Abstract
The steroid hormone estrogen and its classical estrogen receptors (ERs), ER-α and ER-β, have been shown to be partly responsible for the short- and long-term uterine endothelial adaptations during pregnancy. The ER-subtype molecular and structural differences coupled with the differential effects of estrogen in target cells and tissues suggest a substantial functional heterogeneity of the ERs in estrogen signaling. In this review we discuss (1) the role of estrogen and ERs in cardiovascular adaptations during pregnancy, (2) in vivo and in vitro expression of ERs in uterine artery endothelium during the ovarian cycle and pregnancy, contrasting reproductive and nonreproductive arterial endothelia, (3) the structural basis for functional diversity of the ERs and estrogen subtype selectivity, (4) the role of estrogen and ERs on genomic responses of uterine artery endothelial cells, and (5) the role of estrogen and ERs on nongenomic responses in uterine artery endothelia. These topics integrate current knowledge of this very rapidly expanding scientific field with diverse interpretations and hypotheses regarding the estrogenic effects that are mediated by either or both ERs and their relationship with vasodilatory and angiogenic vascular adaptations required for modulating the dramatic physiological rises in uteroplacental perfusion observed during normal pregnancy.
Keywords: estrogen receptor, uterine endothelium, vasodilatation, angiogenesis, pregnancy
The vascular actions of estrogens are mediated via the classical estrogen receptors (ERs), ER-α and ER-β.1 Although these two ERs exhibit substantial homology, they are structurally and functionally distinct.2 Vascular endothelial estrogenic responses are classified as genomic (classical/nuclear) and nongenomic (nonclassical/membrane). Estrogen binding to the substantially more abundant nuclear receptors regulates gene transcription, whereas the less abundant membrane receptors (3 to 5% of the total ERs) control very rapid activation of endothelial nitricoxide synthase (eNOS) and thus nitricoxide (NO)-mediated vasodilatation.3,4 Several recent reports have elucidated ER subtype–specific regulation of complex pregnancy-associated cardiovascular adaptations, especially at the level of the uterine vascular bed. In this review we specifically focus our attention on ER-α- and/or ER-β-associated uterine vasodilator NO-mediated rapid events as well as the longer term uterine angiogenic responses that are both collectively responsible for dramatically elevating uteroplacental blood flow during gestation.5–7 We evaluate and synthesize information related to the (1) role of estrogen and ERs in the cardiovascular adaptations during pregnancy; (2) in vivo and in vitro expression of ERs in uterine artery endothelium during the ovarian cycle and pregnancy, contrasting reproductive and nonreproductive arterial endothelia; (3) structural basis for functional diversity of the ERs and estrogen subtype selectivity; (4) the role of estrogen and ERs on genomic responses of uterine artery endothelial cells; and (5) the role of estrogen and ERs on nongenomic responses in uterine artery endothelia. The focus of this review, therefore, is to summarize and integrate the evidence supporting the vital role for endothelial ERs in pregnancy-induced uterine vascular adaptations as well as the structural, functional, and spatial heterogeneity of the ERs.
Role of Estrogen and Estrogen Receptors in the Cardiovascular Adaptations During Pregnancy
During normal gestation, uterine blood flow (UBF) increases substantially by as much as 50-fold to provide sufficient nutrient and oxygen supply for the growth and healthy function of the developing placenta and fetus.8 This dramatic vascular adaptation is biologically and clinically significant because insufficient elevations of UBF in pathological pregnancies leads to a shortage of nutrient and oxygen delivery/extraction that are associated with intrauterine growth restriction of the fetus and higher prenatal and neonatal morbidity, or even mortality.9,10 A fundamental functional and mechanistic role for estrogen in elevating UBF has been definitively established using both in vivo and in vitro models. These include a concomitant increase in circulating estrogen levels with UBF during the follicular phase of the ovarian cycle and during normal pregnancy11–15 as well as the very dramatic increase in UBF in nonpregnant and pregnant sheep following a bolus injection of exogenous estrogen.6,10,16–18 There is also a substantial body of literature showing that estrogen-induced and pregnancy-associated rises in UBF are, to a great extent, mediated by an upregulation of the endothelial production of the potent vasodilator NO7,19,20 via increasing eNOS protein expression,21–23 and by increasing eNOS activity.4,24–27 However, additional mechanisms that underline the estrogen- and pregnancy-associated rises in UBF also include stimulation of various angiogenesis processes. Both the estrogen-mediated vasodilatory and angiogenic responses of the uterine vasculature are driven to a large extent by ER-mediated processes. Estrogen-induced rises in UBF via these processes has been postulated to be mediated by one or both of the specific ERs.28,29 In ovariectomized sheep, we reported that exogenous estrogen-induced rises in UBF are to a great extent (~70%) inhibited by the nonsubtype-specific ER antagonist ICI 182,780,5 thus demonstrating that the ER-induced rises in UBF are partly mediated by one or more ER-dependent mechanisms. In two physiological states of high endogenous estrogen levels, we also showed that unilateral uterine artery infusions of ICI 182,780 locally reduced UBF from its maximum during the follicular phase of the ovarian cycle and pregnancy. To our knowledge there is only one reported preliminary physiological study in the ovine model showing that selective ER-α ligands are more potent than ER-β ligands for increasing UBF, thus supporting the premise that estrogen-mediated uterine vasodilatation may be mediated more by ER-α than by ER-β.30 This question needs more rigorous testing in the uterine vascular bed.
Classically, the biological functions of estrogen in target tissues are believed to be mediated by specific high-affinity nuclear ERs that function as ligand-activated transcription factors to regulate gene expression.31 However, the rapid activation of signaling mechanisms such as extracellular signal-regulated kinases (ERKs) might be an additional mechanism responsible for acute activation of eNOS by estrogen to produce NO in uterine artery endothelial cells (UAECs) in vitro. Previously we demonstrated that the rapid activation of the eNOS-NO pathway by estrogen in UAECs was indeed mediated by ER localized on the plasma membrane.4 Regardless of the cellular location, two types of ERs have been identified so far, the originally described ER-α32,33 and the subsequently discovered ER-β.26,34,35 In the current review we present detailed information about ER-α and ER-β expression in the endothelium of uterine arteries (UAs) and their regulation in endogenous estrogen and pregnancy-associated rises in UBF. We evaluated both ER-α and ER-β mRNAs and proteins in sheep UA endothelia (UAendo) in vivo and also in in vitro cultured UAECs.
In Vivo and In Vitro Expression of Estrogen Receptors in Uterine Artery Endothelium
We have established several informative assays to study the expression profiles of both ER-α and ER-β in UAs during the ovine ovarian cycle and pregnancy. We suggested that because expression levels of ER-α and/or ER-β are likely to vary under different physiological conditions and clinical settings, 36,37 understanding their regulations is important as it relates to specific functional biological and clinical settings. Several groups have documented the presence of ERs in uterine and other reproductive and nonreproductive arteries, 38–42 including our own laboratories.4,26,35,43 We therefore tested the hypothesis that ER-α and ER-β, receptors present in ovine UAendo and vascular smooth muscle (VSM) are regulated by the reproductive state via alterations in ovarian/placental steroid (estrogen/progesterone) levels.
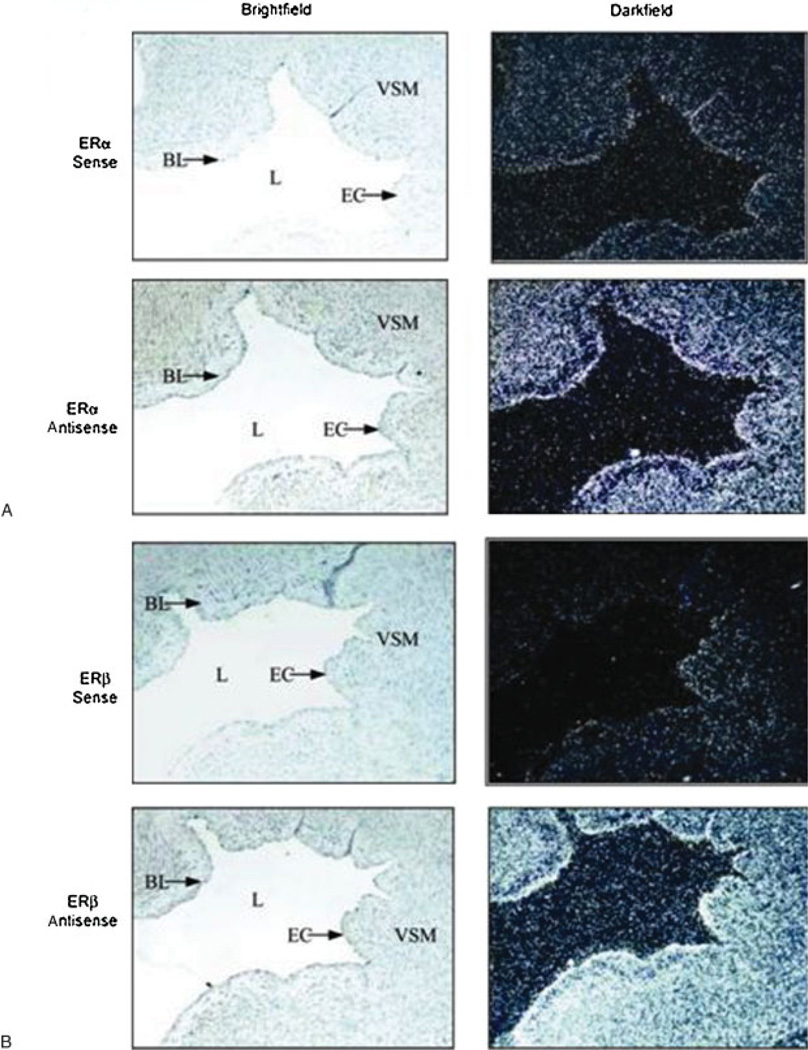
To determine the cellular (endothelium versus VSM) localization of ER-α and ER-β mRNAs in UAs, we first performed in situ hybridization using35 S-labeled sense (control) or antisense riboprobes synthesized from specific ovine ER-α and bovine ER-β cDNAs (Fig. 1A and 1B, respectively). Hybridization with antisense probes revealed that ER-α and ER-β mRNA are both strongly expressed in the ovine UA endothelium along the tunica intima (i.e., the lumen of the vessels). As expected, expression of the ERs was also observed throughout the tunica media. Tissue sections subjected to hybridization with sense probes showed minimal background levels of ER-α and ER-β mRNAs.
Figure 1.
(A) Location of estrogen receptor (ER)-α and (B) ER-β mRNAs in ovine uterine arteries. Uterine arteries from pregnant sheep were fixed and paraffin embedded, and whole uterine artery sections (5 µm) were cut. Localization of ER-α and ER-β mRNAs was determined by in situ hybridization using35 S-labeled sense (control) or antisense riboprobes synthesized from a specific ovine ER-α cDNA or bovine ER-β cDNA. Silver grains shown in darkfield images represent positive mRNA labeling. Images were taken representative of n=7 at 10× magnification. BL, basal lamina; EC, endothelial cell; L, lumen; VSM, vascular smooth muscle.35 (With permission from Byers MJ, et al. J Physiol 2005;565:85–99.)
