Key Points
CXCL13 and CXCL12 mediate chemotaxis of CNS lymphoma cells, and CXCL13 concentration in CSF is prognostic.
CXCL13 plus IL-10 is highly specific for the diagnosis of CNS lymphoma.
Abstract
Establishing the diagnosis of focal brain lesions in patients with unexplained neurologic symptoms represents a challenge. The goal of this study is to provide evidence supporting functional roles for CXC chemokine ligand (CXCL)13 and interleukin (IL)-10 in central nervous system (CNS) lymphomas and to evaluate the utility of each as prognostic and diagnostic biomarkers. We demonstrate for the first time that elevated CXCL13 concentration in cerebrospinal fluid (CSF) is prognostic and that CXCL13 and CXCL12 mediate chemotaxis of lymphoma cells isolated from CNS lymphoma lesions. Expression of the activated form of Janus kinase 1 supported a role for IL-10 in prosurvival signaling. We determined the concentration of CXCL13 and IL-10 in CSF of CNS lymphoma patients and control cohorts including inflammatory and degenerative neurologic disease in a multicenter study involving 220 patients. Bivariate elevated CXCL13 plus IL-10 was 99.3% specific for primary and secondary CNS lymphoma, with sensitivity significantly greater than reference standard CSF tests. These results identify CXCL13 and IL-10 as potentially important biomarkers of CNS lymphoma that merit further evaluation and support incorporation of CXCL13 and IL-10 into diagnostic algorithms for the workup of focal brain lesions in which lymphoma is a consideration.
Introduction
Determination of the pathological basis of focal brain lesions in patients with unexplained neurologic symptoms is a major clinical challenge. Persistent symptoms or rapid neurologic decline often mandates stereotactic brain biopsy, a highly invasive procedure with a 10% to 35% rate of diagnostic failure.1-3 Moreover, many lesions are not amenable to biopsy because of small size, location in deep brain structures, risk of hemorrhage, and other comorbidities.
The diagnosis of central nervous system (CNS) involvement of non-Hodgkin lymphoma is a particular challenge because of lesional response to glucocorticoids and features on magnetic resonance imaging (MRI) that are shared with other pathologies including astrocytic neoplasms, demyelination, neurosarcoid, vasculitis, infections, and leptomeningeal dissemination of systemic cancer. Although flow-cytometric and cytological analysis of cerebrospinal fluid (CSF) is useful in the evaluation of leptomeningeal disease, these tests are usually insensitive to pathological processes based in deep brain structures and rarely provide information that eliminates the need for brain biopsy; the sensitivity of CSF cytological analysis in the evaluation of primary CNS lymphoma (PCNSL) is ∼15%.4 Advances that facilitate diagnosis and early treatment of CNS lymphoma would likely be cost-effective, minimize repeat diagnostic CSF and MRI evaluations and brain biopsies, and also lead to improved outcomes.5-7
The molecular constituents of CSF can be applied as biomarkers to facilitate diagnosis of pathological processes within brain. At least 2 types of CSF biomarkers are used routinely: Epstein-Barr viral DNA in the diagnosis of AIDS-related CNS lymphoma and the germ cell markers α-fetoprotein and/or β–human chorionic gonadotropin in the diagnosis of childhood CNS germinoma. In the proper context, detection of high concentrations of these biomarkers in CSF may eliminate the need for brain biopsy.8
A recent study involving 23 patients with CNS lymphoma and 30 subjects with nonmalignant neurologic conditions provided evidence that the differential expression of micro RNAs in the CSF can be used to diagnose CNS lymphoma9; however, additional studies have shown that the micro RNAs evaluated (miR-21, miR-19, and miR-92a) are also expressed in common brain tumors including malignant glioma, as well as in lung and breast carcinoma, tumors that frequently metastasize to the brain and thus are not specific to CNS lymphoma.10-12 Large-scale proteomic profiling using two-dimensional liquid chromatography and mass spectrometry illuminated the spectrum of CSF proteins that are differentially expressed, both upregulated and downregulated, in CNS lymphoma.13 Candidate peptides hypothesized to have a role in the pathogenesis of CNS lymphomas have also been evaluated in preliminary studies, including interleukin (IL)-10, also identified using proteomic methods,13 and the CXC chemokine ligand (CXCL)13, a mediator of B-cell migration. Recently, Sasayama et al14 found elevated IL-10 in CSF specimens from 31 immunocompetent patients with PCNSL compared with 59 patients with other types of brain tumors, mainly malignant gliomas. Fischer et al15 detected elevated CXCL13 concentration in CSF from 30 lymphoma patients relative to 40 control subjects. Of note, however, neither of these 2 studies evaluated CSF from patients with inflammatory and/or infectious neurologic conditions including focal brain lesions that mimic the presentation of CNS lymphoma. In particular, the diagnostic accuracy of CXCL13 as a CSF biomarker of CNS lymphoma has not previously been evaluated.
We are pursuing the hypothesis that molecules involved in the pathobiology of CNS lymphoma will yield biomarkers with requisite specificity for clinical application. The goal of this study is to substantiate functional roles for CXCL13 and IL-10 in CNS lymphomas and to compare the potential utility of CXCL13 and IL-10 as diagnostic biomarkers, alone and in combination, which discriminates CNS lymphoma from the constellation of neuro-inflammatory conditions, primary and metastatic brain tumors, and other conditions for which CSF evaluation is standard practice.
Methods
Study population
CSF specimens from 220 subjects were obtained after informed consent in accordance with the Declaration of Helsinki and Institutional Review Board–approved protocols at the University of California, San Francisco and the Clinic of Infectious Diseases, San Raffaele Hospital, Milan, Italy. CSF was frozen within 2 hours of collection and stored at −70°C, as described.13 Exclusion criteria included age <12 years, traumatic CSF collection, and therapeutic intervention within 3 weeks.
We evaluated 4 populations of patients with CNS lymphoma (N = 83, median age 58; supplemental Table 1, see the Blood Web site): (1) 43 newly diagnosed patients with PCNSL (5 with HIV-associated PCNSL); (2) 10 patients with secondary CNS lymphoma (SCNSL; 1 HIV); (3) 17 patients with recurrent PCNSL; and (4) 13 patients with recurrent SCNSL. We studied 4 populations with diagnoses other than CNS lymphoma for comparison (N = 137, median age 46; supplemental Tables 2 and 3): (1) 46 benign controls with tumors or infection outside of the brain who underwent staging lumbar punctures, including 9 with aggressive extra-CNS non-Hodgkin lymphoma; (2) 71 patients with neuro-inflammatory conditions including multiple sclerosis, CNS vasculitis, neurosarcoid, encephalitis, cerebrovascular disease, toxoplasmosis, coccidioidal meningitis, cryptococcal meningitis, progressive multifocal leukoencephalopathy, and neurodegenerative conditions; (3) 8 patients with primary brain tumors including glioblastoma, medulloblastoma, and CNS germinoma; and (4) 12 patients with metastatic brain tumors including breast and lung cancer.
