Abstract
Sirtuin enzymes are a family of highly conserved protein deacetylases that depend on nicotinamide adenine dinucleotide (NAD+) for their activity. There are seven sirtuins in mammals and these proteins have been linked with caloric restriction and aging by modulating energy metabolism, genomic stability and stress resistance. Sirtuin enzymes are potential therapeutic targets in a variety of human diseases including cancer, diabetes, inflammatory disorders and neurodegenerative disease. Modulation of sirtuin activity has been shown to impact the course of several aggregate-forming neurodegenerative disorders including Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and spinal and bulbar muscular atrophy. Sirtuins can influence the progression of neurodegenerative disorders by modulating transcription factor activity and directly deacetylating proteotoxic species. Here, we describe sirtuin protein targets in several aggregate-forming neurodegenerative diseases and discuss the therapeutic potential of compounds that modulate sirtuin activity in these disorders.
Keywords: sirtuin, histone deacetylase, Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, spinal and bulbar muscular atrophy
Introduction
Silent Information Regulator 2 (Sir2) proteins, also known as sirtuins, were originally identified as genetic silencing factors1,2 and were later found to prolong lifespan in yeast3,4. Sir2 is a deacetylase that acts on histones and other proteins in the presence of nicotinamide adenine dinucleotide (NAD+)5 and it also possesses mono-ADP-ribosyltransferase activity6, functions that are conserved in eukaryotic organisms7. NAD-dependent deacetylation by sirtuins was later linked with caloric restriction and aging in several organisms4,8. These findings have launched a new field within the discipline of biology with an increasing number of laboratories devoted to studying the role of mammalian sirtuins during normal cellular senescence and in aging-related diseases9.
In mammals, there are seven members of the sirtuin family and they have been associated with protection against diseases of aging by a variety of mechanisms such as regulation of stress response, apoptosis and DNA repair10,11,12,13,14. Sirtuins are categorized as class III histone deacetylases (HDACs); however, it is worth noting that not all sirtuin substrates are histones and several members of this protein family do not have deacetylase activity15,16. Phylogenetic analysis suggests that HDACs evolved before histones and a major function of these enzymes is the deacetylation of non-histone substrates15,16. Some researchers have proposed that sirtuins should be renamed NAD-dependent deacylases to reflect the repertoire of enzymatic activities performed by these proteins17.
Based on sequence similarity, the sirtuins from eubacteria, archaea and eukaryotes are categorized into five groups that have varied enzymatic activities18. Class I sirtuins (SIRT1, SIRT2, and SIRT3) have robust deacetylase activity in the presence of NAD+19, whereas Class II sirtuins (SIRT4) have ADP-ribosyltransferase activity20. Class III sirtuins (SIRT5) have NAD-dependent demalonylase and desuccinylase activities in addition to deacetylase activity19,21,22. The class IV sirtuins (SIRT6 and SIRT7) are deacetylase enzymes that may be weaker and more substrate-specific than the class I deacetylases in vitro19,23,24,25,26,27, and SIRT6 has also been shown to have ADP-ribosyltransferase activity23,24,25. A fifth group of sirtuins, class U, has been identified in bacteria and is phylogenetically intermediate between class I and class IV sirtuins18.
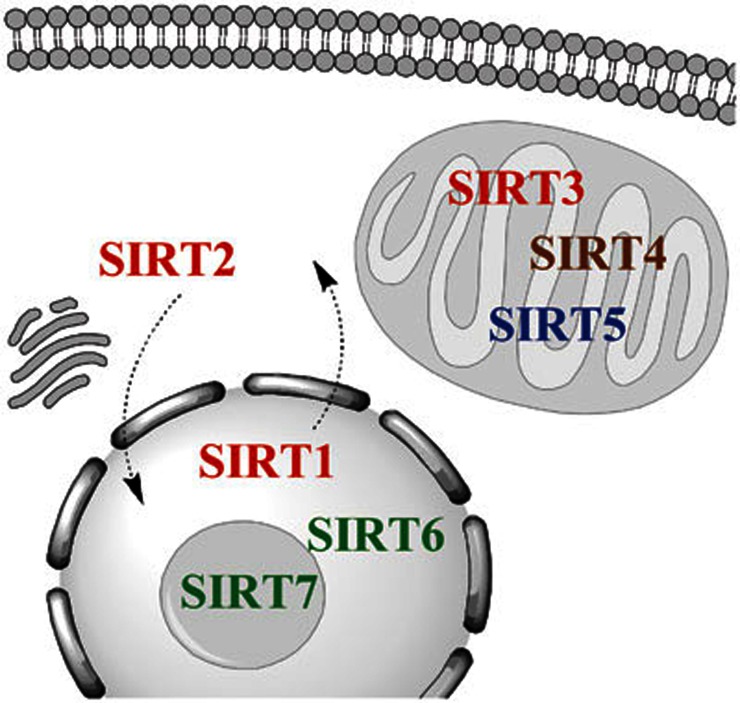
The seven mammalian sirtuins have different subcellular localizations, a significant consideration when evaluating their in vivo substrates. SIRT1 is generally thought to have a nuclear localization, although cytoplasmic SIRT1 has also been reported and may be associated with apoptosis, differentiation and oncogenic transformation28,29,30,31,32. SIRT2 is a predominantly cytoplasmic protein that has been shown to deacetylate tubulin, but may also shuttle to the nucleus where it functions as a mitotic checkpoint protein33,34. SIRT3, SIRT4 and SIRT5 are localized to the mitochondria, but have different enzymatic activities19,20,22,35. SIRT6 is a chromatin-associated nuclear protein24,25, and SIRT7 is localized to nucleoli36. The subcellular localizations and enzymatic activities for the mammalian sirtuins are diagrammed in Figure 1.
Figure 1.
