Abstract
Thymidylate synthase (TSase) produces the sole intracellular de novo source of thymidine (i.e. the DNA base T) and thus is a common target for antibiotic and anticancer drugs. Mg2+ has been reported to affect TSase activity, but the mechanism of this interaction has not been investigated. Here we show that Mg2+ binds to the surface of Escherichia coli TSase and affects the kinetics of hydride transfer at the interior active site (16 Å away). Examination of the crystal structures identifies a Mg2+ near the glutamyl moiety of the folate cofactor, providing the first structural evidence for Mg2+ binding to TSase. The kinetics and NMR relaxation experiments suggest that the weak binding of Mg2+ to the protein surface stabilizes the closed conformation of the ternary enzyme complex and reduces the entropy of activation on the hydride transfer step. Mg2+ accelerates the hydride transfer by ca. 7-fold but does not affect the magnitude or temperature-dependence of the intrinsic kinetic isotope effect. These results suggest that Mg2+ facilitates the protein motions that bring the hydride donor and acceptor together, but it does not change the tunneling ready state of the hydride transfer. These findings highlight how variations in cellular Mg2+ concentration can modulate enzyme activity through long-range interactions in the protein, rather than binding at the active site. The interaction of Mg2+ with the glutamyl-tail of the folate cofactor and nonconserved residues of bacterial TSase may assist in designing antifolates with poly-glutamyl substitutes as species-specific antibiotic drugs.
Keywords: Enzyme Kinetics, Enzyme Dynamics, Hydride Transfer, Kinetic Isotope Effect, X-ray Crystallography, NMR spectroscopy, Thymidylate Synthase, Magnesium
Introduction
Metal ions are known to stabilize physiologically active conformations of biomolecules by various mechanisms in cells.1,2 The divalent magnesium cation (Mg2+) is an essential mineral nutrient for all living organisms, which is involved in many cellular functions including energy metabolism, cell growth and proliferation, etc.3 Owing to the high intracellular magnesium content (i.e. as high as 20 mM),4 cells can exploit the transport of Mg2+ between organelles to vary its local concentrations and regulate enzyme activities.3,5 Previous studies suggested that the intracellular magnesium content reaches its maximum during the S phase of the cell cycle, concomitant with DNA replication.6 In exponentially growing Escherichia coli B cells, the intracellular concentration of total Mg2+ can be higher than 100 mM.7,8
One of the enzymes crucial for DNA replication is thymidylate synthase (TSase), which is one of the most conserved enzymes in evolution.9 TSase catalyzes the reductive methylation of 2’-deoxyuridine-5’-monophosphate (dUMP) to form 2’-deoxythymidine-5’-monophosphate (dTMP), using N5,N10-methylene-5,6,7,8-tetrahydrofolate (CH2H4fol) as the cofactor (Scheme 1).10 This reaction provides the sole de novo source of thymidine (i.e. DNA base T) in the vast majority of organisms, including some bacteria and DNA viruses.11-13 TSase is over-expressed in tumor cells to facilitate the faster DNA replication.14,15 Consequently, TSase has been an attractive target for both antibiotic and anticancer drugs. Classical drugs that target TSase are structural analogues of either dUMP (e.g. 5-flurouracil) or CH2H4fol (e.g. raltitrexed and other antifolates), which often interfere with the metabolic pathways of nucleosides/nucleotides or folates, leading to toxicity and development of resistance in cells.16,17 Therefore, current drug designs focus on avoiding drug resistance and selectively targeting TSase activity in malignant tumor cells or in specific pathogenic species. Careful investigations in the catalytic mechanism of TSase can aid these drug design efforts.16,18
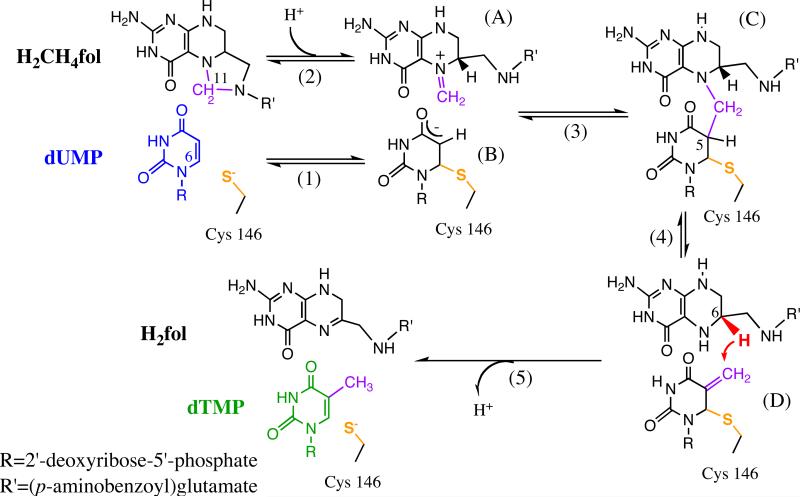
Scheme 1.
The proposed mechanism for thymidylate synthase10 (adapted from Ref 19 with copyright permission from ACS). This reaction involves a series of chemical conversions (steps 1-5) with multiple intermediates (A-D), and the hydride transfer (red, step 5) is rate limiting for the catalytic turnover.20
TSase is a homodimer with two active sites, each of which is composed of residues from both protomers, and previous experiments suggested that TSase has “half-of-the-sites” activity, i.e. only one active site is competent at a time.21-23 The large collection of structural and kinetic studies of TSase revealed that protein segments move concertedly throughout the many-step reaction,24,25 which has attracted experimental and computational examinations of protein motions that contribute to activation of chemical bonds.19,26-32 However, the complex catalytic mechanism of TSase makes it difficult to expose information on the microscopic chemical conversions (e.g. steps 1-5 in Scheme 1). We have measured the kinetic isotope effect (KIE) on the hydride transfer (step 5 in Scheme 1) in Escherichia coli TSase (ecTSase),26 which is rate limiting for the catalytic turnover in the absence of Mg2+.20 Similar to H-transfer reactions in many other wild-type enzymes,26,33-38 the hydride transfer in ecTSase exhibits a temperature-dependent rate but a temperature independent KIE. The recently-developed Marcus-like model affords an interpretation of those kinetic results, which suggests that protein motions can facilitate an enzymatic H-transfer in three aspects.37,39-45 First, conformational fluctuations of the protein pre-organize an electrostatic environment that is favorable for formation of the reactive complexes for the H-transfer step.37,46-49 Secondly, fine-tuning of the conformations of reactive complexes further reorganize the active site to accommodate structural changes in the substrates going from the reactant state to the tunneling ready state (TRS) of the H-transfer. Thirdly, the fluctuation of donor-acceptor distance (DAD) at the TRS affects the H-tunneling probability. While the DAD fluctuation at TRS determines the magnitude and temperature dependence of the intrinsic KIE, pre- and re-organization affects the rate (and the activation parameters) of the H-transfer and thus the kinetic commitment factor in experimental KIE measurements.50,51 To explore the effects of those three categories of protein motions on the hydride transfer, we recently conducted kinetic and structural analysis of a remote mutant of ecTSase, Y209W (9 Å away from the hydride transfer site). This remote mutation affected both the rate and KIE of the hydride transfer through a long range of interactions, suggesting that TSase may exploit a “network of coupled motions” to facilitate activation of this C-H bond.19
Two previous studies reported that Mg2+ variably affected TSase activities from different species,52,53 but the mechanism of those effects has never been investigated. Here we report detailed kinetic and structural analysis of how Mg2+ affects the catalytic mechanism of ecTSase and particularly the hydride transfer step. Our results suggest that, although Mg2+ binds weakly to the surface of ecTSase, this interaction stabilizes the productive conformations of both the protein and the bound folate cofactor, thereby accelerating the hydride transfer at the active site (16 Å away).