Gene expression and immunohistochemistry
Quantitative reverse transcription–polymerase chain reaction (RT-PCR) for CXCL13, IL-10, and IL-10 receptor A was performed as described using GUS (β-glucuronidase) as control. Immunohistochemical analysis was performed using the avidin-biotin complex technique, as described.16 Antibodies to IL-10 and CXCL13 were supplied by R&D, and antiphospho–JAK-1 (phosphorylation at tyrosine 1022) antibody by Abcam. Isotype controls confirmed specificity. Photomicroscopy was performed using an Olympus BH45 microscope and an Olympus DP72 camera with Olympus DP2-BSW acquisition software.
Enzyme-linked immunosorbent assay
Determination of CXCL13, IL-10, and albumin concentration in CSF was performed in duplicate and quantified by standard curve. Enzyme-linked immunosorbent assay kits for CXCL13 were supplied by R&D Systems; for IL-10, by Becton Dickinson; and for albumin, by Bethyl Laboratories.
Chemotaxis
Non-Hodgkin lymphoma cells were isolated from CNS metastatic lesions obtained by brain biopsy or from CSF during staging lumbar punctures, in accordance with patient consent and an Institutional Review Board–approved protocol. To enable the generation of adequate numbers of cells for in vitro assays, patient-derived lymphoma cells were implanted into the cerebrum of NOD-SCID γ mice whereupon they expanded to form visible tumors within 4 weeks, according to an Institutional Animal Care and Use Committee–approved protocol.17 At necropsy, intracranial lymphoma xenografts were isolated, and malignant B cells purified by CD19+ selection by MACS (Miltenyi Biotech) for in vitro migration assays. Each of the patient-derived lymphoma cell lines evaluated in chemotaxis assays contained aberrations in DNA copy number that are characteristic of diffuse large B-cell lymphoma (DLBCL),18 as demonstrated by array-based comparative genomic hybridization (data not shown).
In vitro migration of primary lymphoma cells was measured by Transwell assay, using Transwell plates (Costar) with 5.0-μm polycarbonate membranes. Chemokines were diluted in serum-free RPMI medium and added to the lower chambers. Primary lymphoma cells were rested in serum-free RPMI for 30 minutes before addition to plates. Lymphoma cells (2 × 105) were resuspended and added to the upper chambers. Plates were incubated at 37°C for 18 hours, and cells that had transmigrated through pores to the lower chamber were collected and counted using an LSR II Flow cytometer at high speed for 60 seconds per sample. Each experiment was performed in triplicate, and data were presented as percentage difference of control.
Statistics
Chemotaxis results were compared by 1-way analysis of variance. The ability of CSF CXCL13 and IL-10 concentration to identify CNS lymphoma was evaluated by receiver-operating characteristic (ROC) curves.19,20 Kaplan-Meier and log rank tests were used to assess differences in outcome according to expression of CXCL13 and IL-10. End point definitions were as defined by the Revised Response Criteria for malignant lymphomas.21 Statistical analyses were performed using Prism and MedCalc software.
Results
CXCL13 in PCNSL and SCNSL
CXCL13 dictates the motility and homing of B cells in the formation of ectopic lymphoid follicles.22,23 Previous studies have localized CXCL13 transcripts and protein within biopsy specimens and CSF from PCNSL patients, raising the possibility that this chemokine may contribute to CNS tropism.24
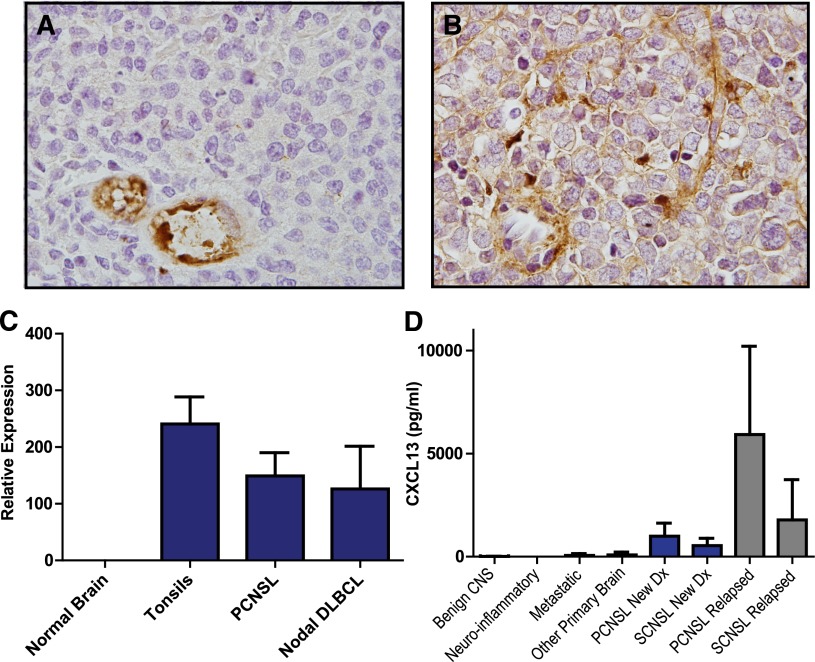
Transcriptional and protein expression of CXCL13 in PCNSL tumors was confirmed (Figure 1), and the mean concentration of CXCL13 protein in CSF from newly diagnosed patients with PCNSL and SCNSL was >50-fold higher than in CSF from patients without CNS lymphoma (P < 1 × 10−7); strikingly, CXCL13 concentrations were 10-fold higher in the CSF of patients with relapsed PCNSL (P < .0005) as compared with the CSF of newly diagnosed patients, perhaps reflecting the higher proportion of leptomeningeal invasion at recurrence in this cohort of patients, of whom >50% had malignant cytology. CXCL13 concentration in CSF was also elevated in 6 of 11 subjects with CNS demyelinating disease (9-116 pg/mL), consistent with previous observations.25 Of note, CXCL13 concentration was also elevated in the CSF of 3 of 6 patients with breast cancer metastatic to the brain (58-508 pg/mL), suggesting a role for this chemokine in directional migration of breast cancer cells to the brain26 (Table 1; supplemental Tables 1-3).
Figure 1.
CXCL13 expression in CNS lymphomas. Expression of CXCL13 in a diagnostic specimen of de novo PCNSL (A) and in relapsed SCNSL (B), as demonstrated by immunohistochemistry (×1000). CXCL13 expression is most evident in stromal elements including tumor blood vessels. (C) Quantitative RT-PCR demonstrates markedly increased expression of CXCL13 in diagnostic specimens of PCNSL (N = 23). CXCL13 transcript levels were similar in PCNSL compared with reactive pharyngeal tonsils (N = 2) and nodal DLBCL (N = 9) but barely detectable in specimens of normal brain (N = 3 cases). (D) CXCL13 protein is markedly increased in CSF in association with both PCNSL and SCNSL compared with the vast majority of neuro-inflammatory conditions and other brain tumors. The highest CSF concentration of CXCL13 was detected in association with relapsed CNS non-Hodgkin lymphoma.
