Abstract
It has been claimed that glutamate excitotoxicity might have a role in the pathogenesis of several retinal degenerative diseases, including glaucoma and diabetic retinopathy. Neuropeptide Y (NPY) has neuroprotective properties against excitotoxicity in the hippocampus, through the activation of Y1, Y2 and/or Y5 receptors. The principal objective of this study is to investigate the potential protective role of NPY against glutamate-induced toxicity in rat retinal cells (in vitro and in an animal model), unraveling the NPY receptors and intracellular mechanisms involved. Rat retinal neural cell cultures were prepared from newborn Wistar rats (P3-P5) and exposed to glutamate (500 μM) for 24 h. Necrotic cell death was evaluated by propidium iodide (PI) assay and apoptotic cell death using TUNEL and caspase-3 assays. The cell types present in culture were identified by immunocytochemistry. The involvement of NPY receptors was assessed using selective agonists and antagonists. Pre-treatment of cells with NPY (100 nM) inhibited both necrotic cell death (PI-positive cells) and apoptotic cell death (TUNEL-positive cells and caspase 3-positive cells) triggered by glutamate, with the neurons being the cells most strongly affected. The activation of NPY Y2, Y4 and Y5 receptors inhibited necrotic cell death, while apoptotic cell death was only prevented by the activation of NPY Y5 receptor. Moreover, NPY neuroprotective effect was mediated by the activation of PKA and p38K. In the animal model, NPY (2.35 nmol) was intravitreally injected 2 h before glutamate (500 nmol) injection into the vitreous. The protective role of NPY was assessed 24 h after glutamate (or saline) injection by TUNEL assay and Brn3a (marker of ganglion cells) immunohistochemistry. NPY inhibited the increase in the number of TUNEL-positive cells and the decrease in the number of Brn3a-positive cells induced by glutamate. In conclusion, NPY and NPY receptors can be considered potential targets to treat retinal degenerative diseases, such as glaucoma and diabetic retinopathy.
Keywords: Retinal cells, neuropeptide Y, NPY receptors, neuroprotection, glutamate
Neuropeptide Y (NPY) is one of the most abundant peptides in the mammalian central nervous system (CNS).1, 2, 3 NPY is a highly conserved peptide containing 36 amino acids. Its biological effects are mediated by six G-protein-coupled receptors Y1, Y2, Y3, Y4, Y5 and y6.3, 4, 5 The retina is a specialized nervous tissue where NPY and its receptors are expressed in the retina of different species.6, 7 The presence of mRNA for Y1, Y2, Y4 and Y5 NPY receptors has been detected in rat retinas8, 9 and in cultured rat retinal neural cells,8 but their distribution in different cell types and their function in the retina is poorly understood.
Glutamate is the main excitatory neurotransmitter in the CNS, including in retina.10 Excitotoxicity, which is considered as an overactivation of glutamate receptors triggering neuronal cell death, has been associated with several acute and chronic neurodegenerative disorders11, 12 and in retinal degenerative disorders, such as glaucoma13, 14, 15 and diabetic retinopathy.16, 17, 18
NPY has been linked to several physiological and pathological functions, such as feeding behaviour, memory processing, pain, anxiety, cell proliferation and many other processes in the central and peripheral nervous systems.19, 20 Some studies have demonstrated putative neuroprotective effects of NPY in various regions of the CNS. In particular, NPY inhibits the glutamate release in rat hippocampus and is neuroprotective in rat hippocampus and striatum.2, 21, 22, 23, 24, 25 Moreover, the activation of NPY Y1, Y2 and Y5 receptors mediates the neuroprotective effect of NPY against AMPA- and kainate-induced excitotoxicity in organotypic rat hippocampal slice cultures.21 It has also been suggested that selective activation of Y1 and Y2 receptors protects mouse hippocampal cells from excitotoxic lesions.24 Similarly, NPY Y2 and Y5 are implicated in the neuroprotective role against kainate-induced excitotoxicity in hippocampus even after delayed application of the respective agonists. Specific activation of NPY Y2 receptor is also effective in a transient middle cerebral artery occlusion model of ischemia.23 Recently, it was shown that NPY, also through NPY Y2 receptor activation, mediates the survival of dopaminergic neurons in Parkinson's disease models.26 In addition, NPY was suggested as a potential neuroprotective agent in Alzheimer's disease by counteracting the toxic effect of β-amyloid in an in vitro model.27, 28
We have also shown that NPY in the retina presents neuroprotective properties. Specifically, NPY protected rat retinal cells in culture against 3,4-methylenedioxy-N-methylamphetamine (MDMA)-induced toxicity,29 although the NPY receptor subtype(s) involved in this neuroprotective effect is unknown.
As the retina is affected by various degenerative diseases, where glutamate excitotoxicity might eventually have a role,13, 17 our major goal in the present work is to evaluate the putative neuroprotective role of NPY and NPY receptors against glutamate excitotoxicity in retinal cells. We have evaluated the involvement of the different NPY receptors, as well as the possible intracellular signaling pathways involved in the neuroprotective effects of NPY in retinal cells, using primary rat retinal neural cell cultures.
Results
NPY protects neurons against necrotic and apoptotic cell death induced by glutamate
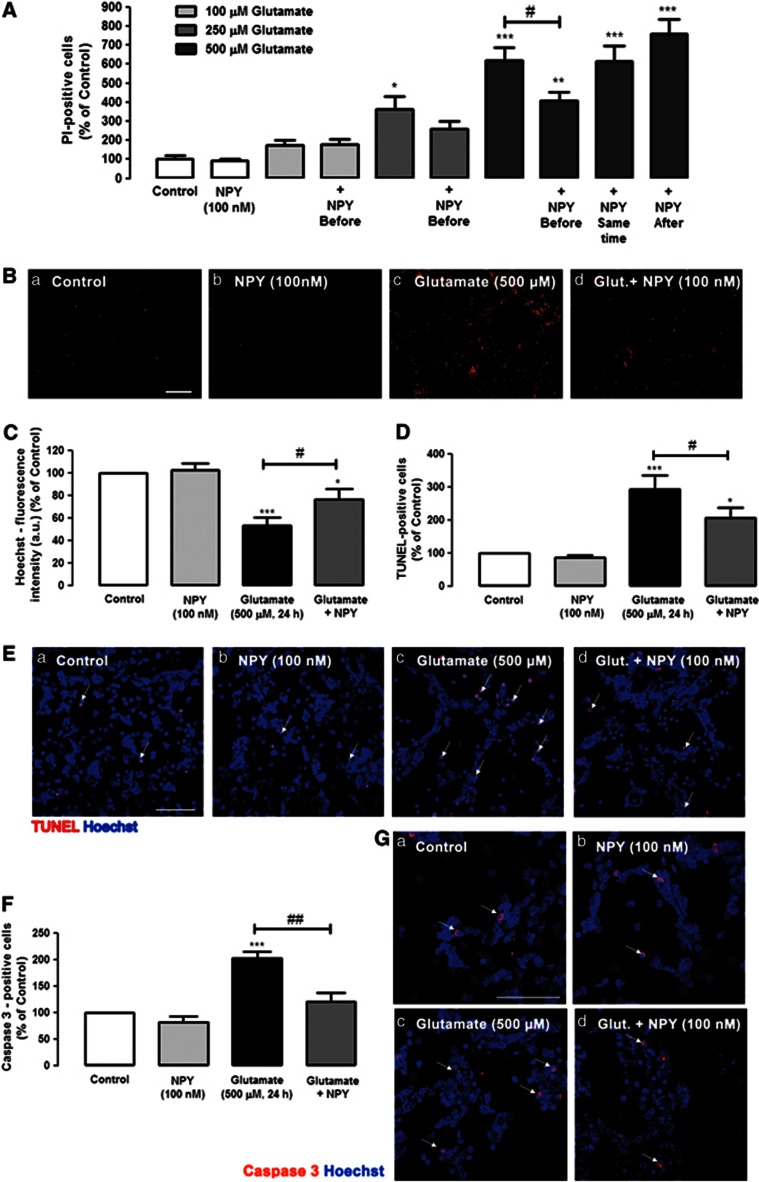
Necrotic and late apoptotic cell death of rat retinal neural cells was evaluated by propidium iodide (PI) uptake assay. Retinal cells were exposed to 100, 250 or 500 μM glutamate for 24 h (Figures 1A and B). The number of PI-positive cells in coverslips exposed to 100, 250 or 500 μM of glutamate was 175.7±27.1%, 364.7±64.4% and 617.3±71.7% of control, respectively. These results indicate that cell viability decreases significantly with increased glutamate concentrations. To investigate the potential neuroprotective role of NPY against glutamate-induced toxicity, retinal cells were incubated with NPY (100 nM) at three different times: 1 h before the incubation with glutamate (100, 250 and 500 μM), simultaneously with the addition of glutamate (500 μM) and 30 min after exposure to glutamate (500 μM). NPY did not affect the increase in the number of PI-positive cells induced by exposure to 100 μM glutamate, as the number of PI-positive cells (179.9± 25.0% of control—NPY applied 1 h before glutamate) was similar to glutamate alone. When cells were exposed to 250 μM glutamate, there was a tendency, although not significant, for a protective effect of NPY when applied before glutamate. A neuroprotective effect of NPY was observed when 500 μM glutamate stimulus was applied. When cells were exposed to 500 μM glutamate, and NPY (100 nM) was added 1 h before glutamate, there was a significant neuroprotective effect of NPY, as shown by a decrease in the number of PI-positive cells to 409.4±41.8% of control (Figures 1A and Bd), which can be compared with the glutamate condition (617.3±71.7% of control), indicating a decrease in the number of PI-positive cells of 34%. However, when cells were exposed to NPY, either simultaneously or 30 min after adding 500 μM glutamate, the neuroprotective effect was lost. Under the two conditions, the number of PI-positive cells was 614.7±80.5% and 756.9±78.0% of control, respectively, similar to percentage found when cells were exposed to 500 μM glutamate (617.3±71.7% of control). Based on these results, NPY was applied 1 h before glutamate (500 μM) for the subsequent experiments.