Results and Discussions
Mg2+ Affects the Entropy of Activation on kcat
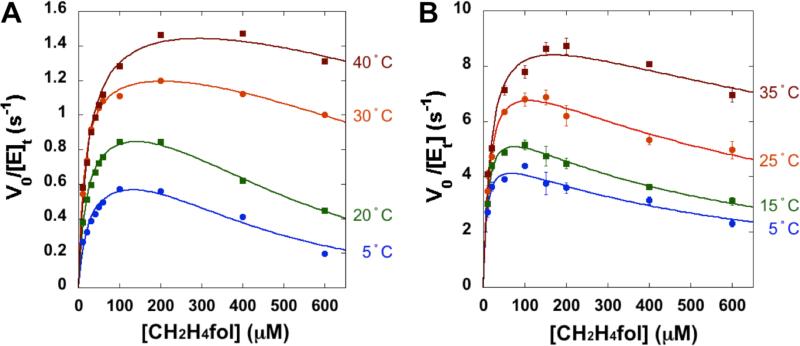
To investigate the effect of Mg2+ on ecTSase activity, we measured the steady-state initial velocities of ecTSase in the absence and presence of 50 mM MgCl2 (i.e. the same concentration used in previous studies52,53). To assess the potential effects of ionic strength on the protein activity, we conducted a control experiment that measured the initial velocities at 25 °C in the absence and presence of 50 mM CaCl2 and found no difference. The cofactor CH2H4fol exhibited substrate inhibition at high concentrations in both the absence and presence of Mg2+ (Figure 1), which is consistent with its alternate unproductive binding mode in crystal structures.54 Based on the analysis of initial velocities (details provided in the Experimental Section), the presence of Mg2+ affects the cooperativity of CH2H4fol binding in the inhibitory mode, suggesting an effect on the interaction between the protein and folate cofactor.
Figure 1.
The initial velocity of ecTSase-catalyzed reaction vs. concentration of CH2H4fol, in the (A) absence and (B) presence of 50 mM MgCl2. Figure A is adapted from Ref 26 with permission from ACS. The detailed data analysis is provided in the Experimental Section.
The analysis of initial velocities above provided the first-order rate constant, kcat, of the reaction at four different temperatures, which were fit to the Eyring equation (Eq 1) to evaluate the activation parameters:
| (1) |
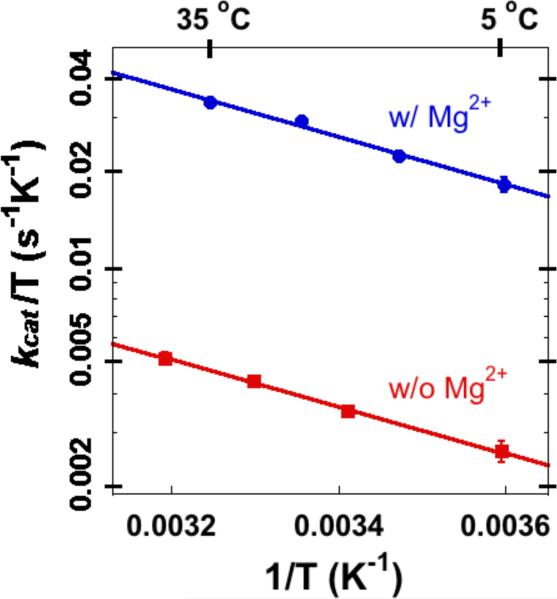
where ΔH‡ is the enthalpy of activation, ΔS‡ is the entropy of activation, T is absolute temperature, and kB, h, and R are Boltzmann constant, Planck constant, and gas constant, respectively. In the temperature range of 5-35 °C, the presence of Mg2+ increases kcat by approximately 7-fold (Figure 2). The temperature dependence of kcat is linear in both the absence and presence of Mg2+, suggesting that a single kinetic step is rate-limiting for the catalytic turnover in each case. Mg2+ reduces the activation free energy and entropy of activation (ΔG‡ and TΔS‡ at 25 °C, respectively, see Table 1) of kcat by ca. 1 kcal/mol, while the enthalpy of activation (ΔH‡) remains the same. These results suggest that Mg2+ affects the conformational fluctuations of the active site that constitute the entropic contribution of the catalysis, which is further supported by our NMR relaxation experiments below. We also found that the presence of Mg2+ increases the Michaelis constant of dUMP by ca. 5-fold at 25 °C (Table 2).
Figure 2.
Eyring plots of the initial velocity of ecTSase-catalyzed reaction in the absence (w/o, red) and presence (w/, blue) of 50 mM MgCl2. Mg2+ accelerates kcat by ca. 7-fold, and only affects the entropy of activation by ca. 1 kcal/mol (Table 1).
Table 1.
Activation parameters from fitting kcat of ecTSase to the Eyring equation in the absence (w/o) and presence (w/) of 50 mM MgCl2.a
| TSase | ΔH‡ kcal/mol | -TΔS‡ at 25 °C kcal/mol | ΔG‡ kcal/mol |
|---|---|---|---|
| w/ Mg2+ | 3.4 ± 0.2 | 12.8 ± 0.2 | 16.2 ± 0.4 |
| w/o Mg2+b | 3.4 ± 0.1 | 13.9 ± 0.1 | 17.3 ± 0.2 |
The KIE experiments suggest that the hydride transfer is rate limiting for kcat under both conditions (see text below), thus, the steady-state kinetic experiments actually exposed the activation parameters of the hydride transfer step. The values of kcat are presented in Table S1 in SI.
Data are from Ref 26.
Table 2.
Michaelis constants and dissociation constants of dUMP and CH2H4fol for ecTSase, in the absence (w/o) and presence (w/) of 50 mM MgCl2 at 25 °C.
Mg2+ Binds to ecTSase and Affects the Affinities of Both Substrates to the Protein
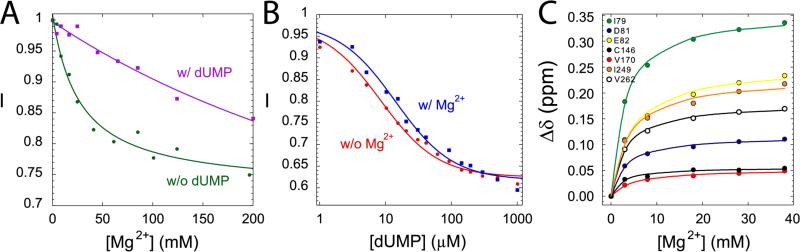
Mg2+ can affect the activity of an enzyme by either chelating with the substrate to form a more active Mg-substrate complex, or binding to the protein to alter its conformations and/or constitute the active site.5 To determine the functional role of Mg2+ in TSase activity, we measured the values of dissociation constant (Kd) that describe the interactions between Mg2+ and apo-ecTSase, between Mg2+ and the binary ecTSase-dUMP complex, and between Mg2+ and the covalent ternary complex ecTSase-(5F-dUMP)-CH2H4fol. The binary and ternary complexes resemble the intermediates B and C, respectively, in the catalyzed reaction (Scheme 1).55 The Kd values were determined to be 27 ± 7 mM for apo-ecTSase, 0.5 ± 0.2 M for ecTSase-dUMP, and 3.7 ± 1.2 mM for ecTSase-(5F-dUMP)-CH2H4fol, respectively (Figure 3, A and C). The relative magnitudes of these Kd values suggest the activation effect is most likely induced by Mg2+ binding to the ternary enzyme complex rather than the binary complex. This finding is in accordance with Mg2+ binding at the folate binding site rather than chelating with dUMP.
Figure 3.
The fluorescence intensity (I, relative to the initial intensity) of ecTSase (A) vs. MgCl2 concentration in the absence (w/o, green) and presence of 100 μM (w/, purple) dUMP; and (B) vs. dUMP concentration in the absence (w/o, red) and presence of 50 mM (w/, blue) of MgCl2. (C) Changes in backbone chemical shifts as Mg2+ is titrated into the solution of ecTSase-(5F-dUMP)-CH2H4fol complex. Different colors represent seven representative residues. The detailed data analysis is provided in the Experimental Section.