Table 1.
Summary of CXCL13 and IL-10 levels in CSF of patients with PCNSL and SCNSL, neuro-inflammatory conditions, other malignant brain tumors, and neoplasms or infection outside of the brain/CSF
| Diagnosis | N | Age | Gender | CXCL13 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|
| New diagnosis PCNSL | 43 | 60 | 19 M/24 F | 996 ± 312 | 282.9 ± 113 |
| New diagnosis SCNSL | 10 | 57 | 5 M/5 F | 539 ± 157 | 57 ± 37 |
| Recurrent PCNSL | 17 | 55 | 6 M/11 F | 5926.2 ± 2030 | 1003 ± 483 |
| Recurrent SCNSL | 13 | 58 | 10 M/3 F | 1783 ± 896 | 302 ± 126 |
| Neuro-inflammatory/infection | 71 | 45 | 44 M/27 F | 44.9 ± 19 | 5.6 ± 2.33 |
| Other primary brain tumors | 8 | 40.5 | 5 M/3 F | 84.5 ± 60.7 | 10.9 ± 5.6 |
| Metastatic brain tumors | 12 | 54.5 | 2 M/10 F | 58.46 ± 41.6 | 5.3 ± 1.5 |
| Neoplasm/infection outside of brain | 46 | 45 | 29 M/17 F | 11 ± 3.6 | 3.6 ± 1.4 |
All CSF from newly diagnosed patients was obtained via lumbar puncture. Age refers to median age of each cohort. CXCL13 and IL-10 levels in CSF from individual patients are provided in supplemental Tables 1-3.
M, male; F, female.
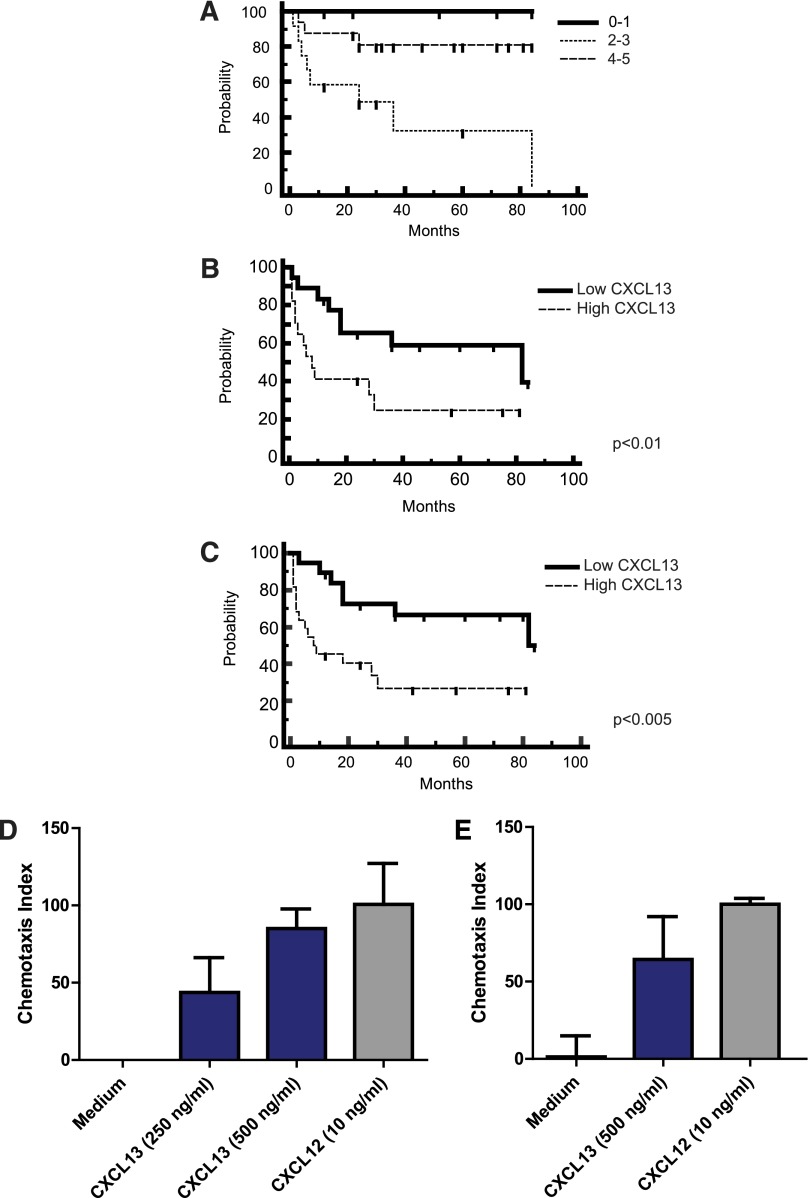
We noted that newly diagnosed patients with CNS involvement of lymphoma (both primary and secondary) with low CSF levels of CXCL13 at diagnosis (below the median concentration of 200 pg/mL) exhibited significantly longer progression-free survival (PFS) with standardized treatment27 compared with newly diagnosed patients with high CSF CXCL13, suggesting a physiological role for this chemokine in CNS lymphoma pathogenesis (Figure 2). Notably, none of the 5 PCNSL patients with undetectable CXCL13 in CSF at the time of diagnosis have progressed after treatment, with median follow-up of 46 months.
Figure 2.