Figure 1.
NPY protects against necrotic and apoptotic retinal cell death induced by glutamate. (A and B) Necrotic cells were assessed by PI incorporation assay. (C) Cell nuclei were stained by Hoechst 33342. Apoptotic cells were assessed by (D and E) TUNEL assay and (F and G) cleaved caspase 3- immunocytochemistry. (A) Quantification of PI-positive cells (percentage of control). Retinal cells were exposed to different concentrations of glutamate (100, 250 and 500 μM) for 24 h and treated with NPY (100 nM) at three different time points: 1 h before, simultaneously, and 30 min after glutamate, as indicated below bars. The results represent the mean±S.E.M of n=4–11 independent experiments; ***P<0.001, **P<0.01, *P<0.05, compared with control; #P<0.05, compared with glutamate (500 μM); one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. (B) Representative images of (a) control and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY (1 h before), showing PI-positive cells (red spots), Bar=100 μm. (C) Quantification of fluorescence intensity (arbitrary units) of cells stained with Hoechst 33342 (nucleus marker), compared with control (no drug). These results represent the mean±S.E.M. of n=21–27 independent experiments; ***P<0.001, *P<0.05, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (D) Quantification of TUNEL-positive cells (percentage of control). Cultured retinal cells were exposed to glutamate and treated with NPY (1 h before glutamate exposure), as indicated below bars. Data represent the mean±S.E.M. of n=5–6 independent experiments; ***P<0.001, *P<0.05, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (E) Representative images of (a) control and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY (1 h before), showing TUNEL-positive cells (purple spots, indicated by white arrows) and cell nuclei stained with Hoechst 33342 (blue); Bar=50 μm. (F) Quantification of cleaved caspase-3 positive cells (red) per field compared with control conditions (100% no drug, Ga). Rat retinal cells were exposed to glutamate and treated with NPY (1 h before glutamate exposure), as indicated below bars. The results represent the mean±S.E.M. of n=5–6 independent experiments; ***P<0.001, *P<0.05, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (G) Representative images of (a) control and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY (1 h before), showing cleaved caspase 3-positive cells (purple spots). Cell nuclei were stained with Hoechst 33342 (blue). NPY per se had no effect on the number of PI-, Hoechst 33342-, TUNEL-, or cleaved caspase 3-positive cells compared with control. Bar=50 μm
The effects of glutamate and/or NPY treatments on the total number of cells were also assessed (Figure 1C). Cells were stained with Hoechst33342, and the fluorescence intensity (arbitrary units) was measured. Glutamate (500 μM, 24 h) was found to decrease the Hoechst33342-fluorescence intensity to 50.8±7.0% of control (untreated cells). NPY partially prevented this effect triggered by glutamate, as the decrease in fluorescence intensity was attenuated by NPY (75.4±9.8% of control).
Apoptotic cell death was assessed using the TUNEL (terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling) assay to obtain a better characterization of the protective role of NPY against retinal cell death caused by exposure to glutamate (Figures 1D and E). Glutamate (500 μM) increased the number of apoptotic cells to 294.1±41.7% of control. When NPY (100 nM) was applied 1 h before glutamate, the increase in the number of TUNEL-positive cells triggered by glutamate was reduced to 206.2±32.6% of control, representing a 30% reduction. In addition, although glutamate (500 μM, 24 h) increased the number of active caspase 3-positive cells to 201.9±12.8% of control (Figures 1F and Gc), NPY pre-treatment reduced the increase in the number of caspase 3-positive cells triggered by glutamate to 120.7±16.7% of control (Figure 1Gd).
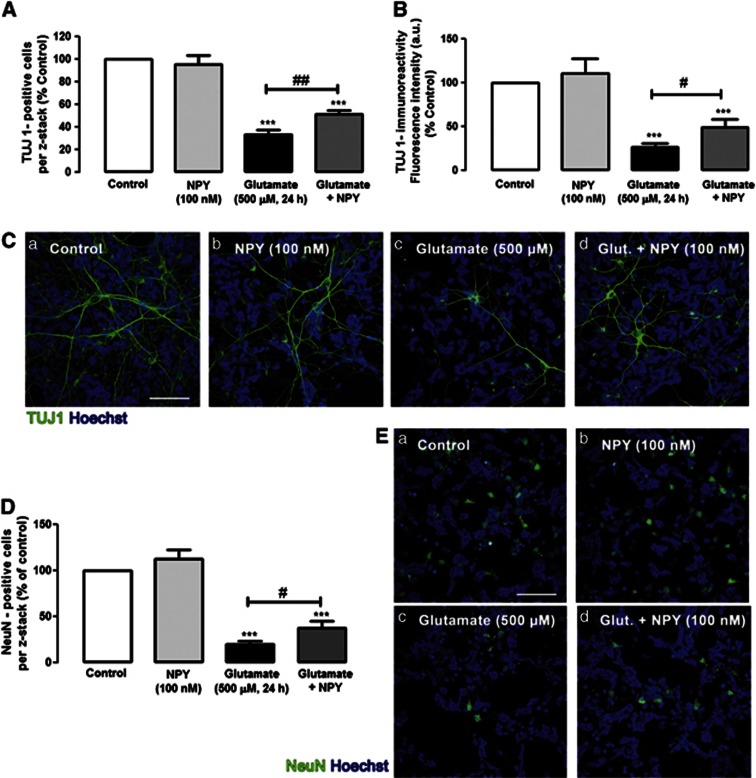
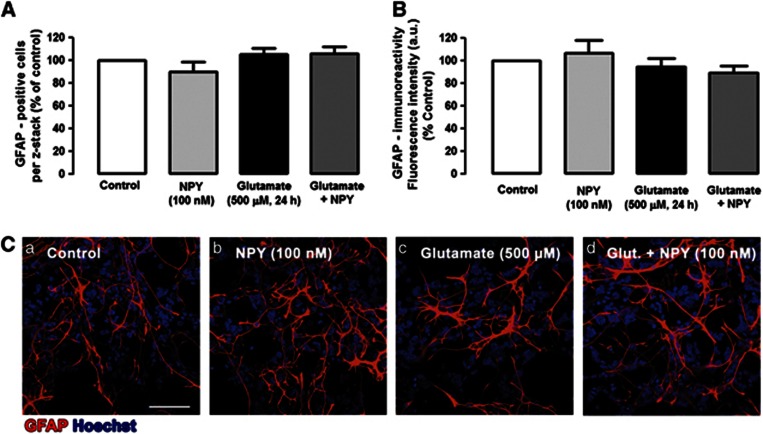
To further elucidate the protective effect of NPY against glutamate-induced cell death and considering that these cell cultures are composed of neurons, macroglial and microglial cells, we evaluated, by immunocytochemistry, which cell types could be most strongly affected by glutamate and, eventually, protected by NPY (Figures 2, 3, 4). To quantify the effects of glutamate and NPY on different cell types, the immunoreactivity (fluorescence intensity) and/or the number of positive cells to different cell markers were evaluated. Under control conditions, a normal distribution of TUJ1-positive neurons was observed (Figures 2Ca). When cells were exposed to 500 μM glutamate for 24 h, the number of neurons decreased and their neurites integrity was dramatically affected (Figure 2Cc). The quantification of TUJ1-positive cells (Figure 2A) revealed that glutamate induced a significant decrease in the number of neurons in culture to 33.4±3.8% of control. The application of NPY before glutamate inhibited significantly the decrease in the number of TUJ1-positive cells to 51.4±3.6% of control. Additionally, by analyzing the TUJ-1-immunoreactivity (Figure 2B), we also found that glutamate induced a significant decrease in the content of this neuronal marker to 26.0±4.9% of control (Figure 2B). In cells incubated with NPY before glutamate application, the decrease in TUJ-1 immunoreactivity was attenuated (49.2±8.5% of control), compared with cells just exposed to glutamate. In rat retinal cell cultures, among several neuronal markers, TUJ1 presents the best immunoreactivity profile. However, TUJ1 is considered an immature neuronal marker, and therefore the expression of NeuN, a marker of mature neurons, was also evaluated in the same conditions. Similar results were found (Figures 2D and E). The number of NeuN-positive cells dramatically decreased in the presence of 500 μM glutamate to 19.8±4.0% of control. The pre-incubation of the retinal cells with NPY inhibited the decrease in the number of NeuN-positive cells triggered by glutamate (38.1±7.2% of control). To evaluate the effects of glutamate and NPY on macroglial cells, we analyzed the immunoreactivity of glial fibrillary acidic protein (GFAP), a macroglial cell (astrocytes and Müller cells) marker (Figure 3). The number of GFAP-positive cells (Figure 3A) and the GFAP immunoreactivity (fluorescence intensity) were evaluated (Figure 3B). We found that exposure of retinal cells to glutamate induced a slight change in the morphology of some GFAP-positive cells, compared with control cells (Figure 3Bc), namely a decrease in the number of cell processes and an increase of their thickness (Figure 3Bc). However, by evaluating the number of GFAP-positive cells and the quantification of GFAP immunoreactivity (fluorescence intensity) revealed no significant differences between cells exposed to glutamate and controls. These small alterations in GFAP-positive cell morphology triggered by glutamate appeared to be partially prevented by NPY (Figure 3Bd). The effects of glutamate and NPY on microglial cells were assessed by analyzing the immunoreactivity of two microglial cell markers: CD11b and CD68/ED1 (Figure 4). CD11b labels resting and activated microglial cells, while ED1 is a marker of activated microglia.30 Two different parameters were evaluated for these markers: the number of CD11b- and CD68/ED1-positive cells per field, and the CD11b or CD68/ED1 immunoreactivity. NPY increased the number of microglial cells (resting and activated; Figures 4Cb and Fb). Similarly, glutamate or NPY plus glutamate also increased the number of CD11b- and CD68/ED1-positive cells. As with the results obtained for the number of CD11b-positive cells, the fluorescence intensity measurements showed that NPY, glutamate and NPY plus glutamate increased the immunoreactivity of CD11b- and CD68/ED1-positive cells (Figures 4B and E).