To investigate the effects of Mg2+ on the interactions between the substrates and enzyme, we measured the dissociation constants for dUMP binding to apo-ecTSase and to the ecTSase-Mg2+ complex, using the fluorescence assay (Figure 3B). We also assessed the dissociation constants for CH2H4fol binding to the ecTSase-dUMP and ecTSase-dUMP-Mg2+ complexes,56 using the equation derived by Klinman and Matthews57 (Eq 5). The presence of Mg2+ increased the value (Table 2), which is consistent with the increase of and implicates the same effects on binding and productive binding of the substrate dUMP. On the contrary, the presence of Mg2+ decreased the value (Table 2), suggesting that Mg2+ ameliorates the interaction between CH2H4fol and the enzyme. The unchanged value is probably due to the compensating effects of decrease in and increase in the hydride transfer rate constant with Mg2+ (kcat is a parameter at the nominator of the term58). The higher affinity of CH2H4fol in the presence of Mg2+ corroborates the reduction of TΔS‡ found in the steady-state kinetic experiments above, since the binding of CH2H4fol induces large conformational changes of TSase that close the active-site cavity and properly align the reactants for catalysis.24,25 Therefore, our kinetic and binding studies suggest that Mg2+ stabilizes the bound CH2H4fol and thus reduces the entropic cost for the conformational changes of the protein that lead to formation of the reactive complexes, thereby accelerating kcat.
Mg2+ Affects the Rate but not the Intrinsic KIE of Hydride Transfer
Previous studies suggested that the hydride transfer is rate limiting for kcat of ecTSase in the absence of Mg2+.20 To examine if Mg2+ changes the rate-limiting step for the catalytic turnover, we measured the intrinsic KIE as well as the KIE on kcat with 6R-xH-CH2H4fol (xH=H, D, or T) at 25 °C in the presence of 50 mM MgCl2 (Figure S2 in Supporting Information, SI). The observed KIE on kcat (Dkcat) is equal to the intrinsic KIE (Table 3), indicating that the hydride transfer is also rate-limiting for kcat in the presence of Mg2+. Therefore, the observed effects on kcat and the activation energy parameters (Figure 2 and Table 1) suggest that Mg2+ accelerates the rate of the hydride transfer and slightly reduces the entropy of activation on this step.
Table 3.
The observed H/D KIEs on kcat (Dkcat) and the intrinsic H/D KIEs (Dk) on the hydride transfer of ecTSase at 25 °C, in the absence (w/o) and presence (w/) of 50 mM MgCl2.
| 6R-xH-CH2H4fol | kcat (s-1) | (μM) | (mM) | D kcat | D k | |
|---|---|---|---|---|---|---|
| w/ Mg2+ | xH = H | 8.7 ± 0.2 | 15 ± 1 | 0.76 ± 0.07 | 3.8 ± 0.3 | 3.9 ± 0.2a |
| xH = D | 2.3 ± 0.2 | 19 ± 5 | 3 ± 1 | |||
| w/o Mg2+ | 3.7 ± 0.1b | 3.8 ± 0.3c | ||||
Determined from the competitive KIE experiment (Table S2 in SI, 25 °C).
From Ref 20.
From Ref 26.
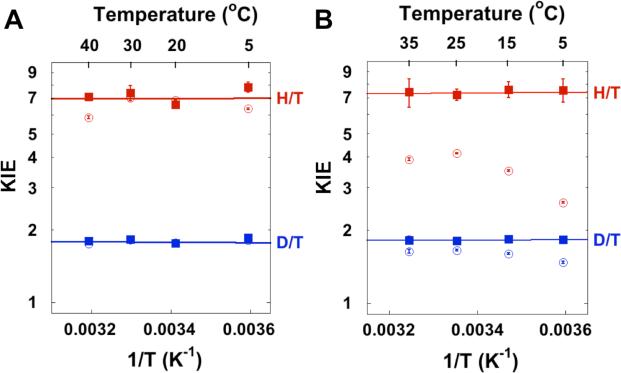
To investigate the effects of Mg2+ on the TRS of the hydride transfer, we determined the temperature dependence of the intrinsic KIE. We measured the observed KIEs on the second-order rate constant (simplified as kcat/KM hereafter),51,59 and used Northrop's method to extract the intrinsic KIEs as described before.26 In the absence of Mg2+, the observed KIEs are similar to the intrinsic values (Figure 4A), implying that the hydride transfer is also (at least partially) rate-limiting for kcat/KM.26 On the contrary, the observed KIEs are smaller than the intrinsic KIEs in the presence of Mg2+ (Figure 4B), indicating that the hydride transfer is no longer rate-limiting for kcat/KM. This observation agrees with the accelerated rate of hydride transfer by Mg2+ and suggests a larger kinetic commitment (Cf in Table S2 in SI) for kcat/KM.50, 51 However, the intrinsic KIE on the hydride transfer has the same value as measured in the absence of Mg2+, and remains temperature-independent (Figure 4 and Table 4). Based on the Marcus-like model, these observations suggest that Mg2+ facilitates protein motions that bring the hydride donor in CH2H4fol into proximity with its acceptor in dUMP (i.e. pre- and re-organization), but it does not alter the TRS of the hydride transfer.
Figure 4.
The observed KIEs on kcat/KM (empty symbols) and the intrinsic KIEs (filled symbols) on the hydride transfer in the (A) absence and (B) presence of 50 mM MgCl2. Figure A is adopted from Ref 26 with permission from ACS. The observed and intrinsic KIE values in the presence of Mg2+ are presented in Table S2 in SI. The lines are nonlinear regression of the intrinsic KIEs to Arrhenius equation (Eq 9).
Table 4.
Isotope effects on the Arrhenius parameters of the hydride transfer of ecTSase in the absence (w/o) and presence (w/) of 50 mM MgCl2.a
| w/o Mg2+b | w/ Mg2+ | |
|---|---|---|
| AH/AT | 6.8 ± 2.8 | 6.6 ± 1.3 |
| ΔEa H-T (kcal/mol) | 0.02 ± 0.25 | -0.06 ± 0.12 |
Structural and Dynamic Effects of Mg2+Binding to ecTSase
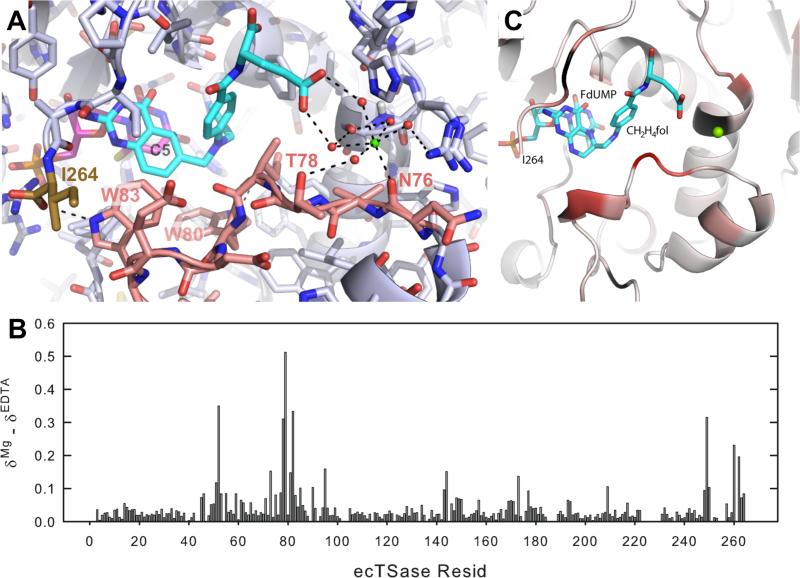
It is generally difficult to discriminate Mg2+ from a water molecule in X-ray diffraction data, due to the similarity between their electron densities. The main difference between water and Mg2+ is the tetrahedral coordination of the first and octahedral coordination of the latter. To investigate the structural origin for our observed kinetic effects, we carefully examined a number of previously solved crystal structures for putative Mg2+ ions that were octahedrally-coordinated via shorter hydrogen bonds (H-bonds) with surrounding water molecules or oxyanions of the protein. A 1.3 Å-resolution crystal structure27 of ecTSase ternary complex with dUMP and a cofactor analogue, 10-propargyl-5,8-dideazafolic acid (CB3717), shows such geometry for water 1092 at the binding cleft for the glutamyl (Glu) tail of CB3717 (Figure 5A, PDB ID: originally 2G8O and now 4IW5). The glutamate side chain of CB3717 forms H-bonds with a cluster of water molecules with octahedral “water 1092” at its base (Figure 5A). Although some magnesium might be present, the crystal of this TSase-dUMP- CB3717 complex was grown against 1.4 M sodium citrate, thus this “water 1092” is most likely a Na+ ion. As Na+ is isoelectronic to Mg2+, has the same size, and also has octahedral coordination geometry, this Na+ binding site could also accommodate Mg2+ with a similar geometry. We also determined another ternary complex of ecTSase with dUMP and a di-Glu antifolate inhibitor (BGC 945, now known as ONX 0801) that was crystalized with 0.2 M MgCl2.60 While this crystallization condition resulted in lower resolution than the one with sodium citrate (1.75 Å60 vs. 1.3 Å27), it shows unambiguous electron density for an octahedrally-hydrated Mg2+ (Figure S5, PDB ID 4ISK). The Mg2+ site in the ecTSase complex with BGC945 and the possible Mg2+ site in the ecTSase ternary complex with CB3717 are different, owing to the different stereochemistry of each inhibitor's Glu moiety; however in both cases the cation establishes hydrogen bond interactions between the Glu moiety and an electronegative protein loop, and stabilizes this interface through electrostatic interactions (Figures 5 and S5). We also used NMR chemical shift changes to map the Mg2+ binding site in the ecTSase-(5F-dUMP)-CH2H4fol complex (Figure 5, panels B and C), and found excellent agreement between the binding sites identified in the crystal structures and in solution. The protein loop that Mg2+ interacts with is involved in closing the active site upon cofactor binding.24,25 Particularly, W83 on this loop forms an H-bond with the C-terminal carboxylic group (residue I264 in ecTSase) as CH2H4fol binds to the protein, which helps to immobilize the C-terminus to seal the active site cavity. This loop also contains W80, which not only orients L143 to protect the active site from bulk solvent but also forms an H-bond with E58, while E58 coordinates the active site water molecules essential for TSase activity.25,32
Figure 5.
