Abstract
In many organisms, attenuation of growth signaling by caloric restriction or mutational inactivation of growth signaling pathways extends lifespan and protects against cancer and other age-related diseases. The focus of many efforts to understand these effects has been on the induction of oxidative stress defenses that inhibit cellular senescence and cell death. Here we show that in the model organism S. cerevisiae, growth signaling induces entry of cells in stationary phase into S phase in parallel with loss of reproductive capacity, which is enhanced by elevated concentrations of glucose. Overexpression of RNR1 encoding a ribonucleotide reductase subunit required for the synthesis of deoxynucleotide triphosphates and DNA replication suppresses the accelerated loss of reproductive capacity of cells cultured in high glucose. The reduced reproductive capacity of these cells is also suppressed by excess threonine, which buffers dNTP pools when ribonucleotide reductase activity is limiting. Caloric restriction or inactivation of the AKT homolog Sch9p inhibits senescence and death in stationary phase cells caused by the DNA replication inhibitor hydroxyurea or by inactivation of the DNA replication and repair proteins Sgs1p or Rad27p. Inhibition of DNA replication stress represents a novel mechanism by which caloric restriction promotes longevity in S. cerevisiae. A similar mechanism may promote longevity and inhibit cancer and other age-related diseases in humans.
Keywords: DNA replication stress, aging, caloric restriction, chronological lifespan, reactive oxygen species, ribonucleotide reductase, senescence
Introduction
Caloric restriction extends the lifespans of diverse eukaryotic organisms and protects against cancer and other age-related diseases in rodents. Many efforts to understand the underlying mechanisms have focused on the reduction in intracellular levels of reactive oxygen species (ROS) and oxidative damage to DNA and other macromolecules that occurs when conserved growth signaling pathways activated by nutrients are downregulated by caloric restriction.1 The results of these studies are consistent with a role for oxidative damage that induces senescence and cell death as an important determinant of lifespan. However, complexities exist in the relationships between ROS and aging and age-related diseases that point to causal factors other than, or in addition to, oxidative stress and oxidative damage.2
DNA replication stress—i.e., inefficient DNA replication—recently emerged as a primary cause of genome instability and oncogene-induced senescence (OIS) at early stages of cancer.3 Replication stress occurs at DNA replication forks, which contain highly recombinogenic regions of unwound template DNA. Unwound DNA at replication forks is uniquely susceptible to single-strand scissions that result in double-strand DNA breaks.4 Earlier studies of OIS were focused on understanding the senescence-promoting effects of ROS-induced oxidative damage to DNA and other macromolecules, which is elevated by sustained oncogenic growth signaling. However, the results of a number of recent studies revealed that in addition to inducing ROS, sustained growth signaling by oncogenes also induces DNA replication stress in preneoplastic cells, which leads to senescence in S phase.3 How replication stress arises downstream of growth signaling by activated oncogenes remains unclear.
DNA replication stress was also recently implicated as a pro-aging factor in the budding yeast Saccharomyces cerevisiae chronological aging model, which shares features with OIS in mammalian cells.5 Chronological lifespan (CLS) is assessed by measuring the reproductive capacity of cells driven into stationary phase by nutrient depletion. Reversible growth arrest (i.e., quiescence) of nutrient-depleted cells in stationary phase requires downregulation of many of the same conserved growth signaling pathways—including those that require RAS and mTOR homologs and members of the AKT/PKB family of kinases6—that cause OIS when inappropriately activated in preneoplastic cells. Budding yeast cells that lose reproductive capacity in stationary phase enter into an irreversible senescent state7 and eventually die by programmed cell death.8
As in earlier studies of OIS in mammals and of aging in many organisms, much of the effort to understand the impact of growth signaling pathways on senescence and death in the budding yeast CLS model has been on their ability to downregulate oxidative stress defenses. However, a strong correlation exists between shorter CLS and a variety of experimental manipulations that inhibit stationary phase growth arrest in G0/G1, where cells cannot develop replication stress. This includes, for example, the shorter CLS and less frequent G0/G1 arrest of stationary phase cells induced by ectopic expression of the cyclin-dependent kinase (CDK) activator Cln3p9 or inactivation of the CDK inhibitor Sic1p.10,11 Both of these experimental manipulations promote entry of cells into S phase. Increasing the concentration of glucose in medium also shortens CLS via a mechanism that depends on the AKT homolog Sch9p and results in less frequent stationary phase growth arrest in G0/G1.10 A strong correlation also exists between longevity in the CLS model and the increased frequency with which stationary phase cells growth arrest in G0/G1, when conserved RAS, TOR and AKT/PKB growth signaling pathways are attenuated by mutations or caloric restriction.9,10 Cells that are budded in stationary phase also senesce12 and/or die10,13 significantly more frequently than unbudded cells, which is consistent with replication stress as a causal factor.
Based on these findings and parallels with the emerging connections between replication stress and OIS in mammalian cells, we proposed earlier that in addition to inducing oxidative stress, sustained growth signaling via conserved AKT-dependent and other growth signaling pathways induces replication stress in cells in stationary phase.9 In this study, we sought to test this hypothesis more directly. Our results show that replication stress induced by the ribonucleotide reductase (RNR) inhibitor hydroxyurea (HU) or by inactivation of genes encoding the replication-related proteins Rad27p or Sgs1p reduces the reproductive capacity of stationary phase cells via a mechanism that depends on growth signaling. Conversely, overexpression of RNR1 encoding a subunit of RNR, which is required for the synthesis of deoxynucleotide triphosphates (dNTPs) and DNA replication, inhibits the loss of reproductive capacity of stationary phase cells. Replication stress-induced senescence and death of cells in stationary phase is inhibited by caloric restriction or by inactivation of the AKT homolog Sch9p. This represents a novel mechanism by which caloric restriction or mutational inactivation of growth signaling promotes longevity of budding yeast cells in stationary phase.
Results
Attenuation of growth signaling by mutational inactivation of Sch9p or by caloric restriction inhibits hydroxyurea-induced replication stress and loss of reproductive capacity in cells in stationary phase
The CLS of budding yeast is substantially shorter in cells exposed during transitions from exponential growth to stationary phase to a low concentration (30 mM) of the RNR inhibitor hydroxyurea14 that does not inhibit the proliferation of cells in exponential cultures or on nutrient agar plates.14-16 In mammalian cells, sensitivity of dividing cells to HU occurs specifically in S phase.17 In budding yeast, HU-induced DNA damage and sensitivity to HU are detected in dividing cells, but not in cells arrested in G1 or G2. This contrasts with the sensitivity of budding yeast cells to the DNA damaging agent methylmethane sulfonate (MMS), which is detected in G1- or G2-arrested cells as well.18 Therefore, sensitivity to HU likely reflects increased replication stress due to a reduction in dNTP pools required for DNA replication rather than DNA repair.
