Abstract
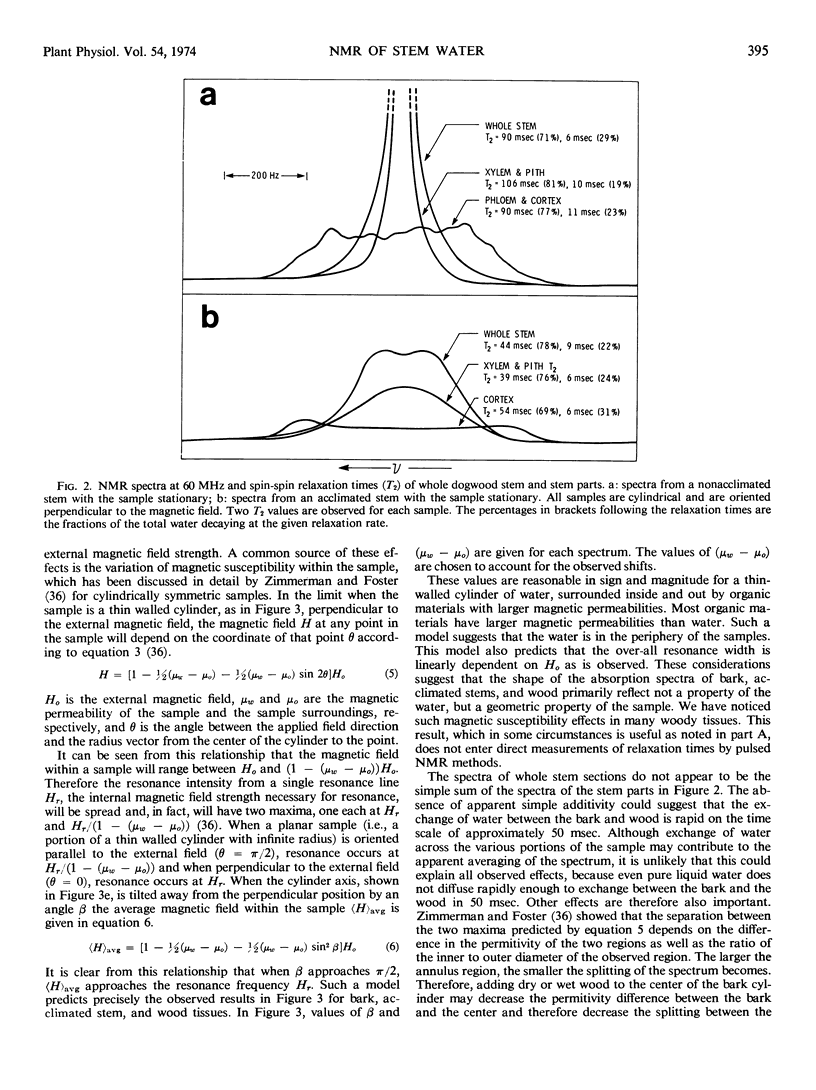
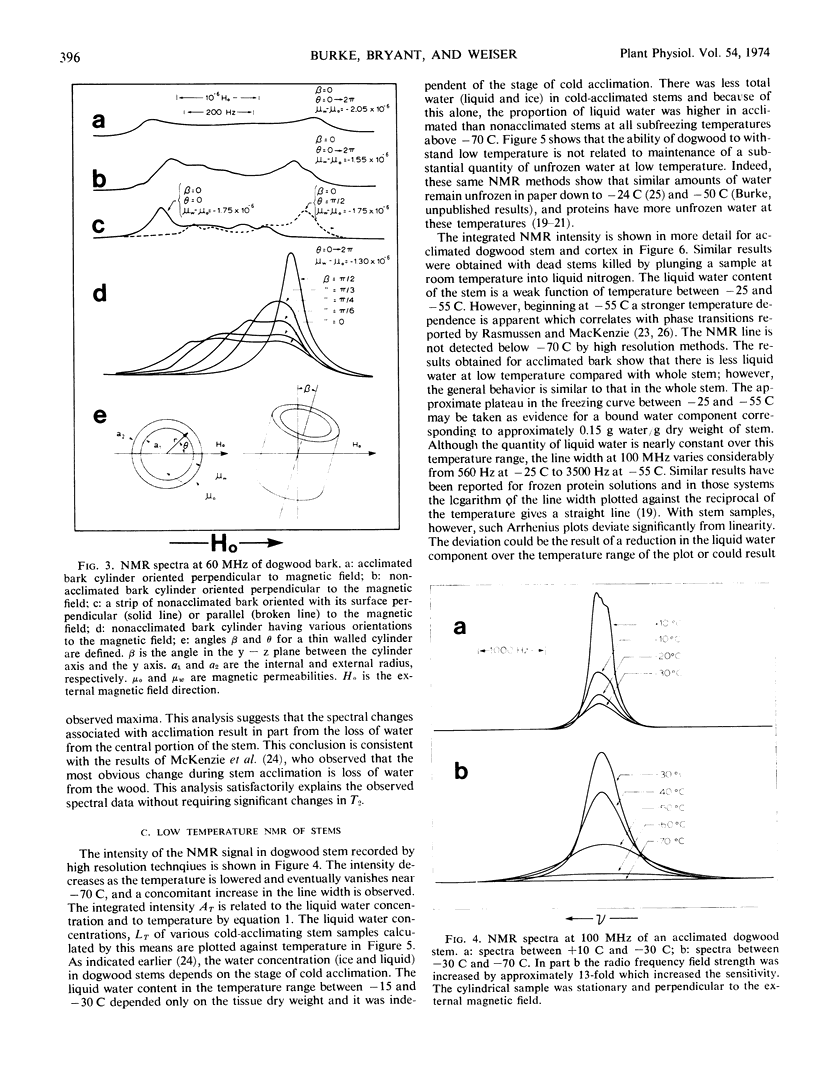
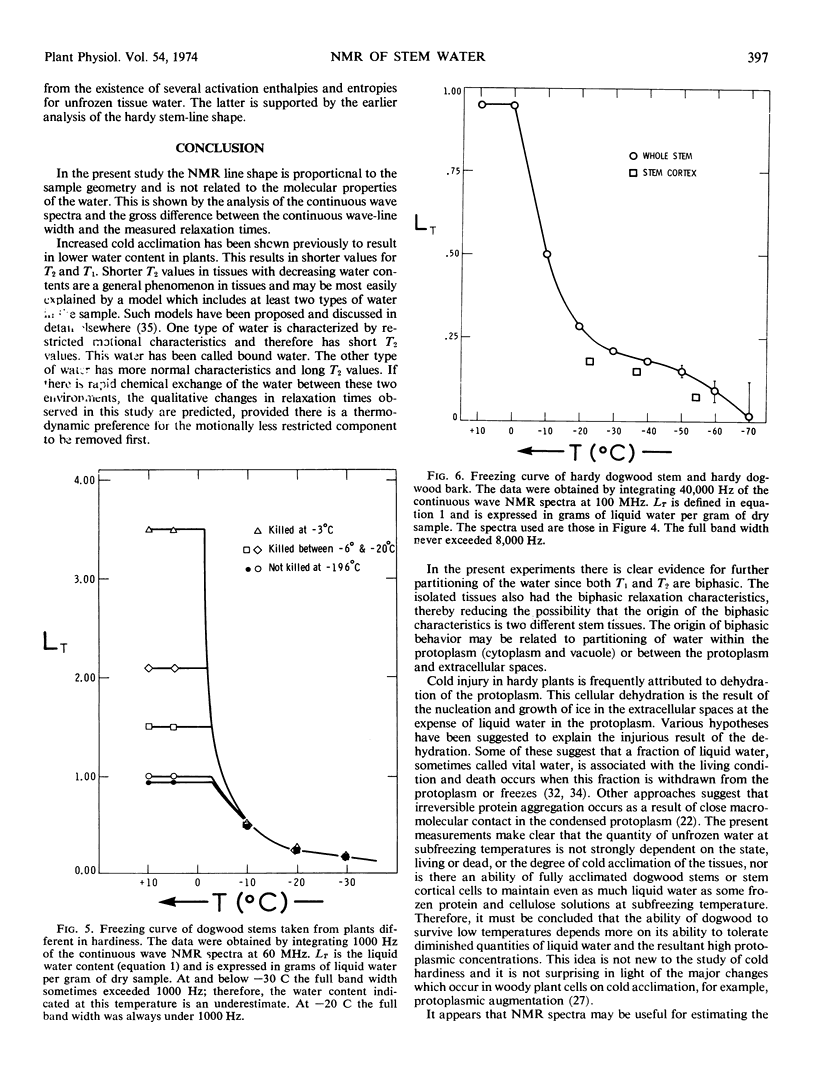
The pulsed and continuous-wave nuclear magnetic resonance of water in cold-acclimating red osier dogwood (Cornus stolonifera Michx) stem showed reduced relaxation times and increased line width. The reduction of relaxation times suggests an over-all restriction in the motional characteristics of the water. The increased line width is not related to a molecular property of the water, but is useful in estimating the initiation of cold acclimation. Biphasic relaxation characteristics may be related to partitioning of the water at the cellular level. The liquid water content of the stem was a weak function of temperature between −25 and −55 C, corresponding to approximately 0.15 gram of water per gram of dry stem. The quantity of unfrozen water at subfreezing temperatures was not strongly dependent on the degree of cold acclimation. It is concluded that the ability of dogwood to survive low temperatures depends on its ability to tolerate diminished quantities of liquid water.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRATTON C. B., HOPKINS A. L., WEINBERG J. W. NUCLEAR MAGNETIC RESONANCE STUDIES OF LIVING MUSCLE. Science. 1965 Feb 12;147(3659):738–739. doi: 10.1126/science.147.3659.738. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Hazlewood C. F., Nichols B. L., Rorschach H. E. Spin echo studies on cellular water. Nature. 1972 Jan 21;235(5334):170–171. doi: 10.1038/235170a0. [DOI] [PubMed] [Google Scholar]