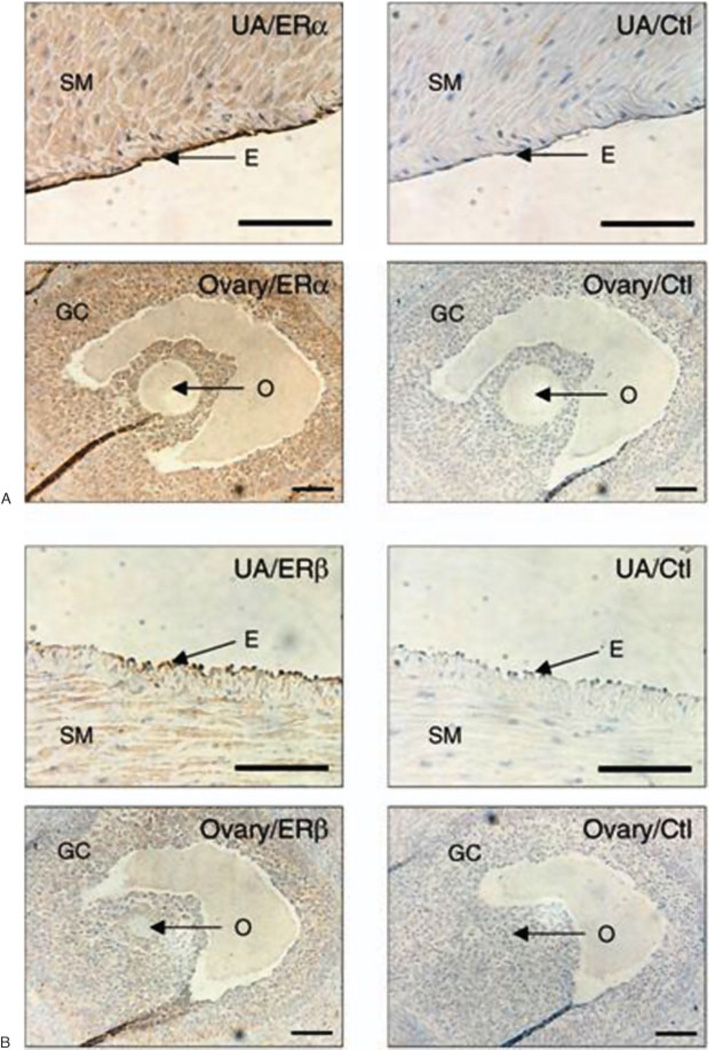
We also reported,4,26 that UA tissue sections from late gestation (days 120 to 130; 0.8 to 0.9 of pregnancy) sheep show intensive immunoreactive signals for both ER-α and ER-β proteins in the nuclei, and to a lesser extent the cytoplasm, of the endothelial and smooth muscle cells (Fig. 2A and 2B, respectively). However, the cytoplasm immunostaining of ER-α and ER-β may also in part be derived from plasma membrane staining because the immunohistochemical techniques such as these do not provide a clear subcellular structure of cytoplasm versus plasma membrane. In subsequent studies, we showed ER-α protein was expressed uniformly throughout the UA vascular cells (nonnuclear and nuclear staining), whereas ER-β exhibited heavy preferential nuclear staining,35 an observation consistent with reports in ovine fetal pulmonary endothelial cells44 and UAECs.26
Figure 2.
Immunohistochemical analysis of both (A) estrogen receptor (ER)-α and (B) ER-β (B) in uterine artery (UA) of pregnant ewes. Ovarian tissue from the same ewes was used as the positive control for ER-α and ER-β showing abundant expression of both ER-α and ER-β protein in the granulosa cells, but not the oocyte. Corresponding concentrations of rabbit and mouse immunoglobulin Gs served as nonspecific binding controls. E, endothelium; GC, granulosa cells; O, oocyte; SM, smooth muscle. Bar = 100 µm.26 (With permission from Liao WX, et al. Biol Reprod 2005;72:530–537.)
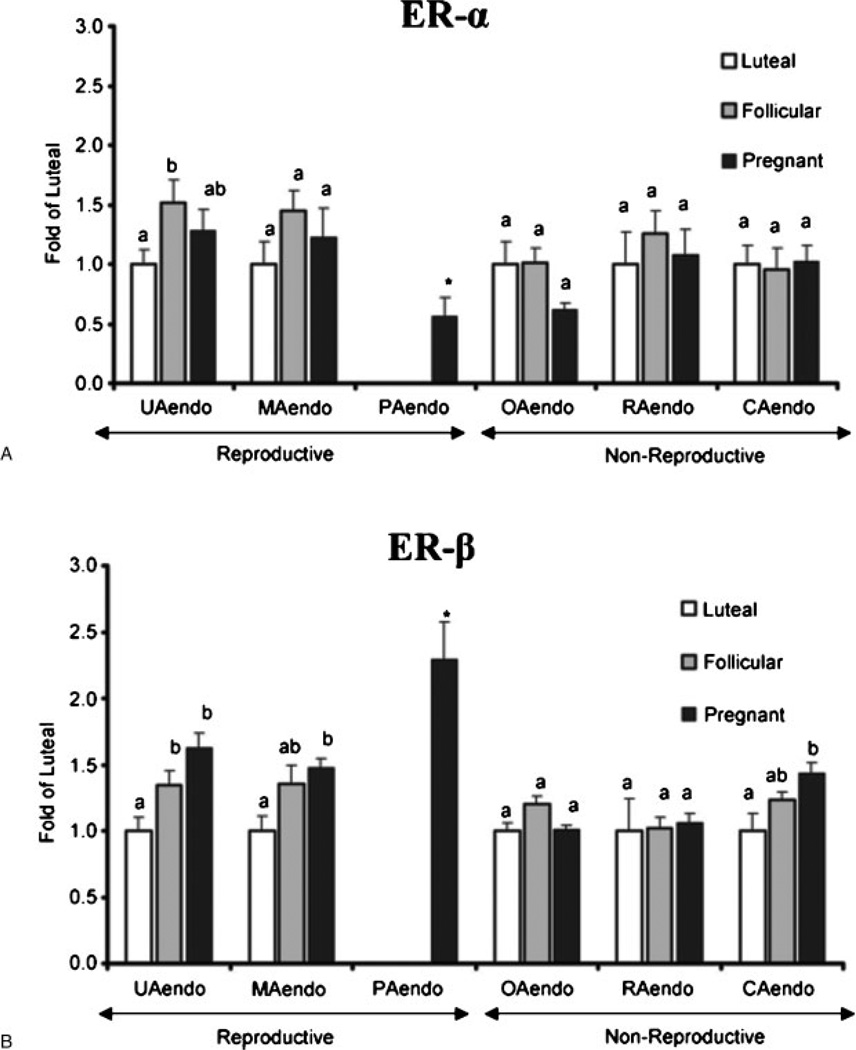
Using Western immunoblot analysis, which unlike the previously described histological techniques is much more quantitative, we performed studies to determine if ER-α and/or ER-β protein expression is modified in the reproductive versus nonreproductive endothelia during physiological states of elevated estrogen (follicular phase and pregnancy) or progesterone (luteal phase and pregnancy) (Fig. 3). Changes in UAendo ER-α and ER-β protein were compared with the endothelium derived from other reproductive endothelia, that is, mammary (MAendo) and placental (PAendo) as well as various nonreproductive endothelia, omental (OAendo), renal (RAendo), and coronary (CAendo). In contrast to the 1.5-fold higher UAendo ER-α in follicular versus luteal UAendo, there was no significant ER-α change in any other endothelial preparation from follicular sheep (Fig. 3A), demonstrating not only specificity of these UAendo ER-α changes, but the local ovarian control of the uterine vasculature. Besides UAendo and may be a small trend in MAendo, when compared with luteal endothelium, ER-β was not significantly changed in any of the follicular endothelial preparations from nonreproductive endothelia that were studied (Fig. 3B). By contrast, during pregnancy, all three reproductive endothelial isolated proteins displayed significantly increased ER-β expression. Specifically, UAendo ER-β from the pregnant group was increased 1.6-fold and MAendo 1.5-fold over luteal controls. Besides the reproductive sources of these vessels, uterine and mammary vascular beds are highly responsive to estrogen-induced vasodilatation. 6,45 It is probable that unlike the lack of change of ER-β during the follicular phase, the prolonged exposure of these endothelia to placental-derived estrogens and progesterone during gestation may account for the rises in ER-β expression. Regarding placental endothelium isolated from another reproductive vessel, ER-β was 2.3-fold higher than luteal UAendo and substantially higher than pregnant UAendo. Because PAendo ER-α was lower than luteal UAendo, it appears that ER-β is the primary ER in the ovine placental vasculature. These observations collectively also point to the potential for a unique role of ER-β to regulate reproductive vascular functions during pregnancy and development. The only nonreproductive tissue in which ER-β changed significantly was CAendo from pregnant sheep, which rose 1.5-fold over luteal. In MAendo and CAendo, follicular phase levels suggest a trend for these vascular beds that are estrogen responsive. Others have shown multiple tissue comparisons46,47 of vascular ER expression.48–55 In this study we reported on endothelial isolated proteins extensively studied with regard to the endocrine status of the animal. Although ER-α was readily detectable in the vessels tested, none of the other reproductive or nonreproductive endothelia surveyed except UAendo and PAendo had ER-α levels that were different relative to luteal phase expression (Fig. 3A). ER-β levels also were not altered by physiological state in RAendo and OAendo, vascular beds traditionally thought of as major controlling centers of blood pressure. The observation that both ERs are not altered in OAendo by the ovarian cycle, ovariectomy with or without hormone replacement (not shown), may have functional significance in that we have demonstrated estrogen profoundly increases UBF 10- to 20-fold in as little as 2 hours, whereas omental blood flow was unaltered.5,6,28 It is noteworthy that ER-β (Fig. 3B) was indeed elevated by pregnancy in the three reproductive endothelia (UA, MA, and PA) but also CAendo and that follicular ER-β levels were similar to pregnancy levels in UA, MA, and CA. Thus CA was the only nonreproductive endothelium to show ER-β regulation and suggests a more ubiquitous role for this receptor subtype in the mammalian vasculature especially in lieu of the observation that ER-β knockout (KO) mice develop age-dependent hypertensive cardiovascular disease.56 The finding that ER-β in CAendo is increased in pregnancy is particularly intriguing, given the substantial increases in cardiac output, heart rate, and stroke volume and the profound decrease in systemic vascular resistance observed during normal pregnancy.6,28,57 Furthermore, blood flows to the various vascular beds we evaluated are differentially affected by exogenous E2β treatment, that is, elevated in the uterine, mammary, and coronary, but not the omental or renal beds.6,17,45 Moreover, estrogen-induced rises in UBF,7,13,20 coronary blood flow,58 and mammary blood flow59 are attenuated by treatment with the NOS inhibitor L-NAME. In this regard, eNOS was substantially upregulated in the UAendo during folliculogenesis and pregnancy, although it is present in all the reproductive and nonreproductive endothelia tested,21,23,35,60 suggesting that elevations in eNOS activation and thus NO production rather than elevations in eNOS capacity may be more important in CA and MA endothelia. These data also reinforce the unique nature of the UAendo versus other vascular beds as a tissue highly responsive to estrogen.