We confirmed that cells exposed to 30 mM HU suffer a substantial loss of reproductive capacity once cultures achieve early stationary phase (Fig. 1A and B). The reduced reproductive capacity of HU-treated cells was not caused by HU inhibition of entry into stationary phase, because HU-treated and control untreated stationary phase cultures achieved similar terminal densities. HU treatment also increased the fraction of budded cells in early stationary phase (Fig. 1F, “2% glu”), which reflects inhibitory effects of HU on DNA replication rather than DNA repair. These findings establish that, in principle, replication stress can reduce the reproductive capacity of cells in stationary phase. However, the increase in the fraction of budded cells (from 22% to 44%) was substantially less than the fraction of the same population of cells that exhibited a HU-induced loss of reproductive capacity at the same time point (from 73% to 3%). Therefore, measuring the fraction of budded cells in stationary phase underestimates the fraction of cells that are undergoing replication stress.
Figure 1. Attenuation of growth signaling inhibits hydroxyurea-induced replication stress and loss of reproductive capacity in cells in stationary phase. (A) Reproductive capacity of wild-type BY4741 cells continuously exposed to 30 mM HU during 3 d of medium depletion. In these experiments, the potential impact of replication stress on reproductive capacity was assessed at one or two time points in stationary phase under conditions that led to a loss of reproductive capacity of less than 60% in one set of conditions. (B) Reproductive capacity of BY4741 cells under the same conditions assessed by serial 10-fold dilutions of cultures spotted on YPD agar plates. Similar 10-fold dilutions were performed in subsequent experiments that assessed reproductive capacity by spot tests. (C) Reproductive capacity of BY4741 cells exposed to 30 mM HU during 3 d of nutrient depletion in medium that initially contained 0.5% glucose. (D and E) Reproductive capacity of DBY746 wild-type (D) or DBY746 sch9Δ (Ε) cells exposed to 30 mM HU during 3 d of medium depletion. (F and G) % visibly budded BY4741 wild-type cells (F) or cells in the DBY746 background (G) exposed to 30 mM HU during 3 d of nutrient depletion. (H) Reproductive capacity of DBY746 wild-type cells cultured in 10% glucose SC buffered medium for 7 d in the presence or absence of 30 mM HU. (I) Reproductive capacity of DBY746 sch9Δ cells cultured in 10% glucose SC buffered medium for 7 d in the presence or absence of 30 mM HU. (J) Reproductive capacity of DBY746 wild-type cells cultured in 10% glucose YPD medium for 7 d in the presence or absence of 30 mM HU. (K) Reproductive capacity of sch9Δ cells in the DBY746 background cultured in 10% glucose YPD medium for 7 d in the presence or absence of 30 mM HU.
Caloric restriction imposed by reducing the initial concentration of glucose in medium from 2–0.5% extends CLS in concert with a reduction in intracellular superoxide anions19 and more frequent G0/G1 arrest of cells in stationary phase.9 Caloric restriction also abolished the sensitivity to HU of cells in early stationary phase (Fig. 1C) in parallel with a reduction in the number of cells with detectable buds (Fig. 1F, “0.5% glu”). Inactivation of the AKT homolog SCH9 also extends CLS in parallel with a reduction in intracellular levels of superoxide anions10,20 and a decrease in the frequency with which stationary phase cells fail to arrest growth in G0/G1 (Fig. 1G, see also ref. 9). Compared with isogenic wild-type cells (Fig. 1D), sch9Δ cells were also less sensitive to HU treatment during transitions to stationary phase (Fig. 1E) and less frequently exhibited buds compared with HU-treated wild-type cells (Fig. 1G). We conclude that attenuation of growth signaling by caloric restriction or genetic inactivation of Sch9p-dependent growth signaling protects against the loss of reproductive capacity caused by HU-induced replication stress in cells in early stationary phase, in part by promoting growth arrest of stationary phase cells in G0/G1.
Acetic acid and other organic acids accumulate in chronological aging experiments that employ SC medium,21 and buffering medium to eliminate the resulting decline in pH extends CLS.21,22 This is likely due to the activation of growth signaling pathways by low pH.22 In fact, many of the longevity-promoting effects of inactivating Sch9p and Ras2p reported previously are related to inhibition of the pro-aging effects of low pH rather than glucose. For example, in 2% glucose YPD medium, which maintains a higher pH, the CLS-extending effects of deleting SCH9 are absent in early stationary phase cultures. However, increasing the concentration of glucose in YPD medium to 10%, which approximates conditions S. cerevisiae cells are frequently exposed to in their natural environment, shortens CLS in a Sch9p-dependent fashion in response to glucose signaling rather than signaling by low pH.10 To determine whether Sch9p-dependent glucose signaling impacts replication stress induced by HU when effects of low pH are eliminated, we asked whether deletion of SCH9 inhibits HU-induced loss of reproductive capacity in 10% glucose SC buffered to pH 6. Cells in these cultures remained sensitive to 30 mM HU (Fig. 1H), although buffering the medium delayed this sensitivity until day 7 of medium depletion. In contrast, sensitivity to HU was absent in sch9Δ cells cultured under similar conditions (Fig. 1I). Similar results were obtained in YPD medium (Fig. 1J and K). We conclude that inactivation of Sch9p-dependent glucose signaling can inhibit HU-induced replication stress independently of low pH.
