Abstract
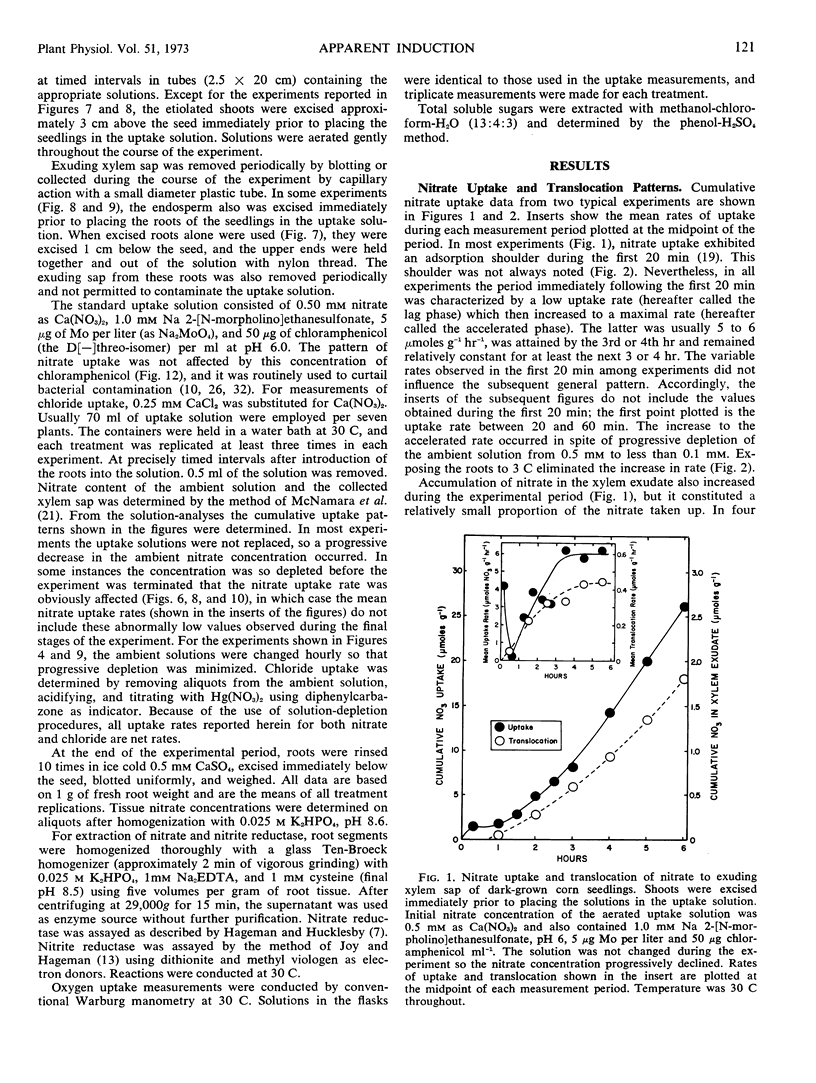
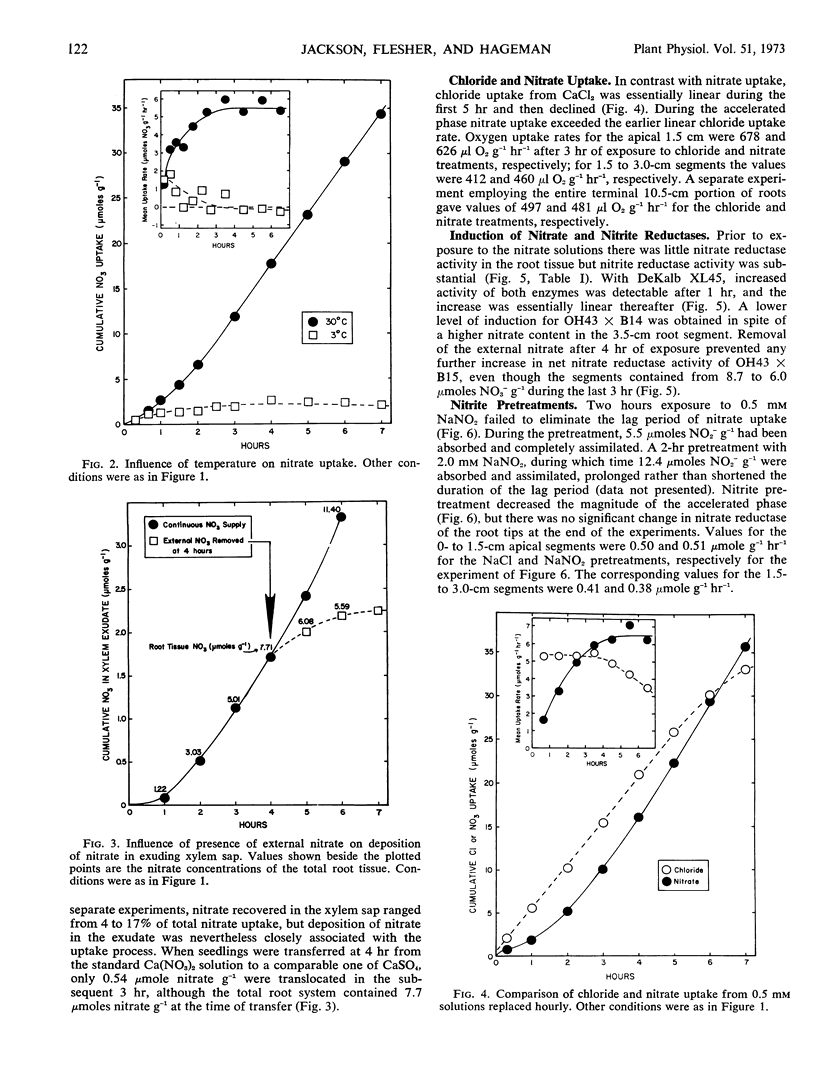
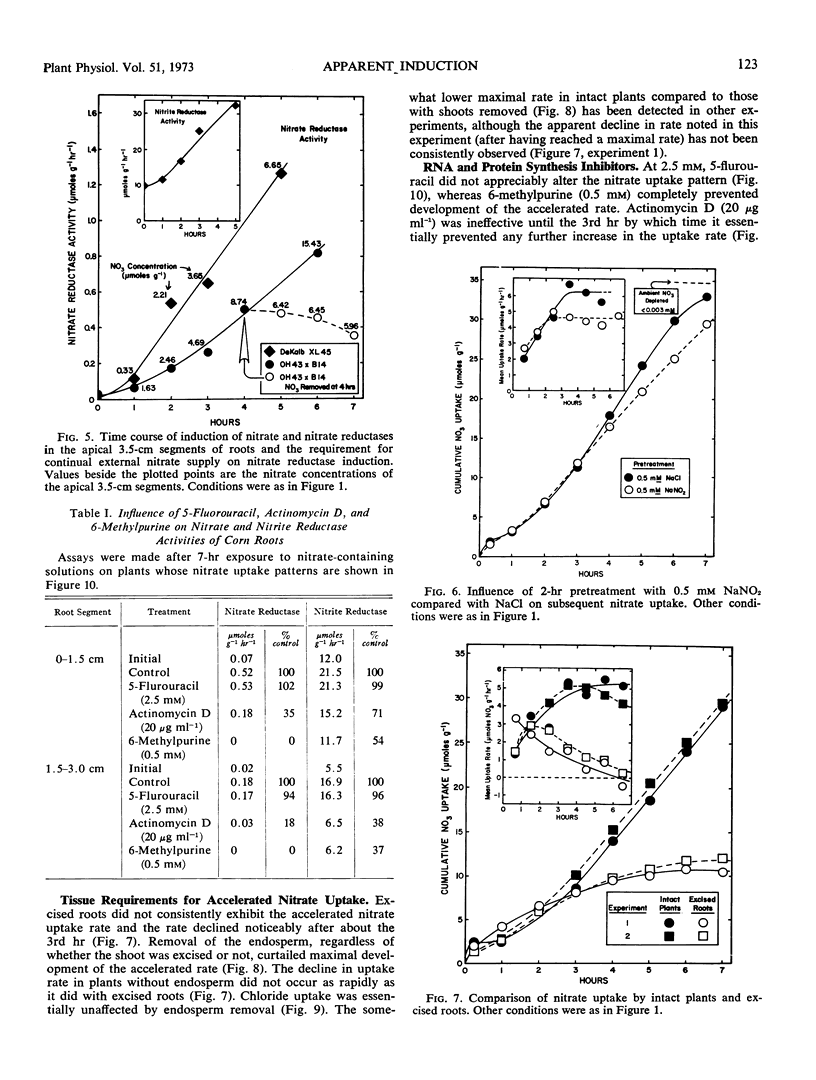
Five-or six-day old seedlings of corn (Zea mays L.) were exposed to 0.25 mm Ca(NO3)2, 1.0 mm sodium 2-[N-morpholino]-ethanesulfonate, 5 μg Mo per liter and 50 μg of chloramphenicol per ml at pH 6. Nitrate uptake was determined from depletion of the ambient solution. The pattern of nitrate uptake was characterized, after the first 20 minutes, by a low rate which increased steadily to a maximal rate by 3 to 4 hours. Transfer of nitrate to the xylem did not totally account for the increase. Development of the maximal accelerated rate did not occur at 3 C with excised roots nor with seedlings whose endosperm had been removed. Use of CaCl2 rather than Ca(NO3)2 resulted in a linear rate of chloride uptake during the first 4 hours, and chloride uptake was not as restricted by endosperm removal as was nitrate uptake.