Subcellular localization and function of the mammalian sirtuins. The seven sirtuins are categorized into four groups based on their sequence homology, and these proteins have varied enzymatic activities and subcellular localizations18. Class I sirtuins (SIRT1, SIRT2, and SIRT3) are depicted with red text. These proteins have robust deacetylase activity in the presence of NAD+19. SIRT1 is generally thought to have a nuclear localization, although cytoplasmic SIRT1 has also been reported in neurons and other cell types28,29,30,31,32, SIRT2 is a predominantly cytoplasmic protein that may shuttle to the nucleus33,34 and SIRT3 is a mitochondrial protein35. The class II sirtuin (SIRT4) is highlighted in brown. This protein has ADP-ribosyltransferase activity and is also a mitochondrial protein20. The class III sirtuin (SIRT5) is depicted in blue and this mitochondrial enzyme has NAD-dependent demalonylase and desuccinylase activities in addition to a weaker deacetylase activity19,21,22. The class IV sirtuins (SIRT6 and SIRT7) are in green text. SIRT6 is a nuclear protein with weak deacetylase activity and ADP-ribosyltransferase activity23,24,25, and SIRT7 is localized to nucleoli and it has deacetylase activity19,26,27.
Over the past few years, sirtuins have been explored in Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and spinal and bulbar muscular atrophy by a variety of techniques including in vitro assays, cell culture, animal models of neurodegenerative disease and studies of human tissue. In this review, we will summarize recent findings in sirtuin neurobiology, highlight the mechanism of action for sirtuins in these neurodegenerative disorders, and discuss the therapeutic potential of compounds that modulate sirtuin activity.
Alzheimer's disease
Alzheimer disease (AD) is the most common neurodegenerative disorder, affecting nearly half of all people over the age of eighty five37. AD is genetically heterogeneous and has been linked with mutations in genes encoding amyloid precursor protein (APP), presenilin 1 (PS1) and presenilin 2 (PS2) as well as the ε4 allele of apolipoprotein E. Amyloid precursor protein cleavage by the β- and γ-secretase complexes leads to the formation of amyloid-β (Aβ) peptides that can aggregate and form amyloid plaques. Amyloid plaques and neurofibrillary tangles comprising hyperphosphorylated tau protein are the pathologic hallmarks of the human disease. Current treatments are not curative; therefore, the validation of new therapeutic targets is crucial38.
The initial clues that modulation of sirtuin activity might affect AD pathology came from studies reporting that a sirtuin agonist, resveratrol, was able to attenuate cell death induced by Aβ and oxidized lipoproteins in cell culture models39,40,41. These cell culture findings were confirmed in several subsequent studies42,43 and resveratrol was found to enhance proteasome-mediated clearance of Aβ44. Several studies examining the effect of resveratrol in vivo found that this compound reduced the plaque burden in the brains of transgenic mice overexpressing APP45,46. The topic of whether resveratrol specifically modulates sirtuin activity via a direct or indirect mechanism that also involves AMP kinase (AMPK), phosphoinositide 3-kinase (PI3K) or other targets is controversial47,48,49,50,51,52,53. Several studies have also reported that resveratrol only potentiates SIRT1 activity in the presence of a fluorescent moiety that is used for in vitro pharmacologic screens49,54,55. A more recent paper suggests that resveratrol activates SIRT1 activity via an allosteric mechanism and the fluorescent tag used in in vitro assays mimics large hydrophobic residues present at positions -1 and -6 of substrates including PGC-1α and Foxo3a56. Therefore, in addition to drug studies, it is important to also use genetic strategies with concomitant evaluation of downstream targets to evaluate whether modulation of sirtuin pathways can account for a specific biological phenotype.
In 2005, a study directly linked neuroprotection with SIRT1 expression by using lentiviral-mediated overexpression, and protection from Aβ-induced neurotoxicity was observed in mixed cortical culture models57. The proposed mechanism was that SIRT1 and resveratrol reduced Aβ-stimulated NF-κB signaling in microglia. The following year, a second group confirmed that SIRT1 is neuroprotective in Aβ models of AD using a combination of cell culture systems and murine models. They found that neuronal SIRT1 expression decreased levels of ROCK1, a serine/threonine Rho kinase previously shown to regulate Aβ metabolism, and this effect enhanced α-secretase activity, thus promoting a non-amyloidogenic pathway for processing APP14,58.
Additional in vivo evidence that SIRT1 may ameliorate Aβ pathology was established in the APP/PS1 model of AD. In this model, overexpression of SIRT1 decreased plaque burden, improved behavioral phenotypes and potentiated α-secretase-mediated processing by deacetylating retinoic acid receptor β, a transcriptional activator of ADAM10. ADAM10 is a component of the α-secretase, which processes APP along an anti-amyloidogenic pathway that decreases formation of toxic Aβ42 species59.
Tau pathology is a major aspect of Alzheimer's disease pathology and neurofibrillary tangles distribution correlates with cognitive impairment in patients60. The first study exploring the effect of SIRT1 on neurodegenerative changes in a mouse model that exhibits tau pathology was published in 200713. This study used resveratrol and lentiviral-mediated SIRT1 overexpression in cell culture models and the p25 mouse model of AD, a transgenic line that recapitulates additional aspects of AD including hyperphosphorylated tau protein, neurofibrillary pathology and neuronal loss61. This paper reported behavioral effects including reduced learning impairment and molecular changes such as deacetylation of PGC-1α and p53 in the presence of active SIRT1, but the effect on tau and amyloid processing was not explored13.
A subsequent study using the sirtuin inhibitor nicotinamide found that tau phosphorylation is ameliorated in a triple transgenic model of AD. Neuroprotection by a sirtuin inhibitor may have been due to the use of a compound that affects multiple sirtuins in a mouse model that displays both tau and amyloid pathologies simultaneously12. Levels of acetylated α-tubulin increased in this study; therefore, the protective effect of nicotinamide may have been partially due to inhibition of SIRT212.