Glucose enhances the effects of replication stress associated with the inactivation of RAD27 or SGS1
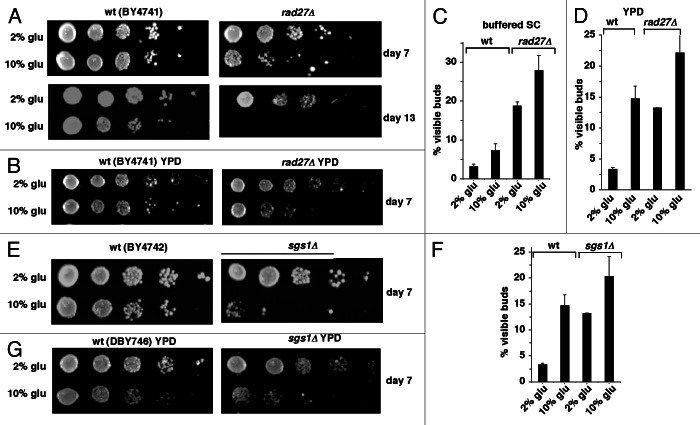
The RAD27 gene encodes the S. cerevisiae homolog of the conserved Fen1 5′ flap endonuclease required for maturation of Okazaki fragments during DNA synthesis.23 rad27Δ cells exhibit an extended S phase24 and arrest in S phase at an elevated temperature.25 Therefore, inactivation of RAD27 causes replication stress, which likely contributes to the shorter CLS detected in rad27Δ compared with wild-type cells.26,27 To determine whether glucose signaling enhances replication stress induced in stationary phase cells in the absence of HU, we compared the reproductive capacity of stationary phase wild-type and rad27Δ cells cultured in buffered SC medium that initially contained either 2 or 10% glucose.
At day 7 of medium depletion, rad27Δ cells cultured in buffered medium prepared with 2% glucose exhibited a similar reproductive capacity compared with wild-type cells. At day 13, the reproductive capacity of rad27Δ cells was reduced compared with wild-type cells under these conditions (Fig. 2A), consistent with prior reports that rad27Δ cells lose reproductive capacity at an earlier time point in CLS experiments in unbuffered medium.26,27 In contrast, rad27Δ cells cultured in buffered SC medium prepared with 10% glucose exhibited a substantially shorter reproductive capacity compared with wild-type cells at day 7, and the reproductive capacity of rad27Δ cells was reduced even further by day 13 (Fig. 2A). A similar reduction in reproductive capacity was observed in rad27Δ compared with wild-type cells cultured for 7 d in 10% glucose YPD medium, but not in YPD medium that initially contained 2% glucose (Fig. 2B). In both buffered SC medium and YPD medium, compared with wild-type cells, rad27Δ cells also arrested growth in stationary phase more frequently with visible buds, and the fraction of budded cells in stationary phase was increased further at the higher concentration of glucose (Fig. 2C and D).
Figure 2. Glucose enhances the effects of replication stress induced by inactivating RAD27 or SGS1. (A) Reproductive capacity after 7 or 13 d medium depletion of BY4741 and isogenic rad27Δ cells cultured in buffered SC medium that initially contained the indicated amounts of glucose. (B) Reproductive capacity of BY4741 and rad27Δ cells cultured for 7 d in YPD medium that initially contained the indicated amount of glucose. (C and D) % visibly budded BY4741 wild-type and rad27Δ cells cultured for 7 d in buffered SC medium (C) or YPD medium (D) that initially contained the indicated amounts of glucose. (E) Reproductive capacity of BY4742 wild-type cells or sgs1Δ cells in the BY4742 background cultured for 7 d in SC medium that initially contained the indicated amount of glucose. (F) % visibly budded cells in the same cultures described in panel (E). (G) Reproductive capacity of BY4742 wild-type cells and sgs1Δ cells in the BY4742 background cultured for 7 d in YPD medium that initially contained the indicated amount of glucose.
SGS1 encodes an ortholog of RecQ helicases that, when defective in humans, causes cancer-predisposing disorders accompanied by accelerated aging. In budding yeast, Sgs1p plays a role in response to replication stress.28 Although sgs1Δ cells have been reported to exhibit a shorter replicative lifespan (RLS)29 and CLS,30 it has also been reported that sgs1Δ cells do not exhibit a shorter CLS.26,31sgs1Δ cells more frequently undergo adaptive regrowth in stationary phase, which complicates CLS measurements. For example, in early stationary phase, sgs1Δ cells initially exhibit a substantial reduction in reproductive capacity compared with wild-type cells, but at later time points differences in reproductive capacity are masked by adaptive regrowth.31
We next asked whether growth signaling in buffered medium containing 10% glucose would exacerbate effects on reproductive capacity associated with the defective response to replication stress in sgs1Δ cells. After 7 d of medium depletion, the reproductive capacity of sgs1Δ cells cultured in buffered 2% glucose SC medium was similar to that of isogenic wild-type cells (Fig. 2E). However, similar to rad27Δ cells (Figs. 2A and C), despite the absence of a significant change in reproductive capacity in sgs1Δ cells cultured under these conditions, compared with wild-type cells, sgs1Δ cells more frequently failed to arrest in G0/G1 (Fig. 2F). This is consistent with the possibility that adaptive regrowth masks the effects of replication stress on reproductive capacity. However, a significant reduction in the reproductive capacity of sgs1Δ compared with wild-type cells was detected when they were cultured in 10% glucose buffered medium (Fig. 2E). This was accompanied by a further increase in the fraction of cells with visible buds (Fig. 2F). Similar effects of deleting SGS1 on reproductive capacity were observed in 10% glucose YPD cultures of cells in a different genetic background (DBY746) (Fig. 2G). These findings establish that glucose signaling enhances replication stress and the effects of replication stress on the reproductive capacity of rad27Δ and sgs1Δ cells in stationary phase independently of effects of low pH. Conversely, reducing the concentration of glucose abrogates these effects in part by enhancing growth arrest of stationary phase cells in G0/G1.
Chronological lifespan extension by overexpression of RNR1
The expression of genes encoding the RNR subunit Rnr1p and other proteins required for DNA replication are downregulated in response to the depletion of nutrients from growth medium as cells enter stationary phase.32 To determine whether downregulation of RNR1 as cells transition into stationary phase might induce replication stress that contributes to the senescence and/or death of stationary phase cells, we asked whether overexpression of RNR1 encoding the large subunit of the RNR complex that catalyzes the rate-limiting step in dNTP synthesis33 would extend CLS. When cultured in synthetic complete (SC) medium containing 2% glucose, wild-type W303 cells transformed with a high copy plasmid that expresses RNR1 exhibited a longer CLS compared with cells transformed with an empty vector (Fig. 3A). The extended CLS of cells overexpressing RNR1 was accompanied by a reduction in the number of cells undergoing programmed cell death marked by DNA degradation that resulted in cells with a sub G1 content of DNA (Fig. 3B).