Figure 2.
NPY protects neuronal cell death induced by glutamate in rat retinal neural cell cultures. Neurons were identified with (C) anti-TUJ1 (green) or (E) anti-NeuN (green) antibodies, respectively. (A) Quantification of TUJ1-positive cells per z-stack. The results were normalized and are presented as percentage of control condition. The results represent the mean±S.E.M. of n=5–7 independent experiments; ***P<0.001, compared with control; ##P<0.01, compared with glutamate; one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. (B) Quantification of TUJ 1-immunoreactivity by fluorescence intensity (arbitrary units), compared with control conditions (100% no drug, Ca). The results represent the mean±S.E.M. of n=4–8 independent experiments ***P<0.001, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (C) Representative images of (a) control cultures and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY, showing TUJ1-positive cells (green). Cell nuclei were identified by Hoechst 33342 staining (blue). (D) Quantification of NeuN-positive cells per z-stack. The results were normalized and are presented as percentage of control condition. The results represent the mean±S.E.M. of n=3–5 independent experiments; ***P<0.001, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (E) Representative images of (a) control cultures and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY, showing NeuN-positive cells (green). Cell nuclei were stained with Hoechst 33342 (blue). NPY per se did not affect the number of TUJ1- or NeuN-positive cells or the TUJ1-immunoreactivity compared with control. Bar=50 μm
Figure 3.
NPY has no effect in glial cells. Microglial cells were identified with (C) anti-GFAP (red) antibody. (A) Quantification of GFAP-positive cells per z-stack. (B) Quantification of GFAP-immunoreactivity by fluorescence intensity (arbitrary units), compared with control conditions (100% no drug, Ca). The results were normalized and are presented as percentage of control condition. (C) Representative images of (a) control and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY, showing GFAP-positive cells (red). Cell nuclei were stained with Hoechst 33342 (blue). NPY per se did not affect the number of GFAP-positive cells or the GFAP-immunoreactivity compared with control. Bar=50 μm
Figure 4.
Glutamate and NPY increase the proliferation and activation of retinal microglial cells. Microglial cells were identified by immunocytochemistry using (C) anti-CD11b (green) and (F) anti-CD68/ED1 (red) antibodies. (A) Quantification of CD11b-positive cells (green) per field, compared with control conditions (no drug, Ca). (B) Quantification of fluorescence intensity (arbitrary units) of CD11b-immunoreactivity, compared with control (100% no drug, Ca). These results (A and B) represent the mean±S.E.M. of n=8 independent experiments, with **P<0.01, *P<0.05, compared with control; one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. (C) Representative images of (a) control and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY, showing CD11b- positive cells (green). Cell nuclei were stained by Hoechst 33342 (blue). Bar=50 μm. (D) Quantification of CD68/ED1-positive cells per field, compared with control (100% no drug, Da). (E) Quantification of fluorescence intensity (arbitrary units) of CD68/ED1-immunoreactivity, compared with control (100% no drug, Da). These results (D and E) represent the mean±S.E.M. of n=5 independent experiments; ***P<0.001, **P<0.01, *P<0.05, compared with control; one-way ANOVA followed by Bonferroni's post-hoc test. (F) Representative images of (a) control, and cultures treated with (b) NPY, (c) glutamate or (d) glutamate+NPY, showing CD 68/ED1-positive cells. Cell nuclei were stained by Hoechst 33342 (blue). Bar=50 μm
Activation of NPY Y2, Y4 or Y5 receptors inhibits the increase in necrotic cell death induced by glutamate
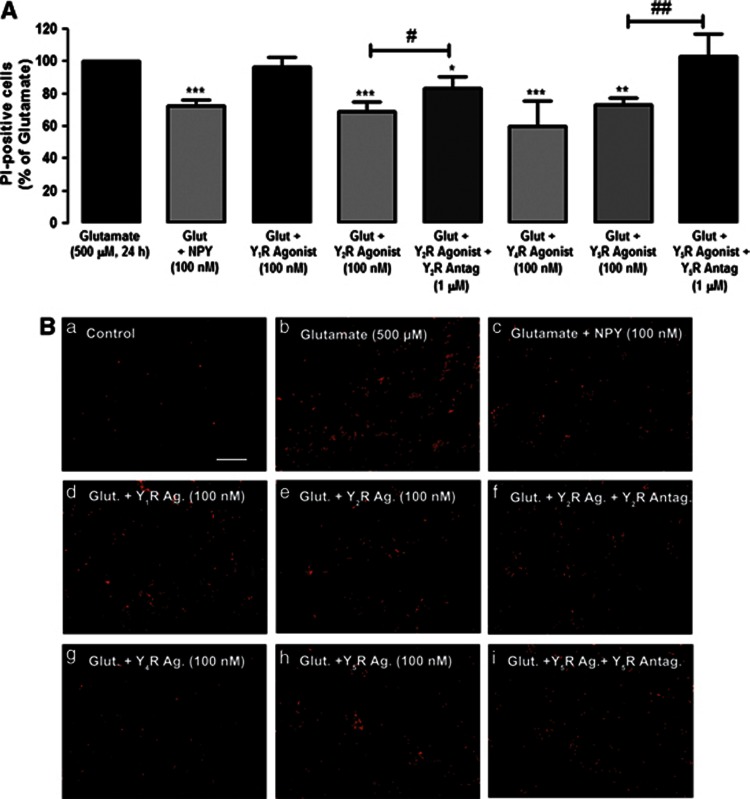
We evaluated the effects of NPY receptor agonists and antagonists to determine which NPY receptors could be mediating the protective role of NPY against necrotic cell death induced by glutamate (Figures 5A and B). In this analysis, we compared the number of PI-positive cells for each experimental condition with the number of PI-positive cells in cultures exposed to glutamate, taken as 100%. NPY decreased the number of PI-positive cells to 72.4±3.7% relative to glutamate. The NPY Y1 receptor agonist ([Leu,31Pro34]NPY) did not inhibit glutamate-induced necrotic cell death (Figures 5A and B). However, the NPY Y2 receptor agonist (NPY13–36) inhibited the increase in PI-positive cells (68.8±6.4%, compared with glutamate; Figure 5A). This protective effect was partially prevented by the NPY Y2 receptor antagonist BIIE0246 (83.4±7.2% compared with glutamate). Furthermore, the NPY Y4 agonist (r-PP, 100 nM) also partially protected retinal cells exposed to glutamate, as shown by the number of PI-positive cells decreasing to 60.2±15.5% relative to glutamate. In addition, NPY Y5 receptor agonist (Gly,1Ser,3,22Gln,4,34Thr,6Arg,19Tyr,21Ala,23,31Aib32)PP also exerted a protective effect, as seen by the increase in the number of PI-positive cells induced by glutamate, which was attenuated to 73.0±4.4%, compared with glutamate (Figures 5A and B). This effect was completely blocked by NPY Y5 receptor antagonist. The NPY receptor agonists or antagonists per se did not increase the number of PI-positive cells, compared with control (data not shown).
Figure 5.