(A) The octahydral cation (green) binds to the surface of the ecTSase complex with dUMP (magenta) and CB3717 (cyan) (PDB ID 4IW5). All the catalyzed chemical transformations occur near C5 of dUMP (labeled in black, see Scheme 1) in the interior active site. Mg2+ mediates an H-bond network between the Glu-tail of the cofactor and an electrophilic part of a loop (residues 76-93 in ecTSase, pink) containing residues W80 and W83, which are important for closing the active site cavity (see text). The C-terminus (I264, brown) is very flexible in apo-TSase, but it is immobilized by an H-bond with W83 in the ternary complex upon the cofactor binding. (B) Difference in backbone amide chemical shift upon Mg2+ binding to the ecTSase complex with 5F-dUMP and CH2H4fol. (C) NMR Data from panel B are painted onto the crystal structure of the corresponding complex (PDB ID 1TSN) using a white to red color scheme representing zero to maximum chemical shift changes. Prolines and unassigned residues are colored black. The binding site of Mg2+ (green) suggested from panel A is indicated in this structure by aligning 1TSN with 4IW5.
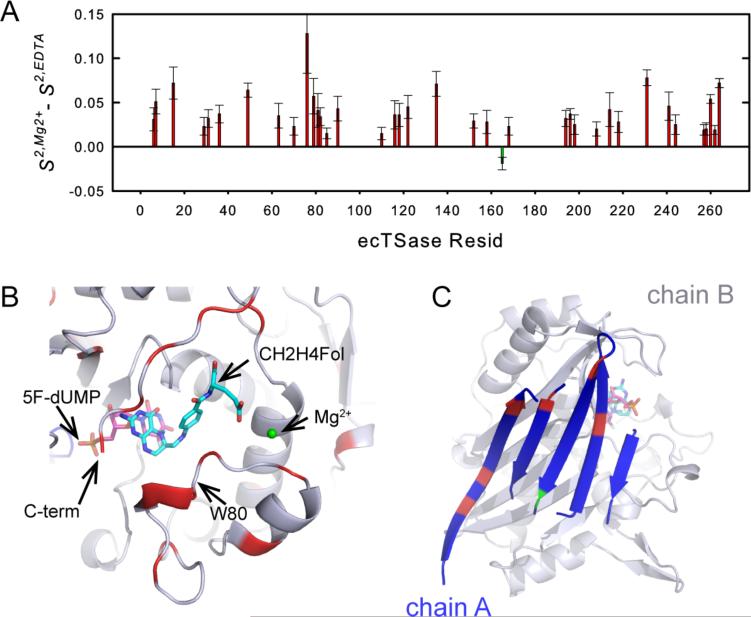
Our kinetic and binding studies above suggest that Mg2+ accelerates kcat by reducing the entropic cost for the conformational changes of the protein that accompanies progression from the ground state to TRS. To investigate the effects of Mg2+ on the protein flexibility, we conducted NMR relaxation experiments to measure the generalized order parameters (S2) for backbone 15N-1H vectors in the ecTSase-(5F-dUMP)-CH2H4fol complex in the absence and presence of Mg2+ (see Experimental Section and Figure S4). S2 represents the rigidity of the structure, which ranges from zero to one indicating complete disorder to fixed bond orientation on the ps-ns timescale.61,62 Importantly, S2 has been shown to be an excellent proxy for conformational entropy in a number of systems.63-68 In our experiments, 37 bond vectors exhibited significant (greater than 2σ) differences in S2 upon Mg2+ binding, of which 36 showed elevated S2 (Figure 6A). This is consistent with a model in which Mg2+ binding lowers the entropic barrier of the hydride transfer by paying some of the conformational entropy penalty in the ground state. Although we cannot measure S2 for the TRS to evaluate differences in the protein flexibility in that state, our KIE experiments above suggest that Mg2+ does not affect the TRS (Figure 4). In addition, Mg2+ binding rigidifies regions that stabilize the closed conformation of the enzyme (e.g. C-terminus and the loop containing W80 and W83, Figure 6, A and B), which corroborates the indications from the X-ray crystallographic data above. Lastly, it is notable that dynamic effects extend beyond the metal binding region, as distal bond vectors, including some at the dimer interface (I29, G31, F36, F152, K158, Q165, and D198), show significant changes (Figure 6C).
Figure 6.
(A) Mg2+ binding rigidifies the ecTSase-(5F-dUMP)-CH2H4fol complex. Significant (greater than 2s) differences in 15N-1H order parameters (ΔS2 = S2, Mg2+-S2, EDTA, see Experimental Section) are shown, where positive ΔS2 indicates rigidification in the Mg2+-bound complex on the ps-ns timescale. S2 data for the two individual states are shown in Figure S4. (B) Residues that become more rigid upon Mg2+ binding are highlighted in red on a ternary complex structure. Residues with no significant change are colored grey, and the single bond vector that becomes more flexible is not visible in this view. The suggested binding site for Mg2+ is shown in green. (C) Mg2+ binding also affects dynamics at the dimer interface. Significant changes in S2 are highlighted on the 5-stranded ß-sheet of one subunit (chain A) that comprises the dimer interface. Rigidified residues are colored in red and Q165 (which becomes more flexible) is colored in green.
Taken together, our kinetic, structural, and NMR data suggest that the weak binding of Mg2+ to the surface of TSase stabilizes the closed conformations of the ternary enzyme complex, which not only enhances the affinity of CH2H4fol but also increases the fraction of reactive complexes for the chemical reactions after CH2H4fol binding. A similar relay between the protein surface and active site has been observed for the TSase domain of the bifunctional TSase-dihydrofolate reductase enzyme in Cryptosporidium hominis. In that case, two nonconserved residues that bind the Glu-tail of CH2H4fol are responsible for the faster kcat of this TSase domain than other TSase enzymes.69 Mutations on those two residues affected the positioning and flexibility of the cofactor, and this effect propagated into the active site and reduced kcat of the TSase domain.70
The binding site of Mg2+ revealed by the current study agrees with previous observations that Mg2+ modulated the selective inhibition of poly-Glu antifolates towards bacterial and viral TSase activities.52 The binding region for the poly-Glu moiety is not conserved among different species,71 and is also quite open to solvent, which would allow Mg2+ to bind and modulate H-bonds at several locations between the poly-Glu tail and the protein loop (Figure 5). The residues in the loop of ecTSase whose side chains contribute to the Mg2+-mediated H-bond network include T78 in both ternary complexes and E82 in the di-Glu inhibitor complex. The corresponding residues in human TSase are lysine and alanine, respectively. These differences may preclude formation of analogous Mg2+-mediated H-bond network between human TSase and the cofactor, explaining the lack of sensitivity of human TSase activity to Mg2+. Future studies can exploit our current findings and design species-specific TSase inhibitors, such as antifolates with a poly-Glu substitute that would create a perfect binding site for Mg2+ in a specific TSase enzyme, which may lead to species-specific drugs that target DNA biosynthesis.