Figure 3.
(A) Estrogen receptor (ER)-α and (B) ER-β protein expression in reproductive versus nonreproductive arterial endothelia. Western blot analysis was performed to evaluate the relative levels of ER-α and ER-β protein in luteal, follicular, and pregnant sheep reproductive and nonreproductive endothelia. Samples from luteal, follicular, and pregnant sheep are expressed as fold of the average of all luteal samples within an artery type, run on the same Western blots. Expression of PAendo is given as fold of the average luteal UAendo was also run on the same blot. Treatment groups: luteal: uterine (n = 12), mammary (n = 5), omental (n = 7), renal (n = 6), and coronary (n = 7). Follicular: uterine (n = 8), mammary (n = 5), omental (n = 8), renal (n = 6), and coronary (n = 8). Pregnant: uterine (n = 12), mammary (n = 6), placental (n = 8) omental (n = 8), renal (n = 8), and coronary (n = 8). Data are means plus or minus standard error of the mean. Means with different letters are statistically different (p < 0.05) within a tissue preparation. For ER-α UAendo: Lut < Fol (p < 0.05), For ER-β UAendo: Lut < Fol (p < 0.05) and Lut < Preg (p < 0.001); MAendo: Lut < Preg (p < 0.01); CAendo: Lut < Preg (p < 0.05). *For ER-α: PAendo < Luteal UAendo but for ER-β PAendo > Luteal UAendo (p < 0.05).35 (With permission from Byers MJ, et al. J Physiol 2005;565:85–99.)
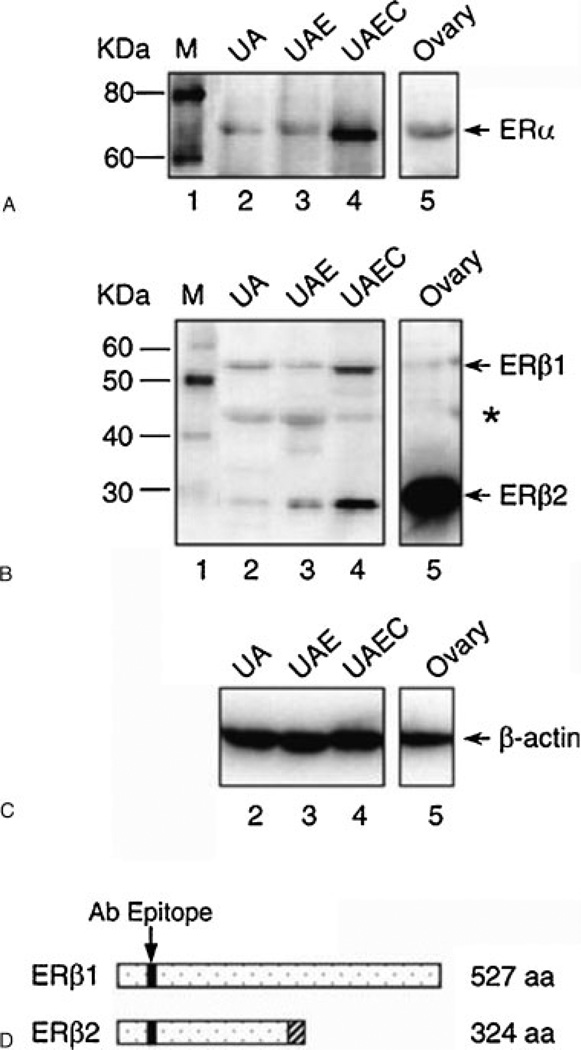
To establish an in vitro model of estrogen actions, we have shown the presence of ERs in cultured UAECs. We first measured ER-α protein expression in isolated UAEC plasma membranes and nuclear extracts by Western blotting and performed binding studies using E2β-BSA-FITC as a ligand, the latter as a membrane-impermeable estrogen agonist. We observed the classical 67-kDa ER-α protein detectable in both plasma membrane and nuclear extracts, whereas, as expected, considerably higher ER-α protein levels were found in the nucleus compared with plasma membranes. In addition, binding studies using E2β-BSA-FITC as a ligand showed that fluorescence labeling was primarily localized on UAEC plasma membrane.4 We then confirmed these data using the immunocytochemical localization of both ER-α and ER-β in cultured UAECs from pregnant sheep (Byers and Magness, unpublished observations). Using Western analysis (Fig. 4) and real-time polymerase chain reaction (not shown), we subsequently demonstrated that both ER-α and ER-β are indeed expressed in cultured UAECs from pregnant sheep. We concluded that these UAECs preparations in primary culture provide a very useful in vitro model for the study of estrogen actions on uterine vascular function and signaling.
Figure 4.
Western immunoblot analysis of (A) estrogen receptor (ER)-α, (B) ER-β, and (C) β-actin in the protein extracts of uterine artery (UA), uterine artery endothelium (UAE), uterine artery endothelial cell (UAEC), and ovary from pregnant ewes. (D) A diagram representing the truncated form of ER-β2 that results from the splicing deletion of exon 5 shown in (B). The shadowed box represents the amino acid sequences encoded by different reading frame. aa, amino acid. Bands marked with asterisk may indicate additional truncated forms of ER-β.26 (With permission from Liao WX, et al. Biol Reprod 2005;72:530– 537.)
Because ER-β appears to be the main receptor subtype modulated in the UA and other reproductive endothelium during gestation, we also evaluated ER-β variants in UAECs from pregnant sheep. Expression of several ER-β variants has been identified in human reproductive tissues, but there was (and remain) very limited functional data currently available. In addition, no data were available on this in uterine artery endothelium; however, the ER-β2 variant has been postulated to act as putative dominant negative regulators of estrogen action.61 We reported that this interesting truncated variant was indeed detected in the UAendo (UAE) and in cultured UAECs, demonstrating that not only was ER-β2 variant present ex vivo but it also was maintained and even elevated in passage 4 UAECs (Fig. 4). From a structural standpoint, ER-β2 is the result of the splicing deletion at exon 5, and it is missing all the C-terminal amino acids and lacks most of the ligand-binding domain (LBD) of ER-β.62 One previous functional study showed that it may serve as a dominant negative receptor capable of blocking both ER-α and ER-β signaling pathways,63 thus conferring a means by which expression levels of both receptors could be modulated. In these studies we did not evaluate ER-β2 variant in UAendo from luteal or follicular phase sheep, although we theorize that the luteal ER-β2 variant levels will be higher than those either in follicular or pregnant UAendo. If this is the case, then falls in this dominant negative ER could help account for the rises in ER-α and/or ER-β in the follicular phase and pregnancy (Fig. 3). Regardless, its potential role in estrogen actions and pregnancy is largely unknown and requires further scrutiny.
The Estrogen Receptor Structure: A Structural Basis for Functional Diversity and Estrogen Subtype Selectivity
Although E2β has affinity for both ER-α and ER-β as well as to several other pharmacological agonists and antagonists (e.g., selective estrogen receptor modulators [SERMs]), the molecular and structural difference between these receptors allow for a wide range of functional heterogeneity. These differences may partly explain selective estrogenic actions in the same or diverse target cells and tissues. ER-α and ER-β are members of a nuclear receptor superfamily sharing conserved regions, named A/B, C, D, E, and F domains as well as the N-terminal transactivation domain, the DNA-binding domain (DBD), the dimerization domain, the nuclear localization sequence (NLS), and the LBD.64,65 However, ER-α and ER-β are the products of distinct and separate genes (ESR1 and ESR2, respectively) found on different chromosomes (locus 6q25.1 and locus 14q23–24.1, respectively).66,67 ER-α and ER-β only share a 12% homology in their A/B regions, a 16% homology in their D region, a 59% homology in their E domain, and a 9% homology in their F regions.68,69 In summary, ER-α and ER-β only share 56% amino acid homology in their LBDs, and they differ greatly in their N-terminal and C-terminal/DNA binding regions.1,64,65,70,71 Similarly, their A/B region, which contains the solvent exposed and negatively charged activation function (AF)-1 domain as well as numerous phosphorylation sites for signal transduction, differs in both length and amino acid sequence.64 ER-α and ER-β additionally share very low amino acid sequence homology in the α-helices of their DNA binding domains.64 Investigation of the dynamic plasticity of the ligand-binding pocket of ERs has shown that in this specific region ER-β differs from ER-α based on a substitution of Leu384/Met421in ER-α corresponding to Met336/Ile373 in ER-β.
Structural differences between ER-α and ER-β specifically suggest a structural basis for selectivity in ligand-dependent differential receptor activation.72 The differences in their structures has led to the development of numerous pharmacological ligands that discriminate between the two ER subtypes on the basis of affinity, efficacy, and inherent differences in their coupling to other signaling components (Table 1). For instance, the diarylethylenes such as diarylpropionitrile (DPN) have been demonstrated to possess selectivity and potency for ER-β over ER-α owing to preferential interaction with a key methionine residue in the LBD.72,73 Consequently, DPN demonstrates a 72-fold higher binding affinity toward ER-β over ER-α.1 In contrast, compounds such as propylpyrazole triol (PPT) demonstrate more than a 410-fold agonistic potency for ER-α over ER-β, exhibiting very high level of ER-α selectivity.74,75 With regard to antagonist ligands, the phenol-containing compounds such as 4-[2-phenyl-5, 7-bis(trifluoromethyl) pyrazolo [1, 5-a] pyrimidin-3-yl] phenol (PHTPP) show considerable affinity and selectivity toward ER-β over ER-α.76 Likewise, 1,3-bis (4-hydroxy-phenyl)-4-methyl-5-[4-(2-piperidinylethoxy) phenol]-1Hpyrazoledi hydrochloride (MPP) demonstrates up to a 200-fold antagonistic selectivity for ER-α over ER-β.77,78 These ER-subtype selective compounds possess specific utility for pharmacological approaches that aim at elucidating the differential and or/distinct roles of ER-α and ER-β in the physiological functions of estrogen. Importantly, these compounds (Table 1) also support the notion that structural differences between ER-α and ER-β may explain the fact that estrogen-induced activation of one ER subtype may fulfill a complete and distinct physiological response independent of the other. Using these specific pharmacological agents coupled with additional molecular approaches such as siRNA or adenoviral overexpression of ER-α and ER-β in cell lines provides quite convincing evidence of the individual and complementary roles for the two ER subtypes.79
Table 1.