Figure 3. High copy expression of RNR1 extends chronological lifespan. (A) CLS determined by measuring colony-forming units (cfus) of wild-type (W303) cells transformed with a high copy plasmid expressing RNR1 or the empty vector. (B) DNA content of wild-type cells transformed with the plasmid expressing RNR1 or the empty vector after 5 d of medium depletion. (C) CLS of wild-type and mec1-21 cells transformed with the plasmid expressing RNR1 or the empty vector. (D) % visibly budded cells in the cultures described in panel (C). Data in panels (A–C) are representative of results from two or more independent experiments.
The essential function of the DNA damage and replication stress response protein Mec1p is to upregulate RNR and dNTP pools.34 Disruption of this function by the mec1-21 mutation causes a reduction in dNTP pools35 and a shorter CLS.9 A shorter CLS has also been reported for mec1Δ cells.30 mec1-21 cells transformed with an empty vector exhibited a slightly shorter CLS compared with wild-type cells transformed with an empty vector, and the shorter CLS of mec1-21 cells was suppressed by overexpression of RNR1 (Fig. 3C). Overexpression of RNR1 also enhanced stationary phase growth arrest in G0/G1 indicated by a reduction in cells with visible buds in stationary phase cultures of wild-type or mec1-21 cells (Fig. 3D). These findings indicate that RNR1 expression is limiting in W303 cells in stationary phase, and limiting RNR1 expression reduces the frequency with which cells arrest growth in G0/G1.
Sustained growth signaling by high glucose enhances a requirement for RNR1 expression and threonine in cells in stationary phase
Mutations or limiting RNR1 expression may lead to a failure to exit S phase as cells enter stationary phase. It is possible that the expression of RNR1 and other replication-related genes also becomes limiting in stationary phase cells that re-enter S phase after these genes have been downregulated. To determine whether cells re-enter S phase after they enter stationary phase, the DNA content of cells at various time points before and after entry into stationary phase was determined by DNA staining and flow cytometry. These measurements indicated that in 2% glucose SC medium, in fact, the fraction of BY4741 wild-type cells in S phase increased after these cultures entered stationary phase (Fig. 4A). However, overexpression of RNR1 did not enhance the reproductive capacity of early stationary phase cells in the BY4741 background cultured in 2% glucose SC medium (Fig. 4B), in contrast to its effect on stationary phase W303 cells cultured under similar conditions (Fig. 3). Therefore, RNR1 expression is not limiting in BY4741 cells under these conditions.
Figure 4. Sustained growth signaling enhances the requirement for RNR1 in cells in stationary phase. (A) Fraction of cells in various cell cycle compartments in 2% glucose cultures determined by measuring DNA content. (B and C) Reproductive capacity after 2 d medium depletion of BY4741 wild-type cells transformed with a high copy plasmid expressing RNR1 or the empty vector and cultured in SC medium prepared with 2% glucose (B) or 10% glucose (C). (D) Fraction of cells in various cell cycle compartments in 10% glucose cultures determined by measuring DNA content. (E) % visibly budded cells after depletion of 10% glucose SC medium for indicated times. Asterisk indicates statistically significant increase in the fraction of visibly budded cells at the 60 and 72 h time points compared with the fraction at the 36 h time point (p < 0.01). (F) Concentration of glucose in medium at indicated time points in 2 or 10% glucose cultures. (G) Concentration of ammonium ions in medium in the same cultures. (H) Reproductive capacity of thr1Δ cells transformed with a high copy plasmid expressing RNR1 or the empty vector after culturing for 3 d in SC medium prepared with 2% glucose. (I and J) Reproductive capacity of BY4741 wild-type cells cultured in medium prepared with 1× or 4× threonine and 10% glucose (I) or 2% glucose (J).
We next asked whether enhanced glucose signaling that accelerates the loss of reproductive capacity of BY4741 cells might increase their requirement for RNR1 expression. The reproductive capacity of wild-type BY4741 cells in early stationary phase (after ~72 h of medium depletion) was substantially diminished in 10% glucose compared with 2% glucose SC cultures (compare Fig. 4B and C “vec”), as reported previously.10 The reduced reproductive capacity of cells in these cultures was not related to increased non-enzymatic glycation of proteins and other macromolecules by high glucose. This is because gal1Δ cells, which cannot metabolize galactose, are significantly less sensitive to excess galactose compared with glucose in early stationary phase (Fig. S1), despite the 10-fold higher rate at which galactose non-enzymatically glycates proteins compared with glucose.36 It was also not related to fewer cells achieving stationary phase in 10% glucose cultures, because after 2 d of medium depletion, the terminal density of 10% glucose cultures was twice that of 2% glucose cultures. Overexpression of RNR1 restored the reproductive capacity of cells cultured in 10% glucose SC medium to levels observed in 2% glucose SC cultures (Fig. 4C). We conclude that limiting RNR1 expression is the cause of the more rapid loss of reproductive capacity of cells in 10% glucose cultures.
The shorter CLS observed in cultures that initially contained 10% glucose occurs in parallel with a 5- to 8-fold increase in the rate at which budded compared with unbudded cells die.10 This is consistent with the possibility that in 10% glucose cultures, limiting RNR1 expression causes replication stress and death in cells that are more frequently driven into S phase after RNR1 expression is downregulated in stationary phase. The fraction of cells with an S phase content of DNA in 10% glucose cultures initially declined to 7% during the first 24 h. The S phase fraction then increased to 57% of the total population at 72 h (Fig. 4D). This increase was accompanied by an increase in the fraction of cells with less than a G1 content of DNA, indicating that DNA degradation was occurring in dying cells (Fig. 4D, “sub G1”). Some cells with an S phase content of DNA detected at later time points in 10% glucose cultures may correspond to cells that were actually in G2/M but suffered a partial loss of DNA due to DNA degradation. However, the fraction of cells with a G2/M content of DNA at 24 h (approximately 20%), which was before DNA degradation began to occur, was substantially less than the fraction of cells with an S phase content of DNA detected at the 72 h time point (57%). Therefore, even if all the G2/M cells in the these cultures suffered DNA degradation that resulted in cells with an S phase content of DNA, this would account for less than half of the increase in cells with an S phase DNA content at 72 h. Furthermore, although the fraction of budded cells in 10% glucose cultures initially decreased during the first 36 h, a statistically significant increase (p < 0.04) in the fraction of budded cells occurred at later time points (Fig. 4E). Therefore, increasing the concentration of glucose increases the frequency with which cells enter S phase in stationary phase cultures, where they die due to insufficient RNR1 expression.