The activation of NPY Y2, Y4 and Y5 receptors inhibits the necrotic cell death induced by glutamate. Necrotic cells were evaluated by PI incorporation assay. Cells were exposed to glutamate, and treated with NPY, or NPY receptor agonists and antagonists, indicated below bars. (A) Quantification of PI-positive cells (percentage of glutamate condition) per field in retinal cell cultures treated with NPY Y1 receptor agonist ([Leu,31Pro34]NPY;100 nM); NPY Y2 receptor agonist (NPY13–36; 100 nM) and antagonist (BIIE 0246; 1 μM); NPY Y4 agonist receptor (r-PP, 100 nM); NPY Y5 receptor agonist ((Gly,1Ser,3,22Gln,4,34Thr,6Arg,19Tyr,21Ala,23,31Aib32)PP) and antagonist (L-152,804; 1 μM). (B) Representative images of (a) control and cultures treated with (b) glutamate, (c) glutamate+NPY, (d) glutamate+Y1R agonist, (e) glutamate+Y2R agonist, (f) glutamate+Y2R agonist+Y2R antagonist, (g) glutamate+Y4R agonist, (h) glutamate+Y5R agonist and (i) glutamate+Y5R agonist+Y5R, showing PI-positive cells (red spots). Bar=100 μm. Values are expressed as the percentage of PI-positive cells per field compared with the glutamate condition. The results represent mean±S.E.M. of n=4–11 independent experiments; ***P<0.001, **P<0.01, compared with glutamate; ###P<0.001, ##P<0.01, compared with glutamate+NPY receptor agonist; one-way analysis of variance followed by Bonferroni's post-hoc test
NPY Y5 receptor activation inhibits apoptotic retinal cell death induced by glutamate
We have evaluated the potential neuroprotective effect of NPY receptor agonists against the increase in apoptotic cell (TUNEL-positive cells) number by exposure to glutamate. NPY reduced 30% the number of apoptotic cells to 69.7±3.8%, compared with glutamate. NPY receptor agonists and antagonists were used to investigate those involved in this neuroprotective effect (Figures 6A and B). The NPY Y5 receptor agonist mimicked the effect of NPY, inhibiting the increase in the number of TUNEL-positive cells triggered by glutamate; the percentage of apoptotic cells decreased to 68.2±6.0%, compared with glutamate. This effect was completely blocked by the NPY Y5 receptor antagonist (L-152,804). The selective NPY Y1, Y2 or Y4 receptors agonists did not decrease the number of TUNEL-positive cells in cultures exposed to glutamate. NPY receptor agonists and antagonists alone did not increase the number of TUNEL-positive cells, compared with control (data not shown).
Figure 6.
The activation of NPY Y5 receptor inhibits the apoptotic cell death induced by glutamate. Apoptotic cells were assessed by TUNEL assay. Cells were exposed to glutamate and treated with NPY, or NPY receptor agonists and antagonists, as indicated below bars. (A) Quantification of TUNEL-positive cells per field compared with glutamate condition (100%) in retinal cell cultures treated with NPY Y1 receptor agonist ([Leu,30Pro31]NPY, 100 nM); NPY Y2 receptor agonist (NPY13–36; 100 nM); NPY Y4 agonist receptor (r-PP, 100 nM); NPY Y5 receptor agonist ((Gly,1Ser,3, 22Gln,4, 31Thr,6Arg,19Tyr,21Ala,23, 30Aib32)PP, 100 nM) and antagonist (L-152,804; 1 μM). (B) Representative images of (a) control and cultures treated with (b) glutamate, (c) glutamate+NPY, (d) glutamate+Y1R agonist, (e) glutamate+Y2R agonist, (f) glutamate+Y4R agonist, (g) glutamate+Y5R agonist and (h) glutamate+Y5R agonist+Y5R antagonist, showing TUNEL-positive cells (purple spots – some examples are indicated by white arrows). Bar=50 μm. Values are expressed as the percentage of TUNEL-positive cells per field compared with the glutamate condition. The results represent mean±S.E.M. of n=5–6 independent experiments, with *P<0.05, compared with glutamate; ##P<0.01, compared with glutamate +Y5R agonist; one-way analysis of variance followed by Bonferroni's post-hoc test
Protein kinase A (PKA) and p38K proteins mediate the neuroprotective effect of NPY against glutamate-induced necrotic retinal neural cell death
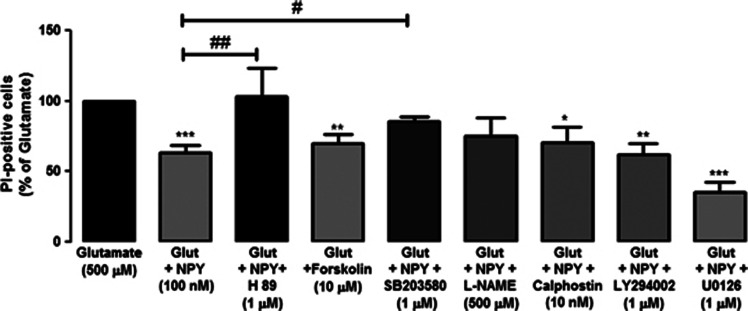
Inhibitors of key proteins in different intracellular pathways were used to elucidate the intracellular pathways that mediate the neuroprotective effect of NPY when cells are exposed to glutamate or/and NPY (Figure 7). The PKA inhibitor, H89 (1 μM), prevented the neuroprotective effect of NPY (63.2±5.5%, compared with glutamate). The number of PI-positive cells exposed to glutamate, or to glutamate plus NPY and H89, was similar. In order to confirm the involvement of PKA in the neuroprotective effect of NPY against glutamate-induced excitotoxicity, we have evaluated the effect of the PKA activator, forskolin (10 μM), with cells exposed to glutamate. Forskolin decreased the number of PI-positive cells (69.6±7.1%, compared with glutamate) to a similar extent as NPY (63.2±5.5%, compared with glutamate). The protective effect of NPY against the increase of PI-positive cells triggered upon exposure to glutamate was also partially prevented (85.6±2.7%, compared with glutamate) by the presence of the p38K inhibitor (SB203580). The inhibitors of nitric oxide synthase (NOS), protein kinase C (PKC), phosphoinositide 3-kinase (PI3K) and MEK1/2, namely L-NG-nitroarginine methyl ester (L-NAME), calphostin C, LY294002 and U0126, respectively, did not affect the neuroprotective effect of NPY against glutamate-induced toxicity (Figure 7). The inhibitors per se did not increase the number of PI-positive cells, compared with control (data not shown).
Figure 7.
PKA and protein 38 kinase (p38K) mediate the neuroprotective effect of NPY against retinal neuronal cell death triggered by glutamate. The involvement of different intracellular pathways in the neuroprotective effect of NPY against glutamate-induced excitotoxicity was assessed by PI uptake (PI-positive cells), using different inhibitors of proteins involved on those pathways. Retinal cell cultures were exposed to NPY (100 nM), glutamate (500 μM) and the inhibitors indicated below bars. Quantification of PI-positive cells (compared with glutamate condition) in retinal cells treated with H89 (1 μM; PKA inhibitor), forskolin (10 μM; PKA activator), SD203580 (1 μM; p38K inhibitor), L-NAME (500 μM; NOS inhibitor), calphostin C (10 nM; PKC inhibitor), LY294002 (1 μM; PI3K inhibitor) and U0126 (1 μM; MEK1/2 inhibitor). Values are expressed as the percentage of PI-positive cells (per field), compared with the glutamate condition. The results represent the mean±S.E.M. of n=7–9 independent experiments, with ***P<0.001, **P<0.01, *P<0.05, compared with glutamate; ##P<0.01, #P<0.05, compared with glutamate+NPY; one-way analysis of variance followed by Bonferroni's post-hoc test
NPY protects rat retina from apoptotic cell death induced by glutamate excitotoxicity
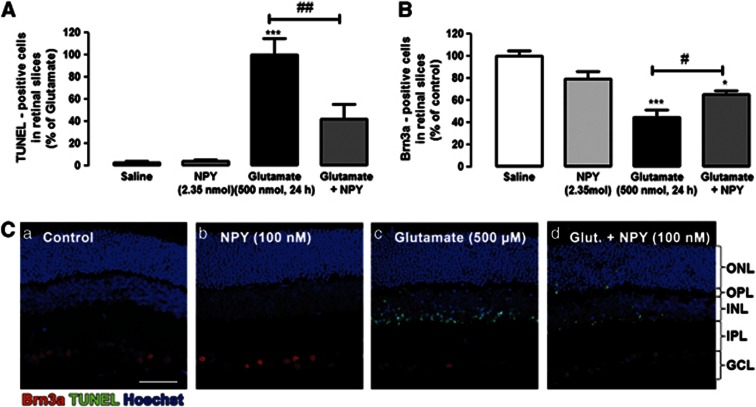
Rat retinas were exposed to 500 nmol glutamate for 24 h (Figure 8). Apoptotic cell death was assessed by TUNEL assay (Figures 8A and C). The number of TUNEL-positive cells in retinal slices obtained from retinas exposed to 500 nmol glutamate was 159.0±23.1 cells per field. The TUNEL-positive cells were mainly located in the inner retina, especially in inner nuclear layer (INL) and ganglion cell layer (GCL). Rarely, few apoptotic cells were found in outer nuclear layer (ONL). To investigate the potential neuroprotective role of NPY against glutamate induced-toxicity, rat eyes were intravitreally injected with NPY (2.35 nmol) 2 h before glutamate injection (500 nmol). When NPY was applied before glutamate, the number of TUNEL-positive cells in retinal slices decreased to 44.2±18.4% compared with glutamate, representing a 55% reduction in the number of TUNEL-positive cells.