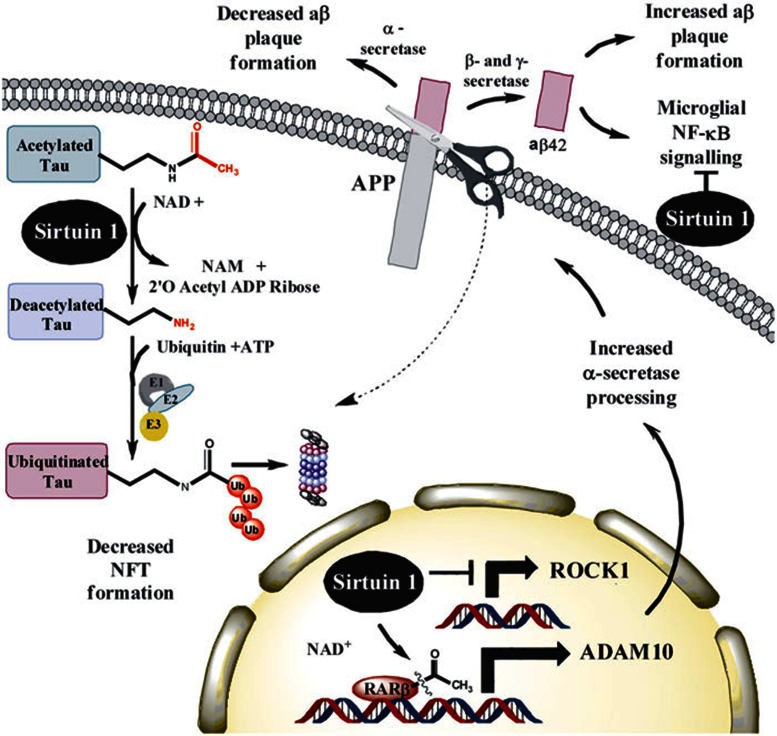
Subsequent work by a third group of researchers has found that SIRT1 deletion causes increased tau acetylation, phosphorylation, cognitive defects and early mortality in the P301L tau mouse model62,63. These observations are supported by in vitro and neuronal culture data indicating that SIRT1 can directly deacetylate tau protein at multiple residues. Studies on human tissue also indicate that tau is acetylated at an early stage during the disease process in patients. The mechanism proposed by these investigators is that removal of acetyl groups may expose lysine residues to ubiquitin ligases so that tau protein could be marked for proteasomal degradation62,64. Major mechanisms that have been proposed for SIRT1 in Alzheimer's disease pathogenesis are summarized in Figure 2.
Figure 2.
Mechanisms of activity for sirtuins in Alzheimer's disease. In vitro and neuronal culture data show that SIRT1 can directly deacetylate tau protein at multiple residues62,63,64. The mechanism proposed by these investigators is that removal of acetyl groups may expose lysine residues to ubiquitin ligases so that tau protein is marked for proteasomal degradation62,63. This process decreases accumulation of hyperphosphorylated PHF tau, cognitive defects and early mortality in the P301L tau mouse model62,63. Overexpression of SIRT1 has also been shown to decrease plaque burden and improve behavioral phenotypes by deacetylating retinoic acid receptor β, a transcriptional activator of ADAM10. ADAM10 is a component of the α-secretase, which processes APP along an anti-amyloidogenic pathway that decreases formation of toxic Aβ42 species59. An independent study has also shown that SIRT1 expression may decrease levels of ROCK1, a serine/threonine Rho kinase previously shown to regulate Aβ metabolism, and this effect also promotes the non-amyloidogenic α-secretase pathway14,58. The SIRT1 agonist resveratrol was also found to enhance proteasome-mediated clearance of Aβ44 and reduce the plaque burden in the brains of transgenic mice overexpressing APP45,46. Experiments using mixed cortical culture models have also shown that SIRT1 acts to reduce Aβ-stimulated NF-κB signaling in microglia57.
Although these preclinical studies provide compelling evidence that SIRT1 and resveratrol may influence both Aβ and neurofibrillary tau pathology in cell culture and animal models, the crucial test will be whether there is a clinical benefit for patients with AD. There are several ongoing or recently completed clinical trials that may address this issue by testing various formulations of resveratrol in AD patients. A phase II double blind, placebo-controlled trial sponsored by the Alzheimer's Disease Cooperative Study is currently recruiting patients with mild-to-moderate dementia who will be treated for 12 months with resveratrol or placebo. Evaluation using brain imaging and cerebrospinal fluid biomarkers are primary outcomes65. In addition, the Department of Veteran's Affairs is sponsoring a phase III trial to investigate the effects of resveratrol in combination with glucose and malate over 12 months in AD patients using cognitive testing as a primary outcome66. A third study evaluates the use of Etanercept, an anti-inflammatory agent that inhibits TNF-α, in combination with nutritional supplements including resveratrol, versus the nutritional supplements alone over a period of 6 weeks using cognitive testing as the primary outcome67. These clinical trials are an important first step in evaluating the safety and efficacy of targeting this pathway in human populations.
Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disorder, affecting 1% of the population over 60 years of age in industrialized countries68. PD is a movement disorder causing tremor, rigidity, bradykinesia and postural instability; however, cognitive and behavioral changes including sleep impairments, olfactory deficits and neuropsychiatric disorders can also manifest69. Neuropathologic analysis shows a substantial loss of dopaminergic neurons in the substantia nigra and accumulation of intracytoplasmic Lewy bodies, inclusions that contain α-synuclein and ubiquitin69. While there is no cure for PD, medications and surgery can improve some of the symptoms.
Activation or overexpression of SIRT1 and its homologues by genetic means or resveratrol treatment has been shown to be protective in cell culture, worm and mouse models of PD11,70,71,72,73. The mechanism for SIRT1 activity in this disease has been supported in multiple studies that have shown a role of SIRT1 in the activation of heat shock factor 1 (HSF1), which affects transcription of molecular chaperones including heat shock protein 70 that regulate homeostasis of cellular proteins11,74,75.