Conclusions
The role of protein motions in the chemical reactions catalyzed by enzymes has been subject of great contemporary interest. Experimental and computational studies of dihydrofolate reductase, for example, have suggested a “network of coupled motions” to rationalize long-range structural and dynamic effects of remote residues on the catalyzed reaction.43,72-75 It is interesting to examine this concept in more complex enzymatic systems, such as TSase. TSase exploits progressive conformational changes to assist the binding and orientation of ligands, and to accommodate structural changes in the ligands during the catalyzed chemical transformations.24,25 Particularly, our recent study of Y209W ecTSase demonstrated that protein motions at various time scales can affect different parameters of the catalyzed hydride transfer step.19 The current study has explored how Mg2+ weakly bound at the protein surface affects the kinetics of wild type ecTSase through long-range interactions throughout the enzyme. Our crystal structures suggest a binding site for Mg2+ near the Glu-moiety of the cofactor, which stabilizes the closed conformations of the ternary enzyme complex of ecTSase. Our kinetic findings suggest that the binding of Mg2+ reduces the entropic cost for protein conformational changes that lead to formation of the reactive complexes, and thereby accelerates the rate of hydride transfer. NMR derived order parameters, which are a proxy for conformational entropy, support this hypothesis. While the hydride transfer is faster and is no longer rate limiting in the presence of Mg2+, the intrinsic KIE and its temperature dependence are unchanged, suggesting that Mg2+ binding does not affect the TRS of the hydride transfer. Since TSase is crucial for DNA replication, these results agree with previously established positive correlation between the intracellular magnesium content and proliferating rate of Escherichia coli cells.7,8 Future studies will continue to incorporate structural and kinetic experiments to better define the Mg2+ binding site and specificity and clarify its effect on TSase catalysis. The possible effect of other biologically relevant divalent metals will also be explored. Most importantly, comparison studies between the human and bacterial TSases may reveal the origin of their different sensitivity to Mg2+ in the kinetics. Understanding those differences can assist designing species-specific antibiotic drugs by exploiting the interaction between Mg2+ and nonconserved residues of bacterial TSases.
Experimental Section
Materials and Instruments
[2-14C] dUMP (specific radioactivity 53 Ci/mol) was purchased from Moravek Biochemicals. [6R-xH]CH2H4folate (xH = D or T) was synthesized according to our published procedure,19 and unlabeled CH2H4folate (xH = H) was a generous gift from EPROVA (Switzerland). Ultima Gold liquid scintillation cocktail reagent was purchased from Packard Bioscience. Liquid scintillation vials were purchased from Research Products International Corp. The ecTSase enzymes were expressed and purified according to the established procedures.76 All other materials were purchased from Sigma. The steady-state kinetic experiments were conducted using a Hewlett-Packard Model 8452A diode-array spectrophotometer equipped with a temperature-controlled cuvette assembly. For the competitive KIE experiments, the radioactive materials were separated with an Agilent Technologies model 1100 HPLC system with a Supelco Discovery® C18 reverse phase column, and analyzed with a Liquid Scintillation Counter (LSC). Figures 5B and 6A were generated with SigmaPlot 10.0, Figures 5A, 5B, 6B and 6C were generated with Pymol v1.5.0.477 and all other figures with KaleidaGraph (Version 4.03).
Experiments and Data Analysis
Steady-State Kinetic Experiments
Our previous experiments measured the steady-state initial velocities of ecTSase in the absence of Mg2+ by monitoring the increase of absorbance at 340 nm that indicates conversion of CH2H4fol to dihydrofolate (Δε340nm = 6.4 mM-1cm-1).26 In the current study, we conducted the same experiments in the presence of 50 mM MgCl2. Addition of ecTSase to a final concentration of 20 nM (concentration in terms of dimer, same below) initiated the reaction. Values of kcat and Km of CH2H4fol were measured with 100 μM dUMP at 5, 15, 25, and 35 °C, and the Km value of dUMP was measured with 150 μM CH2H4fol at 25 °C. To check the potential effects of ionic strength on the activity of TSase, we also conducted the control experiment at 25 °C, in which MgCl2 was replaced by 50 mM CaCl2, and found no effects on the initial velocities.
Analysis of Steady-State Initial Velocities
Analysis of the initial velocities at each temperature employed the least-squares nonlinear regression available in KaleidaGraph (Version 4.03). The initial velocities of ecTSase vs. concentration of dUMP can be fit with the standard Michaelis-Menten equation. In contrast, high concentrations of CH2H4fol inhibit the activity of ecTSase, and the data were analyzed by the general equation for uncompetitive substrate inhibition (Eq 2), which incorporates Hill coefficients to account for the potential cooperativity in substrate binding:78
| (2) |
where [E]t is the total enzyme concentration; [S] is the concentration of the substrate (e.g. CH2H4fol in this case); kcat(i) accounts for the activity when the substrate binds in the inhibitory mode (if products can still form when the substrate binds in the inhibitory mode); KM and KI are the Michaelis constant and inhibition constant of the substrate, respectively; n and x are Hill coefficients for the cooperativity of substrate binding in the productive and inhibitory mode, respectively. Previous crystallographic studies suggested that the alternate binding mode of CH2H4fol is unproductive due to improper orientation for the catalyzed reaction, i.e. kcat(i) = 0.54 Analysis of the initial velocities using Eq 2 with kcat(i) = 0 shows that the integer value of n = 1 provides the best fit in both the absence and presence of Mg2+. This result agrees with the previously suggested “half-of-the-sites” activity for ecTSase, i.e. one competent active site per dimer with no cooperativity in the productive binding mode (n = 1).21-23 To reduce errors on the kinetic parameters in the statistical analysis, we fixed n =1 in the following data analysis, which simplifies Eq 2 to Eq 3:
| (3) |
Analysis of the initial velocities using Eq 3 shows that the integer value of x =2 provides the best fit in the absence of Mg2+, while x =1 is optimal in the presence of Mg2+. These results imply that Mg2+ alters the interaction between the protein and CH2H4fol, which eliminates the cooperativity of CH2H4fol binding in the inhibitory mode. Further analysis on the kinetic parameters used x=2 for the initial velocities in the absence of Mg2+, and x=1 in the presence of Mg2+, and the results are presented in Figure 1 and Table S1.
Equilibrium Dissociation Constants
Fluorescence Assay Equilibrium dissociation constants of the enzyme complexes were measured by titrating Mg2+ or dUMP into a solution of ecTSase, following the previously established fluorescence assay.20 The excitation wavelength was 290nm, and the maximum emission of fluorescence was at 334 nm. Each experiment contained at least a duplicate of datasets. To correct for fluorescence changes that are not associated with the protein, we conducted parallel titrations in which ecTSase was replaced by a quantity of tryptophan with the same initial fluorescence intensity. We also conducted control experiments in which dUMP was titrated into the solution that contained all the reagents except the protein/tryptophan in the absence and presence of Mg2+. The control experiments did not produce detectable changes in fluorescence signals, suggesting that the interactions between the ligands (if exist) do not affect the fluorescence intensity measured for the protein.