Selectivity of Pharmacological Ligand Compounds for Estrogen Receptor-α and Estrogen Receptor-β
| Ligand Compound | ER-α/ER-β Selectivity |
Agonist/Antagonist | Relative Binding Selectivity |
Reference |
|---|---|---|---|---|
| Estradiol-17β | ER-α/ER-β | Agonist | N/A | Kuiper et al46 |
| ICI 182,780 | ER-α/ER-β | Antagonist | N/A | Wakeling et al126 |
| *4-OH Tamoxifen | ER-α/ER-β | Antagonist* | N/A | Wakeling et al127 |
| ZK 164015 | ER-α/ER-β | Antagonist | N/A | Walter et al128 |
| PPT | ER-α | Agonist | ~410-fold | Kraichely et al129 |
| Y 134 | ER-α | Agonist | ~125-fold | Yang et al130 |
| *(R,R)-THC | ER-α | Agonist* | ~120-fold | Sun et al131 |
| Raloxifene | ER-α | Agonist | ~15-fold | Sun et al131 |
| MPP | ER-α | Antagonist | ~200-fold | Sun et al77 |
| Genistein | ER-β | Agonist | ~26-fold | Malamas et al43 |
| DPN | ER-β | Agonist | ~70-fold | Meyers et al1 |
| FERb 033 | ER-β | Agonist | ~62-fold | Minutolo et al132 |
| WAY 200070 | ER-β | Agonist | ~68-fold | Compton et al76 |
| PHTPP | ER-β | Antagonist | ~36-fold | Compton et al76 |
| *(R,R)-THC | ER-β | Antagonist* | ~4-fold | Sun et al131 |
| *RU486 | ER-β | Antagonist* | ~5-fold | Fang et al133 |
Denotes ligand compounds with both known partial ER agonist and antagonist activities.
ER, estrogen receptor; N/A, not applicable; unknown or negligible binding selectivity or equal binding affinity for both ER-α and ER-β.
These are the systematic (IUPAC) names: 1. Estradiol-17β: (17β)-estra-1,3,5(10)-triene-3,17-diol; 2. ICI 182,780: 7α,17β-[9-[(4,4,5,5,5-Pentafluoropentyl) sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol; 3. 4-OH Tamoxifen: (Z)-2-[4-(1,2-diphenylbut-1-enyl)phenoxy]-N,N-dimethylethanamine; 4. ZK 164015: 2-(4-Hydroxyphenyl)-3-methyl-1-[10-(pentylsulfonyl)decyl]-1H-indol-5-ol; 5. PPT: 4,4',4''-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol; 6. Y 134: [6-Hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl]-[4-[4-(1-methylethyl)-1-piperazinyl]phenyl]methanone; 7. (R,R)-THC: (R,R)-5,11-Diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol; 8. MPP: 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride; 9. DPN: 2,3-bis(4-Hydroxyphenyl)-propionitrile. Alternative name: Diarylpropionitrile; 10. FERb 033: 2-Chloro-3′-fluoro-3,4'-dihydroxy-[1,1-biphenyl]-4-carboxaldehyde oxime; 11. WAY 200070: 7-Bromo-2-(4-hydroxyphenyl)-1,3-benzoxazol-5-ol; 12. PHTPP: 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo [1,5-a]pyrimidin-3-yl]phenol; 13. RU486: 11β-[p-(Dimethylamino)phenyl]-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one.
The molecular mechanisms that underlie the physiological selectivity of endogenous estradiol-17β for classical ER-α and/or ER-β remain unknown and are the focus of significant fundamental research. For example, three-dimensional crystallography binding studies have demonstrated that estradiol-17β actually binds “upside-down” to ER-β compared with its binding to ER-α, implying differential conformation of the receptor complex, and perhaps this accounts in part for the differential signaling activation described for these two receptors when exposed to the same ligand.80 For the purposes of this review, it is thus conceivable that this differential rotated binding orientation enables estrogen to induce diverse and differential genomic and/or nongenomic biological outcomes based on selective structural and conformational changes of the ER-α and/or ER-β receptor complex.
The emerging evidence for the considerable functional diversity between ER-α and ER-β derives from the observation that there are distinct endothelium-dependent genomic and/or nongenomic processes that are impaired in ER-null mice. For example, compared with wild-type or ER-α deficient mice, the ER-β KO mice exhibit altered long-term genomic adaptations leading to severely impaired endothelial proliferation and migration.81 However, compared with wild-type or ER-β KO, the rapid endothelium-dependent NO-mediated vasodilatation is significantly reduced in ER-α deficient mice.82,83 In this regard, it is possible that although both ERs are important for proper vascular homeostasis, functional heterogeneity allows for more diverse mediation of selective estrogenic actions. Additionally, these possibilities also suggest that the spatial and subcellular localization of membrane versus nuclear ER-α or ER-β also confers additional and distinct functions attributed to the acute or prolonged ER actions. However, little is known about whether there are possible structural differences in these receptors subpopulations at the plasma membrane or whether this may represent a more complex diversity of ER-α or ER-β on estrogen-mediated physiological effects.
Role of Estrogen and Estrogen Receptors on Genomic Responses of Uterine Artery Endothelial Cells
Genomic estrogen-ER effects in the uterine vascular system include (1) maintenance of vasodilation via upregulation of key enzyme gene/protein expression (e.g., eNOS), (2) promotion of angiogenesis by enhancement of proliferation, migration, and tube formation, and (3) blood vessel remodeling in proportion to the growth of the uteroplacental unit.
Prolonged increases in estrogen such as that described earlier during the follicular phase (1 to 3 days), but even more so in pregnancy (>100 days), lead to altered expression of numerous genes/proteins associated with a balance of vasodilatation relative to vasoconstriction, which collectively serves to regulate vascular tone.84 For example, these locally produced endothelial vasoactive products include endothelin-1,85 eNOS,86–89 and multiple components of the renin-angiotensin system.90,91 MacRitchie et al88 demonstrated in ovine fetal pulmonary endothelial cells that in vitro physiological elevations of estrogen for 2 days resulted in upregulation of eNOS mRNA abundance, protein levels, and its activity. Other reports suggest a role for both ER-α and ER-β in long-term vasodilatory effects. Estrogen in endothelium modulates eNOS expression through its interaction with ER-α and/or ER-β.52,92,93 In contrast, estrogen augments vasoconstriction in blood vessels from ER-β deficient mice.56 However, the mechanism regulating this seemingly contradictory estrogenic response remains unresolved. There is also a report on a role for estrogen-related receptors (ERRs) in that ERRα1 upregulates eNOS mRNA and protein expression and stimulates eNOS activity in bovine pulmonary artery endothelial cells. We observed that compared with luteal phase sheep, ex vivo UAendo eNOS was elevated by over one-to threefold (p < 0.001) during the follicular phase and by four- to sixfold (p < 0.001) during pregnancy,21,23,35,60 and these levels related well to the increases in UBF during these physiological states. In summary, estrogen-ER regulates gene and protein expression of diverse function associated with the maintenance of vascular tone within the vasculature.
The angiogenesis paradigm begins with activation of endothelial cells by an angiogenic stimulus followed by degradation of the basement membrane and subsequent proliferation and/or migration of endothelial cells to form new vessels from the preexisting vasculature.94,95 Subsequent to the new vessel formation, there is stabilization of the vessel by extracellular matrix and adhesion factor production to line the intimal surface of the vessel wall.94,95 As a result, it is important to evaluate the role of estrogen and the ERs in inducing these aspects of endothelial cell biology.
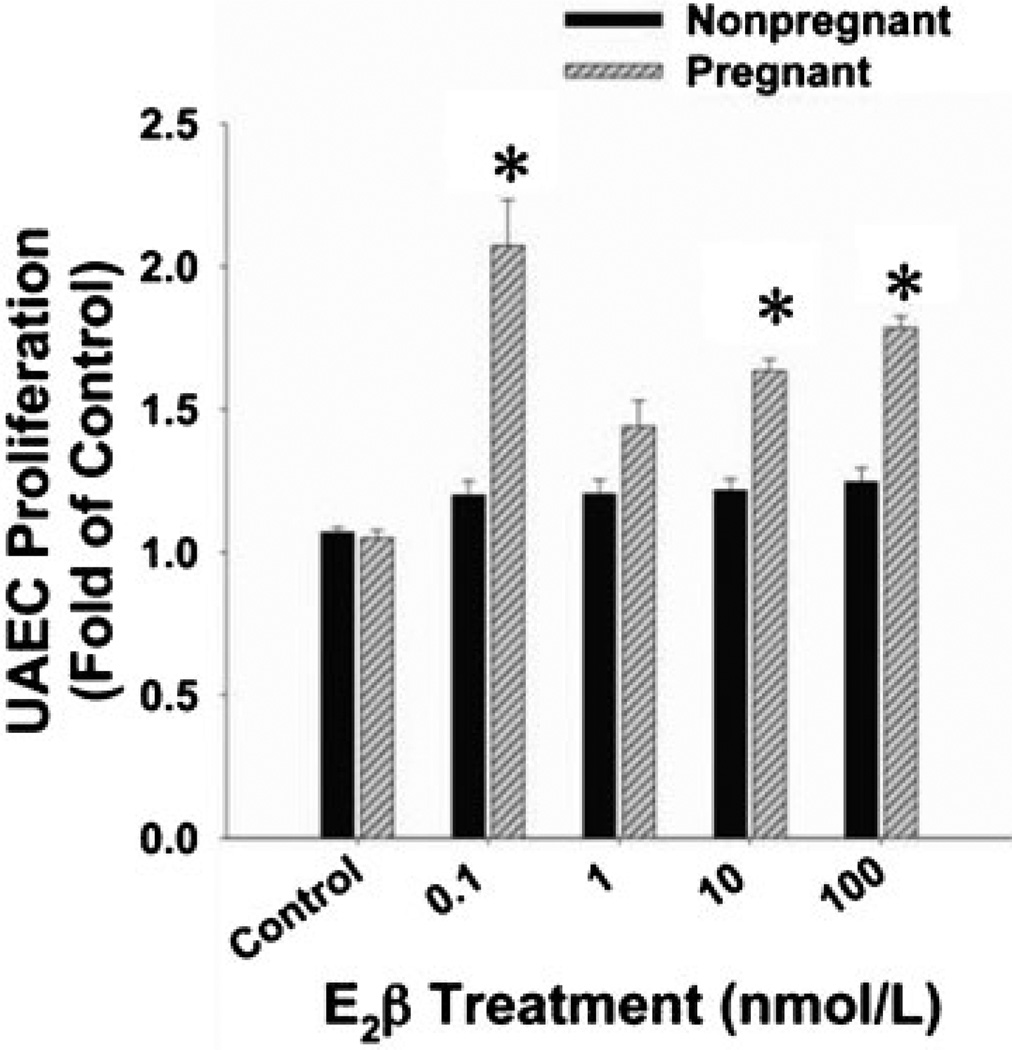
With regard to the estrogen-mediated angiogenic regulation of UBF during gestation, we first review the role of estrogen-ER in the proliferation of endothelial cells in general. In cultured human umbilical vein endothelial cells (HUVECs) and ovine uterine artery endothelial cells (both reproductive ECs) derived from the pregnant state (P-UAECs), low concentrations of estradiol-17β causes proliferation via classical ER-α and/or ER-β, demonstrating a role for ERs in pregnancy-induced angiogenesis.95–97 In this regard, the nonspecific ER antagonist ICI 182,780 completely abrogated the mitogenic actions of estradiol-17β on these endothelial cells.96,97 Findings from our laboratory have also shown (Fig. 5) that estradiol-17β does not induce proliferation in ovine uterine artery endothelial cells derived from the nonpregnant state (NP-UAECs), demonstrating both a pregnancy-induced uterine vascular endothelial cell adaptation as well as estrogenic response programming during pregnancy.96
Figure 5.