- Cooke R., Wien R. Nuclear magnetic resonance studies of intracellular water protons. Ann N Y Acad Sci. 1973 Mar 30;204:197–209. doi: 10.1111/j.1749-6632.1973.tb30780.x. [DOI] [PubMed] [Google Scholar]
- Cooke R., Wien R. The state of water in muscle tissue as determined by proton nuclear magnetic resonance. Biophys J. 1971 Dec;11(12):1002–1017. doi: 10.1016/S0006-3495(71)86274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope F. W. Nuclear magnetic resonance evidence using D2O for structured water in muscle and brain. Biophys J. 1969 Mar;9(3):303–319. doi: 10.1016/S0006-3495(69)86388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPIREUX J., WILLIAMS D. Influence of deoxyribonucleic acid on the intermolecular structure of water. Nature. 1962 Aug 18;195:699–700. doi: 10.1038/195699b0. [DOI] [PubMed] [Google Scholar]
- DOUGLASS D. C., FRISCH H. L., ANDERSON E. W. Self-diffusion of water in tobacco mosaic virus solutions. Biochim Biophys Acta. 1960 Nov 18;44:401–403. doi: 10.1016/0006-3002(60)91595-x. [DOI] [PubMed] [Google Scholar]
- Fuchigami L. H., Weiser C. J., Evert D. R. Induction of Cold Acclimation in Cornus stolonifera Michx. Plant Physiol. 1971 Jan;47(1):98–103. doi: 10.1104/pp.47.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. E., Curnutte B., Jr, Lark K. G. Water structure and the denaturation of DNA. J Mol Biol. 1965 Sep;13(2):571–585. doi: 10.1016/s0022-2836(65)80118-8. [DOI] [PubMed] [Google Scholar]