CXCL13 is prognostic and mediates chemotaxis of lymphoma cells isolated from CNS lymphoma lesions. (A) Survival for the cohort of PCNSL patients by International Extranodal Lymphoma Study Group score (N = 33 total patients), demonstrating representation of the prognostic subgroups. Kaplan-Meier analysis; y-axis indicates percent overall survival (OS). The International Extranodal Lymphoma Study Group prognostic score is based on 5 variables: age >60, Performance Status >1, elevated lactate dehydrogenase, elevated CSF protein, and involvement of deep regions in the brain. (B) Newly diagnosed patients with PCNSL (N = 34 patients) with elevated CXCL13 at diagnosis (>200 pg/mL, the median CSF concentration among all CNS lymphoma patients) exhibited shorter time to progression (TTP) after treatment with methorexate-based induction. P < .01 (hazard ratio = 2.96). The median TTP of PCNSL patients with low CSF CXCL13 (N = 16) is 82 months vs 7 months for patients who presented with elevated CSF CXCL13. (C) PCNSL and SCNSL (N = 41) with elevated CSF CXCL13 at diagnosis exhibit shorter PFS (defined as disease progression or death as a result from any cause).21 P < .005 (hazard ratio = 3.12). The median PFS of PCNSL and SCNSL patients with low CSF CXCL13 (N = 19) is 82 months vs 9 months for patients who presented with high CXCL13. The OS of PCNSL and SCNSL patients with low CSF CXCL13 at diagnosis is also longer than for patients with high CSF CXCL13 at diagnosis. P < .04 (hazard ratio = 3.03), although the median OS for both cohorts has not been reached (not shown). All patients were treated with a high-dose methotrexate-based induction regimen without whole brain irradiation consolidation. Patients with stage IV DLBCL with CNS involvement (C) received cyclophosphamide, vincristine, adriamycin, and prednisone instead of temozolomide, as described.27 (D) Directed chemotaxis of CNS lymphoma cells (isolated from a brain parenchymal lesion of relapsed CNS lymphoma) in response to chemokines CXCL13 and CXCL12. The y-axis depicts chemotaxis index (% compared with medium control). P < .05. (E) CXCL13- and CXCL12-mediated chemotaxis of meningeal lymphoma cells isolated from the CSF of a CNS lymphoma patient with refractory disease. P < .05. There was no evidence for synergy or additive effects when CXCL13 and CXCL12 were used in combination, and there was no chemotaxis in response to C3a anaphylatoxin or ephrin A4 peptides.
We tested the hypothesis that CXCL13 may contribute to tropism of CNS lymphoma by evaluating specific chemotactic responses of patient-derived DLBCL cells isolated from CNS metastatic lesions and from CSF of CNS lymphoma patients. Here we demonstrate that lymphoma cells transmigrated through a 5.0-μm polycarbonate membrane at a significantly higher rate toward CXCL13 or stromal-derived factor 1α (CXCL12) compared with control medium (Figure 2). These results, reproducible in CNS lymphoma tumors isolated from 3 consecutive patients, represent the first direct evidence that CXCL13 and CXCL12 are chemotactic to tumor cells isolated from CNS lymphoma lesions, and they suggest a role for these chemokines as mediators of CNS tropism of systemic DLBCL (Figure 1). Notably, although expression of CXCL12 has also previously been demonstrated within PCNSL diagnostic specimens,28 we were unable to detect elevated CXCL12 in the CSF of CNS lymphoma patients and therefore pursued further evaluation of CXCL13 as a candidate biomarker.
IL-10 in PCNSL and SCNSL
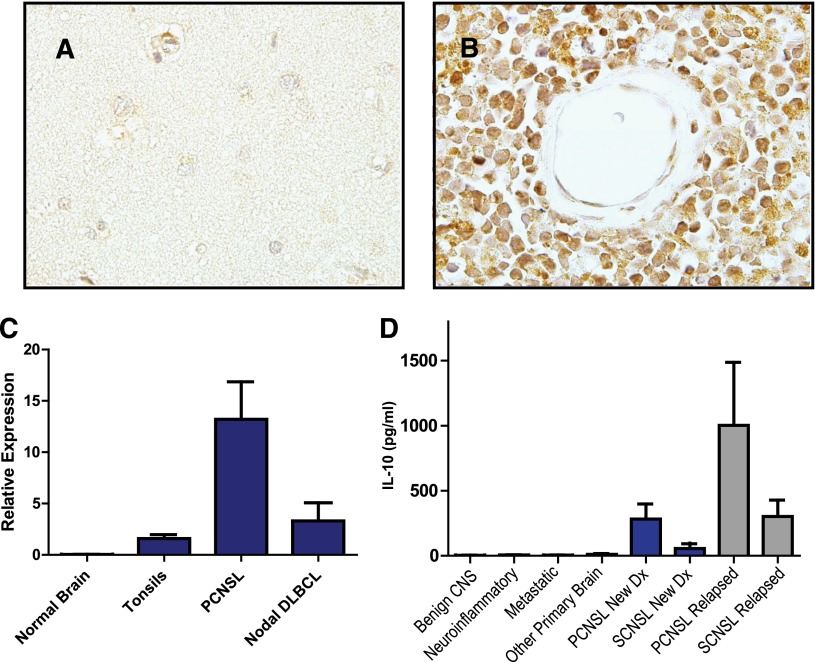
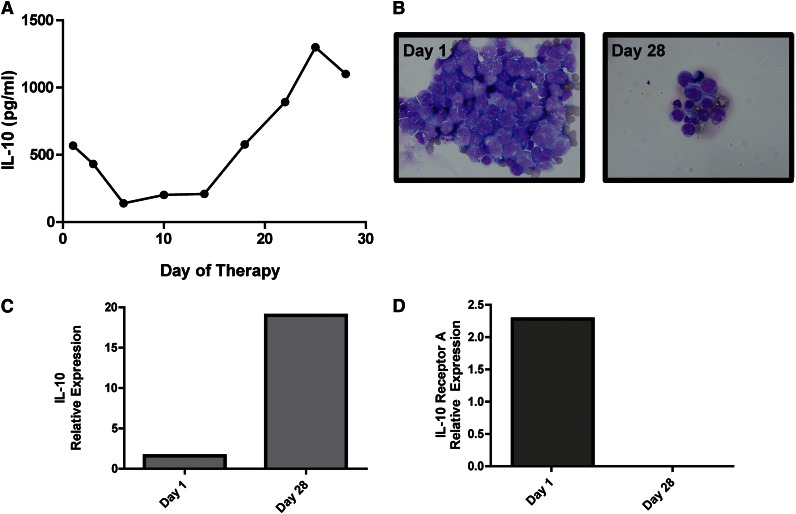
Immunohistochemical analysis demonstrated expression of IL-10 and its receptor (not shown) in 5 consecutive cases of PCNSL (Figure 3). IL-10 transcripts were detected in diagnostic specimens of PCNSL at high levels relative to normal brain, reactive tonsils, and nodal germinal center–type DLBCL; notably, however, the 1 nodal-activated B-cell DLBCL in this series contained a normalized IL-10 transcript level of 12, similar to the mean-normalized IL-10 transcript level in PCNSL cases. The concentration of IL-10 in CSF from PCNSL and SCNSL was also markedly elevated compared with CSF from populations of control subjects with inflammatory or granulomatous disease, other types of brain tumors, and extra-CNS malignancy (P < 2.3 × 10−5); the highest CSF IL-10 levels were also detected in relapsed PCNSL (Figure 3, Table 1, and supplemental Tables 1-3). Notably, newly diagnosed patients with CNS involvement of lymphoma (both primary and secondary) with low CSF levels of IL-10 at diagnosis exhibited significantly longer PFS compared with patients with high CSF IL-10 (Figure 4). These results are consistent with previous observations regarding the relationship between elevated IL-10 levels and adverse outcome in DLBCL.14,29 Moreover, serial analysis demonstrated that CSF IL-10 concentration within the lumbar sac and in the brain ventricle reproducibly correlated with clinical course, both at diagnosis and in PCNSL and SCNSL patients at relapse. CSF levels of IL-10 also correlated with clinical response or progression in CNS lymphoma patients who participated in a phase 1 trial of intraventricular rituximab plus methotrexate.30 (The result is representative of 6 consecutive trial patients analyzed.) Quantitative RT-PCR of RNA transcripts purified from B cells isolated from the CSF by flow cytometry demonstrated IL-10 expression by lymphoma cells, and dynamic changes in CSF protein concentration were reflected by a change in the relative IL-10 transcript levels expressed by the meningeal lymphoma cells (Figure 5; supplemental Figure 1).