SIRT1 is not the only class III HDAC linked to a neuroprotective phenotype in PD. In 2007, researchers identified a compound that increases the inclusion size of α-synuclein aggregates and examined this molecule's in vitro activity. This small molecule was a SIRT2 inhibitor, and a secondary screen of structural analogues to identify more potent molecules was performed. The SIRT2 inhibitors showed dose-dependent rescue of α-synuclein-mediated toxicity in cell culture systems and were also able to protect dopaminergic neurons from cell death in a Drosophila model of PD76. While the exact mechanism for reducing α-synuclein-A53T-mediated cell death is not known, these compounds decreased the number but increased the size of α-synuclein aggregates in a cellular model. A recent study validated these observations showing that genetic deletion of SIRT2 is protective in a chemically induced mouse model of PD using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)77. The proposed mechanism is that SIRT2 becomes active as a response to MPTP-induced stress causing Foxo3a deacetylation, which leads to increased levels of the pro-apoptotic factor Bim and neuronal death77. Major mechanisms that have been proposed for SIRT1 and SIRT2 in Parkinson's disease pathogenesis are diagrammed in Figure 3.
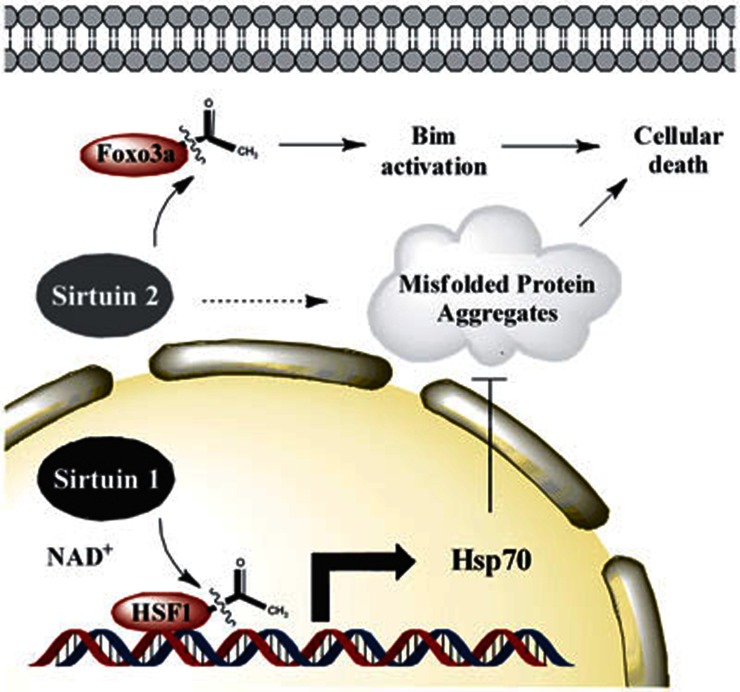
Figure 3.
Sirtuin-mediated deacetylation in Parkinson's disease. In PD, SIRT1 can deacetylate heat shock factor 1 (HSF1), which increases hsp70 transcription and decreases the formation of abnormal protein aggregates11,74,75. Genetic deletion of SIRT2 is protective in a chemically induced MPTP-model of PD77. It is proposed that SIRT2-mediated Foxo3a deacetylation leads to increased levels of the pro-apoptotic factor Bim and neuronal death77. Studies using small molecule inhibitors have shown that SIRT2 modulates the size and number of α-synuclein aggregates76.
The experimental evidence that SIRT1 and SIRT2 have opposing effects on PD models of neurodegeneration in vivo means that target specificity within this class of histone deacetylases is important. The identification of multiple compounds that affect sirtuin activity and improve PD pathogenesis is encouraging. With sufficient evidence from preclinical studies and more information about the safety of using these compounds, it will be possible to test whether these molecules will help patients suffering from these neurodegenerative disorders in the upcoming years.
Huntington's disease
Huntington's disease (HD) is an autosomal dominant neurological disorder characterized by cognitive dysfunction, personality changes, and loss of coordination and motor functions. It is caused by the expansion of a CAG repeat that codes for a stretch of glutamine residues, affecting the conformation and aggregation propensity of the huntingtin protein78. This disease causes increasing disability over many years and current treatments alleviate symptoms but are not curative.
Sirtuin deacetylases have been investigated as pharmacologic targets to slow the progression of HD in cell culture and animal models that recapitulate elements of the human disease. In nematode models of polyglutamine cytotoxicity, both resveratrol treatment and genetic overexpression of SIRT1 were shown to be neuroprotective79,80. This finding was confirmed in primary neuronal culture derived from knock-in mice carrying a mutant huntingtin protein79. However, the converse is true in Drosophila models where genetic or pharmacological reduction of either SIRT1 or SIRT2 homologues was shown to be neuroprotective81. It is not clear why sirtuins have different effects on HD pathogenesis in these animal systems.
Studies using pharmacologic approaches to altering sirtuin activity have also reported widely differing effects in mouse models of HD. In a chemically-induced model of HD, cognitive and motor deficits caused by 3-nitropropionic acid administration were improved by pretreating the animals with resveratrol82. However, treatment with resveratrol did not improve survival in the N171-82Q mouse model of HD, a transgenic line that overexpresses a truncated huntingtin protein, although there was some improvement in peripheral tissues83. A third study used the HDR6/1mouse model, which overexpresses a moderately sized fragment of N-terminal huntingtin. These mice were treated with nicotinamide, a chemical inhibitor that may affect multiple sirtuin proteins. This treatment increased BDNF and PGC-1α gene expression and improved HD-associated motor deficits, but did not reduce huntingtin aggregation or weight loss in these mice. All three studies taken together suggest that resveratrol and nicotinamide may be useful therapeutic compounds; however, pharmacokinetic and pharmacodynamics studies should be performed to confirm that active metabolites indeed penetrate disease-relevant tissues and cause changes in HD-relevant molecular targets.