Binding of Mg2+ We measured the dissociation constants that describe the interactions between Mg2+ and ecTSase apoenzyme, and between Mg2+ and the binary ecTSase-dUMP complex. The initial solution contained 25 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraacetate (EDTA), and 0.8 μM ecTSase pre-incubated with 0 or 100 μM dUMP at 25 °C in 100 mM tris(hydroxymethyl)aminomethane (Tris)/HCl buffer (pH 7.5). Titrating a concentrated solution of MgCl2 into the initial solution caused a decrease in protein fluorescence (after correcting for the dilution factor), which indicates protein conformational changes induced by Mg2+ binding. Although Mg2+ can possibly bind into multiple sites of TSase, we fit the data to the simplest model (i.e. one-site binding, Eq 4) to compare the affinity of this ion for the apoenzyme and the binary complex:
| (4) |
where I is the observed fluorescence intensity; IE and IEL are the molar fluorescence intensities for the apoenzyme and binary complex, respectively; [L] is the ligand concentration; and Kd is the dissociation constant for L.
Binding of dUMP We also measured the dissociation constants that describe the interactions between dUMP and apo-ecTSase, and between dUMP and the binary ecTSase-Mg2+ complex. The initial solution contained 25 mM DTT, 1 mM EDTA, and 0.8 μM ecTSase pre-incubated with 0 or 50 mM MgCl2 in 100 mM Tris/HCl buffer (pH 7.5). Titrating a concentrated solution of dUMP into the initial solution caused a decrease in protein fluorescence due to dUMP binding. Previous studies suggested dUMP only binds to one active site per ecTSase dimer in the absence of CH2H4fol.20 Thus, we fit the data to the one-site binding model (Eq 4) to evaluate the dissociation constants of dUMP. Binding of CH2H4fol Due to non-specific quenching of fluorescence by formaldehyde (HCHO), which is used to stabilize CH2H4fol in solution, we cannot directly measure the dissociation constant of CH2H4fol by the fluorescence assay. Instead, we used the equation derived by Klinman and Matthews57 (Eq 5) to calculate the dissociation constants of CH2H4fol:
| (5) |
where KM is obtained from the steady state kinetic experiments (Table S1); Dkcat is H/D KIE measured on kcat by the noncompetitive KIE experiment (Table 3); and D(kcat/KM)H is the H/D KIE on kcat / KM, which is calculated from the intrinsic H/D KIE and kinetic commitment factor on the hydride transfer (Table S2).
NMR Titration Experiments We used NMR spectroscopy to measure the Kd for Mg2+ binding to the ecTSase-(5F-dUMP)-CH2H4fol complex. Resonance assignments for the metal-free complex are deposited in the BMRB (accession 19082), and are described elsewhere.79 MgCl2 was titrated into the ternary complex and the chemical shift changes of backbone amide groups were monitored by TROSY 1H-15N HSQC spectra. It is important to note that only one set of resonances is observed in HSQC spectra, which indicates symmetric binding of 5F-dUMP and CH2H4fol to both active sites of the homodimer. The initial solution contained 0.5 mM ecTSase-FdUMP-CH2H4fol, 25 mM HEPES, 100 mM NaCl, 10 mM TCEP-HCl, 0.01% NaN3, pH 7.5. Chemical shift changes in both 1H and 15N were taken into account based on Eq 6:
| (6) |
A Kd value for each resolved resonance showing a change in chemical shift upon Mg2+ addition was obtained by fitting Δδ to Eq 7:
| (7) |
where [LT] and [ET] are the total Mg2+ and ecTSase concentrations, respectively. The reported Kd of 3.7 ± 1.2 mM represents the mean and standard deviation of the fits from 29 separate amide resonances.
Noncompetitive KIE Experiments
Spencer et al have measured the KIEs on kcat at 25 °C using the noncompetitive experiments in the absence of Mg2+.20 We conducted the experiments under the same conditions in the presence of 50 mM MgCl2, with 100 μM dUMP and various concentrations of 6R-xH-CH2H4fol (xH = H or D) in 100 mM Tris/HCl buffer (pH 7.5). Addition of ecTSase to the final concentration of 20 or 80 nM initiated the reaction for 6R-H-CH2H4fol or 6R-D-CH2H4fol, respectively.
Competitive KIE Experiments
Our previous study used the competitive method to measure the KIEs on kcat/KM and the temperature dependence of intrinsic KIE on the hydride transfer step in the absence of Mg2+.26 In the current study, we conducted the experiments under the same conditions in the presence of 50 mM MgCl2. Following the previously published procedure,26 we used the modified Northrop method to extract the intrinsic KIE from the observed H/T and D/T KIEs on kcat / KM (Eq 8).45,50,51
| (8) |
where T(kcat / KM)H and T(kcat / KM)D are the observed H/T and D/T KIEs on kcat / KM, respectively; and Tk is the intrinsic H/T KIE on the hydride transfer (Tk = kH/kT). We have developed an online program, as well as a Mathematica script, to solve Eq 8 numerically for Tk at each temperature (http://ccs14.chem.uiowa.edu/faculty/kohen/group/tools.html). Fitting the intrinsic KIE values to the Arrhenius equation (Eq 9) allows analysis of their temperature dependence.45
| (9) |
where the subscripts L and T denote the light (H or D) and heavy (T) isotopes of hydrogen, respectively; ΔEa is the difference in the activation energies of the hydride transfer between the light and heavy isotopes. Since the hydride transfer is irreversible, the comparison between the observed and intrinsic KIEs provides the forward kinetic commitment (Cf) on kcat / KM (Eq 10).50,51 Table S2 summarizes the observed and intrinsic KIEs, as well as Cf, at the four experimental temperatures in the presence of Mg2+.
| (10) |
Similarly, for H/D KIEs,
| (11) |
Eq 11 was used to calculate the H/D KIE on kcat /KM at 25 °C from Dk and Cf (Table S2).
Identifying Mg2+ in the Crystal Structures
Divalent Mg2+ ion has an identical number of electrons (10) as water and thus is difficult to distinguish in electron-density maps, particularly if the position is partially occupied or the maps are obtained at low resolution. We examined the water molecules in a 1.3 Å-resolution crystal structure of the ternary ecTSase-dUMP-CB3717 complex (PDB ID: 2G8O) for putative Mg2+ ions with an approximate octahedral arrangement of hydrogen bonding (H-bonding) partners. A few candidate water molecules were on the periphery of the protein outside the binding region for the ligands, while the only candidate water (i.e. putative Mg2+) that can potentially affect catalysis was in the binding groove for the Glu-moiety of the cofactor (Figure 5A, new PDB ID: 4IW5). This putative Mg2+ formed H-bonds with five other water molecules and with the backbone carbonyl of N76. Interatomic distances of five of the H-bonds were 2.4 Å, and the sixth was 2.7 Å, which are shorter than the average H-bond length between water molecules. Two of the coordinated water molecules formed H-bonds with the carboxyl oxygen of the Glu of the cofactor, while four of them (including one water that was also H-bonded with the cofactor Glu) formed H-bonds with the protein backbone carbonyl or side chains (Figure 5A). This water cluster was only seen in the more highly-resolved protomer (i.e. with lower isotropic B-factors) of the ecTSase dimer. Since this crystal was grown against 1.4 M sodium citrate, this putative Mg2+ is most likely a Na+ ion. In contrast, the electron density maps for both protomers of the ecTSase ternary complex (PDB ID 4ISK)with dUMP and a di-Glu antifolate inhibitor (BGC945) has strong, unambiguous density for Mg2+ that is octahedrally coordinated with six water molecules in the poly-Glu binding sites (Figure S5).60 This density can be fit with the ideal geometry for an octahedrally coordinated Mg2+, where the distances between Mg2+ and coordinated water molecules are approximately 2.1 Å.