Figure 3.
IL-10 expression in CNS lymphomas. Absent IL-10 expression in normal brain (A) with strong expression of IL-10 by lymphoma cells in PCNSL (B), as demonstrated by immunohistochemistry. There was weak or absent IL-10 expression by tumor vessels (×1000). (C) Quantitative RT-PCR demonstrates markedly increased expression of IL-10 in diagnostic specimens of PCNSL (N = 23) compared with reactive tonsils and specimens of normal brain. The average IL-10 transcriptional expression was also higher in PCNSL compared with 9 cases of nodal DLBCL, of which 7 were of germinal center B-cell phenotype. Notably, the normalized IL-10 transcript level, 12, in the 1 nodal activated B-cell-type DLBCL specimen was similar to the mean-normalized IL-10 expression in the PCNSL cases. (D) Mean CSF IL-10 protein is >70-fold higher in patients with both PCNSL and SCNSL compared with neuro-inflammatory conditions and other brain tumors (P < 2.3 × 10−5). The CSF concentration of IL-10 was highest in relapsed cases.
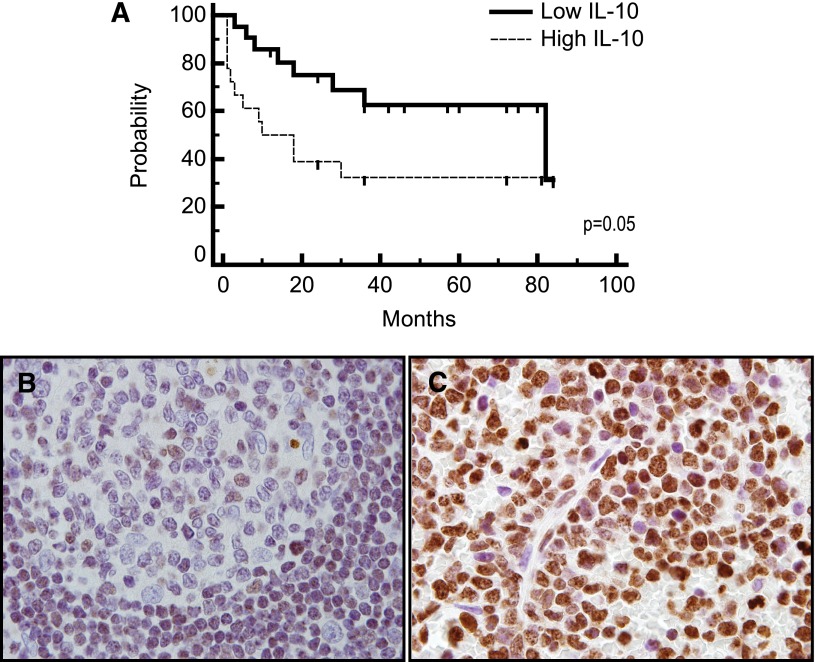
Figure 4.
IL-10 is prognostic and may be associated with JAK-1 activation. (A) Patients with non-HIV–associated PCNSL and SCNSL with elevated concentration of IL-10 in CSF at diagnosis (N = 18) (above 45 pg/mL, the median concentration among all CNS lymphoma patients) exhibited significantly shorter TTP compared with patients with low CSF IL-10 (N = 21). P = .05 (hazard ratio = 2.33). The median TTP (as well as PFS) of PCNSL and SCNSL patients with low CSF IL-10 at diagnosis is 82 months vs 10 months for patients with elevated CSF IL-10. The median OS of CNS lymphoma patients with low CSF IL-10 at diagnosis was 84 months, whereas the median OS of patients with elevated CSF IL-10 at diagnosis has not been reached. P < .057 (hazard ratio = 3.6) (not shown). All patients were treated with a high-dose methotrexate-based induction regimen without whole brain irradiation consolidation. Patients with stage IV DLBCL with CNS involvement received cyclophosphamide, vincristine, adriamycin, and prednisone instead of temozolomide, as described.27 Germinal center B cells in reactive tonsils (B) exhibited weak to absent immunoreactivity for JAK-1 activation (phosphorylation of JAK-1 at tyrosine 1022), whereas strong intratumoral JAK-1 activation (>30% lymphoma cells) was detected in 70% of diagnostic specimens of PCNSL (N = 30) (C) (×1000).
Figure 5.
IL-10 gene expression and CSF concentration correlate with disease course in a patient with recurrent CNS lymphoma. (A) A decline in CSF concentration of IL-10 correlates with initial cytological response followed by tumor progression in a representative patient with recurrent SCNSL who participated in a phase 1 trial of intraventricular rituximab plus methotrexate.30 (The result is representative of 6 consecutive trial patients analyzed.) (B) Cytological appearance of lymphoma cells in CSF at baseline and persistent disease at completion of intraventricular therapy (28 days). (C) Quantitative RT-PCR of RNA transcripts purified from B cells isolated from the CSF by flow cytometry demonstrated upregulated IL-10 expression in refractory CNS lymphoma after treatment with rituximab plus methotrexate. The increased relative expression of IL-10 transcripts by the meningeal lymphoma cells corresponded to increased concentration of IL-10 protein in CSF at day 28 (A). (D) In contrast, transcriptional expression of IL-10 receptor A decreased after therapy.
Given recent demonstrations of increased expression of JAK-1 in PCNSL,16,31 we evaluated the activated (ie, phosphorylated) form of this kinase to assess signal transduction downstream of the IL-10 receptor, using an antibody specific for phospho–JAK-1. Expression of phospho–JAK-1 was reproducibly demonstrated by immunohistochemical analysis of diagnostic specimens of PCNSL using an antibody specific for phospho–JAK-1, particularly in aggressive cases (Figure 4). Among the 9 cases in which matched CSF and diagnostic biopsies were available, there was an 88.8% rate of concordance between high or low IL-10 concentration in CSF and the JAK-1 activation state (P < .02, χ2 test); of the 7 PCNSL cases with elevated CSF IL-10 at diagnosis, 6 were associated with high intratumoral phospho–JAK-1. High phospho–JAK-1 expression was also detected in each of 2 biopsy specimens of SCNSL available for analysis. By contrast, as a control, germinal center B cells from reactive tonsils consistently exhibited weak JAK-1 activation. Taken together, in addition to supporting IL-10 as a potential mediator of survival signaling in CNS lymphomas, our results suggest that intratumoral phospho–JAK-1 may be a novel biomarker of prosurvival cytokine signaling in CNS lymphomas.