Genetic models of SIRT1 overexpression have provided more clarity with multiple mouse models showing that SIRT1 is protective against mutant huntingtin neurotoxicity. Using the R6/2 model, a transgenic mouse that overexpresses truncated N-terminal huntingtin, Jeong et al.84 showed that deleting the catalytic exon of SIRT1 exacerbated the disease, while overexpressing SIRT1 by 2-3-fold improved survival and reduced protein aggregation in HD. A second study used a truncated huntingtin N171-82Q model in addition to a full-length huntingtin protein model and found that SIRT1 overexpression improved motor function and attenuated brain atrophy in these transgenic mouse lines10.
Although both studies showed a clear improvement in HD symptoms, several mechanisms for the role of SIRT1 were proposed. In the disease state, mutant huntingtin interacts directly with SIRT1, inhibiting its enzymatic activity. One possible mechanism is that SIRT1 deacetylates TORC1, facilitating its interaction with CREB and transcription of BDNF, a neurotrophic factor that is protective in HD84. Other possible consequences of the inhibition of SIRT1 activity by mutant huntingtin include decreased activation of TrkB, the receptor for BDNF, and Foxo3a, a transcription factor linked to BDNF and DARPP32 expression in neurons10,78,84. While there are several plausible explanations, the basic observation that SIRT1 is protective has been described in three genetic mouse models of HD. Further validation studies will examine the regulation of transcriptional networks by SIRT1 and its use as a therapeutic target in HD.
SIRT2 has also been investigated in the context of HD and pharmacologic inhibition has been shown to decrease neurodegeneration in Drosophila, cell culture and mouse models85,86. The mechanism for this phenomenon has been linked with SIRT2 inhibitor-mediated decrease in sterol biosynthesis, a pathway that has been shown to be dysfunctional in HD models85. Cholesterol metabolism may affect myelination and synapse maintenance, however, the mechanism by which cholesterol homeostasis affects HD pathophysiology has not been fully elucidated87. Genetic deletion of SIRT2 did not affect disease progression, α-tubulin acetylation, or biosynthesis of cholesterol in the R6/2 mouse model of HD88. It is plausible that compensatory changes in other proteins may occur when SIRT2 is deleted in the mouse germline, whereas SIRT2 inhibitors produce an acute reduction in the protein's activity. It is also possible that changes in sterol biosynthetic pathways induced by SIRT2 inhibitors may be due to off-target effects from these compounds. More recently, viniferin, a naturally occurring resveratrol derivative, was found to be protective in cell culture models of HD. This compound increases SIRT3 levels, causing deacetylation of MnSOD and LKB89. As a result, activation of AMPK by its upstream kinase LKB promotes mitochondrial biogenesis and increases cell survival in HD models89. The major mechanisms that have been proposed for sirtuins in HD pathogenesis are diagrammed in Figure 4. While the mechanism for neuroprotection in HD is still unclear, multiple research groups have identified compounds that target three distinct sirtuins and appear to be protective in mouse models of HD. Additional preclinical studies will need to explore their safety profiles and whether they may also be of benefit in patients.
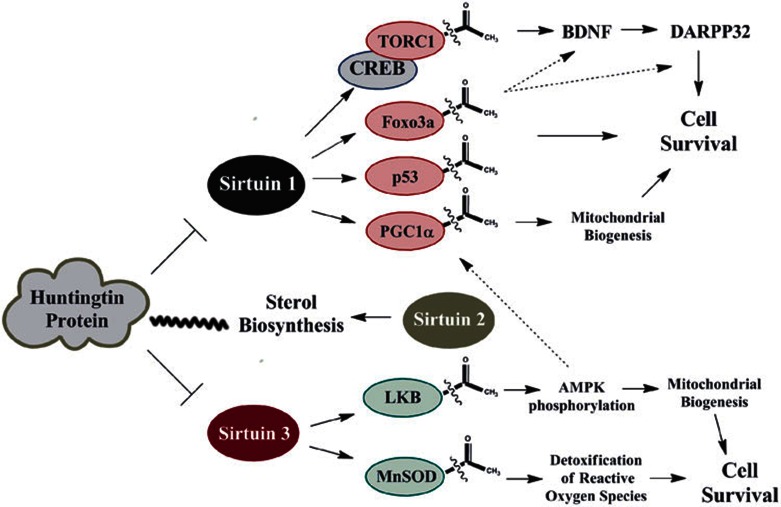
Figure 4.
Sirtuin targets in Huntington's disease. Mutant huntingtin protein can directly inhibit SIRT1 activity, affecting multiple downstream targets. According to one model, SIRT1 deacetylates TORC1, facilitating its interaction with CREB, which is linked to BDNF and DARPP32 expression in neurons10,78,84. Other possible consequences of the inhibition of SIRT1 activity by mutant huntingtin include the decreased deacetylation and altered activities of Foxo3a, p53 and PGC-1α10. In addition, sterol biosynthesis is dysregulated in HD (indicated by the wavy line) and studies using SIRT2 inhibitors have shown decreased neurodegeneration accompanied with decreased sterol biosynthesis in HD models85,86,87,127. It has been proposed that SIRT2 affects cholesterol biosynthetic pathways, which may affect myelination and synapse maintenance in HD mouse models85,86,87. More recently, viniferin, a naturally occurring resveratrol derivative, was shown to be protective in cell culture models of HD. This compound increases SIRT3 levels, affecting LKB and MnSOD acetylation89.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is the most common form of motor neuron disease, a rapidly progressive condition that affects muscle strength and coordination. Over the past 20 years, causal mutations in a functionally diverse set of genes including SOD190, TDP4391,92, FUS93, UBQLN214 and C9ORF7294,95,96,97 have been identified, and there are also many proposed etiologies that focus on distinct aspects of disease pathogenesis98. In addition to playing a role in familial ALS, recent studies have also shown that these proteins may be altered in sporadic forms of the disease14,95,96,99,100,101,102,103,104,105. People on an average live 2-5 years following diagnosis, and there are no known cures for the disease; therefore, the investigation of new therapeutic targets is crucial.