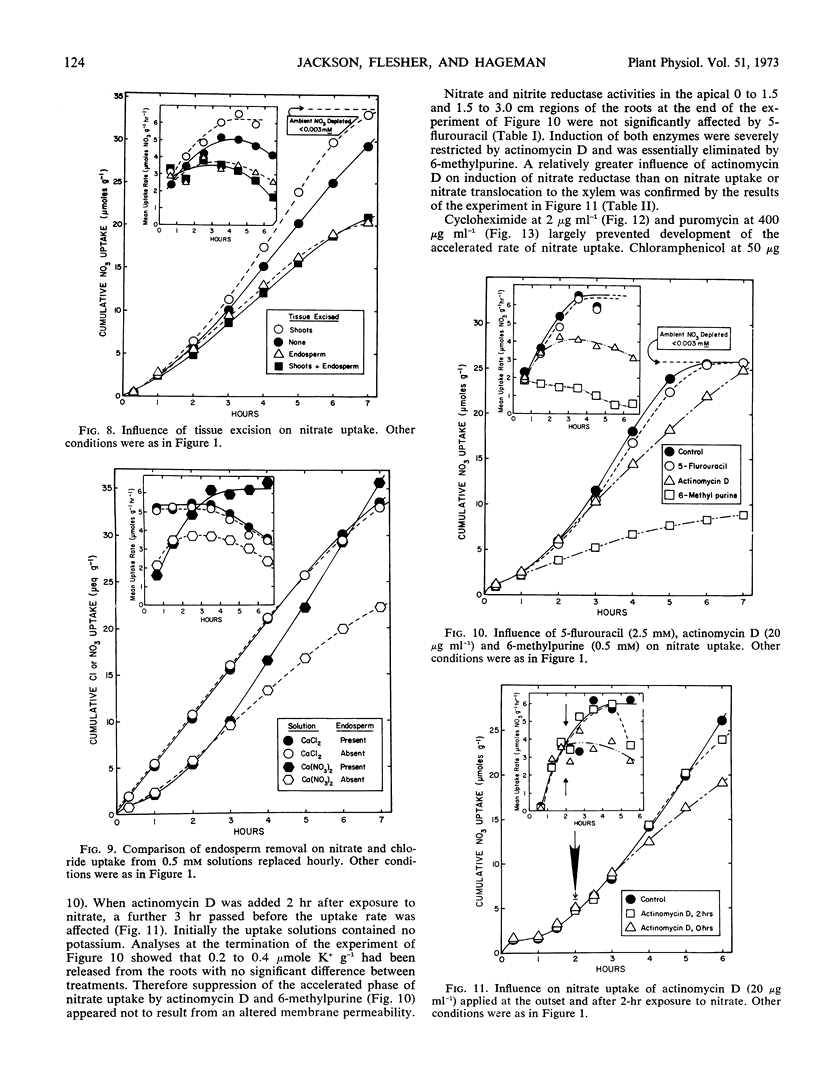
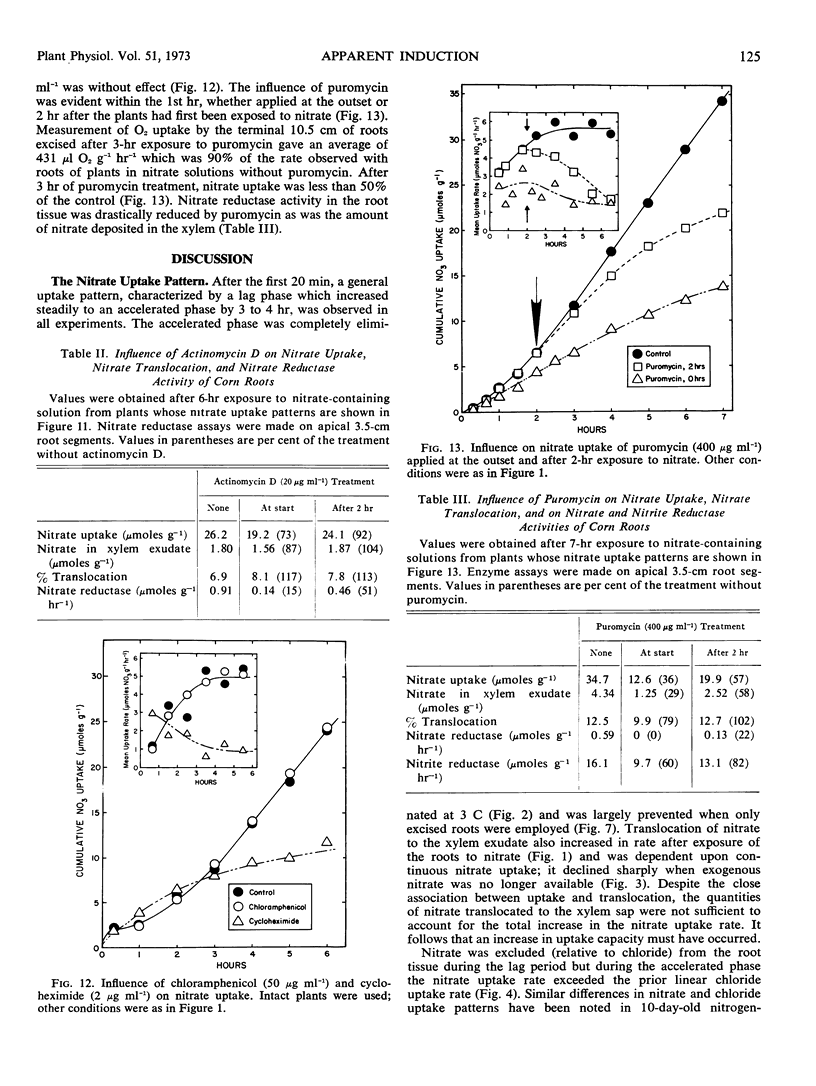
Nitrite pretreatments or the addition of cycloheximide (2 μg ml−1), puromycin (400 μg ml−1) and 6-methylpurine (0.5 mm) restricted maximal development of the accelerated nitrate uptake rate. Actinomycin D (20 μg ml−1) inhibited the rate only after about three hours exposure. The RNA and protein synthesis inhibitors also restricted nitrate reductase induction in the apical segments of the root tissue. The data suggest that development of the maximal accelerated rate of nitrate uptake depended upon continuous protein synthesis, and the hypothesis that synthesis of a specific nitrate transport protein must occur is advanced. But the alternative hypothesis, i.e., that induction of nitrate reductase (and/or a consequence of the act of nitrate reduction) provided the required stimulus, remains tenable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne W. F., Miflin B. J. An ATP dependent reduction of nitrate to ammonia by a cell free particulate system from barley roots. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1305–1310. doi: 10.1016/0006-291x(70)90008-2. [DOI] [PubMed] [Google Scholar]
- EPSTEIN E. Mechanism of ion absorption by roots. Nature. 1953 Jan 10;171(4341):83–84. doi: 10.1038/171083a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. [Chloroplast ribosomes: stereospecificity of inhibition by chloramphenicol]. Science. 1969 Jan 31;163(3866):477–478. doi: 10.1126/science.163.3866.477. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Holm R. E., Key J. L. Inhibition of Auxin-induced Deoxyribonucleic Acid Synthesis and Chromatin Activity by 5-Fluorodeoxyuridine in Soybean Hypocotyl. Plant Physiol. 