Coexpression of IL-10 and the IL-10 receptor was also directly demonstrated by quantitative RT-PCR of meningeal B-lymphoma cells purified by flow cytometry from CSF in patients with recurrent CNS lymphoma, supporting IL-10 as an autocrine growth factor in this setting (Figure 5). Notably, we were unable to detect CXCL13 transcripts in lymphoma cells isolated from the CSF of these patients, in agreement with our immunohistochemical results, which demonstrated the strongest expression of CXCL13 by stromal cells. These data support a predominantly paracrine model of CXCL13 interaction between CNS lymphoma and its microenvironment.
Diagnostic utility of CXCL13 and IL-10
Given the marked elevation of CXCL13 in CSF of CNS lymphoma patients, we evaluated its potential utility as a diagnostic biomarker in CNS lymphoma patients using ROC curve analysis. CXCL13 concentrations >90 pg/mL were 69.9% sensitive and 92.7% specific for CNS lymphoma, and an IL-10 concentration in CSF >16.15 pg/mL was 65.4% sensitive and 92.6% specific for all CNS lymphomas.
Elevated CXCL13 and/or IL-10 concentration in CSF was present in 83% of CNS lymphomas cases, as defined by these thresholds. Given that similar elevation of these peptides was detected in only 13% of CSF from non-CNS lymphoma cases (Table 1; supplemental Tables 1-3), we considered the potential utility of CXCL13 in combination with IL-10 as a discriminator. Using the respective thresholds of 90 pg/mL and 16 pg/mL, defined for all CNS lymphomas, we determined that the combined elevation of both peptides was 99% specific for CNS lymphoma. Similar results using bivariate analysis were obtained in a test set involving 150 patients (98.9% specificity)32 and a multicenter validation set of 70 distinct patients, analyzed 2 years later (100% specificity). Only 1 non-CNS lymphoma case was misclassified using this model, a patient with recurrent CNS germinoma, in which elevated CSF levels of α-fetoprotein plus β–human chorionic gonadotropin combined with clinical history confirmed the pretest impression of germ cell tumor. Confirmatory brain biopsy was not necessary given this information.
The diagnostic utility of CXCL13 plus IL-10 was particularly impressive in the discrimination of non-HIV–associated PCNSL at diagnosis, the most common presentation and one for which Epstein-Barr viral DNA has no established utility as a biomarker. By ROC curve analysis, slightly higher CSF concentrations of CXCL13 (>116 pg/mL) and IL-10 (>23 pg/mL) yielded optimal diagnostic accuracy in this population. The detection of elevated concentration of either CXCL13 or IL-10 above these cut points was 84.2% sensitive and 90.5% specific for the diagnosis of PCNSL. Elevation of both peptides in CSF above these thresholds was 50% sensitive and 99.3% specific for PCNSL (Figure 6; Table 2).
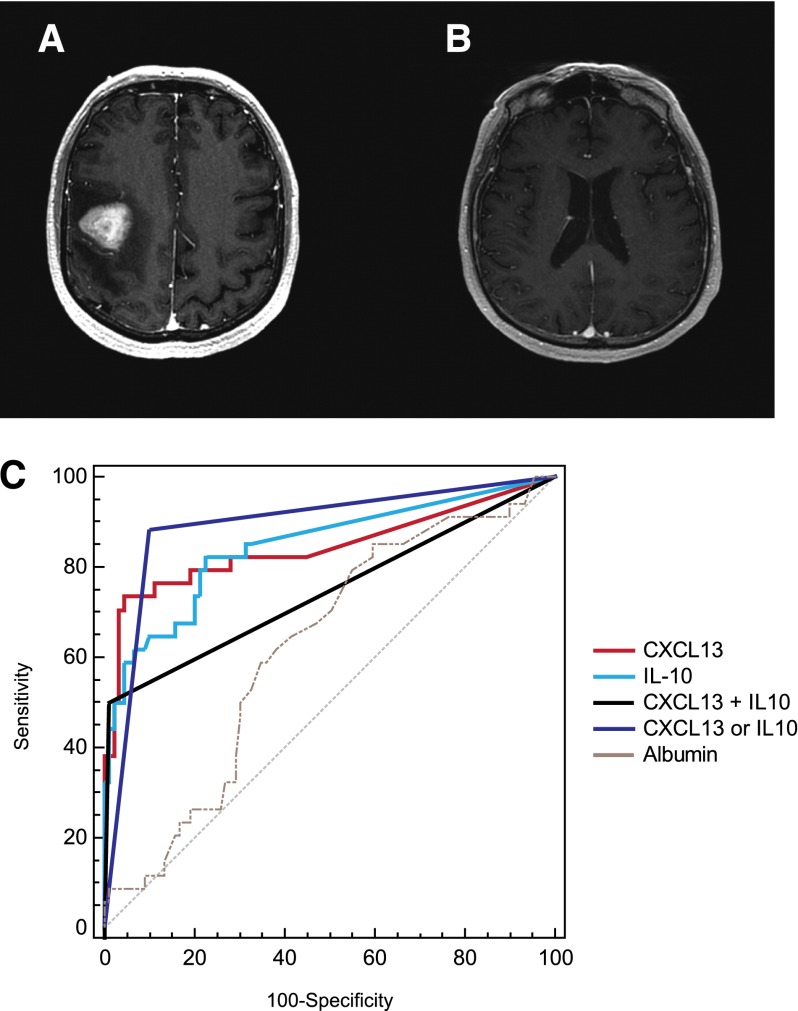
Figure 6.
CXCL13 and IL-10 are highly specific for the diagnosis of PCNSL. (A-B) MRI features of PCNSL in 2 patients at diagnosis. (A) MRI depicts a homogeneously contrast-enhancing mass with vasogenic edema. At the time of diagnosis established by brain biopsy, the CSF contained CXCL13 concentration of 170 pg/mL and IL-10 concentration of 61 pg/mL. (B) Normal-appearing MRI of a patient with progressive neurologic symptoms who was aggressively treated with steroids before a diagnosis could be elicited. Four CSF collections and 1 brain biopsy were unrevealing, and the diagnosis of disseminated PCNSL was made at autopsy. The CSF collected and stored from this patient was later determined to contain CXCL13 concentration of 6236 pg/mL and IL-10 concentration of 76 pg/mL. (C) ROC analysis demonstrated that high concentrations of CXCL13 and IL-10 are each highly sensitive and specific for CNS lymphomas. A CSF concentration of CXCL13 >116 pg/mL is 71% sensitive and 94.9% specific for PCNSL (area under the curve [AUC], 0.841; 95% confidence interval [CI], 0.779-0.892). A CSF concentration of IL-10 >23 pg/mL is 63.9% sensitive and 94.1% specific for CNS involvement of lymphoma (AUC, 0.851; 95% CI, 0.789-0.901). Bivariate elevation of both CXCL13 plus IL-10 in CSF was 50% sensitive and 99.3% specific for CNS lymphoma (AUC, 0.746; 95% CI, 0.675-0.809). Elevation of either CXCL13 or IL-10 in CSF was 84.2% sensitive and 90.5% specific for the diagnosis of PCNSL (AUC, 0.874; 95% CI, 0.815-0.919). By contrast, the AUC of CSF albumin was 0.604 and significantly lower than CXCL13 or IL-10 (P < .0001).