In 2% glucose cultures, the increase in cells in S phase occurred after both glucose (Fig. 4F) and the nitrogen source ammonium (Fig. 4G) were completely depleted. Ammonium was also completely depleted from 10% glucose cultures after 36 h (Fig. 4G), which coincided with the time these cultures entered stationary phase marked by the absence of a continued increase in cell number (data not shown). Although the concentration of glucose also declined initially, it did not decline below approximately 3% during the first several days of stationary phase (Fig. 4F), during which large numbers of cells were entering S phase.
In exponentially proliferating cells, inactivation of the THR1 gene in the threonine biosynthesis pathway confers sensitivity to HU,16,37 and the sensitivity of thr1Δ cells to HU can be suppressed by exogenous threonine.37 thr1Δ sensitivity to HU is related to the role of threonine biosynthesis in buffering dNTP pools when RNR activity is limiting.37 Threonine biosynthesis depends on oxaloacetate, an intermediate of the tricarboxylic acid (TCA) cycle, which is repressed by glucose. For example, the MDH1 gene encoding malate dehydrogenase, which catalyzes the conversion of malate to oxaloacetate in the TCA cycle, is substantially downregulated when glucose is exhausted in 2% glucose cultures.38 Consistent with a role for threonine biosynthesis in maintaining dNTP pools in stationary phase cells cultured in 2% glucose SC medium, thr1Δ cells, which are predicted to have a very short CLS,39 rapidly lost reproductive capacity within the first few days of stationary phase when cultured under these conditions (compare Fig. 4H and B “vec”), and this phenotype was suppressed by overexpression of RNR1 (Fig. 3H). Therefore, threonine biosynthesis protects against replication stress-induced loss of reproductive capacity in stationary phase. We next asked whether in wild-type cells, the deficiency in RNR1 expression imposed by increasing the glucose concentration to 10% might be related to inhibition of threonine biosynthesis downstream of repression by residual glucose of oxaloacetate and other TCA cycle intermediates. Consistent with this hypothesis, similar to the effect of overexpressing RNR1 (Fig. 4C), the addition of excess threonine to 10% glucose cultures of wild-type cells suppressed the loss of reproductive capacity observed in these cultures after a few days of medium depletion (Fig. 4I). As expected, the addition of excess threonine did not significantly impact the reproductive capacity of wild-type cells cultured in 2% glucose medium (Fig. 4J). We conclude that the inadequate levels of RNR1 expression to maintain reproductive capacity in stationary phase cells cultured in 10% glucose medium is due to re-entry of cells into S phase combined with insufficient levels of threonine to support efficient dNTP synthesis, most likely due to repression of the TCA cycle by residual glucose.
Discussion
Replication stress inhibits the reproductive capacity of cells in stationary phase
Mutations in RecQ helicases and other DNA replication and repair proteins that induce replication stress or create defects in responses to replication stress are well-established causes of premature aging in many organisms. Our findings indicate that in S. cerevisiae, replication stress-induced senescence is also a significant factor in normal aging that is promoted by growth signaling and inhibited by caloric restriction. Inhibition of replication stress-induced senescence represents a novel, largely unexplored mechanism by which caloric restriction inhibits chronological aging in this organism.
The absence of budded cells or of a continued increase in cell number when nutrients are depleted are defining hallmarks of stationary phase cultures and the basis for the yeast chronological lifespan model of aging of post-mitotic cells in complex eukaryotes. Consequently, events related to DNA replication and other aspects of cell division have not been broadly considered as factors in the senescence and death of budding yeast cells in stationary phase. However, the increase in the number of cells in S phase after entry into stationary phase (Fig. 4) establishes that contrary to long-prevailing assumptions, budding yeast cells in stationary phase frequently re-enter the cell cycle without restoration of nutrients to medium. This is consistent with a recent report that in chronological aging experiments performed in 2% glucose SC medium, cells that die in stationary phase exhibit a progressively larger DNA content at later time points.13 It is not consistent with the argument based on the relatively small fraction of budded cells detected in stationary phase cultures that replication stress does not exert a significant impact on chronological lifespan.40 The fraction of cells that suffered replication stress-induced loss of reproductive capacity in early stationary phase in our experiments was substantial and was substantially larger than the fraction of cells that exhibited detectable buds in the same cultures at the same time points (for example, compare Fig. 1A and F). Therefore, measuring the number of budded cells underestimates the fraction of cells that suffer replication stress in stationary phase.
A requirement for efficient DNA replication to avoid loss of reproductive capacity in stationary phase is consistent with the results of a prior genome-wide screen by Powers et al. for genetic determinants of chronological lifespan.39 The results of this screen predict that the CLS of a rnr1Δ strain and the rad27Δ strain employed in our experiments is shorter than 91% of all 4759 haploid deletion strains examined in this screen. They also predict that the CLS of sic1Δ strains, which undergo aberrant entry into S phase41 and have a substantially shorter CLS compared with wild-type cells,10,11 and of sgs1Δ strains is shorter than 82% (sic1Δ) and 75% (sgs1Δ) of all 4759 of these deletion strains. lsm1Δ cells harboring a defect in mRNA capping that causes replication stress42 also exhibit a substantially shorter CLS compared with wild-type cells.14 The Powers et al. screen predicts that the CLS of lsm1Δ cells is shorter than 94% of all the strains they examined. Importantly, although our data indicate that DNA replication stress can have a substantial impact on CLS, it is not the only determinant of CLS modulated by growth signaling pathways. In addition to oxidative stress, other factors known to limit CLS downstream of growth signaling include mitochondrial dysfunction, reduced autophagy and factors that increase genetic instability independently of replication stress.43
Growth signaling-induced replication stress and its inhibition by caloric restriction or inactivation of SCH9
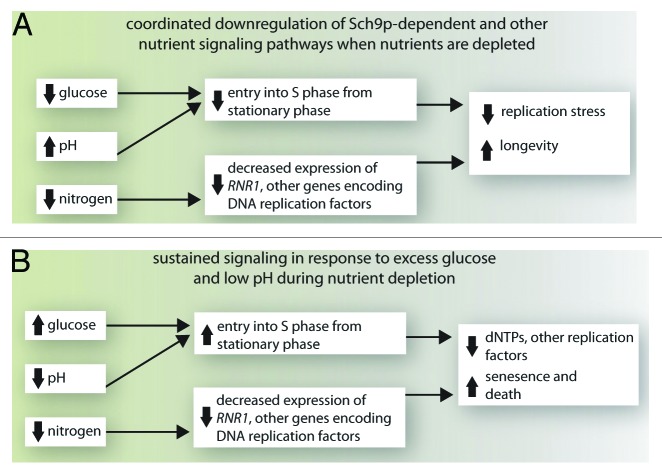
The depletion of nutrients from yeast cultures triggers the downregulation of growth signaling pathways and entry into stationary phase in parallel with the reduced transcription of a large number of genes, including genes encoding Rnr1p and other proteins required for DNA replication.32 Our findings indicate that maintaining the reproductive capacity of cells in stationary phase requires coordination of these events, such that cells entering stationary phase are no longer in S phase or are blocked from entering S phase from stationary phase, where reduced expression of RNR1 and other replication-related genes creates suboptimal conditions for replicating DNA (Fig. 5A). The expression of RNR1 and other replication-related genes is downregulated in response to nitrogen depletion.32 Consequently, sustained growth signaling after nitrogen is depleted likely drives cells into S phase in the absence of sufficient RNR and/or other components of the replication machinery to support efficient DNA replication (Fig. 5B).