Figure 8.
NPY protects against apoptotic cell death in the rat retina induced by glutamate. Cells undergoing apoptosis were identified by TUNEL assay, and ganglion cells were identified by immunohistochemistry against Brn3a (ganglion cell marker). (A) Quantification of TUNEL-positive cells in rat retinal slices (presented as percentage of glutamate condition). Retinas were exposed to glutamate (500 nmol; intravitreal injection) and treated (or not) with NPY (2.35 nmol, 2 h before intravitreal injection of glutamate), as indicated below bars. Data represent the mean±S.E.M. of n=3–5 independent experiments (animals); ***P<0.001 compared with control; #P<0.05, compared with glutamate; one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. (B) Quantification of Brn3a-positive cells in rat retinal slices (presented as percentage of control). Retinas were exposed to glutamate (500 nmol; intravitreal injection) and treated (or not) with NPY (2.35 nmol, 2 h before glutamate exposure), as indicated below bars. Data represent the mean±S.E.M. of n=3–5 independent experiments; ***P<0.001, *P<0.05, compared with control; #P<0.05, compared with glutamate; one-way ANOVA followed by Bonferroni's post-hoc test. (C) Representative images of retinal slices obtained from eyes exposed to different conditions (intravitreal injection): (a) saline (0.9% NaCl), treated with (b) NPY, (c) glutamate (500 nmol) or (d) glutamate+NPY (2.35 nmol, 2 h before glutamate), showing TUNEL-positive cells (green), Brn3a-positive cells (red), and cell nuclei stained with Hoechst 33342 (blue). NPY per se had no effect on the number of TUNEL- or Brn3a-positive cells compared with control. IPL, inner plexiform layer; OPL, outer plexiform layer. Bar=50 μm
The number of Brn3a-positive cells, a specific marker of ganglion cells in the retina, was also evaluated in the rat retinal slices (Figures 8B and C). Exposure of the retina to glutamate decreased the number of Brn3a-positive cells to 44.8±6.7% of control. Conversely, when NPY (2.35 nmol) was applied 2 h before glutamate injection in the rat vitreous, there was a protective effect as the number of Brn3a-positive cells increased (from 44.8±6.7 to 65.7±3.1% of control).
Discussion
In this study, we investigated the protective role of NPY and NPY receptors against glutamate-induced neural cell death in rat retinal neural cells. Glutamate triggered necrosis and apoptosis in retinal cells, and NPY was able to inhibit both processes. Moreover, we have demonstrated that NPY Y2, Y4 and Y5 receptors mediate the protective effect of NPY against necrotic cell death caused by glutamate and that NPY Y5 receptor mediated the NPY protective effect against apoptotic cell death induced by glutamate. Additionally, we have shown that the neuroprotective effect of NPY is mediated by PKA and p38K. Finally, using an animal model, we have demonstrated that NPY also has a protective role against glutamate-induced excitotoxicity in the retina. These findings suggest that NPY can be viewed as a potential new target to protect retinal cells in retinal corresponding degenerative diseases, such as glaucoma or diabetic retinopathy.
Previous studies have shown that NPY can exert neuroprotective effects against excitotoxicity triggered by glutamate or glutamate receptor agonists in various regions of the CNS, such as hippocampus and striatum.2, 21, 23, 25, 26 In addition, we have previously shown that NPY protects against MDMA (ecstasy) toxicity in cultured rat retinal cells.29 We extend this to show, for the first time, that NPY is able to protect necrotic and apoptotic cell death induced by glutamate in retinal cells. However, the neuroprotective effect of NPY only occurs when the peptide is applied before the excitotoxic stimulus and is not present when it is added simultaneously or after glutamate. This is consistent with the majority of studies describing a protective role of NPY, where the peptide was applied before the toxic stimulus.21, 29, 31 However, some reports have also indicated that NPY is effective when it is applied a few hours after the excitotoxic stimulus.23, 25, 32 Similarly, NPY was also able to protect against cell death induced by glutamate in the retina. We have shown, for the first time, that NPY exerts neuroprotective effects in the retina in vitro and using an animal model.
Glutamate excitotoxicity is characterized morphologically by a decrease in the number of neurons and a reduction in the length of neuronal processes.33, 34 It induced a similar effect in rat retinal cells, which was partially prevented by NPY, specifically in neurons. In fact, NPY protected immature (TUJ1-positive cells) and mature (NeuN-positive cells) neurons in culture. Some studies indicate that Müller cells have a dual role under toxic conditions. When threatened, these cells can be either neuroprotective or contribute to exacerbate the excitotoxic stimuli (reviewed in Bringmann and Wiedemann35). In the present study, glutamate slightly changed the morphology of few GFAP-positive cells, increasing the thickness of their processes and decreasing the number of cell processes.
The microglial cell response to glutamate exposure was completely different. Glutamate increased the number of CD11b- and CD68/ED1-positive cells, as well as the immunoreactivity of these two markers. Glutamate and glutamate receptor agonists are known to activate microglial cells in CNS, such as the hippocampus.36, 37 In the present work, both glutamate and NPY increased microglial cell proliferation, as well as microglia activation. When NPY and glutamate were present, the effect on microglia proliferation and activation was not enhanced. In contrast, other groups have reported inhibition of microglia phagocytosis and cell motility by NPY upon inflammatory challenge through the activation of NPY Y1 receptor.38, 39 In addition, NPY, via Y2 receptors, has a protective role against methamphetamine-induced microgliosis.40 In the early stages of neurodegenerative processes, the activation of microglia contributes to neuronal protection and tissue regeneration. However, continuous retinal microglial overactivation may lead to chronic inflammation, loss of autoregulatory mechanisms, irreversible neuronal loss and photoreceptor apoptosis.41, 42, 43, 44 Microglial activation is involved in the initiation and perpetuation of degenerative process in many diseases, such as retinal dystrophies.42, 45
Using pharmacological tools, we have shown that NPY protects retinal cells against necrotic cell death induced by glutamate through the activation of NPY Y2, Y4 and Y5 receptors. In another study, using hippocampal slice cultures, the anti-necrotic effect of NPY was also seen to be mediated by Y2 and Y5 receptors. However, NPY Y1 receptors contributed to the neuroprotective effect of NPY as well.21 Although other studies have suggested that only the NPY Y1 or Y2 receptors are involved in the rescue of neurons from excitotoxic cell death,24, 25 the involvement of NPY Y4 receptor has not been evaluated in majority of these.
In the present study, we show that only the NPY Y5 receptor activation protects against glutamate-induced apoptotic cell death. Another study has linked the antiapoptotic effect of NPY in the hippocampus to the activation of NPY Y2 and Y5 receptors.23 The difference between ours and these results might be due to the differential expression of NPY receptors in retinal and hippocampal cultures, as well as to the involvement of different signaling pathways underlying the neuroprotective effects.
We have also found that three different NPY receptors are involved in the neuroprotective effect against necrotic cell death induced by glutamate in rat retinal cell cultures. Similarly, other groups have also shown that activation of different NPY receptors can induce the same biological effect.23, 25, 46, 47 For example, NPY inhibits KCl-evoked [Ca2+]i increase in retinal neurons through the activation of NPY Y1,Y4 and Y5 receptors. There are two possible main explanations for this: (1) the formation of homo- or hetero-dimers between different NPY receptors;48, 49, 50, 51 and (2) heterogenous distribution of these receptors through the different cell types present in the culture.
To obtain a better understanding of the intracellular mechanisms underlying the NPY neuroprotective role against necrotic cell death induced by glutamate, we have looked at the possible involvement of various pathways. The NPY protective effect may be linked to its inhibitory effect on glutamate release, as found previously in hippocampus.21, 22 In rat retinal cultures, NPY inhibits both the [Ca2+]i increase induced by KCl46 and the aspartate release in these cultures (unpublished observations). NPY neuroprotection has also been associated with the involvement of ERK1/2 and Akt pathways in a Parkinson's disease model.26 In this study, we have suggested that the NPY neuroprotective role is mediated by PKA and p38K activation. The PKA inhibitor, H89, blocked the neuroprotective effect of NPY, while forskolin, a PKA activator, presented a similar protective effect to NPY, suggesting the involvement of this particular kinase in the neuroprotective effect of NPY against glutamate-induced necrotic cell death in rat retinal cells. PKA activation by NPY has been previously shown. For example, NPY has a biphasic modulatory effect on [Ca2+]i increases induced by ATP, mediates the upregulated mRNA expression of gonadotropin-releasing hormone in a neuroblastoma cell line and induces cathecolamine release in human adrenal chromaffin cells, through the activation of PKA.52, 53, 54 However, there is also evidence showing that NPY inhibits PKA. The activation of NPY receptors inhibits both the axonal transport in sensory neurons and cell proliferation in vascular smooth muscle cells, with these effects being mediated by PKA inhibition.4, 47, 55, 56, 57
We also show that NPY activates p38K, and this enzyme, as PKA, appears to mediate, at least partially, the neuroprotective role of NPY against glutamate-induced cell death. In retinal Müller cells, the activation of NPY Y1 receptors activates p38 MAPK.58 Moreover, p38K activation protects ARPE-19 cells (retinal pigment epithelium cells) against cell death triggered by pro-oxidant conditions.59
In conclusion, NPY can have a neuroprotective role against necrotic and apoptotic cell death induced by glutamate in rat retinal cells both in cultured cells and in situ in the retina. NPY, by activating NPY Y2, Y4 and Y5 receptors, protects retinal cells against glutamate-induced necrosis and is also able to protect against retinal cell apoptosis by activating NPY Y5 receptors. In addition, PKA and p38K mediate the neuroprotective effects of NPY. We believe these results might be useful to devise novel pharmacologic targets and therapies to treat retinal degenerative diseases, such as glaucoma and diabetic retinopathy.