The first study linking SIRT1 with ALS pathogenesis used both resveratrol and lentiviral injection and found that these interventions provided short term neuroprotection, although clinical outcomes documenting an improvement in motor function or lifespan of the whole animal were not assessed13. Several subsequent studies used tissue culture to demonstrate that resveratrol protects neurons in a cell-based model of ALS106,107. Further analysis found that resveratrol elicits protection from neurotoxic factors in patient cerebrospinal fluid (CSF) using rat brain cortical motor neurons. This study found that incubating neuronal cultures with CSF from ALS patients was more damaging than exposing neuronal cells to CSF from control subjects. Furthermore, resveratrol protected cultured neurons from cytotoxic factors present in CSF whereas riluzole, the only FDA approved medication for the treatment of ALS, was not beneficial in this cellular model107. These findings suggest that resveratrol may act via a different mechanism from riluzole, a medication that is believed to work by reducing glutamate excitotoxicity or blocking voltage-gated sodium channels108.
Two papers have investigated the effect of resveratrol in the G93A mouse model of ALS, the most commonly used transgenic line that develops ALS pathology due to overexpression of a mutant SOD1109. While dietary resveratrol treatment was not sufficient to effect disease outcomes110, intraperitoneal injection of resveratrol was sufficient to produce a significant improvement in both symptoms and survival of G93A mice111. The proposed mechanism was that SIRT1 can deacetylate HSF1, inducing the transcription of molecular chaperones such as hsp70 and hsp25, and decreasing motor neuron death11,70,75,111. Resveratrol is reported to have a short half-life in vivo112, and the different administration routes used in these studies may account for their different findings. SIRT1 levels have been shown to change both in G93A mouse models113 as well as in patient tissue114, suggesting that this protein may be a relevant disease target both in mouse models and human disease.
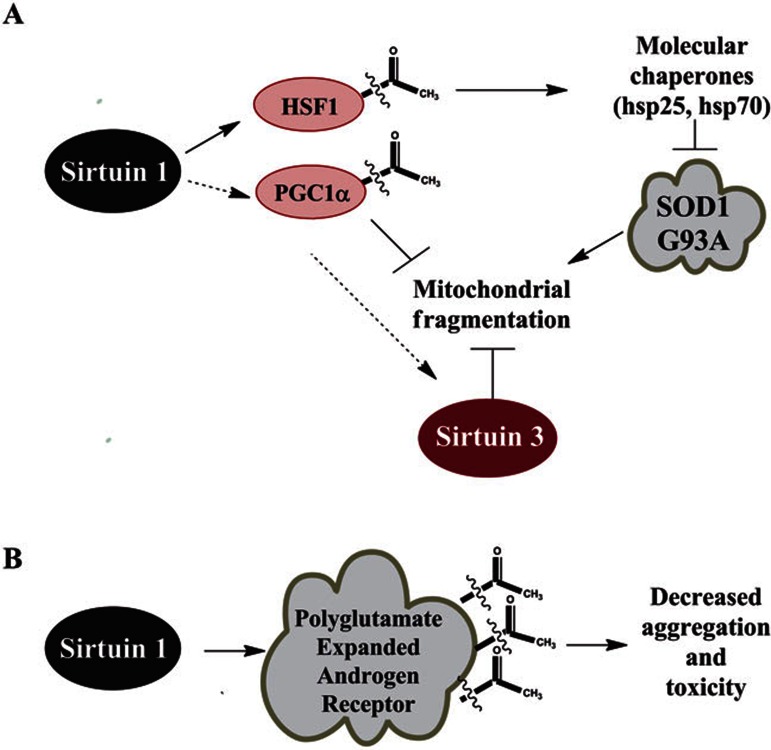
A recent paper identified SIRT3 as a relevant player in ALS pathogenesis using a cell-based model. SIRT3 was shown to protect against mitochondrial fragmentation and neuronal cell death induced by SOD1 G93A overexpression115. The major mechanisms that have been proposed for sirtuins in ALS pathogenesis are diagrammed in Figure 5A. Additional research confirming the effect of pharmacologic intervention in animal models of ALS will be important in evaluating whether sirtuin modulatory compounds should enter the human clinical trial pipeline.
Figure 5.
Sirtuin targets in motor neuron diseases. (A) In the SOD1 G93A mouse model of amyotrophic lateral sclerosis, intraperitoneal injection of resveratrol is protective. The proposed mechanism is that SIRT1 can deacetylate HSF1, inducing transcription of hsp70 and hsp25. Induction of these heat shock proteins decrease proteotoxic stress from misfolded protein aggregates11,70,75,111. SIRT3 and PGC-1α were also shown to protect against mitochondrial fragmentation and neuronal cell death induced by SOD1 G93A overexpression in cell culture115. (B) In spinal and bulbar muscular atrophy (SBMA), the polyglutamine-expanded androgen receptor can be directly deacetylated by SIRT1 at lysines 630, 632, and 633. It has been shown that deacetylation of the androgen receptor at these residues can decrease aggregation and toxicity in cellular models of SBMA124.
Spinal and bulbar muscular atrophy
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy's disease, is a slowly progressive neurodegenerative disease affecting motor and sensory neurons116,117. SBMA is a polyglutamine repeat disorder caused by the expansion of a CAG trinucleotide repeat in the N-terminal region of the androgen receptor (AR)116,117,118. This disorder causes muscle weakness in males between the age of 30 and 50 and is also associated with endocrine problems including testicular atrophy, infertility and gynecomastia due to androgen insensitivity117. Surgical or pharmacological reduction of testosterone has been shown to ameliorate the SBMA disease phenotype in animal models119,120; however, this therapy has not yet shown a clear benefit in human clinical trials121,122.