Table 2.
Bivariate elevation of both CXCL13 and IL-10
| Biomarker | Sensitivity | Specificity | AUC | CI |
|---|---|---|---|---|
| CXCL13 | 71% | 95% | 0.841 | 0.779-0.892 |
| IL-10 | 64% | 94.1% | 0.851 | 0.789-0.901 |
| CXCL13 or IL-10 | 84.2% | 90.5% | 0.874 | 0.815-0.919 |
| CXCL13 plus IL-10 | 50% | 99.3% | 0.746 | 0.675-0.809 |
| Cytology | 14% | Reference standard | Reference standard | Reference standard |
| Flow cytometry | 20% | Reference standard | Reference standard | Reference standard |
Bivariate elevation of both CXCL13 and IL-10 is more than twice as sensitive as cytology (P < .001) and flow cytometry (P < .05), with equivalent specificity, in the detection of non-HIV–associated PCNSL at diagnosis (Fisher’s exact test [2-tailed]). An algorithm based on detection of elevation of either CXCL13 or IL-10 in CSF is more than 3 times as sensitive compared with the reference standard tests (P < .0001).
Elevated CSF concentrations of CXCL13 and IL-10 were highly specific in discriminating CNS lymphomas from a spectrum of relevant disorders including extra-CNS diffuse large cell lymphoma and lesions that mimic the radiographic appearance of CNS lymphoma including leptomeningeal carcinoma, multiple sclerosis, neurosarcoid, CNS vasculitis, encephalitis, and toxoplasmosis. At least 14 patients with neuro-inflammatory disorders in this cohort had CSF flow cytometry performed specifically to evaluate suspected CNS B-cell lymphoma; flow cytometric evaluations were repeated at least 2 to 3 times in 6 patients with highly suspicious lesions, which ultimately were diagnosed by brain biopsy as multiple sclerosis, granulomatous angiitis, neurosarcoid, amyloid, transverse myelitis, and encephalitis (supplemental Table 4).
Cytological evaluation of CSF was conducted in 97.3% of newly diagnosed immunocompetent patients with PCNSL in this series and was positive in only 14%; adjunctive flow cytometric analysis of CSF was also done in 52.6% of newly diagnosed patients and was positive in only 20%. Of note, 92% of newly diagnosed patients were taking dexamethasone during diagnostic CSF evaluations. Nearly 1 in 5 newly diagnosed PCNSL patients (19%) experienced significant diagnostic delays (median 18 weeks, range 10-120 weeks) attributed to lymphocytotoxic effects of glucocorticoids. Nine patients had nondiagnostic CSF evaluations performed before brain biopsy, and 2 patients were subjected to brain biopsies that were nondiagnostic, likely because of the effect of steroids. In 1 patient, the diagnosis of PCNSL could be established only at autopsy because of the effects of long-term steroid administration prescribed in the primary care setting. Among the 9 patients in whom lumbar punctures were performed as part of the diagnostic workup before brain biopsy, 8 had CSF that contained elevated CXCL13, and 5 had CSF that contained elevated CXCL13 plus IL-10, including the patient with PCNSL diagnosed at autopsy (Figure 6; Table 2). The most stringent protein CSF biomarker, bivariate elevation of both CXCL13 and IL-10, was significantly more sensitive than cytology (P < .001) and flow cytometry (P < .05) in the detection of PCNSL at diagnosis (Fisher’s exact test (2-tailed) (Figure 6; Table 2).
The positive predictive value of bivariate elevation of CXCL13 and IL-10 in CSF was 95% in the identification of newly diagnosed HIV-negative PCNSL, with 88% negative predictive value. Importantly, absent elevation of both biomarkers in the CSF had a negative predictive value of 95% in excluding the diagnosis of PCNSL. These findings illustrate the significant utility of the combinatorial application of these 2 biomarkers within a diagnostic algorithm for PCNSL.
Discussion
Our study builds on a foundation of evidence that implicates CXCL13 and IL-10 in the pathogenesis of intraocular and PCNSL.15,33,34 We provide the first demonstration of CXCL13 and CXCL12 as mediators of CNS lymphoma cell chemotaxis, raising the possibility that these chemokines may contribute to CNS tropism of B lymphoma. In addition, we provide the first evidence that high expression of CXCL13 is associated with inferior outcome in CNS lymphoma patients, raising the possibility that this chemokine may mediate prosurvival signals,35 possibly via B-cell activation through the B-cell receptor,36 and thus implicating the CXCL13:CXCR5 interaction as a potential target for pharmacologic inhibition in patients with high-risk disease. Previous evidence for autocrine IL-10 production and signaling in lymphoma has been generated by analysis of clonal cell lines, including DLBCL.29,37,38 Here we provide direct evidence for transcriptional and protein expression of IL-10 by CNS lymphomas and demonstrate that IL-10 concentration in CSF reflects disease activity in response to methotrexate and rituximab therapy. In addition, we identify phospho–JAK-1 as a potential transducer of prosurvival signals mediated by cytokines such as IL-10 and IL-4, supporting further evaluation of this molecule as a biomarker and a potential therapeutic and pharmacodynamic target to be evaluated in future clinical studies.
It is important to note that Chan and colleagues initially demonstrated the diagnostic utility of the intravitreal and CSF measurement of IL-10 in the evaluation of a small cohort of patients with intraocular and CNS lymphoma.39 In a multicenter study involving discovery and validation sets of relevant patients, we demonstrate that CXCL13 and IL-10 constitute a complementary biomarker pair that yields diagnostic information in most PCNSL cases with sensitivity significantly greater than reference standard CSF tests, cytology and flow cytometry, and equivalent to the sensitivity of results obtained from small-needle brain biopsies.1-3
Although this is the largest and most comprehensive analysis of candidate CSF biomarkers of CNS lymphoma to date, it is notable that CXCL13 is also elevated in the setting of the inflammatory response that accompanies acute Lyme neuroborreliosis.40 However, the pretest probability of neuroborreliosis is dramatically influenced by geography, history, results of serological testing, and characteristic MRI findings, which are typically distinct from CNS lymphoma.