Materials and Methods
Primary rat retinal neural cell cultures
Three-to-five-day old Wistar rat pups were used to prepare primary rat retinal cell cultures, as previously described.8, 60 All procedures involving animals were in agreement with the Association for Research in Vision and Ophthalmology (ARVO) statement on vision and ophthalmic research for experimental models. Briefly, rat retinas were dissected under sterile conditions, using a light microscope, in Ca2+- and Mg2+-free Hanks' balanced salt solution (in mM: 137 NaCl, 5.4 KCl, 0.45 KH2PO4, 0.34 Na2HPO4, 4 NaHCO3, 5 glucose, pH 7.4) and digested with 0.1% trypsin (w/v, Gibco, Life Technologies Corporation, Paisley, UK) for 15 min at 37 °C. Cells were plated on glass coverslips coated with poly-D-lysine (0.1 mg/ml, Sigma-Aldrich Co. LLC, St. Louis, MO, USA) using Minimum Essential Medium Eagle (Sigma-Aldrich), supplemented with 25 mM HEPES (Sigma-Aldrich), 26 mM NaHCO3, 10% fetal bovine serum (Gibco) and penicillin (100 U/ml)–streptomycin (100 mg/ml, Gibco) for 8/9 days (37 °C, 5% CO2), at a density of 2 × 106cells/cm2.
Animals
Adult male Wistar rats (250–300 g bodyweight, Charles River, France) were housed in a temperature- and humidity-controlled environment and were provided with standard rodent diet and water ad libitum, while kept on a 12 h light/12 h dark cycle. All procedures involving the animals were in agreement to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Four different groups of animals were used: control (saline) injected, glutamate injected, glutamate+NPY injected, and NPY injected.
Intravitreal injections
The rats were anesthetized by isoflurane inhalation using a gas-anesthetizing system (VetEquip, Pleasanton, CA, USA). Then, oxybuprocaine (4 mg/ml; Laboratórios Edol, Linda-a-Velha, Portugal) anesthetic was applied topically to the eyes and the pupils were dilated with tropicamide (10 mg/ml; Laboratórios Edol). Using a Hamilton syringe (Hamilton, Reno, NV, USA) with 33-gauge needle, 3 μl of 0.78 mM NPY (total amount 2.35 nmol) or 3 μl of 167 mM glutamate (total amount 500 nmol) or sterile saline solution (0.9% sodium chloride; Fresenius Kabi, Carnaxide, Portugal) were intravitreally injected. Control group was injected with saline solution while NPY group was injected with 2.35 nmol NPY. Glutamate group was injected with saline and 2 h later with 500 nmol glutamate. Finally, glutamate+NPY group was injected with 2.35 nmol NPY and 2 h later with 500 nmol glutamate. Fusidic acid (10 mg/g; Leo Pharmaceutical, Ballerup, Denmark) ointment was applied in the conjunctival sac at the end of the experiment. The animals were killed 24 h after glutamate (or saline) injection.
Frozen retinal sections
Under deep anesthesia (75 mg/kg ketamine and 10 mg/kg xylazine), rats were transcardially perfused with phosphate-buffered saline (PBS; pH 7.4), followed by 4% (w/v) paraformaldehyde (PFA) in PBS. The eyes were enucleated, washed in PBS and then transferred to PFA for 1 h. The cornea was removed and the eye cup was further fixed for 1 h in PFA. After washing in PBS, the eyes were cryopreserved by placing the eye cup in 15% (w/v) sucrose in PBS for 1 h and then in 30% (w/v) sucrose in PBS overnight at 4 °C. Finally, the eye cup was embedded in tissue-freezing medium (OCT; Sakura Finetek Europe B.V., AJ Alphen aan den Rijn, The Netherlands), the frozen blocks were cut into 10 μm thickness sections in a cryostat and the cryosections were then collected on SuperFrost Plus glass slides (Menzel-Glaser, Braunschweig, Germany) and stored at −20 °C.
Immunocytochemistry
After treatment, cells cultured on glass coverslips were washed twice with PBS and fixed in 4% PFA (20 min; room temperature (RT)). The cells were then permeabilized with 1% Triton X-100 for 5 min, and blocked with 3% (w/v) fatty acid-free bovine serum albumin (BSA, Sigma-Aldrich), supplemented with 0.2% Tween 20, to prevent nonspecific binding, for 1 h at RT. Cells were incubated with primary antibodies for 90 min at RT: rabbit anti-GFAP (1 : 400; Dako, Glostrup, Denmark); mouse anti-GFAP protein (1 : 500, Sigma-Aldrich); rat anti-CD11b or mouse anti-CD11b (1 : 200; AbD Serotec, Kidlington, UK); mouse anti-TUJ1 (1 : 500, Covance Research Products Inc, Berkeley, CA, USA); anti-vimentin (1 : 400, Thermo Fisher Scientific, Waltham, MA, USA); rabbit anti-cleaved caspase 3 (1 : 1600, Cell Signaling Technology, Danvers, MA, USA); mouse anti-CD68/ED1 (1 : 200, AbD Serotec); mouse anti-NeuN (1 : 400, Merck Millipore, Billerica, MA, USA). All antibody solutions were prepared in 3% fatty acid-free BSA solution.
After washing, the cells were incubated for 1 h at RT with secondary antibodies: Alexa 488 anti-mouse IgG, Alexa 594 anti-rat IgG or Alexa 594 anti-rabbit IgG (1 : 200, Invitrogen, Life Technologies Corporation, Paisley, UK). Finally, after 5 min washing, cell nuclei were stained with Hoechst 33342 (1 mg/ml in PBS, Molecular Probes, Eugene, OR, USA) for 5 min, and, following rinsing twice with PBS, the coverslips were mounted on glass slides using Dako Fluorescent mounting medium (Dako). Cells were visualized using a fluorescence microscope (Zeiss Axioshop 2 Plus) coupled to a digital camera (Axiocam HRc) and a scanning laser confocal microscope LSM 510 META (Zeiss, Jena, Germany). Images were analyzed using Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA, USA) or ImageJ (National Institutes of Health, Bethesda, MD, USA), as indicated in figure legends.
The number of cleaved caspase 3-positive cells was counted in 10–12 random fields on each coverslip, while CD11b and CD68/ED 1-positive cells were counted in 6 random fields. The number of TUJ1-, GFAP- and NeuN-positive cells was counted in 10 random z-stacks. The average number of cleaved caspase 3-, CD11b- or CD68/ED 1-, TUJ1-, GFAP- and NeuN-positive cells per random field was determined for each condition tested (control – no drug; NPY; glutamate; and glutamate+NPY).
Immunoreactivity was quantified on micrographs taken after immunocytochemical experiments. Images were acquired using identical settings. The fluorescence levels (arbitrary units) were quantified using image analysis software (Image J), considering the mean grey value in six random fields per coverslip of at least three independent experiments. Negative controls were stained without primary antibodies per each immunocytochemistry performed.
Immunohistochemistry
Eye sections were fixed with acetone for 10 min at −20 °C, permeabilized in PBS containing 0.25% Triton X-100 (Sigma) for 30 min, blocked in PBS containing 10% newborn goat serum (Gibco) and 1% BSA for 30 min and incubated with a mouse anti-Brn3a (retinal ganglion cell marker; 1 : 200; Millipore) overnight at 4 °C, in a closed humidified plastic container. After washing, slices were incubated with Alexa 568 anti-mouse IgG (1 : 200, Invitrogen) for 1 h at RT.
Cell viability studies
Hoechst staining
The Hoechst33342 marker was used to label cell nuclei in rat retinal cells in culture. The Hoechst33342 fluorescence intensity was evaluated in images captured in a confocal microscope (LSM 510 Meta; Zeiss) using identical settings and image analysis software (Image J). The fluorescence intensity was obtained by the mean grey value in six random fields per coverslip of at least three independent experiments. The average of mean grey value was determined in arbitrary units in each experimental condition, and the results were expressed as a percentage of control.