- Hansen J. R. Pulsed NMR study of water mobility in muscle and brain tissue. Biochim Biophys Acta. 1971;230(3):482–486. doi: 10.1016/0304-4165(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Hazlewood C. F., Nichols B. L., Chamberlain N. F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969 May 24;222(5195):747–750. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
- JARDETZKY C. D., JARDETZKY O. The effect of tobacco mosaic virus (TMV) on the proton magnetic resonance spectrum of water. Biochim Biophys Acta. 1957 Dec;26(3):668–669. doi: 10.1016/0006-3002(57)90132-4. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Brassfield T. S., Law G. D., Purcell G. V. Hydration of macromolecules. Science. 1969 Mar 21;163(3873):1329–1331. doi: 10.1126/science.163.3873.1329. [DOI] [PubMed] [Google Scholar]
- Rasmussen D. H., MacKenzie A. P. The glass transition in amorphous water. Application of the measurements to problems arising in cryobiology. J Phys Chem. 1971 Apr 1;75(7):967–973. doi: 10.1021/j100677a022. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., Rheaume B., Pomeroy K., Lepage M. Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology. 1968 Nov-Dec;5(3):202–225. doi: 10.1016/s0011-2240(68)80164-6. [DOI] [PubMed] [Google Scholar]
- Sussman M. V., Chin L. Liquid water in frozen tissue: study by nuclear magnetic resonance. Science. 1966 Jan 21;151(3708):324–325. doi: 10.1126/science.151.3708.324. [DOI] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]