NMR Spin Relaxation79
Backbone dynamics were assessed using TROSY versions of 15N R1, R1ρ, and {1H}-15N heteronuclear NOE pulse sequences.80 Data were collected at 500 and 600 MHz on Bruker Avance III spectrometers equipped with TCI cryogenic probeheads. For the metal free complex, the conditions were 0.5 mM ecTSase-FdUMP-CH2H4fol, 25 mM NaPO4, 150 mM NaCl, 1 mM EDTA, 10 mM DTT, pH 7.5, 298 K. For the Mg2+-bound complex, the conditions were 0.5 mM ecTSase-FdUMP-CH2H4fol, 25 mM HEPES, 50 mM NaCl, 40 mM MgCl2, 10 mM TCEP-HCl, pH 7.5, 298 K. HEPES was used in the presence of Mg2+ due to the low solubility product of magnesium phosphate. In the absence of metal, HSQC spectra were identical in HEPES vs. phosphate and DTT vs. TCEP-HCl. 15N R1 data were collected with the fids interleaved using relaxation delays of 0, 400, 800, 1600, 2400, and 3200 ms (the underlined time points were measured twice for error estimation). 15N R1ρ data were also collected with the fids interleaved using relaxation delays of 1, 5, 10, 20, 30, 50, and 70 ms. The strength of the spin-lock field was 1.4 kHz. R1 and R1ρ decay rates were fit to peak intensities using expfit2 (in-house written software). Pure R2 rates were obtained from R1 and R1ρ rates as described previously.80 The raw relaxation rates are plotted in Figure S3.
We used TENSOR281 in conjunction with PDB ID 1TSN to select the optimal global tumbling models. The best fit isotropic global tumbling times were 29 ns/rad and 31 ns/rad for the metal-free and metal-bound complexes, respectively. However, for both the Mg2+ -bound and metal-free cases, an axially symmetric anisotropic model was chosen based on statistical criteria. Model selection and data fitting were carried out as described before.82 Model-free order parameters are shown in Figure S4.79.
Supplementary Material
Acknowledgement
This work was supported by NIH GM065368 (AK), NIH GM083059 (ALL), NIH GM51232 (RMS). ZW was also supported by a fellowship from the University of Iowa Center for Biocatalysis and Bioprocessing, and by activities of the Predoctoral Training program in Biotechnology, NIH grant 2T32GM008365.
Footnotes
Supporting Information Available. Steady state initial velocities vs. dUMP concentrations; initial velocities vs. concentration of 6R-xH-CH2H4fol (xH = H or D); steady state kinetic parameters; the KIE and Cf values at different temperatures; raw 15N R1, R2, and {1H}-15N heteronuclear NOE spin relaxation data; and NMR order parameters; Table with Data collection and refinement statistics and a figure that shows the binding site of Mg2+ in the ecTSase complex with BGC945. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Dokmanic I, Sikic M, Tomic S. Acta Crystallogr. D. 2008;64:257–263. doi: 10.1107/S090744490706595X. [DOI] [PubMed] [Google Scholar]
- 2.Harding MM, Nowicki MW, Walkinshaw MD. Crystallogr. Rev. 2010;16:247–302. [Google Scholar]
- 3.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Clin. Chim. Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 4.Romani AM, Scarpa A. Front Biosci. 2000;5:D720–D734. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- 5.Cowan JA. Biometals. 2002;15:225–235. doi: 10.1023/a:1016022730880. [DOI] [PubMed] [Google Scholar]
- 6.Wolf FI, Fasanella S, Tedesco B, Torsello A, Sgambato A, Faraglia B, Palozza P, Boninsegna A, Cittadini A. Front Biosci. 2004;9:2056–2062. doi: 10.2741/1389. [DOI] [PubMed] [Google Scholar]
- 7.Moncany ML, Kellenberger E. Experientia. 1981;37:846–847. doi: 10.1007/BF01985672. [DOI] [PubMed] [Google Scholar]
- 8.Chang CF, Shuman H, Somlyo AP. J. Bacteriol. 1986;167:935–939. doi: 10.1128/jb.167.3.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry KM, Fauman EB, Finer-Moore JS, Montfort WR, Maley GF, Maley F, Stroud RM. Proteins. 1990;8:315–333. doi: 10.1002/prot.340080406. [DOI] [PubMed] [Google Scholar]
- 10.Carreras CW, Santi DV. Annu. Rev. Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 11.Pozzi C, Ferrari S, Cortesi D, Luciani R, Stroud RM, Catalano A, Costi MP, Mangani S. Acta Crystallogr. D. 2012;68:1232–1241. doi: 10.1107/S0907444912026236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen CJ, Liu YJ, Lu CP. J. Virol. 2012;86:10900. doi: 10.1128/JVI.01882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colson P, Gimenez G, Boyer M, Fournous G, Raoult D. PLoS ONE. 2011;6:e18935. doi: 10.1371/journal.pone.0018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L. Cancer Res. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 15.Chen C-Y, Chang Y-L, Shih J-Y, Lin J-W, Chen K-Y, Yang C-H, Yu C-J, Yang P-C. Lung Cancer. 2011;74:132–138. doi: 10.1016/j.lungcan.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Costi MP. Med. Res. Rev. 1998;18:21–42. doi: 10.1002/(sici)1098-1128(199801)18:1<21::aid-med2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Longley DB, Johnston PG. In: Apoptosis, Cell Signaling, and Human Diseases: Molecular Mechanisms. Srivastava R, editor. Vol. 1. Humana Press; 2007. pp. 263–278. [Google Scholar]
- 18.Costi MP, Tondi D, Rinaldi M, Barlocco D, Pecorari P, Soragni F, Venturelli A, Stroud RM. Biochim. Biophys. Acta. 2002;1587:206–214. doi: 10.1016/s0925-4439(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Abeysinghe T, Finer-Moore JS, Stroud RM, Kohen A. J. Am. Chem. Soc. 2012;134:17722–17730. doi: 10.1021/ja307859m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer HT, Villafranca JE, Appleman JR. Biochemistry. 1997;36:4212–4222. doi: 10.1021/bi961794q. [DOI] [PubMed] [Google Scholar]
- 21.Maley F, Pedersenlane J, Changchien LM. Biochemistry. 1995;34:1469–1474. doi: 10.1021/bi00005a001. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AC, O'Neil RH, DeLano WL, Stroud RM. Biochemistry. 1999;38:13829–13836. doi: 10.1021/bi991610i. [DOI] [PubMed] [Google Scholar]
- 23.Saxl RL, Changchien LM, Hardy LW, Maley F. Biochemistry. 2001;40:5275–5282. doi: 10.1021/bi002925x. [DOI] [PubMed] [Google Scholar]
- 24.Stroud RM, Finer-Moore JS. Biochemistry. 2003;42:239–247. doi: 10.1021/bi020598i. [DOI] [PubMed] [Google Scholar]
- 25.Finer-Moore JS, Santi DV, Stroud RM. Biochemistry. 2003;42:248–256. doi: 10.1021/bi020599a. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal N, Hong B, Mihai C, Kohen A. Biochemistry. 2004;43:1998–2006. doi: 10.1021/bi036124g. [DOI] [PubMed] [Google Scholar]
- 27.Newby Z, Lee TT, Morse RJ, Liu Y, Liu L, Venkatraman P, Santi DV, Finer-Moore JS, Stroud RM. Biochemistry. 2006;45:7415–7428. doi: 10.1021/bi060152s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Kohen A. J. Am. Chem. Soc. 2010;132:9820–9825. doi: 10.1021/ja103010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanaan N, Roca M, Tunon I, Marti S, Moliner V. J. Phys. Chem. B. 2010;114:13593–13600. doi: 10.1021/jp1072457. [DOI] [PubMed] [Google Scholar]
- 30.Kanaan N, Roca M, Tunon I, Marti S, Moliner V. Phys. Chem. Chem. Phys. 2010;12:11657–11664. doi: 10.1039/c003799k. [DOI] [PubMed] [Google Scholar]