1971 May;47(5):606–608. doi: 10.1104/pp.47.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Laties G. G. Bicarbonate Fixation and Malate Compartmentation in Relation to Salt-induced Stoichiometric Synthesis of Organic Acid. Plant Physiol. 1971 Apr;47(4):525–531. doi: 10.1104/pp.47.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Barnett N. M., Lin C. Y. RNA and protein biosynthesis and the regulation of cell elongation by auxin. Ann N Y Acad Sci. 1967 Aug 9;144(1):49–62. doi: 10.1111/j.1749-6632.1967.tb34000.x. [DOI] [PubMed] [Google Scholar]
- Key J. L. Effect of purine and pyrimidine analogues on growth and RNA metabolism in the soybean hypocotyl-the selective action of 5-fluorouracil. Plant Physiol. 1966 Oct;41(8):1257–1264. doi: 10.1104/pp.41.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E. A., Mengel K. Ionic balance in different tissues of the tomato plant in relation to nitrate, urea, or ammonium nutrition. Plant Physiol. 1967 Jan;42(1):6–14. doi: 10.1104/pp.42.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C., Sakore T. D., Nordman C. E. Stereochemistry of actinomycin--DNA binding. Nat New Biol. 1971 Jun 16;231(24):200–205. doi: 10.1038/newbio231200a0. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M. CO(2) Metabolism in Corn Roots. I. Kinetics of Carboxylation and Decarboxylation. Plant Physiol. 1967 May;42(5):712–718. doi: 10.1104/pp.42.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Bacteria, antibiotics and amino Acid incorporation into maize endosperm protein bodies. Plant Physiol. 1966 Feb;41(2):325–327. doi: 10.1104/pp.41.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]