PI staining
PI [3,8-Diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide, Sigma-Aldrich] is a polar substance that only stains the nucleus of dead or dying cells with disrupted cell membranes. In cells undergoing necrosis or late apoptosis, PI binds to DNA, emitting a bright red fluorescence (630 nm) when excited by blue–green light (493 nm). Cells plated on coverslips were exposed to 100, 250 or 500 μM glutamate (Sigma-Aldrich) for 24 h at 37 °C. In order to test for a potential protective role of NPY, cells were incubated with 100 nM NPY (Novabiochem, Laufelfingen, Switzerland) at three different times: 1 h before exposure to glutamate (500 μM), simultaneously with the addition of glutamate and 30 min after exposure to glutamate. The agonists for NPY receptors (Y1 ([Leu31Pro34]NPY), Y2 (NPY13–36), Y4 (r-PP) and Y5 ((Gly,1Ser,3,22Gln,4,34Thr,6Arg,19Tyr,21Ala,23,31Aib32)PP), 100 nM, Bachem, Bubendorf, Switzerland) were also tested 1 h before exposure to glutamate. Cells were incubated with antagonists of NPY receptors (Y1 (BIBP3226); Y2 (BIIE0246) and Y5 (L-152,804), 1 μM, Tocris Bioscience, Bristol, UK) 30 min before incubation with the agonists of these receptors.
Inhibitors of key proteins in important signaling pathways were used to elucidate the signaling pathways mediating the neuroprotective effect of NPY against glutamate. The inhibitors were introduced 1 h before glutamate addition. H89 (1 μM, Tocris Bioscience was used as a PKA inhibitor. SB203580 (1 μM), L-NAME (500 μM), calphostin C (10 nM), LY294002 (1 μM) and U0126 (1 μM) were used as inhibitors of p38K inhibitor, NOS, PKC, PI3K and MEK1/2 proteins (Merck Millipore), respectively. After 24 h exposure to glutamate, cells were washed twice and incubated with PI (7.5 μM) for 10 min, washed again twice and fixed with 4% PFA for 20 min. Cells were then observed with a fluorescence microscope (Zeiss Axioshop 2 Plus) coupled to an Axiocam HRc camera. The number of PI-positive cells was counted in six random fields on each coverslip (two per condition), and the average number of PI-positive cells per random field was determined for each condition tested.
TUNEL assay
In primary cell cultures
Cells cultured on coverslips were exposed to 500 μM glutamate for 24 h, with the drugs described above. After incubation, cells were washed twice and then incubated for 1 h at 37 °C with the TUNEL mix (in situ cell death kit; Roche Applied Science, Mannheim, Germany), washed again and, finally, nuclei were stained with Hoechst33342 for 5 min. Coverslips were mounted in Dako mounting media and images were acquired on a Zeiss PALM Microscope. The number of TUNEL-positive cells was counted in six random fields on each coverslip (two per condition), and the average number of TUNEL-positive cells per random field was determined for each condition tested.
In frozen retinal slices
After immunostaining with an anti-Brn3a antibody and the corresponding secondary antibody, TUNEL assay was performed in retinal sections according to the manufacturer's instructions (Promega, Madison, WI, USA). Nuclei were counterstained with DAPI (1 : 2000). The sections were coverslipped using Glycergel mounting medium (Dako) and visualized in a fluorescence microscope (Leica DM IRE2, Wetzlar, Germany). Images were acquired from the four retinal sections distanced 50 μm from each other per each group. Images from six random fields were taken along each retinal section. The number of TUNEL-positive cells in the ONL, INL and GCL was counted and expressed as an average number of TUNEL-positive cells per random field from the four retinal sections. The number of Brn3a immunoreactive cell bodies was determined in images from six random fields per retinal section of a total of four retinal sections, and expressed as an average number of Brn3a-positive cells per random field from the four retinal sections.
Statistical analysis
All data are presented as mean±S.E.M. Statistical analysis was performed using analysis of variance followed by Bonferroni's post-hoc test, as indicated in the figure legends.
Table 1. Proportion of overall variation in viral fitness and virulence explained by main effects of host genotype and virus genotype versus the host-genotype × virus-genotype interaction effect.
|
% Variance in viral fitness explained by |
% Variance in disease virulence explained by |
|||||
|---|---|---|---|---|---|---|
| Host genotype | Virus genotype | H × V interaction | Host genotype | Virus genotype | H × V interaction | |
| Unpassaged virus | 56% | N.A. | N.A. | 82% | N.A. | N.A. |
| Post-passage virus | 9% | 11% | 51% | 32% | 11% | 59% |
GLM analyses (i.e., three individual GLM models), including either host genotype, virus genotype, or the host-genotype by virus-genotype (H × V) interaction effect, were used to estimate the percentage of overall variation in viral fitness and virulence explained by these independent variables. Thus, the above table summarizes pooled results from nine independent GLM analyses (i.e., three models for each effect used to analyze data sets from our three dependent variables (proviral load, infectious particle counts, and splenomegaly)). “N.A.” refers to the fact that there is only one virus genotype (i.e., the unpassaged virus stock). Therefore, there is no effect of virus genotype or a host-genotype × virus-genotype interaction effect during infection with unpassaged virus. The percentages reported for viral fitness represent the average between proviral load and infectious particle estimates. See Materials and Methods for a description on how percentage of variation estimates were calculated. One caveat to the variance estimates summarized above is that differences in the numbers of degrees of freedom between models can influence the accuracy with which each model estimates the relative contribution of each effect on overall variation. We would like to acknowledge that the degrees of freedom available for each of our model effects are different (host genotype effect (d.f.=4), virus genotype effect (d.f.=8), host-genotype × virus-genotype interaction model (d.f.=23)). Therefore, compared with the interaction effect, models estimating the contribution of host genotype and virus genotype are likely to reflect less accurate estimates of the true contribution of each of these variables. Results of independent GLM analyses are provided in Supplementary Tables 6–9.
Acknowledgments
This work was supported by the Portuguese Foundation for Science and Technology, FEDER, and COMPETE (SFRH/BD/45311/2008, PTDC/SAU-NEU/73119/2006; PTDC/SAU-NEU/099075/2008; PTDC/NEU-OSD/1113/2012; PEst-/SAU/LA0001/2011; PEst-C/SAU/UI3282/2011).
Glossary
- GCL
ganglion cell layer
- GFAP
glial fibrillary acidic protein
- INL
inner nuclear layer
- L-NAME
L-NG-nitroarginine methyl ester
- CNS
central nervous system
- MDMA
3,4-methylenedioxy-N-methylamphetamine
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- ONL
outer nuclear layer
- PI
propidium iodide
- RT
room temperature
- BSA
bovine serum albumin
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- TUNEL
terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling
The authors declare no conflict of interest.