Concentration-dependent cell proliferation responses of nonpregnant uterine artery endothelial cells (NP-UAECs) and pregnant (P)-UAECs to E2β. A biphasic proliferative response was observed in P-UAECs in response to E2β compared with control with maximum responses at a physiological concentration of 0.1 nmol/L (two-way analysis of variance; pregnancy times concentration effect; E2β, F4, 40= 8.16, p < 0.0001. NP-UAECs did not respond to E2β. Asterisk indicates increase (p < 0.05; n= 6) in P-UAEC proliferation compared with both the respective NP-UAEC (n = 7) group and untreated control.96 (With permission from Jobe SO, et al. Hypertension 2010;55:1005–1011.)
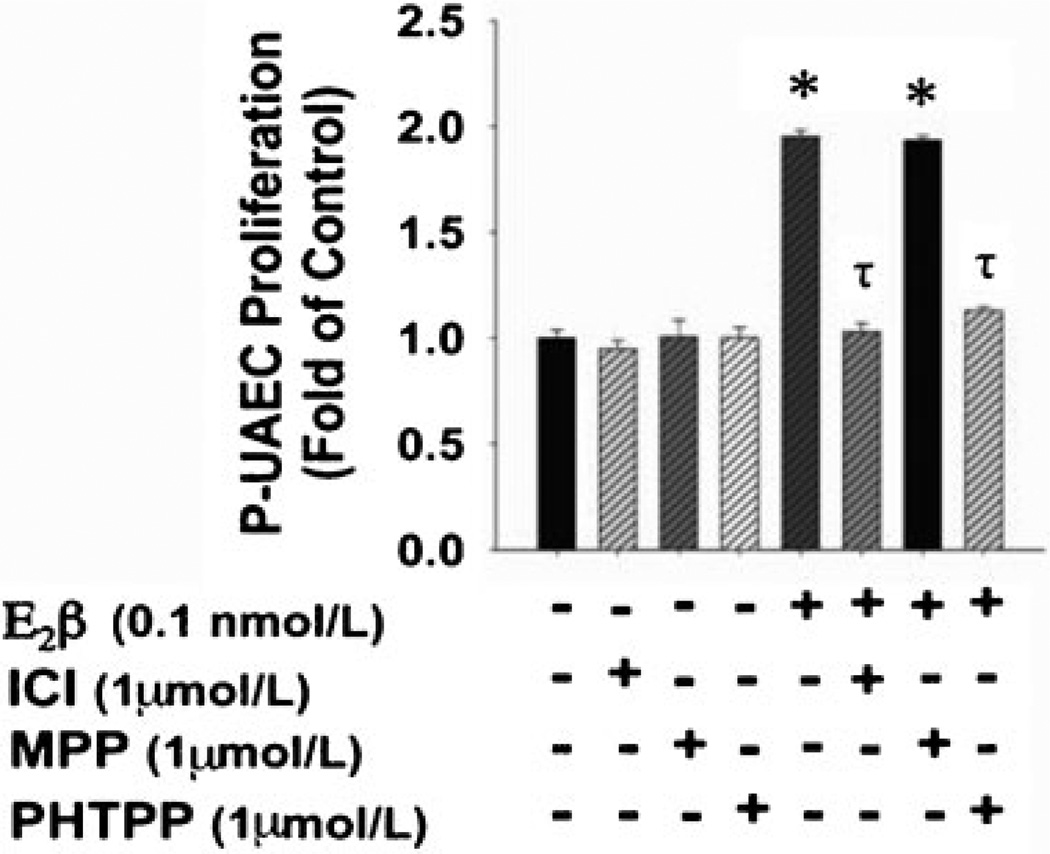
Because of the potential relevance of ER subtype selectivity in vascular function, there is considerable interest in investigating whether the classical ERs exhibit functional heterogeneity in the regulation of estradiol-17β-induced endothelial proliferation. We exploited several of the ER-subtype-specific pharmacological agonists and antagonists described in Table 1. Our studies showed that estradiol-17β-induced proliferation of P-UAECs is unaltered by the specific ER-α inhibitor MPP (Fig. 6), which has >200-fold antagonistic selectivity for ER-α over ER-β.1,96 These results suggest that ER-α does not play a role in pregnancy-induced uterine artery endothelial angiogenesis.96 Equally important and consistent with the MPP antagonist data, the specific ER-α agonist PPT, which demonstrates a 410-fold greater affinity for ER-α over ER-β (Table 1), does not stimulate proliferation of P-UAECs at any concentration studied (Fig. 7), further validating the lack of a role for ER-α in estradiol-17β-induced proliferation of P-UAECs.96 In contrast, the ER-β selective antagonist PHTPP completely inhibits estradiol-17β-induced proliferation of P-UAECs demonstrating ER-β selectivity (Fig. 6).We also observed that the selective ER-β selective agonist DPN induces proliferation in P-UAECs (Fig. 7), further validating that estrogen-induced proliferation of P-UAECs occurs primarily via ER-β and completely independent of ER-α. These findings demonstrate a clear physiological functional heterogeneity of ER-β in estrogen-induced endothelial proliferation and thus the regulation of angiogenesis. This assertion is further supported by observations that ER-β KO mice develop abnormal vascular function and hypertension associated with endothelial dysfunction and impaired angiogenesis.56 Along these lines, it has also been demonstrated that ER-β is the primary estrogen receptor expressed in the primate endometrial vascular and perivascular endothelium, and thus it may be the sole mediator of genomic estrogenic angiogenic actions in uterine vascular endothelium.98
Figure 6.
The effects of 1 µmol/L of ICI, MPP, and PHTPP on pregnant uterine artery endothelial cell (P-UAEC) proliferative responses to 0.1 nmol/L of E2β. ICI and PHTPP, but not MPP, abrogated the response of P-UAECs to E2β (two-way analysis of variance; antagonist times group effect; F5,60 = 25.272, p < 0.001). Asterisks indicate increase (p < 0.05, n = 6) in P-UAEC proliferation compared with untreated control; τ indicates inhibition (p < 0.05) of P-UAEC proliferation with ICI and PHTPP.96 (With permission from Jobe SO, et al. Hypertension 2010;55:1005–1011.)
Figure 7.
Concentration-dependent effects of (A) estrogen receptor (ER)-α agonist PPT, (B) ER-β agonist DPN, and (C) their combination on cell proliferation responses of P-UAECs. Blockade of ER-β with PHTPP (1 µmol/L) before treatment with ER-β agonist DNP is also shown in (C). * Increase (p < 0.05; n = 7) in P-UAEC proliferation compared with untreated controls. λ indicates a difference (p < 0.05) in P-UAEC proliferation in response to DPN or the combination of DNP and PPT compared with E2β-only responses; τ inhibition (p < 0.05) of P-UAEC proliferation with PHTPP.96 (With permission from Jobe SO, et al. Hypertension 2010;55:1005–1011.)
Apart from inducing endothelial cell proliferation, estrogen induces migration, tube formation, and extracellular matrix as well as adhesion factor production in endothelial cells via an ER-mediated mechanism; collectively, these functions are integral components of the angiogenesis paradigm. In this context, the nonspecific ER antagonist ICI 182,780 abrogates the migration, H3 thymidine incorporation, tube formation, and “wound” closure effects of estradiol-17β in cultured HUVECs and UAECs.95,97,99 The ERs also mediate estradiol-17β-induced enhanced HUVEC adhesion to various matrix proteins possibly via an increase in integrin expression and function.95,100 The blockade of these angiogenic responses via the ER antagonists demonstrates that estrogen-induced angiogenesis in the uterine vascular endothelium is mediated specifically via its classical receptors. These data also illustrate that estrogen via the classical ERs plays an important role in endothelial proliferation, migration, and adhesion that are closely involved in uterine angiogenesis, an important vascular adaptation leading to rises in UBF during normal pregnancy. However, presently it is not clear whether estradiol-17β demonstrates subtype specificity via either ER-α or ER-β to induce these other angiogenic components other than endothelial proliferation, and thus this requires further investigation.
Role of Estrogen and Estrogen Receptors on Nongenomic Responses in Uterine Artery Endothelial Cells
The maintenance of vascular tone is regulated by numerous potent endothelium-derived vasodilators. The most studied are NO and prostanoids like prostacyclin (PGI2). These two vasodilators have been shown to be increased during pregnancy; however, in vivo experiments have not confirmed a major contribution of PGI2 to the maintenance of elevated UBF during late gestation.101,102 Although PGI2 is a potent vasodilator, its role in pregnancy is not as well understood as that of NO, which has been shown to have a definitive role in partly regulating uteroplacental blood flow during gestation. Therefore, the following section concentrates on the E2β-ER-NO effects during pregnancy.