A recent publication has shown that the polyglutamine-expanded androgen receptor can be directly deacetylated by SIRT1. Although SIRT1 was previously shown to repress androgen receptor activity via deacetylation123, the role of this process in SBMA had not previously been explored. SIRT1 was found to protect motor neurons with polyglutamine-expanded androgen receptors by deacetylating lysines 630, 632, and 633124. The role of SIRT1 in SBMA is illustrated in Figure 5B. This work is an important finding with great therapeutic potential, because targeting AR acetylation via SIRT1 activation is a treatment strategy that may be safer and have fewer side effects than other avenues that are being explored such as long-term androgen withdrawal.
Conclusions
Numerous sirtuin targets are altered in these neurodegenerative disorders, and compounds modulating the activity of sirtuins are promising therapeutic strategies. There are three major mechanisms through which SIRT1 has been shown to affect neurodegenerative disorders. SIRT1 has been shown to deacetylate transcription factors and coactivators, whose downstream targets influence aggregate formation. One example of this mechanism is the deacetylation of HSF1, which induces the transcription of molecular chaperones that may be relevant to the pathogenesis of both PD and ALS11,70,75,111. In AD, overexpression of SIRT1 affects the acetylation of retinoic acid receptor β, a transcriptional activator of ADAM10, which influences the processing of APP along an anti-amyloidogenic pathway59.
A second mechanism through which SIRT1 has been shown to influence neurodegenerative disease is by modulating the protein turnover of proteotoxic species by direct deacetylation. An example of this mechanism is observed in AD where direct deacetylation of tau protein by SIRT1 may promote protein clearance by removing acetyl groups from lysine residues that are subsequently marked by ubiquitination to signal for protein degradation by the proteasome system62. This mechanism has also been employed to explain the effect of SIRT1 on the stability of some of its other targets125,126.
The enzymatic activity of SIRT1 can also be reduced by direct interaction with proteotoxic species. An example of this phenomenon is observed in HD where mutant huntingtin protein directly interacts with SIRT1, interfering with its ability to activate downstream targets such as TORC1, Foxo3a and PGC-1α10,78,84. Overexpression of SIRT1 by genetic means is protective and compounds that either increase SIRT1 activity or disrupt the interaction between SIRT1 and mutant huntingtin may be of clinical benefit.
In the past few years, it has become clear that modulation of mammalian sirtuins may be a valuable therapeutic strategy in several neurodegenerative diseases. However, many research questions remain. Given the large number of biological substrates affected by sirtuins, the identification of precise and disease-relevant molecular targets is a challenging goal. Multiple HDACs have been shown to influence the course of diseases; however, the degree to which their molecular activities can be coordinated has not been fully characterized. Evaluating the safety and efficacy of small molecules that can modulate sirtuin activity will be important elements in the development of new therapies to treat neurodegenerative diseases.
Acknowledgments
We would like to thank Angeliki Chalkiadaki, Eric Bell, Christin Glorioso, Hung-Chun Chang and Eric Williams for critical review of the manuscript.
References
- Rine J, Strathern JN, Hicks JB, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ, Fogel S, Macleod K. MAR1—a regulator of the HMa and HMα loci in Saccharomyces cerevisiae. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Benguria A, Lai CY, Jazwinski SM. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:3125–3136. doi: 10.1091/mbc.10.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med. 2011;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J Neurosci. 2012;32:124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Green KN, Steffan JS, Martinez-Coria H, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Hirschey MD. Old enzymes, new tricks: sirtuins are NAD(+)-dependent de-acylases. Cell Metab. 2011;14:718–719. doi: 10.1016/j.cmet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Barber MF, Michishita-Kioi E, Xi Y, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487:114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Byles V, Chmilewski LK, Wang J, et al. Aberrant cytoplasm localization and protein stability of SIRT1 is regulated by PI3K/IGF-1R signaling in human cancer cells. Int J Biol Sci. 2010;6:599–612. doi: 10.7150/ijbs.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- Sugino T, Maruyama M, Tanno M, Kuno A, Houkin K, Horio Y. Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett. 2010;584:2821–2826. doi: 10.1016/j.febslet.2010.04.063. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci USA. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hiratsuka M, Osaki M, et al. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. 2007;26:945–957. doi: 10.1038/sj.onc.1209857. [DOI] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association. 2012 Alzheimer's disease facts and figures Alzheimers Dement 20128131–168. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Wong BK, Hayden MR. Convergent pathogenic pathways in Alzheimer's and Huntington's diseases: shared targets for drug development. Nat Rev Drug Discov. 2011;10:853–867. doi: 10.1038/nrd3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on β-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from β-amyloid toxicity by resveratrol and catechin. Brain Res Bull. 2003;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Sun AY, Draczynska-Lusiak B, Sun GY. Oxidized lipoproteins, β amyloid peptides and Alzheimer's disease. Neurotox Res. 2001;3:167–178. doi: 10.1007/BF03033189. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against β-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Effect of resveratrol and catechin on PC12 tyrosine kinase activities and their synergistic protection from β-amyloid toxicity. Drugs Exp Clin Res. 2003;29:243–255. [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-β peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Gertz M, Nguyen GT, Fischer F, et al. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One. 2012;7:e49761. doi: 10.1371/journal.pone.0049761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, et al. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Aβ42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prayson RA.NeuropathologyPhiladelphia: Elsevier, 2005.
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Sohn P, Devidze N, et al. Sirt1 deficiency in the brain increases tau acetylation and exacerbates cognitive deficits in a mouse model of tauopathy Society for Neuroscience 2012. Neuroscience Meeting Planner: New Orleans, LA 2012.