- 31.Kanaan N, Ferrer S, Marti S, Garcia-Viloca M, Kohen A, Moliner V. J. Am. Chem. Soc. 2011;133:6692–6702. doi: 10.1021/ja1114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Ferrer S, Moliner V, Kohen A. Biochemistry. 2013 doi: 10.1021/bi400267q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandaria JN, Cheatum CM, Kohen A. J. Am. Chem. Soc. 2009;131:10151–10155. doi: 10.1021/ja902120t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohen A, Cannio R, Bartolucci S, Klinman JP. Nature. 1999;399:496–499. doi: 10.1038/20981. [DOI] [PubMed] [Google Scholar]
- 35.Sikorski RS, Wang L, Markham KA, Rajagopalan PT, Benkovic SJ, Kohen A. J. Am. Chem. Soc. 2004;126:4778–4779. doi: 10.1021/ja031683w. [DOI] [PubMed] [Google Scholar]
- 36.Fan F, Gadda G. J. Am. Chem. Soc. 2005;127:17954–61. doi: 10.1021/ja0560377. [DOI] [PubMed] [Google Scholar]
- 37.Nagel ZD, Klinman JP. Chem. Rev. 2010;110:PR41–PR67. doi: 10.1021/cr1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pudney CR, Johannissen LO, Sutcliffe MJ, Hay S, Scrutton NS. J. Am. Chem. Soc. 2010;132:11329–11335. doi: 10.1021/ja1048048. [DOI] [PubMed] [Google Scholar]
- 39.Borgis DC, Lee SY, Hynes JT. Chem. Phys. Lett. 1989;162:19–26. [Google Scholar]
- 40.Kuznetsov AM, Ulstrup J. Can. J. Chem. 1999;77:1085–1096. [Google Scholar]
- 41.Marcus RA. J. Chem. Phys. 2006;125:194504. doi: 10.1063/1.2372496. [DOI] [PubMed] [Google Scholar]
- 42.Marcus RA. J. Phys. Chem. B. 2007;111:6643–6654. doi: 10.1021/jp071589s. [DOI] [PubMed] [Google Scholar]
- 43.Hammes GG, Benkovic SJ, Hammes-Schiffer S. Biochemistry. 2011;50:10422–10430. doi: 10.1021/bi201486f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hay S, Scrutton NS. Nat. Chem. 2012;4:161–168. doi: 10.1038/nchem.1223. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Roston D, Kohen A. Adv. Protein Chem. Struct. Biol. 2012;87:155–180. doi: 10.1016/B978-0-12-398312-1.00006-8. [DOI] [PubMed] [Google Scholar]
- 46.Warshel A. J. Biol. Chem. 1998;273:27035–27038. doi: 10.1074/jbc.273.42.27035. [DOI] [PubMed] [Google Scholar]
- 47.Cannon WR, Benkovic S. J. J. Biol. Chem. 1998;273:26257–60. doi: 10.1074/jbc.273.41.26257. [DOI] [PubMed] [Google Scholar]
- 48.Rajagopalan PT, Benkovic SJ. Chem. Rec. 2002;2:24–36. doi: 10.1002/tcr.10009. [DOI] [PubMed] [Google Scholar]
- 49.Hammes-Schiffer S, Benkovic SJ. Annu. Rev. Biochem. 2006;75:519–541. doi: 10.1146/annurev.biochem.75.103004.142800. [DOI] [PubMed] [Google Scholar]
- 50.Northrop DB. Biochemistry. 1975;14:2644–2651. doi: 10.1021/bi00683a013. [DOI] [PubMed] [Google Scholar]
- 51.Cook PF, Cleland WW. Enzyme Kinetics and Mechanism. Garland Science; London ; New York: 2007. pp. 253–324. [Google Scholar]
- 52.Maley GF, Maley F, Baugh CM. J. Biol. Chem. 1979;254:7485–7. [PubMed] [Google Scholar]
- 53.Rode W, Jastreboff MM. Mol. Cell Biochem. 1984;60:73–76. doi: 10.1007/BF00226300. [DOI] [PubMed] [Google Scholar]
- 54.Birdsall DL, Finer-Moore J, Stroud RM. J. Mol. Biol. 1996;255:522–535. doi: 10.1006/jmbi.1996.0043. [DOI] [PubMed] [Google Scholar]
- 55.Hyatt DC, Maley F, Montfort WR. Biochemistry. 1997;36:4585–4594. doi: 10.1021/bi962936j. [DOI] [PubMed] [Google Scholar]
- 56.Since the bound dUMP in the active site elaborates a large hydrophobic surface to accommodate cofactor binding, ecTSase binds dUMP and CH2H4fol sequentially (Ref 9,10).
- 57.Klinman JP, Matthews RG. J. Am. Chem. Soc. 1985;107:1058–1060. [Google Scholar]
- 58.Cook PF, Cleland WW. Enzyme Kinetics and Mechanism. Garland Science; London ; New York: 2007. pp. 35–58. [Google Scholar]
- 59.Kohen A, Roston D, Stojković V, Wang Z. In: Encyclopedia of Analytical Chemistry. Vol. S1-S3. Meyers RA, editor. John Wiley & Sons, Ltd; Chichester, UK: 2011. pp. 77–99. [Google Scholar]
- 60.Tochowicz A, Dalziel S, Eidam O, O'Connell JD, 3rd, Griner S, Finer-Moore JS, Stroud RM. J Med. Chem. 2013 doi: 10.1021/jm400490e. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipari G, Szabo A. J. Am. Chem. Soc. 1982;104:4559–4570. [Google Scholar]
- 62.Lipari G, Szabo A. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]
- 63.Popovych N, Sun S, Ebright RH, Kalodimos CG. Nat. Struct. Mol. Biol. 2006;13:831–838. doi: 10.1038/nsmb1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frederick KK, Marlow MS, Valentine KG, Wand AJ. Nature. 2007;448:325–329. doi: 10.1038/nature05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marlow MS, Dogan J, Frederick KK, Valentine KG, Wand AJ. Nat. Chem. Biol. 2010;6:352–358. doi: 10.1038/nchembio.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzeng SR, Kalodimos CG. Nature. 2012;488:236–240. doi: 10.1038/nature11271. [DOI] [PubMed] [Google Scholar]
- 67.Wand AJ. Curr. Opin. Struct. Biol. 2012 In Press http://www.ncbi.nlm.nih.gov/pubmed/23246280.
- 68.Petit CM, Zhang J, Sapienza PJ, Fuentes EJ, Lee AL. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18249–18254. doi: 10.1073/pnas.0904492106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doan LT, Martucci WE, Vargo MA, Atreya CE, Anderson KS. Biochemistry. 2007;46:8379–8391. doi: 10.1021/bi700531r. [DOI] [PubMed] [Google Scholar]
- 70.Martucci WE, Vargo MA, Anderson KS. Biochemistry. 2008;47:8902–8911. doi: 10.1021/bi800466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamb A, Finer-Moore J, Calvert AH, Stroud RM. Biochemistry. 1992;31:9883–9890. doi: 10.1021/bi00156a005. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2794–2799. doi: 10.1073/pnas.052005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong KF, Selzer T, Benkovic SJ, Hammes-Schiffer S. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6807–6812. doi: 10.1073/pnas.0408343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nashine VC, Hammes-Schiffer S, Benkovic SJ. Curr. Opin. Chem. Biol. 2010;14:644–651. doi: 10.1016/j.cbpa.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Goodey NM, Benkovic SJ, Kohen A. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15753–15758. doi: 10.1073/pnas.0606976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Changchien L-M, Garibian A, Frasca V, Lobo A, Maley GF, Maley F. Prot. Expres. Pur. 2000;19:265–270. doi: 10.1006/prep.2000.1245. [DOI] [PubMed] [Google Scholar]
- 77.Schrodinger . LLC; 2010. [Google Scholar]
- 78.LiCata VJ, Allewell NM. Biophys. Chem. 1997;64:225–234. doi: 10.1016/s0301-4622(96)02204-1. [DOI] [PubMed] [Google Scholar]
- 79.Sapienza PJ, Lee AL. Biomol. NMR Assigm. 2013 Submitted. [Google Scholar]
- 80.Lakomek NA, Ying J, Bax A. J Biomol. NMR. 2012;53:209–221. doi: 10.1007/s10858-012-9626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dosset P, Hus JC, Blackledge M, Marion D. J. Biomol. NMR. 2000;16:23–28. doi: 10.1023/a:1008305808620. [DOI] [PubMed] [Google Scholar]
- 82.Sapienza PJ, Mauldin RV, Lee AL. J. Mol. Biol. 2011;405:378–394. doi: 10.1016/j.jmb.2010.10.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.