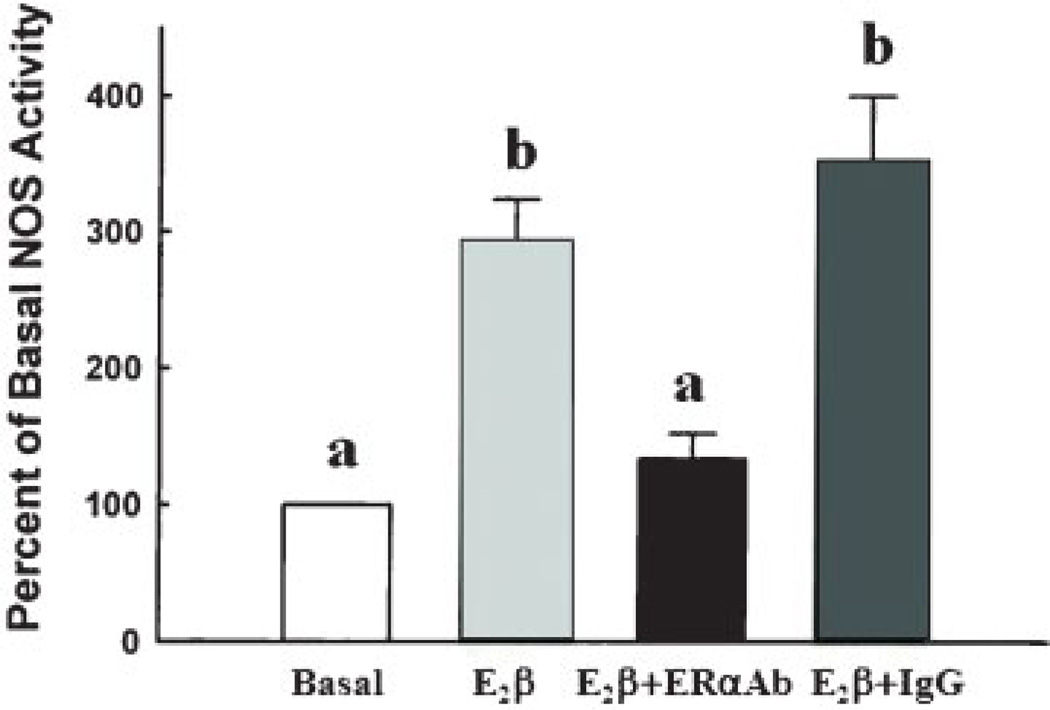
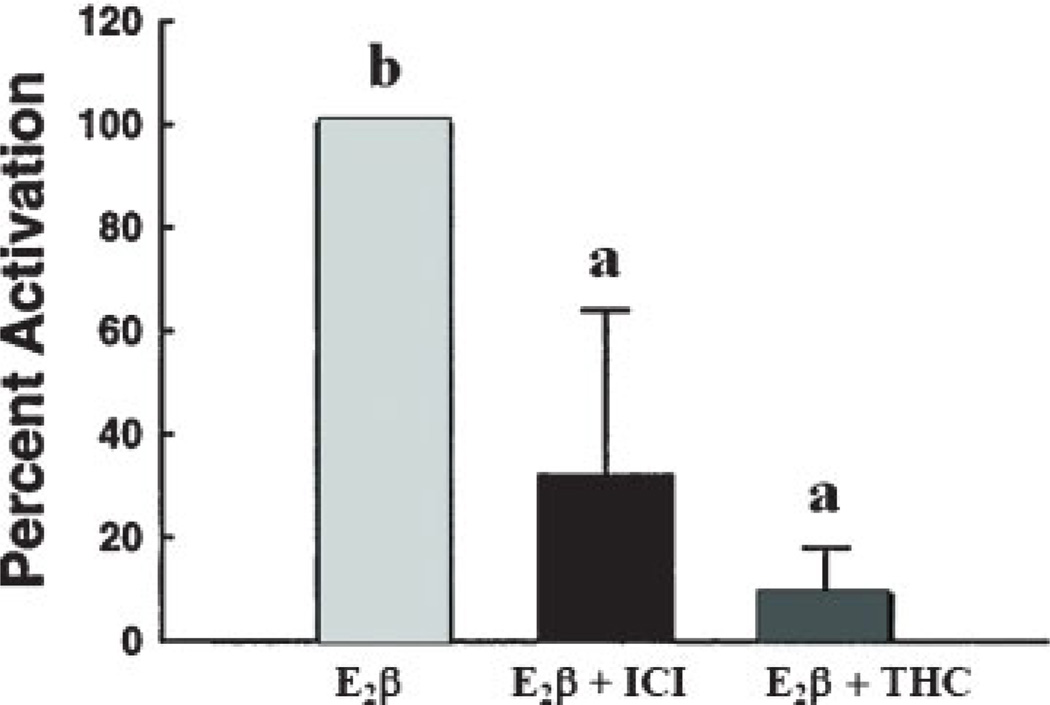
It is generally accepted that rapid ER-mediated estrogenic responses contribute to NO production in numerous vascular beds including aortic,103 pulmonary artery,104,105 and umbilical vein106,107 endothelial cells leading to vasodilatation and maintenance of perfusion. Membrane ERs colocalize and are functionally linked to distinct subpopulations of specialized dynamic cholesterol-rich plasma membrane domains called caveolae that compartmentalize many receptors and signaling molecules to create a specific “functional signaling module.”108,109 Within these caveolae, ERs (both ER-α and ER-β) and eNOS are colocalized with caveolin (Cav)-1, the main resident scaffolding protein.26,44,110 Cav-1 and eNOS interaction occurs through the caveolin scaffolding domain (CSD) on Cav-1, and the CSD expands the calmodulin binding domain on eNOS that is necessary for Ca2+/calmodulin-dependent eNOS activation.111 Stimulation of endogenous levels of ER-α and ER-β as well as overexpression of either receptor are capable of initiating eNOS activation.112–114 Although the details of the E2β-ER-NO mechanism are still under investigation, much work has focused on understanding ER-α signaling cascade activation.3,115 However, little is known about ER-β signaling. Chambliss et al reported an increase in eNOS activity after estrogen treatment and its abrogation when isolated endothelial membranes were preincubated with ICI-182,780 or ER-α antibody but not immunoglobulin G controls112 (Fig. 8). Additionally, in a later study from the same laboratory, these investigators demonstrated that purified endothelial caveolar membranes lost eNOS activity when ICI-182,780 or THC, an ER-β antagonist, was used in combination with estrogen113 (Fig. 9). These seemingly conflicting results partly may be explained by the overexpression technique used during these studies; however, they demonstrated that ER-β can initiate the required signaling cascade that activates eNOS.112,116 It is important to note, however, that THC, as shown in Table 1, possesses antagonistic properties toward ER-β while acting as an agonist toward ER-α. We also recently showed that that S-nitrosylation of proteins were upregulated via an E2β-ER-NO mechanism in P-UAECs.79 However, endogenous levels of ER-α or ER-β, evaluated with specific agonists PPT and DPN, respectively, alone were incapable of initiating these NO-mediated proteomic changes. In contrast, combination PPT plus DPN treatment together induced the same overall S-nitrosylation of protein effect as seen with estrogen treatment alone. These data are indirectly suggestive that both ER-α and ER-β are capable and required for NO production.
Figure 8.
Effect of estrogen receptor (ER)-α antibody on the nitric oxide synthase (NOS) activity response to E2β. Isolated endothelial plasma membrane incubations were performed over 15 minutes in the absence (basal) or presence of E2β with or without antibody to ER-α (TE111) or unrelated immunoglobulin G (IgG) added. Different letters are significantly different (p < 0.05).112 (Adapted from Chambliss KL, et al. Circ Res 2000;87:e44–e52.)
Figure 9.
Effect of ICI 182,780 (10 nM) and THC (1 nM), the ER-β antagonist, on E2β-mediated endothelial nitric oxide synthase (eNOS) activation in isolated endothelial cell caveolae membranes. 3H-L-arginine conversion to 3H-L citrulline was measured over 60 minutes. In caveolae, stimulated NOS activity ranged from 0.5 to 0.9 pmol citrulline per milligram protein* min in separate studies. Different letters are significantly different (p < 0.05).113 Adapted from Chambliss KL, et al. Mol Endocrinol 2002;16:938–946.)
Although several research groups have established that ERs can be localized to the endothelial membrane or caveolae and that they induce rapid NO production,44,106,110,117,118 the trafficking mechanism of ERs to the caveolae remains poorly understood. It has been postulated that even though ERs may not possess transmembrane domains,44,106,110,117,118 ERs have been linked to fatty acid modification via palmitoylation, as a posttranslational change responsible for their presence within the caveolae and their association with regulatory proteins such as Cav-1.69,118,119 In pursuit of further understanding the trafficking mechanism(s) of ERs to the plasma membrane, Pedram and coworkers118 identified a highly conserved Cav-1 binding sequence found in ER-α, ER-β, progesterone receptor(s), and androgen receptors. This very highly conserved sequence is located within the E domain, which contains the ligand-binding pocket, activator, and repressor binding sites. Serine 522 and cysteine 447 are required for ER-α and Cav-1 protein–protein interaction; cysteine 447 was also identified as the site of palmitoylation. 118 Furthermore, Acconcia et al demonstrated that ER-α was palmitoylated by palmitoyl acyl transferase (PAT) and that point mutation of C447A abolished PAT’s palmitoylation recognition site. C447A mutation also abolished rapid activation of the ERK/MAPK signaling pathway,120 required for eNOS activation in these endothelial cell lines.112,120 Interestingly, ER-β also possesses this same recognition site, and mutation on cysteine 418 impaired ER-β’s palmitoylation and completely abolished the rapid estrogen-mediated activation through the p38b signaling pathway.118 However, no specific explanations or evidence have been put forth that would explain the low abundance of ERs in the caveolae (3 to 5%) compared with other cellular locations (~90%) or their physical orientation at the membrane (intracellular or extracellular). This area is currently under investigation. A regulatory protein that may facilitate palmitoylation on ER-α and ER-β is Hsp27. Razandi et al showed recently that downregulation of Hsp27 using siRNA significantly reduced ER-β palmitoylation and ER-β to Cav-1 protein–protein interaction.121
Although the precise steps underlying estrogen-induced eNOS activation is not fully known, eNOS is known to be regulated by complex multisite phosphorylation events.122 eNOS is phosphorylated at least in four serine/threonine sites that have been linked to eNOS activation: Ser 635 located within the NADPH binding domain, ser 1179 located in the FMN binding domain, ser 116 located in the oxygenase domain, and thr 495 located in the calmodulin binding domain (CaM).122 The two most extensively sites studied are the stimulatory phosphorylation ser 1179 and the inhibitory phosphorylation 495.115,123,124 Phosphorylation at position 1179 and/or 635 has been associated with an increase in NO production at resting levels of [Ca2+]. Ser 1179 is thought to increase NO production by removing the auto-inhibitory tail of eNOS,111 whereas ser 635’s location at the CaM auto inhibitory sequence is thought to maintain eNOS activity after the initial rise of intracellular [Ca2+] concentration. 111 Phosphorylation at thr 495 is observed at the basal eNOS activity, and reports have observed downregulation of this phosphorylation after agonist stimulation. The mechanism by which this phosphorylation site is thought to occur is by interfering with Ca2+/CaM binding on eNOS.111 Phosphorylation at ser 114 is currently controversial where some groups observed increased or decreased NO production depending on the agonist used. Mount et al provides a comprehensive list of kinases and phosphatases that have been shown to have or might have a role in eNOS phosphorylation status at the resting and active forms.122 However, a comprehensive study on estrogen-induced eNOS multisite phosphorylation via ER-α and/or ER-β in different subcellular domains is warranted.