- Cohen TJ, Guo JL, Hurtado DE, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resveratrol for Alzheimer's Disease. Available at: : http://clinicaltrials.gov/ct2/show/NCT01504854 Accessed: December 31, 2012
- Randomized Trial of a Nutritional Supplement in Alzheimer's Disease. Available at: : http://www.clinicaltrials.gov/ct2/show/NCT00678431 Accessed: January 1, 2013
- Short term efficacy and safety of perispinal administration of etanercept in mild to moderate Alzheimer's disease. Available at: : http://www.clinicaltrials.gov/ct2/show?term=resveratrol&cond=%22Alzheimer+Disease%22&rank=4 Accessed: January 1, 2013
- de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson's disease. J Biomed Biotechnol. 2012. 2012. p. 845618. [DOI] [PMC free article] [PubMed]
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1ndash;42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- Blanchet J, Longpre F, Bureau G, et al. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Raynes R, Leckey BD, Jr, Nguyen K, Westerheide SD. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J Biol Chem. 2012;287:29045–29053. doi: 10.1074/jbc.M112.353714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Liu L, Arun A, Ellis L, Peritore C, Donmez G. Sirtuin 2 (SIRT2) enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via deacetylating forkhead box O3a (Foxo3a) and activating Bim protein. J Biol Chem. 2012;287:32307–32311. doi: 10.1074/jbc.C112.403048. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- La Spada AR. Finding a sirtuin truth in Huntington's disease. Nat Med. 2012;18:24–26. doi: 10.1038/nm.2624. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Parker JA, Vazquez-Manrique RP, Tourette C, et al. Integration of β-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity. J Neurosci. 2012;32:12630–12640. doi: 10.1523/JNEUROSCI.0277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallos J, Bodai L, Lukacsovich T, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington's disease. Hum Mol Genet. 2008;17:3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–492. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp Neurol. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Jeong H, Cohen DE, Cui L, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Taylor DM, Pallos J, et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci USA. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Quinti L, Kim J, et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington's disease mouse models. Cell Rep. 2012;2:1492–1497. doi: 10.1016/j.celrep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Cattaneo E. Emerging roles for cholesterol in Huntington's disease. Trends Neurosci. 2011;34:474–486. doi: 10.1016/j.tins.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PLoS One. 2012;7:e34805. doi: 10.1371/journal.pone.0034805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jin J, Cichewicz RH, et al. trans-(-)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. J Biol Chem. 2012;287:24460–24472. doi: 10.1074/jbc.M112.382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Morita M, Al-Chalabi A, Andersen PM, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Al-Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3 Brain 2006129(Pt 4):868–876. [DOI] [PubMed] [Google Scholar]
- Gladman M, Cudkowicz M, Zinman L. Enhancing clinical trials in neurodegenerative disorders: lessons from amyotrophic lateral sclerosis. Curr Opin Neurol. 2012;25:735–742. doi: 10.1097/WCO.0b013e32835a309d. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Maetzler W, Grehl T, et al. Screening in ALS and FTD patients reveals 3 novel UBQLN2 mutations outside the PXX domain and a pure FTD phenotype. Neurobiol Aging. 2012;33:2949.e13–2949.e17. doi: 10.1016/j.neurobiolaging.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzman A, Wood WL, Alpert E, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Tang L, Zhang N, Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci Lett. 2011;503:250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- Yáñez M, Galán L, Matías-Guiu J, Vela A, Guerrero A, García AG. CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 2011;1423:77–86. doi: 10.1016/j.brainres.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Gold Standard, Inc. Riluzole. Clinical Pharmacology [online database]. Available at: : http://www.clinicalpharmacology.com Accessed: January 27, 2013
- Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Markert CD, Kim E, Gifondorwa DJ, Childers MK, Milligan CE. A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J Med Food. 2010;13:1081–1085. doi: 10.1089/jmf.2009.0243. [DOI] [PubMed] [Google Scholar]
- Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 2012;1483:112–117. doi: 10.1016/j.brainres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations. J Control Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- Lee JC, Shin JH, Park BW, et al. Region-specific changes in the immunoreactivity of SIRT1 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 2012;1433:20–28. doi: 10.1016/j.brainres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Körner S, Böselt S, Thau N, Rath KJ, Dengler R, Petri S. Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS. Neurodegener Dis. 2013;11:141–152. doi: 10.1159/000338048. [DOI] [PubMed] [Google Scholar]
- Song W, Song Y, Kincaid B, Bossy B, Bossy-Wetzel E. Mutant SOD1(G93A) triggers mitochondrial fragmentation in spinal cord motor neurons: Neuroprotection by SIRT3 and PGC-1α. Neurobiol Dis. 2013;51:72–81. doi: 10.1016/j.nbd.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry DE. Attacking the flank: targeting new pathways in SBMA. Nat Med. 2012;18:1461–1463. doi: 10.1038/nm.2967. [DOI] [PubMed] [Google Scholar]
- Brinkmann AO. Molecular mechanisms of androgen action--a historical perspective. Methods Mol Biol. 2011;776:3–24. doi: 10.1007/978-1-61779-243-4_1. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Kume A, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Doyu M, et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003;9:768–773. doi: 10.1038/nm878. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rhodes LE, Kokkinis AD, White MJ, et al. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol. 2011;10:140–147. doi: 10.1016/S1474-4422(10)70321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno M, Banno H, Suzuki K, et al. Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:875–884. doi: 10.1016/S1474-4422(10)70182-4. [DOI] [PubMed] [Google Scholar]
- Fu M, Liu M, Sauve AA, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie HL, Pestell RG, Merry DE. SIRT1 modulates aggregation and toxicity through deacetylation of the androgen receptor in cell models of SBMA. J Neurosci. 2011;31:17425–17436. doi: 10.1523/JNEUROSCI.3958-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2012;31:1546–1557. doi: 10.1038/onc.2011.347. [DOI] [PubMed] [Google Scholar]
- Kahyo T, Mostoslavsky R, Goto M, Setou M. Sirtuin-mediated deacetylation pathway stabilizes Werner syndrome protein. FEBS Lett. 2008;582:2479–2483. doi: 10.1016/j.febslet.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Valenza M, Leoni V, Tarditi A, et al. Progressive dysfunction of the cholesterol biosynthesis pathway in the R6/2 mouse model of Huntington's disease. Neurobiol Dis. 2007;28:133–142. doi: 10.1016/j.nbd.2007.07.004. [DOI] [PubMed] [Google Scholar]