOI] [PubMed] [Google Scholar]
- 44.Kulkarni RN. Jhala US. Winnay JN. Krajewski S. Montminy M. Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson JM. Arany EJ. Hill DJ. Changes in islet microvasculature following streptozotocin- induced beta-cell loss and subsequent replacement in the neonatal rat. Exp Biol Med (Maywood). 2010;235:189–198. doi: 10.1258/ebm.2009.009316. [DOI] [PubMed] [Google Scholar]
- 46.Porat S. Weinberg-Corem N. Tornovsky-Babaey S. Schyr-Ben-Haroush R. Hija A. Stolovich-Rain M, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Eberhard D. Kragl M. Lammert E. ‘Giving and taking’: endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab. 2010;21:457–463. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 48.El-Gohary Y. Sims-Lucas S. Lath N. Tulachan S. Guo P. Xiao X, et al. Three-dimensional analysis of the islet vasculature. Anat Rec (Hoboken). 2012;295:1472–1481. doi: 10.1002/ar.22530. [DOI] [PubMed] [Google Scholar]
- 49.Agudo J. Ayuso E. Jimenez V. Casellas A. Mallol C. Salavert A, et al. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass. Diabetes. 2012;61:2851–2861. doi: 10.2337/db12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitambi SS. McCulloch KJ. Peterson RT. Malicki JJ. Small molecule screen for compounds that affect vascular development in the zebrafish retina. Mech Dev. 2009;126:464–477. doi: 10.1016/j.mod.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghunath M. Sy Wong Y. Farooq M. Ge R. Pharmacologically induced angiogenesis in transgenic zebrafish. Biochem Biophys Res Commun. 2009;378:766–771. doi: 10.1016/j.bbrc.2008.11.127. [DOI] [PubMed] [Google Scholar]