Estrogen-ER-induced NO production also exhibits specificity with reference to underlying signaling pathways at least in the uterine vasculature. The two widely implicated cascades in the uterine vasculature are the ERK and PI3K/AKT pathways. Chen et al4 demonstrated that estradiol-17β-BSA agonist, a plasma membrane impermeable estrogen, induces rapid activation of ERK1/2 in a time- and concentration-dependent manner along with the upstream activators Raf-1 and MEK-1. However, estrogen did not activate the PI3K/Akt pathway, suggesting that in UAECs, ERK1/2 is the more predominant signaling cascade. In summary, we hypothesize that ERs possibly mediate NO production through the activation of signaling cascades that alter eNOS multisite phosphorylation state, protein–protein interactions, and its spatial distribution.
Conclusion
We have presented data supporting the notion that estrogen and ERs are involved in the short- and long-term endothelial adaptations during pregnancy. UBF increases are partly controlled by endogenous cycling levels of estrogen. In vivo studies demonstrated that ER-α and ER-β proteins are expressed in reproductive and nonreproductive arterial endothelia and their expression pattern within reproductive endothelial changes as estrogen levels change. Ex vivo studies demonstrated the presence of only ER-β in the endometrial endothelium, in all phases of the ovarian cycle in human and nonhuman primate, which points to genomic and nongenomic estrogenic affects orchestrated by ER-β in the absence of ER-α.125
Although both ER-α and ER-β are activated by estrogen, their molecular and structural differences coupled with differential effects of estrogen in target cells and tissues suggest a functional heterogeneity of the ERs in estrogen signaling. To gain this effect, structural and functional analyses of ER-α and ER-β have led to the discovery of ligand compounds that discriminate between the two ERs and provide insights into the complexity of differential estrogen signaling. Furthermore, subcellular membrane or nuclear localization of the ER-α and ER-β subtypes also point to distinct functions with regard to the ERs. These findings, at least in part, mean that investigation of estrogen-signaling requires specific appreciation of ER-subtype functional heterogeneity as well as localization, which may underline the divergent effects of estrogen in target cells and tissues.
Our data have shown that in vitro proliferation of uterine artery endothelial cells was strictly mediated primarily by ER-β when cells were derived from late pregnant ewes as opposed to endothelial cells derived from nonpregnant ewes.96 The differences in response may be due to the differences in the structure-function characteristic of each receptor; therefore, functional heterogeneity of the receptor subtypes, in turn, allows for the induction of a multitude of diverse biological outcomes by estrogen on the uterine vasculature. Additionally, as part of the vasodilatory effects of estrogen, many research groups, including our own, have shown that NO production is mediated via rapid nongenomic action of both ER-α and ER-β. However, detailed explanations on whether eNOS phosphorylation and protein–protein interaction required for NO production may occur via a specific ER subtype are limited. It is important to note that both ER subtypes and eNOS are localized to the membrane caveolae in most endothelium, suggesting that both ER-α and ER-β may be capable of inducing eNOS multisite phosphorylations that consecutively initiate estrogen-induced NO production signaling events.
Thus a question of considerable interest is why the ERs exhibit subtype specificity in differentially regulating long-term genomic versus rapid nongenomic effects of estrogen on the endothelium and if this represents unappreciated estrogen signaling complexity. Nevertheless, we cannot rule out a convergence/combination of both ERs in mediating genomic versus nongenomic signaling mechanisms in the uterine vasculature during pregnancy. It is reasonable to hypothesize on the selective effects of the ERs. Therefore, based on the evidence provided here, we propose the hypothesis that the nongenomic ER-α and/or ER-β signaling mechanisms may represent rapidly activated cellular functionalities needed for acute dynamic vasodilatation, whereas genomic ER-β-mediated signaling may mediate long-term cellular programming for chronic adaptations needed for maintenance of estrogen-induced UBF increases during pregnancy.
Acknowledgments
Funding
Supported by National Institutes of Health grants R25-GM083252 (MBP, SOJ – ML Carnes, PI); T32-HD041921–07 (SOJ – IM Bird, PI); AA19446 (JR, PI); and HL49210, HD38843, HL89144 (RRM, PI).
We wish to thank Gladys E. Lopez, Benjamin C. Hofeld, Timothy J. Morschauser, Mary Y. Sun, Cindy L. Goss, Terrance M. Phernetton, and Jason L. Austin for their assistance with these studies and manuscript preparation. These studies are in partial fulfillment of Ph.D. training (Mayra B. Pastore and Sheikh O. Jobe) in the Endocrinology and Reproductive Physiology Training Program (both have contributed equally to this manuscript).
References
- 1.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 3.Chambliss KL, Yuhanna IS, Mineo C, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87(11):E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 4.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145(1):113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 5.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565(Pt 1):71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol. 1998;275(3 Pt 2):H731–H743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98(9):2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232(3):H231–H235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- 9.Lang U, Baker RS, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow—a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S55–S61. doi: 10.1016/s0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 10.Magness RR, Rosenfeld CR. The role of steroid hormones in the control of uterine blood flow. The Uterine Circulation. In: Rosenfeld CR, editor. Reproductive and Perinatal Medicine. Vol. 10. Ithaca, NY: Perinatology Press; 1989. pp. 239–271. [Google Scholar]
- 11.Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. 1982;55(Suppl 2):32–42. [PubMed] [Google Scholar]
- 12.Ford SP, Christenson RK. Blood flow to uteri of sows during the estrous cycle and early pregnancy: local effect of the conceptus on the uterine blood supply. Biol Reprod. 1979;21(3):617–624. doi: 10.1095/biolreprod21.3.617. [DOI] [PubMed] [Google Scholar]
- 13.Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod. 2004;70(6):1886–1894. doi: 10.1095/biolreprod.103.019901. [DOI] [PubMed] [Google Scholar]
- 14.Greiss FC, Jr, Anderson SG. Uterine vescular changes during the ovarian cycle. Am J Obstet Gynecol. 1969;103(5):629–640. doi: 10.1016/0002-9378(69)90560-2. [DOI] [PubMed] [Google Scholar]
- 15.Harvey CA, Owen DA. Changes in uterine and ovarian blood flow during the oestrous cycle in rats. J Endocrinol. 1976;71(3):367–369. doi: 10.1677/joe.0.0710367. [DOI] [PubMed] [Google Scholar]
- 16.Greiss FC, Jr, Anderson SG. Effect of ovarian hormones on the uterine vascular bed. Am J Obstet Gynecol. 1970;107(6):829–836. doi: 10.1016/s0002-9378(16)34033-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld CR, Killam AP, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17 on the magnitude and distribution of uterine blood flow in nonpregnant, oophorectomized ewes. Pediatr Res. 1973;7(3):139–148. doi: 10.1203/00006450-197303000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld CR, Morriss FH, Jr, Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5(5–6):252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 19.Miller SL, Jenkin G, Walker DW. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol. 1999;180(5):1138–1145. doi: 10.1016/s0002-9378(99)70607-1. [DOI] [PubMed] [Google Scholar]
- 20.Van Buren GA, Yang DS, Clark KE. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gynecol. 1992;167(3):828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- 21.Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x) Am J Physiol Heart Circ Physiol. 2001;280(4):H1692–H1698. doi: 10.1152/ajpheart.2001.280.4.H1692. [DOI] [PubMed] [Google Scholar]
- 22.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001;280(4):H1699–H1705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- 23.Vagnoni KE, Shaw CE, Phernetton TM, Meglin BM, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. III. Ovarian and estrogen effects on NO synthase. Am J Physiol. 1998;275(5 Pt 2):H1845–H1856. doi: 10.1152/ajpheart.1998.275.5.H1845. [DOI] [PubMed] [Google Scholar]
- 24.Magness RR, Rosenfeld CR, Hassan A, Shaul PW. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am J Physiol. 1996;270(6 Pt 2):H1914–H1923. doi: 10.1152/ajpheart.1996.270.6.H1914. [DOI] [PubMed] [Google Scholar]
- 25.Salhab WA, Shaul PW, Cox BE, Rosenfeld CR. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am J Physiol Heart Circ Physiol. 2000;278(6):H2134–H2142. doi: 10.1152/ajpheart.2000.278.6.H2134. [DOI] [PubMed] [Google Scholar]
- 26.Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005;72(3):530–537. doi: 10.1095/biolreprod.104.035949. [DOI] [PubMed] [Google Scholar]
- 27.Yi FX, Boeldt DS, Gifford SM, et al. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod. 2010;82(1):66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256(4 Pt 1):E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol. 2002;38(2):115–125. doi: 10.1016/s0306-3623(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 30.Baker RS, Hirth J, Friedman A, Clark KE. ER alpha receptors mediate estrogen induced increases in uterine blood flow. J Soc Gynecol Investig. 2003;10(Suppl 2):332. [Google Scholar]
- 31.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 33.Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 34.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol. 2005;565(Pt 1):85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto J, Hirose R, Sakaguchi H, Tamaya T. Clinical significance of expression of estrogen receptor alpha and beta mRNAs in ovarian cancers. Oncology. 2000;58(4):334–341. doi: 10.1159/000012121. [DOI] [PubMed] [Google Scholar]
- 37.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res. 1998;83(2):224–229. doi: 10.1161/01.res.83.2.224. [DOI] [PubMed] [Google Scholar]
- 38.Giambiagi N, Pasqualini JR, Greene G, Jensen EV. Recognition of two forms of the estrogen receptor in the guinea-pig uterus at different stages of development by a monoclonal antibody to the human estrogen receptor. Dynamics of the translocation of these two forms to the nucleus. J Steroid Biochem. 1984;20(1):397–400. doi: 10.1016/0022-4731(84)90241-3. [DOI] [PubMed] [Google Scholar]
- 39.Batra S, Iosif S. Nuclear estrogen receptors in human uterine arteries. Gynecol Obstet Invest. 1987;24(4):250–255. doi: 10.1159/000298810. [DOI] [PubMed] [Google Scholar]
- 40.Wu WX, Ma XH, Smith GC, Nathanielsz PW. Differential distribution of ERalpha and ERbeta mRNA in intrauterine tissues of the pregnant rhesus monkey. Am J Physiol Cell Physiol. 2000;278(1):C190–C198. doi: 10.1152/ajpcell.2000.278.1.C190. [DOI] [PubMed] [Google Scholar]
- 41.Weihua Z, Saji S, Mäkinen S, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97(11):5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang K, Zhang L. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15(4):336–348. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malamas MS, Manas ES, McDevitt RE, et al. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem. 2004;47(21):5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- 44.Chambliss KL, Shaul PW. Rapid activation of endothelial NO synthase by estrogen: evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids. 2002;67(6):413–419. doi: 10.1016/s0039-128x(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld CR, Morriss FH, Jr, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17beta on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol. 1976;124(6):618–629. doi: 10.1016/0002-9378(76)90064-8. [DOI] [PubMed] [Google Scholar]
- 46.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 47.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24(1):145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 48.Orimo A, Inoue S, Ikegami A, et al. Vascular smooth muscle cells as target for estrogen. Biochem Biophys Res Commun. 1993;195(2):730–736. doi: 10.1006/bbrc.1993.2106. [DOI] [PubMed] [Google Scholar]
- 49.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89(5):1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- 50.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89(4):1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 51.Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94(4):727–733. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- 52.Kim-Schulze S, McGowan KA, Hubchak SC, et al. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94(6):1402–1407. doi: 10.1161/01.cir.94.6.1402. [DOI] [PubMed] [Google Scholar]