Figure 5. Replication stress and senescence in budding yeast cells. (A) Coordinated downregulation of growth signaling pathways in cells in stationary phase reduces replication stress and promotes longevity. (B) Downregulation of some, but not all growth signaling pathways when nutrients are depleted induces replication stress that causes senescence and cell death. See text for details.
In the standard conditions employed in most chronological aging experiments (unbuffered 2% glucose SC medium), cells in stationary phase may be driven into S phase by sustained growth signaling by low pH. This is suggested by the observations that buffering the medium to maintain a higher pH or the use of YPD medium—which also maintains a higher pH—reduces the fraction of stationary phase cells that are budded10,13,22 as well as the number of dead cells harboring an S phase content of DNA.13 Although, in contrast to W303 cells, the senescence and death of stationary phase BY4741 cells under these conditions is not due to limiting RNR1 expression, many other genes required for efficient DNA replication are also downregulated as cells transition into stationary phase; presumably, the product of a replication-related gene other than RNR1 becomes limiting in this strain background. In 10% glucose medium, sustained signaling by residual glucose is likely responsible for the increased fraction of cells that enter S phase from stationary phase. Our data indicate that RNR1 expression becomes limiting under these conditions due to a deficiency in threonine that likely leads to the depletion of dNTP pools (Fig. 4).
Caloric restriction19 or inactivation of Sch9p10,20 enhance the reproductive capacity of cells in stationary phase in part by reducing intracellular levels of superoxide anions. The data reported here indicate that attenuation of growth signaling by caloric restriction or inactivation of SCH9 also enhances reproductive capacity by promoting more frequent growth arrest of stationary phase cells in G0/G1, where they avoid replication stress. They also predict that Sch9p and other proteins can impact chronological lifespan by interacting with the cell cycle machinery, in addition to regulating stress responses. Consistent with this prediction, it was recently reported that PKA and Sch9p phosphorylate the cell cycle-regulating ubiquitin ligase Cdc34p, and expression of a mutant form of Cdc34p that mimics its constitutive phosphorylation by Sch9p significantly shortens CLS.44 Phosphorylation of Cdc34p by Sch9p leads to destabilization of the cyclin-dependent kinase inhibitor Sic1p, which when stabilized, blocks entry into S phase. sic1Δ cells exhibit a short chronological lifespan in concert with accelerated entry into S phase from stationary phase.10 Together these findings argue that Sch9p impacts chronological aging, in part via effects on the cell cycle machinery that lead to replication stress.
Relevance to complex eukaryotes
The loss of reproductive capacity of yeast cells in stationary phase cultures is usually considered to model events that impact the aging of quiescent, postmitotic cells of higher eukaryotes. Our findings suggest that chronological aging experiments also model events that lead to the senescence of cells in humans and other metazoans as they enter into a quiescent state, in addition to events that occur after the quiescent state is achieved. They are likely relevant to a recent report that as cultured mouse cells approach contact inhibition-induced quiescence in medium containing low levels of glutamine, sustained growth signaling by oncogenic Ras, which requires glucose to elicit oncogenic phenotypes,45 induces abortive S phase entry in the absence of sufficient dNTPs to efficiently replicate DNA.46 Similarly, activated Ras induces senescence in normal human fibroblasts by downregulating RNR and thymidylate synthase, which leads to a deficiency in dNTP pools and DNA damage.47 It was also recently reported that the uncoordinated activation of growth signaling pathways that promote entry into S phase leads to depletion of dNTPs and genome instability in cultured human cells transformed by ectopic expression of cyclin E or oncogenic viral proteins.48
Similar to mutations that activate oncogenes, elevated glucose activates PI3K/AKT/mTOR signaling and other oncogenic signaling pathways in cultured mammalian cells. Glucose activation of these pathways leads to the induction of cyclin D1, cyclin E and other proteins that promote entry into S phase, as well as downregulation of p27, the mammalian homolog of Sic1p.49,50 Elevated glucose that mimics chronic hyperglycemia in humans also induces DNA damage51 and senescence52 in cultured mammalian cells. In budding yeast, inactivation of the conserved AMP kinase Snf1p, which is activated by reduced levels of glucose, shortens CLS in concert with a large increase in the number of cells that fail to arrest in G0/G1 when driven into stationary phase by nutrient depletion.10 The mammalian AMP kinase similarly drives cells out of S phase and into a G1 arrest in response to low glucose.53,54 Drugs that mimic the effects of caloric restriction by activating AMPK protect against cancer.55 We propose that these and other effects are related in part to glucose-induced replication stress and its inhibition by caloric restriction and caloric restriction mimetics. This model and its relevance to dietary factors that promote aging and age-related diseases in humans remain to be explored.
Materials and Methods
Strains and plasmids are described in Table 1.