Footnotes
Edited by A Verkhratsky
References
- Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005;4:331–347. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Michel MC. Receptors for neuropeptide Y: multiple subtypes and multiple second messengers. Trends Pharmacol Sci. 1991;12:389–394. doi: 10.1016/0165-6147(91)90610-5. [DOI] [PubMed] [Google Scholar]
- Silva AP, Cavadas C, Grouzmann E. Neuropeptide Y and its receptors as potential therapeutic drug targets. Clin Chim Acta. 2002;326:3–25. doi: 10.1016/s0009-8981(02)00301-7. [DOI] [PubMed] [Google Scholar]
- Bruun A, Tornqvist K, Ehinger B. Neuropeptide Y (NPY) immunoreactive neurons in the retina of different species. Histochemistry. 1986;86:135–140. doi: 10.1007/BF00493378. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Chalupa LM. Development of neuropeptide Y immunoreactive amacrine and ganglion cells in the pre- and postnatal cat retina. J Comp Neurol. 1995;361:152–164. doi: 10.1002/cne.903610112. [DOI] [PubMed] [Google Scholar]
- Alvaro AR, Rosmaninho-Salgado J, Santiago AR, Martins J, Aveleira C, Santos PF, et al. NPY in rat retina is present in neurons, in endothelial cells and also in microglial and Muller cells. Neurochem Int. 2007;50:757–763. doi: 10.1016/j.neuint.2007.01.010. [DOI] [PubMed] [Google Scholar]
- D'Angelo I, Brecha NC. Y2 receptor expression and inhibition of voltage-dependent Ca2+ influx into rod bipolar cell terminals. Neuroscience. 2004;125:1039–1049. doi: 10.1016/j.neuroscience.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Kishida K, Naka KI. Amino acids and the spikes from the retinal ganglion cells. Science. 1967;156:648–650. doi: 10.1126/science.156.3775.648. [DOI] [PubMed] [Google Scholar]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- Dkhissi O, Chanut E, Wasowicz M, Savoldelli M, Nguyen-Legros J, Minvielle F, et al. Retinal TUNEL-positive cells and high glutamate levels in vitreous humor of mutant quail with a glaucoma-like disorder. Invest Ophthalmol Vis Sci. 1999;40:990–995. [PubMed] [Google Scholar]
- Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–2243. [PubMed] [Google Scholar]
- Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- Santiago AR, Hughes JM, Kamphuis W, Schlingemann RO, Ambrosio AF. Diabetes changes ionotropic glutamate receptor subunit expression level in the human retina. Brain Res. 2008;1198:153–159. doi: 10.1016/j.brainres.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, et al. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Silva AP, Pinheiro PS, Carvalho AP, Carvalho CM, Jakobsen B, Zimmer J, et al. Activation of neuropeptide Y receptors is neuroprotective against excitotoxicity in organotypic hippocampal slice cultures. FASEB J. 2003;17:1118–1120. doi: 10.1096/fj.02-0885fje. [DOI] [PubMed] [Google Scholar]
- Silva AP, Xapelli S, Pinheiro PS, Ferreira R, Lourenco J, Cristovao A, et al. Up-regulation of neuropeptide Y levels and modulation of glutamate release through neuropeptide Y receptors in the hippocampus of kainate-induced epileptic rats. J Neurochem. 2005;93:163–170. doi: 10.1111/j.1471-4159.2004.03005.x. [DOI] [PubMed] [Google Scholar]
- Smialowska M, Domin H, Zieba B, Kozniewska E, Michalik R, Piotrowski P, et al. Neuroprotective effects of neuropeptide Y-Y2 and Y5 receptor agonists in vitro and in vivo. Neuropeptides. 2009;43:235–249. doi: 10.1016/j.npep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Xapelli S, Bernardino L, Ferreira R, Grade S, Silva AP, Salgado JR, et al. Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: a role for microglia. Eur J Neurosci. 2008;27:2089–2102. doi: 10.1111/j.1460-9568.2008.06172.x. [DOI] [PubMed] [Google Scholar]
- Xapelli S, Silva AP, Ferreira R, Malva JO. Neuropeptide Y can rescue neurons from cell death following the application of an excitotoxic insult with kainate in rat organotypic hippocampal slice cultures. Peptides. 2007;28:288–294. doi: 10.1016/j.peptides.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Decressac M, Pain S, Chabeauti PY, Frangeul L, Thiriet N, Herzog H, et al. Neuroprotection by neuropeptide Y in cell and animal models of Parkinson's disease. Neurobiol Aging. 2012;33:2125–2137. doi: 10.1016/j.neurobiolaging.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Croce N, Dinallo V, Ricci V, Federici G, Caltagirone C, Bernardini S, et al. Neuroprotective effect of neuropeptide Y against beta-amyloid 25-35 toxicity in SH-SY5Y neuroblastoma cells is associated with increased neurotrophin production. Neurodegener Dis. 2011;8:300–309. doi: 10.1159/000323468. [DOI] [PubMed] [Google Scholar]
- Rose JB, Crews L, Rockenstein E, Adame A, Mante M, Hersh LB, et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J Neurosci. 2009;29:1115–1125. doi: 10.1523/JNEUROSCI.4220-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro AR, Martins J, Costa AC, Fernandes E, Carvalho F, Ambrosio AF, et al. Neuropeptide Y protects retinal neural cells against cell death induced by ecstasy. Neuroscience. 2008;152:97–105. doi: 10.1016/j.neuroscience.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Streit WJ.Neuroglia2nd edn.Kettenmann H, Ransom BR, (eds). Oxford University Press: New York, NY, USA; 2005 [Google Scholar]
- Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, et al. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YF, Li SB. Neuropeptide Y expression in mouse hippocampus and its role in neuronal excitotoxicity. Acta Pharmacol Sin. 2005;26:63–68. doi: 10.1111/j.1745-7254.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P. Muller glial cells in retinal disease. Ophthalmologica. 2011;227:1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- Christensen RN, Ha BK, Sun F, Bresnahan JC, Beattie MS. Kainate induces rapid redistribution of the actin cytoskeleton in ameboid microglia. J Neurosci Res. 2006;84:170–181. doi: 10.1002/jnr.20865. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Skutella T, Hailer NP, Haas D, Nitsch R. Activated microglial cells migrate towards sites of excitotoxic neuronal injury inside organotypic hippocampal slice cultures. Eur J Neurosci. 1998;10:3284–3290. doi: 10.1046/j.1460-9568.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Cortes L, Cochaud S, Agasse F, Silva AP, et al. Neuropeptide Y inhibits interleukin-1 beta (IL-1beta)-induced microglia motility. J Neurochem. 2011;120:93–105. doi: 10.1111/j.1471-4159.2011.07541.x. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Viegas M, Cortes L, Bernardino L, Vieira OV, et al. Neuropeptide Y inhibits interleukin-1beta-induced phagocytosis by microglial cells. J Neuroinflammation. 2011;8:169. doi: 10.1186/1742-2094-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Ribeiro CF, Malva JO, Silva AP. Protective role of neuropeptide Y Y(2) receptors in cell death and microglial response following methamphetamine injury. Eur J Neurosci. 2012;36:3173–3183. doi: 10.1111/j.1460-9568.2012.08232.x. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Zeiss CJ, Johnson EA. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Invest Ophthalmol Vis Sci. 2004;45:971–976. doi: 10.1167/iovs.03-0301. [DOI] [PubMed] [Google Scholar]
- Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci. 2005;46:2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215:685–691. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Alvaro AR, Rosmaninho-Salgado J, Ambrosio AF, Cavadas C. Neuropeptide Y inhibits [Ca2+]i changes in rat retinal neurons through NPY Y1, Y4, and Y5 receptors. J Neurochem. 2009;109:1508–1515. doi: 10.1111/j.1471-4159.2009.06079.x. [DOI] [PubMed] [Google Scholar]
- Son MY, Kim MJ, Yu K, Koo DB, Cho YS. Involvement of neuropeptide Y and its Y1 and Y5 receptors in maintaining self-renewal and proliferation of human embryonic stem cells. J Cell Mol Med. 2011;15:152–165. doi: 10.1111/j.1582-4934.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinger MC, Bader JE, Kobor AD, Kretzschmar AK, Beck-Sickinger AG. Homodimerization of neuropeptide y receptors investigated by fluorescence resonance energy transfer in living cells. J Biol Chem. 2003;278:10562–10571. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- Berglund MM, Schober DA, Esterman MA, Gehlert DR, Neuropeptide Y. Y4 receptor homodimers dissociate upon agonist stimulation. J Pharmacol Expe Ther. 2003;307:1120–1126. doi: 10.1124/jpet.103.055673. [DOI] [PubMed] [Google Scholar]
- Movafagh S, Hobson JP, Spiegel S, Kleinman HK, Zukowska Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J. 2006;20:1924–1926. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- Silva AP, Carvalho AP, Carvalho CM, Malva JO. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology. 2003;44:282–292. doi: 10.1016/s0028-3908(02)00382-9. [DOI] [PubMed] [Google Scholar]
- Dhillon SS, Gingerich S, Belsham DD. Neuropeptide Y induces gonadotropin-releasing hormone gene expression directly and through conditioned medium from mHypoE-38 NPY neurons. Regul Pept. 2009;156:96–103. doi: 10.1016/j.regpep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J, Araujo IM, Alvaro AR, Mendes AF, Ferreira L, Grouzmann E, et al. Regulation of catecholamine release and tyrosine hydroxylase in human adrenal chromaffin cells by interleukin-1beta: role of neuropeptide Y and nitric oxide. J Neurochem. 2009;109:911–922. doi: 10.1111/j.1471-4159.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- Soares Lemos V, Bucher B, Takeda K. Neuropeptide Y modulates ATP-induced increases in internal calcium via the adenylate cyclase/protein kinase A system in a human neuroblastoma cell line. Biochem J. 1997;321 (Pt 2:439–444. doi: 10.1042/bj3210439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J, Kitlinska J, Jacques D, Perreault C, Nader M, Everhart L, et al. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol. 2008;86:438–448. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma H, Saito A, Kusakabe T, Takenaka T, Kawakami T. Neuropeptide Y inhibits axonal transport of particles in neurites of cultured adult mouse dorsal root ganglion cells. J Physiol. 2002;543 (Pt 1:85–97. doi: 10.1113/jphysiol.2002.020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellieux C, Sauthier T, Domenighetti A, Marsh DJ, Palmiter RD, Brunner HR, et al. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc Natl Acad Sci USA. 2000;97:1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. Neuropeptide Y-evoked proliferation of retinal glial (Muller) cells. Graefes Arch Clin Exp Ophthalmol. 2004;242:944–950. doi: 10.1007/s00417-004-0954-3. [DOI] [PubMed] [Google Scholar]
- Pocrnich CE, Liu H, Feng M, Peng T, Feng Q, Hutnik CM. p38 mitogen-activated protein kinase protects human retinal pigment epithelial cells exposed to oxidative stress. Can J Ophthalmol. 2009;44:431–436. doi: 10.3129/i09-109. [DOI] [PubMed] [Google Scholar]
- Santiago AR, Pereira TS, Garrido MJ, Cristovao AJ, Santos PF, Ambrosio AF. High glucose and diabetes increase the release of [3H]-d-aspartate in retinal cell cultures and in rat retinas. Neurochem Int. 2006;48:453–458. doi: 10.1016/j.neuint.2005.10.013. [DOI] [PubMed] [Google Scholar]