Based on this series, CXCL13 alone can be used to discriminate >70% of PCNSL cases with 94.9% specificity; diagnostic sensitivity of the algorithm can be improved to 84% by parallel evaluation of IL-10, which also provides prognostic information while maintaining >90% specificity. Although the precision of the cutoff points for maximal diagnostic accuracy will need to be prospectively defined, based on this data, CXCL13 and IL-10 may be incorporated within diagnostic algorithms in clinical practice to facilitate the workup of potential CNS lymphoma in patients for whom brain biopsy may be of heightened risk or of low diagnostic yield. Given the very poor sensitivity of currently available CSF-based diagnostic tools to evaluate focal brain lesions in which CNS lymphoma is a consideration, implementation of a biomarker test based on these peptides may prove to be cost-effective, minimize repeat neuroimaging and other expensive diagnostic interventions, and yield improved outcomes.
Supplementary Material
Acknowledgments
The authors are grateful for the guidance of Richard W. Price, University of California, San Francisco Neurology Service.
This research was supported by the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), National Institutes of Health (R01CA139-83-01A1) (J.L.R.), the University of California, San Francisco Brain Tumor Specialized Program of Research Excellence (P50CA097257), and the Leukemia & Lymphoma Society.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.L.R. designed and performed research, analyzed data, and wrote the manuscript; V.S.W. and C.K. performed research, analyzed data, and cowrote the manuscript; H.-X.G. performed research and cowrote the manuscript; R.B. analyzed data and cowrote the manuscript; L.C. performed research and analyzed data; V.D., B.S., M.M., and L.D.K. performed research and cowrote the manuscript; J.H.H. performed research and statistical analysis and cowrote the manuscript; S.A.J., S.C., P.A.T., P.C., and J.G.C. performed research and cowrote the manuscript; and C.L. performed research, analyzed data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James L. Rubenstein, Division of Hematology/Oncology, University of California, San Francisco, M1282, Box 1270, San Francisco, CA 94143; e-mail: jamesr@medicine.ucsf.edu.
References
- 1.Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106(1):72–75. doi: 10.3171/jns.2007.106.1.72. [DOI] [PubMed] [Google Scholar]
- 2.Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82(9):1749–1755. doi: 10.1002/(sici)1097-0142(19980501)82:9<1756::aid-cncr23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Javedan SP, Tamargo RJ. Diagnostic yield of brain biopsy in neurodegenerative disorders. Neurosurgery. 1997;41(4):823–828, discussion 828-830. doi: 10.1097/00006123-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118(3):510–522. doi: 10.1182/blood-2011-03-321349. [DOI] [PubMed] [Google Scholar]
- 5.Hormigo A, Abrey L, Heinemann MH, DeAngelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126(2):202–208. doi: 10.1111/j.1365-2141.2004.05028.x. [DOI] [PubMed] [Google Scholar]
- 6.Abrey LE, Batchelor TT, Ferreri AJ, et al. International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 7.Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44(7):728–734. doi: 10.1080/02841860500256272. [DOI] [PubMed] [Google Scholar]
- 8.Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17(2):554–560. doi: 10.1200/JCO.1999.17.2.554. [DOI] [PubMed] [Google Scholar]
- 9.Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117(11):3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- 10.Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Chadalapaka G, Lee SO, et al. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31(8):1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20(3):539–550. doi: 10.1111/j.1750-3639.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, Josephson SA, Fridlyand J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol. 2008;26(1):96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasayama T, Nakamizo S, Nishihara M, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro-Oncol. 2012;14(3):368–380. doi: 10.1093/neuonc/nor203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer L, Korfel A, Pfeiffer S, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15(19):5968–5973. doi: 10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107(9):3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadoch C, Dinca EB, Voicu R, et al. Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model. Clin Cancer Res. 2009;15(6):1989–1997. doi: 10.1158/1078-0432.CCR-08-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offit K, Parsa NZ, Gaidano G, et al. 6q deletions define distinct clinico-pathologic subsets of non-Hodgkin’s lymphoma. Blood. 1993;82(7):2157–2162. [PubMed] [Google Scholar]
- 19.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 20.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 22.Cyster JG, Ngo VN, Ekland EH, Gunn MD, Sedgwick JD, Ansel KM. Chemokines and B-cell homing to follicles. Curr Top Microbiol Immunol. 1999;246:87–92, discussion 93. doi: 10.1007/978-3-642-60162-0_11. [DOI] [PubMed] [Google Scholar]
- 23.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 24.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101(3):815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 25.Sellebjerg F, Börnsen L, Khademi M, et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73(23):2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- 26.Panse J, Friedrichs K, Marx A, et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer. 2008;99(6):930–938. doi: 10.1038/sj.bjc.6604621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18(4):1146–1155. doi: 10.1158/1078-0432.CCR-11-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JR, Falkenhagen KM, Coupland SE, Chipps TJ, Rosenbaum JT, Braziel RM. Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol. 2007;127(4):633–641. doi: 10.1309/NUQHJ79BHWYD9TAF. [DOI] [PubMed] [Google Scholar]
- 29.Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119(12):2844–2853. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubenstein JL, Li J, Chen L, et al. Multicenter phase I trial of intraventricular immuno-chemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745–751. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung CO, Kim SC, Karnan S, et al. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. 2011;117(4):1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- 32.Wang V.S., Chen L., Kadoch C., et al. Multivariate analysis of cerebrospinal fluid biomarkers in central nervous system lymphoma and controls [abstract]. Cancer Res. 2010;70(8, suppl 1):2728. Abstract 2728. [Google Scholar]
- 33.Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120(5):671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 34.Buggage RR, Whitcup SM, Nussenblatt RB, Chan CC. Using interleukin 10 to interleukin 6 ratio to distinguish primary intraocular lymphoma and uveitis. Invest Ophthalmol Vis Sci. 1999;40(10):2462–2463. [PubMed] [Google Scholar]
- 35.Chunsong H, Yuling H, Li W, et al. CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J Immunol. 2006;177(10):6713–6722. doi: 10.4049/jimmunol.177.10.6713. [DOI] [PubMed] [Google Scholar]
- 36.Sáez de Guinoa J, Barrio L, Mellado M, Carrasco YR. CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118(6):1560–1569. doi: 10.1182/blood-2011-01-332106. [DOI] [PubMed] [Google Scholar]
- 37.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9(1):316–326. [PubMed] [Google Scholar]
- 38.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s lymphoma to apoptosis. Clin Cancer Res. 2001;7(3):709–723. [PubMed] [Google Scholar]
- 39.Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115(9):1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 40.van Burgel ND, Bakels F, Kroes AC, van Dam AP. Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J Clin Microbiol. 2011;49(5):2027–2030. doi: 10.1128/JCM.00084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.