Table 1. Strains and plasmids employed in this study.
| Strain | Genotype | Source |
|---|---|---|
| DBY746 |
MATa leu2-3,112 his3∆1 trp1-2889 ura3-52 |
V. Longo |
| DBY746 sch9Δ |
MATa leu2-3,112 his3∆1 trp1-2889 ura3-52 sch9::URA |
V. Longo |
| BY4741 |
MATa his3Δ leu2Δ met15Δ ura3Δ |
Open Biosystems |
| BY4741 rad27Δ |
|
ATCC |
| BY4741 sgs1Δ |
|
ATCC |
| BY4741 gal1Δ |
|
Research Genetics |
| W303-1A |
MATa ade2-1 ura3-1 his3-11 trp1-1 leu2-3 can1-100 |
Bruce Stillman |
| Y604 (W303 mec1-21) |
MATa ade2-1 ura3-1 his3-11 trp1-1 leu2-3 can1-100 mec1-21 |
|
|
Plasmids |
Description |
Source |
| YEP24 |
2µ based URA3 selectable shuttle vector |
ATCC |
| YEP24-RNR1 | RNR1 cloned with its native promoter into YEP24 | Elizabeth Vallen |
Cell culture conditions
Cells were cultured at 30°C with rotary shaking for the indicated times in YPD medium,56 or synthetic complete (SC) medium supplemented with excess amino acids and bases,57 with the exception that uracil was absent from medium in experiments that employed plasmids. SC and YPD medium contained 2 or 10% glucose as a carbon source. In experiments that employed buffered medium, citrate buffer pH 6 was added to a final concentration of 50 mM. Chronological lifespan and budded cell measurements were performed as described previously9 and in more detail below. Reproductive capacity was assessed by spotting 10-fold serial dilutions of triplicate cultures on YPD agar plates followed by incubation at 30°C for 2 or 3 d. Hydroxyurea was purchased from Sigma-Aldrich.
Chronological lifespan measurements
Cells from exponentially proliferating cultures were inoculated into 50 mLs. of SC medium containing 2 or 10% (weight/volume) glucose in 250 mL. flasks at an initial density of 5 × 107 cells/mL. and continuously cultured at 30°C with rotary shaking for the indicated times. Aliquots removed from cultures at the indicated times were plated in triplicate on YPD agar to determine colony-forming units. Data are representative of measurements made in two or more biological replicas.
Measurements of budded cells
To determine the fraction of cells with visible buds, cells in aliquots taken at each time point were pelleted by centrifugation and resuspended in water. At least 500 cells from each culture were examined for buds using a Nikon Eclipse E600 microscope with a 40× phase contrast objective. Just before examining cells, cell clumps were dissociated by sonication using a Model 60 Sonic Dismembrator sonicator (Fisher Scientific) for 10 sec at a power setting of 5.
Glucose and ammonia measurements
Glucose and ammonia measurements were performed on aliquots of cultures at the indicated time points using a glucose oxidase (GOD) assay (Roche Diagnostics GmbH) and an ammonia assay kit (Sigma) following the manufacturer’s instructions.
Cell cycle analysis
For these experiments cells were cultured at 26°C to limit DNA degradation. Aliquots of cultures were collected at the indicated time points, and cells were pelleted, washed and fixed with ethanol (70% v/v) for 30 min at 4°C. Cells were then resuspended in sodium citrate buffer (50 mM sodium citrate, pH 7.5), sonicated and treated with RNase for 1 h at 50°C followed by subsequent incubation with proteinase K (0.02 mg/107 cells) for several hours. DNA was then labeled with SYBR Green (Molecular Probes/Invitrogen) diluted in Tris-EDTA (pH 8.0) and incubated overnight at 4°C. Before flow cytometry analysis, samples were diluted 1:4 in sodium citrate buffer. Flow cytometry measurements were made using a BD LSR II™ (Becton Dickinson) and data analyzed with BD FACSDiva Software 6.0 (Becton Dickinson). The percentage of cells in each phase of the cell cycle was determined offline with ModFit LT software (Verity Software House).
Reproducibility and statistical analysis
Spot test data are representative of data collected in three or more biological replicas. Error bars in bar graphs represent standard deviations calculated from the results of at least three independent experiments. Statistical significance was assessed where indicated using Student’s unpaired t-test.
Supplementary Material
Acknowledgments
We wish to thank Molly Burhans for preparing plasmid DNA and Figure 5. This research was supported by a National Cancer Institute Support Grant (P30CA016056) to Roswell Park Cancer Institute and by FCT - Fundação para a Ciência e Tecnologia (PTDC/BIA-MIC/114116/2009), Portugal. B.S.M. received a fellowship from FCT (SRFH/BD/41674/2007).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/24232
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24232
References
- 1.Page MM, Robb EL, Salway KD, Stuart JA. Mitochondrial redox metabolism: aging, longevity and dietary effects. Mech Ageing Dev. 2010;131:242–52. doi: 10.1016/j.mad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–8. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 4.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35:7545–56. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burhans WC, Weinberger M. DNA Damage and DNA Replication Stress in Yeast Models of Aging. Subcell Biochem. 2011;57:187–206. doi: 10.1007/978-94-007-2561-4_9. [DOI] [PubMed] [Google Scholar]
- 6.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 7.Aragon AD, Rodriguez AL, Meirelles O, Roy S, Davidson GS, Tapia PH, et al. Characterization of differentiated quiescent and nonquiescent cells in yeast stationary-phase cultures. Mol Biol Cell. 2008;19:1271–80. doi: 10.1091/mbc.E07-07-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–7. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger M, Feng L, Paul A, Smith DL, Jr., Hontz RD, Smith JS, et al. DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS ONE. 2007;2:e748. doi: 10.1371/journal.pone.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger M, Mesquita A, Caroll T, Marks L, Yang H, Zhang Z, et al. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging (Albany NY) 2010;2:709–26. doi: 10.18632/aging.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinzalla V, Graziola M, Mastriani A, Vanoni M, Alberghina L. Rapamycin-mediated G1 arrest involves regulation of the Cdk inhibitor Sic1 in Saccharomyces cerevisiae. Mol Microbiol. 2007;63:1482–94. doi: 10.1111/j.1365-2958.2007.05599.x. [DOI] [PubMed] [Google Scholar]
- 12.Laporte D, Lebaudy A, Sahin A, Pinson B, Ceschin J, Daignan-Fornier B, et al. Metabolic status rather than cell cycle signals control quiescence entry and exit. J Cell Biol. 2011;192:949–57. doi: 10.1083/jcb.201009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami C, Delaney JR, Chou A, Carr D, Schleit J, Sutphin GL, et al. pH neutralization protects against reduction in replicative lifespan following chronological aging in yeast. Cell Cycle. 2012;11:3087–96. doi: 10.4161/cc.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palermo V, Cundari E, Mangiapelo E, Falcone C, Mazzoni C. Yeast lsm pro-apoptotic mutants show defects in S-phase entry and progression. Cell Cycle. 2010;9:3991–6. doi: 10.4161/cc.9.19.13210. [DOI] [PubMed] [Google Scholar]