- 53.Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98(1):36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101(15):1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 55.Andersson C, Lydrup ML, Fernö M, Idvall I, Gustafsson J, Nilsson BO. Immunocytochemical demonstration of oestrogen receptor beta in blood vessels of the female rat. J Endocrinol. 2001;169(2):241–247. doi: 10.1677/joe.0.1690241. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y, Bian Z, Lu P, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295(5554):505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 57.Libby P, Bonow RO, Mann DL, Zipes DP. Pregnancy and cardiovascular disease. In: Libby P, Bonow RO, Mann DL, Zipes DP, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Saunders Elsevier; 2008. p. 1967. [Google Scholar]
- 58.Lang U, Baker RS, Braems GA, Clark KE. Inhibition of nitric oxide (NO) synthesis antagonises the oestrogen-induced increase in coronary blood. Eur J Cancer. 2000;36(Suppl 4):111. doi: 10.1016/s0959-8049(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 59.Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283(3):H1169–H1180. doi: 10.1152/ajpheart.00397.2000. [DOI] [PubMed] [Google Scholar]
- 60.Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol. 1997;272(4 Pt 2):H1730–H1740. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- 61.Cárdenas H, Burke KA, Bigsby RM, Pope WF, Nephew KP. Estrogen receptor beta in the sheep ovary during the estrous cycle and early pregnancy. Biol Reprod. 2001;65(1):128–134. doi: 10.1095/biolreprod65.1.128. [DOI] [PubMed] [Google Scholar]
- 62.Hirata S, Shoda T, Kato J, Hoshi K. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab. 2003;14(3):124–129. doi: 10.1016/s1043-2760(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 63.Inoue S, Hoshino S, Miyoshi H, et al. Identification of a novel isoform of estrogen receptor, a potential inhibitor of estrogen action, in vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;219(3):766–772. doi: 10.1006/bbrc.1996.0308. [DOI] [PubMed] [Google Scholar]
- 64.Kumar R, Johnson BH, Thompson EB. Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essays Biochem. 2004;40:27–39. doi: 10.1042/bse0400027. [DOI] [PubMed] [Google Scholar]
- 65.Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64(5):310–319. doi: 10.1016/s0039-128x(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 66.Gosden JR, Middleton PG, Rout D. Localization of the human oestrogen receptor gene to chromosome 6q24——q27 by in situ hybridization. Cytogenet Cell Genet. 1986;43(3–4):218–220. doi: 10.1159/000132325. [DOI] [PubMed] [Google Scholar]
- 67.Krust A, Green S, Argos P, et al. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27(4):299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Zhou W, Liu Z, Wu J, et al. Identification and characterization of two novel splicing isoforms of human estrogen-related receptor beta. J Clin Endocrinol Metab. 2006;91(2):569–579. doi: 10.1210/jc.2004-1957. [DOI] [PubMed] [Google Scholar]
- 71.Kumar R, Thompson EB. Transactivation functions of the N-terminal domains of nuclear hormone receptors: protein folding and coactivator interactions. Mol Endocrinol. 2003;17(1):1–10. doi: 10.1210/me.2002-0258. [DOI] [PubMed] [Google Scholar]
- 72.Katzenellenbogen JA, Muthyala R, Katzenellenbogen BS. Nature of the ligand-binding pocket of estrogen receptor alpha and beta: The search for subtype-selective ligands and implications for the prediction of estrogenic activity. Pure Appl Chem. 2003;75(11–12):2397–2403. [Google Scholar]
- 73.DeLisle RK, Yu SJ, Nair AC, Welsh WJ. Homology modeling of the estrogen receptor subtype beta (ER-beta) and calculation of ligand binding affinities. J Mol Graph Model. 2001;20(2):155–167. doi: 10.1016/s1093-3263(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 74.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2(5):335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 76.Compton DR, Sheng S, Carlson KE, et al. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor beta antagonist activity. J Med Chem. 2004;47(24):5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- 77.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143(3):941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 78.Krom YD, Pires NM, Jukema JW, et al. Inhibition of neointima formation by local delivery of estrogen receptor alpha and beta specific agonists. Cardiovasc Res. 2007;73(1):217–226. doi: 10.1016/j.cardiores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 79.Zhang HH, Feng L, Wang W, Magness RR, Chen DB. Estrogen-responsive nitroso-proteome in uterine artery endothelial cells: role of endothelial nitric oxide synthase and estrogen receptor-β. J Cell Physiol. 2012;227(1):146–159. doi: 10.1002/jcp.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Hoorn WP. Identification of a second binding site in the estrogen receptor. J Med Chem. 2002;45(3):584–589. doi: 10.1021/jm0109661. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100(2):703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubanyi GM, Freay AD, Kauser K, et al. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99(10):2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100(2):703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bilsel AS, Moini H, Tetik E, Aksungar F, Kaynak B, Ozer A. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res. 2000;46(3):579–584. doi: 10.1016/s0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- 86.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Upregulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360(3):291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
- 87.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Förstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension. 1998;31(2):582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- 88.MacRitchie AN, Jun SS, Chen Z, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81(3):355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 89.Weiner CP, Knowles RG, Moncada S. Induction of nitric oxide synthases early in pregnancy. Am J Obstet Gynecol. 1994;171(3):838–843. doi: 10.1016/0002-9378(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi A, Kobayashi A, Takahashi R, Suzuki F, Nakagawa T, Kimotro K. Effects of voluntary running exercise on blood pressure and renin-angiotensin system in spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Nutr Sci Vitaminol (Tokyo) 2000;46(4):165–170. doi: 10.3177/jnsv.46.165. [DOI] [PubMed] [Google Scholar]
- 91.Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126(6):1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- 92.Abbott CR, Rossi M, Kim M, et al. Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869(1–2):203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 93.Sumi D, Ignarro LJ. Estrogen-related receptor alpha 1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci U S A. 2003;100(24):14451–14456. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furcht LT. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986;55(5):505–509. [PubMed] [Google Scholar]
- 95.Morales DE, McGowan KA, Grant DS, et al. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91(3):755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 96.Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55(4):1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oviedo PJ, Sobrino A, Laguna-Fernandez A, et al. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol Cell Endocrinol. 2011;335(2):96–103. doi: 10.1016/j.mce.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 98.Critchley HO, Brenner RM, Henderson TA, et al. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86(3):1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- 99.Matsubara K, Matsubara Y, King AG, et al. Regulation of endothelial cell proliferation by estrogen in reproductive organs. In: Kimura D, editor. Cell Growth Processes: New Research. Hauppauge, NY: Nova Science Publishers Inc; 2008. pp. 159–182. [Google Scholar]
- 100.Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 101.Magness RR, Rosenfeld CR, Faucher DJ, Mitchell MD. Uterine prostaglandin production in ovine pregnancy: effects of angiotensin II and indomethacin. Am J Physiol. 1992;263(1 Pt 2):H188–H197. doi: 10.1152/ajpheart.1992.263.1.H188. [DOI] [PubMed] [Google Scholar]
- 102.Naden RP, Iliya CA, Arant BS, Jr, Gant NF, Jr, Rosenfeld CR. Hemodynamic effects of indomethacin in chronically instrumented pregnant sheep. Am J Obstet Gynecol. 1985;151(4):484–494. doi: 10.1016/0002-9378(85)90275-3. [DOI] [PubMed] [Google Scholar]
- 103.Klinge CM, Blankenship KA, Risinger KE, et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280(9):7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 104.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273(1 Pt 1):L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 105.Shaul PW, Smart EJ, Robinson LJ, et al. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271(11):6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 106.Haynes MP, Sinha D, Russell KS, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87(8):677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 107.Haynes MP, Li L, Sinha D, et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278(4):2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 108.Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275(25):19416–19421. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 109.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275(5 Pt 1):L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 110.Chen D, Zangl AL, Zhao Q, et al. Ovine caveolin-1: cDNA cloning, E. coli expression, and association with endothelial nitric oxide synthase. Mol Cell Endocrinol. 2001;175(1–2):41–56. doi: 10.1016/s0303-7207(01)00403-8. [DOI] [PubMed] [Google Scholar]
- 111.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- 112.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 113.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16(5):938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 114.Zhang HH, Feng L, Livnat I, et al. Estradiol-17beta stimulates specific receptor and endogenous nitric oxide-dependent dynamic endothelial protein S-nitrosylation: analysis of endothelial nitrosyl-proteome. Endocrinology. 2010;151(8):3874–3887. doi: 10.1210/en.2009-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103(3):401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286(17):14737–14743. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101(7):2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 119.Acconcia F, Ascenzi P, Bocedi A, et al. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem Biophys Res Commun. 2004;316(3):878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 121.Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30(13):3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42(2):271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 123.Boo YC, Hwang J, Sykes M, et al. Shear stress stimulates phosphorylation of eNOS at SeR635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283(5):H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 124.Michell BJ, Griffiths JE, Mitchelhill KI, et al. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9(15):845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 125.Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT. Human oestrogen receptors: differential expression of ER alpha and beta and the identification of ER beta variants. Steroids. 2002;67(12):985–992. doi: 10.1016/s0039-128x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 126.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51(15):3867–3873. [PubMed] [Google Scholar]
- 127.Wakeling AE, Valcaccia B, Newboult E, Green LR. Non-steroidal antioestrogens—receptor binding and biological response in rat uterus, rat mammary carcinoma and human breast cancer cells. J Steroid Biochem. 1984;20(1):111–120. doi: 10.1016/0022-4731(84)90197-3. [DOI] [PubMed] [Google Scholar]
- 128.Walter G, Liebl R, von Angerer E. Synthesis and biological evaluation of stilbene-based pure estrogen antagonists. Bioorg Med Chem Lett. 2004;14(18):4659–4663. doi: 10.1016/j.bmcl.2004.06.098. [DOI] [PubMed] [Google Scholar]
- 129.Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology. 2000;141(10):3534–3545. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- 130.Yang C, Xu G, Li J, et al. Benzothiophenes containing a piperazine side chain as selective ligands for the estrogen receptor alpha and their bioactivities in vivo. Bioorg Med Chem Lett. 2005;15(5):1505–1507. doi: 10.1016/j.bmcl.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 131.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999;140(2):800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 132.Minutolo F, Bertini S, Granchi C, et al. Structural evolutions of salicylaldoximes as selective agonists for estrogen receptor beta. J Med Chem. 2009;52(3):858–867. doi: 10.1021/jm801458t. [DOI] [PubMed] [Google Scholar]
- 133.Fang X, Wong S, Mitchell BF. Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology. 1997;138(7):2763–2768. doi: 10.1210/endo.138.7.5247. [DOI] [PubMed] [Google Scholar]