- 15.Chang M, Bellaoui M, Boone C, Brown GW. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc Natl Acad Sci USA. 2002;99:16934–9. doi: 10.1073/pnas.262669299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–81. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair WK. Hydroxyurea: differential lethal effects on cultured mammalian cells during the cell cycle. Science. 1965;150:1729–31. doi: 10.1126/science.150.3704.1729. [DOI] [PubMed] [Google Scholar]
- 18.Galli A, Schiestl RH. Hydroxyurea induces recombination in dividing but not in G1 or G2 cell cycle arrested yeast cells. Mutat Res. 1996;354:69–75. doi: 10.1016/0027-5107(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 19.Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leão C, et al. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci USA. 2010;107:15123–8. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y, Shadel GS. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009;1:131–45. doi: 10.18632/aging.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–70. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burhans WC, Weinberger M. Acetic acid effects on aging in budding yeast: are they relevant to aging in higher eukaryotes? Cell Cycle. 2009;8:2300–2. doi: 10.4161/cc.8.14.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Shen B. Okazaki fragment maturation: nucleases take centre stage. J Mol Cell Biol. 2011;3:23–30. doi: 10.1093/jmcb/mjq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koren A, Soifer I, Barkai N. MRC1-dependent scaling of the budding yeast DNA replication timing program. Genome Res. 2010;20:781–90. doi: 10.1101/gr.102764.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reagan MS, Pittenger C, Siede W, Friedberg EC. Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide excision repair gene. J Bacteriol. 1995;177:364–71. doi: 10.1128/jb.177.2.364-371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringvoll J, Uldal L, Roed MA, Reite K, Baynton K, Klungland A, et al. Mutations in the RAD27 and SGS1 genes differentially affect the chronological and replicative lifespan of yeast cells growing on glucose and glycerol. FEMS Yeast Res. 2007;7:848–59. doi: 10.1111/j.1567-1364.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- 27.Laschober GT, Ruli D, Hofer E, Muck C, Carmona-Gutierrez D, Ring J, et al. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2010;9:1084–97. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashton TM, Hickson ID. Yeast as a model system to study RecQ helicase function. DNA Repair (Amst) 2010;9:303–14. doi: 10.1016/j.dnarep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.McVey M, Kaeberlein M, Tissenbaum HA, Guarente L. The short life span of Saccharomyces cerevisiae sgs1 and srs2 mutants is a composite of normal aging processes and mitotic arrest due to defective recombination. Genetics. 2001;157:1531–42. doi: 10.1093/genetics/157.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S, Zhang XE, Chen G, Liu W. Compromised cellular responses to DNA damage accelerate chronological aging by incurring cell wall fragility in Saccharomyces cerevisiae. Mol Biol Rep. 2012;39:3573–83. doi: 10.1007/s11033-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 31.Madia F, Gattazzo C, Wei M, Fabrizio P, Burhans WC, Weinberger M, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180:67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 34.Desany BA, Alcasabas AA, Bachant JB, Elledge SJ. Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev. 1998;12:2956–70. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasullo M, Tsaponina O, Sun M, Chabes A. Elevated dNTP levels suppress hyper-recombination in Saccharomyces cerevisiae S-phase checkpoint mutants. Nucleic Acids Res. 2010;38:1195–203. doi: 10.1093/nar/gkp1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ledesma-Osuna AI, Ramos-Clamont G, Vázquez-Moreno L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim Pol. 2008;55:491–7. [PubMed] [Google Scholar]
- 37.Hartman JL., 4th Buffering of deoxyribonucleotide pool homeostasis by threonine metabolism. Proc Natl Acad Sci USA. 2007;104:11700–5. doi: 10.1073/pnas.0705212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–6. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 39.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–84. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabrizio P, Longo VD. Chronological aging-induced apoptosis in yeast. Biochim Biophys Acta. 2008;1783:1280–5. doi: 10.1016/j.bbamcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lengronne A, Schwob E. The yeast CDK inhibitor Sic1 prevents genomic instability by promoting replication origin licensing in late G(1) Mol Cell. 2002;9:1067–78. doi: 10.1016/S1097-2765(02)00513-0. [DOI] [PubMed] [Google Scholar]
- 42.Herrero AB, Moreno S. Lsm1 promotes genomic stability by controlling histone mRNA decay. EMBO J. 2011;30:2008–18. doi: 10.1038/emboj.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cocklin R, Goebl M. Nutrient sensing kinases PKA and Sch9 phosphorylate the catalytic domain of the ubiquitin-conjugating enzyme Cdc34. PLoS ONE. 2011;6:e27099. doi: 10.1371/journal.pone.0027099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiaradonna F, Sacco E, Manzoni R, Giorgio M, Vanoni M, Alberghina L. Ras-dependent carbon metabolism and transformation in mouse fibroblasts. Oncogene. 2006;25:5391–404. doi: 10.1038/sj.onc.1209528. [DOI] [PubMed] [Google Scholar]
- 46.Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE. 2009;4:e4715. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannava S, Moparthy KC, Wheeler LJ, Natarajan V, Zucker SN, Fink EE, et al. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. Am J Pathol. 2013;182:142–51. doi: 10.1016/j.ajpath.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145:435–46. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu JM, Lee MY, Yun SP, Han HJ. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J Cell Physiol. 2010;224:59–70. doi: 10.1002/jcp.22091. [DOI] [PubMed] [Google Scholar]
- 50.Kim YH, Heo JS, Han HJ. High glucose increase cell cycle regulatory proteins level of mouse embryonic stem cells via PI3-K/Akt and MAPKs signal pathways. J Cell Physiol. 2006;209:94–102. doi: 10.1002/jcp.20706. [DOI] [PubMed] [Google Scholar]
- 51.Ksiazek K, Passos JF, Olijslagers S, von Zglinicki T. Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. Biochem Biophys Res Commun. 2008;366:793–9. doi: 10.1016/j.bbrc.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Ksiazek K, Korybalska K, Jörres A, Witowski J. Accelerated senescence of human peritoneal mesothelial cells exposed to high glucose: the role of TGF-beta1. Lab Invest. 2007;87:345–56. doi: 10.1038/labinvest.3700519. [DOI] [PubMed] [Google Scholar]
- 53.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 54.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 55.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 56.Guthrie C, Fink G. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–863. [PubMed] [Google Scholar]
- 57.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, et al. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–67. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.