Abstract
G2/M checkpoint activation after DNA damage results in G2/M cell cycle arrest that allows time for DNA repair before the entry of cells into mitosis. Activation of G2/M checkpoint involves a series of signaling events, which include activation of ataxia telangiectecia-mutated and Rad3-related (ATR) and Chk1 kinases and inhibition of Cdc2/Cyclin B activity. Studies presented in this report show that serine (Ser)/threonine (Thr) protein phosphatase 2A (PP2A) has an important role in G2/M checkpoint activation in response to γ-irradiation (IR) exposure. Using PP2A inhibitors, as well as siRNA targeting various forms of Ser/Thr protein phosphatases, results presented in this report show that specific PP2A inhibition abrogates IR-induced activation of ATR and Chk1 kinases, as well as phosphorylation of Cdc2-Tyr15, and attenuates IR-induced G2/M arrest. These results suggest an important regulation of PP2A on IR-induced G2/M checkpoint signaling response.
Keywords: PP2A, ATM/ATR, Chk1/2, irradiation and G2/M arrest
Introduction
The DNA damage induced by ionizing irradiation triggers rapid activation of DNA-damage checkpoints, resulting in cell cycle arrest and DNA repair. The G2/M checkpoint prevents cells from initiating mitosis when they experience DNA damage during G2, or when they progress into G2 with some unrepaired damage inflicted during previous S or G1 phases (Kastan and Bartek, 2004). The critical target of the G2/M checkpoint is the Cdc2/Cyclin B complex, the activity of which is required for the progress of the cell cycle from the G2 phase to the mitotic phase (Smits and Medema, 2001). In response to DNA damage, ataxia telangiectecia-mutated (ATM) and ATM and Rad3-related (ATR) kinases are rapidly activated through phosphorylation, which in turn leads to the phosphorylation/activation of their downstream targets Chk2 and Chk1 kinases, respectively. Activation of Chk1/Chk2 kinases results in subcellular sequestration, degradation and/or inhibition of the Cdc25 family of phosphatases that normally activate Cdc2/Cyclin B at the G2/M boundary (Kastan and Bartek, 2004).
In contrast to the plethora of studies focusing on the role of protein kinases in the regulation of DNA-damage checkpoint response, only a limited number of studies have examined the role of protein phosphatases in the regulation of this process. Protein phosphatases are necessary components of all signal transduction pathways, as they reverse protein phosphorylation produced by kinases (Cohen, 1992). The highly conserved PPP family of Ser/Thr protein phosphatases is found to regulate many important cellular processes, including cell cycle progression, cellular differentiation and programmed cell death (McConnell and Wadzinski, 2009). The PPP family includes the protein phosphatase PP1, PP2A, PP2B, PP4, PP5, PP6 and PP7. Within the phosphatase family, several members have “PP2A-like” protein phosphatase activity that distinguishes them from other protein phosphatases in the family, which include PP2A, PP4, PP5 and PP6. Several highly specific inhibitors of the members of PPP family have been used for the study of these phosphatases. Among those inhibitors, okadaic acid (OA) is cell permeable and potently inhibits PP1 and PP2A phosphatase activities in intact cells (Favre et al., 1997; Yan and Mumby, 1999). In vitro, PP2A and PP2A-like phosphatases are highly sensitive to OA but insensitive to either inhibitor-1 or inhibitor-2, which are potent inhibitors of PP1 (Cohen et al., 1989; Cohen, 1991). Although OA can inhibit both PP1 and PP2A, because of its higher affinities toward PP2A than to PP1 (Ki=0.032 nM for PP2A vs 147 nM for PP1) (Takai et al., 1995) and higher efficacy for inhibiting PP2A than PP1 (IC50=0.1–0.3 nM for PP2A vs 15–30 nM for PP1) (Swingle et al., 2007), previous studies indicate that incubation of cells with OA is highly selective for inhibiting PP2A activity in intact cells (Favre et al., 1997; Yan and Mumby, 1999).
PP2A is a major cellular Ser/Thr protein phosphatase that has been implicated in the regulation of many cellular processes including cell cycle progression, DNA replication, gene transcription/translation and cell differentiation (Janssens and Goris, 2001; Janssens et al., 2005). PP2A consists of a catalytic subunit (C), a scaffold subunit (A) and a regulatory subunit (B). In mammalian cells, PP2A exists as either an AC core heterodimer or ABC heterotrimer (Janssens et al., 2008). There are two isoforms of the PP2A catalytic subunit, PP2A (Cα) and PP2A (Cβ), and two isoforms of the PP2A scaffold subunit, PP2A (Aα) and PP2A (Aβ). PP2A (Cα) and PP2A (Cβ) share 97% sequence identity, whereas PP2A (Aα) and PP2A (Aβ) share 87% sequence identity (Janssens et al., 2008). Furthermore, in mammalian cells, PP2A (Cα) and PP2A (Aα) are more abundant than PP2A (Cβ) and PP2A (Aβ), respectively (Janssens et al., 2008). Previous studies indicate that the diversity of PP2A holoenzymes is mainly controlled by the PP2A (B) regulatory subunit. The B subunits of PP2A are classified into four distinct families, B/PR55, B′/PR61, B″/PR72 and PTP/PR53, which share no sequence similarity, apart from a few conserved amino acids that allow the interaction with the N-terminal of the PP2A (A) scaffold subunit (Mayer-Jaekel and Hemmings, 1994; Janssens et al., 2008). In addition, each B subunit family consists of at least four members (Mayer-Jaekel and Hemmings, 1994; Janssens et al., 2008).
Recent studies have implicated PP2A in the regulation of DNA-damage response. In response to DNA damage induced by doxorubicin and γ-irradiation (IR), PP2A dephosphorylates and inhibits polo-like kinase 1, the function of which is required for the initiation of mitosis by phosphorylation/activation of Cdc25C and mitotic cyclin at the G2/M boundary (Jang et al., 2007). Furthermore, PP2A is required for IR- and UV-induced dephosphorylation of p53-Thr55 and stabilization of p53 (Li et al., 2004, 2007a). Moreover, recent studies have shown that PP2A is involved in the regulation of ATM and ATR signaling pathways. For example, studies have shown that PP2A binds to ATM and antagonizes ATM-Ser1981 autophosphorylation in the absence of DNA damage. Similarly, PP2A inhibition by OA or siRNA in the absence of DNA damage was found to induce phosphorylation of Chk1-Ser317 in HeLa cervical cancer cells (Leung-Pineda et al., 2006). However, as the activity of Chk1 kinase in those cells was not examined, whether the induction of Chk1– Ser317 phosphorylation by PP2A inhibition resulted in the activation of Chk1 kinase was not studied. In addition, other studies have shown that PP2A is required for HIV-1 Vpr viral protein-induced G2/M cell cycle arrest, through its direct effect on Vpr-induced activation of ATR and Chk1 kinases (Li et al., 2007a). Finally, the PP2A catalytic subunit has been shown to coimmunoprecipitate with γ-H2AX in vitro and colocalizes with γ-H2AX in DNA-damage foci (Chowdhury et al., 2005). Additional evidence indicates that the recruitment of PP2A (C) to DNA-damage foci is H2AX dependent and that the presence of PP2A facilitates DNA repair (Chowdhury et al., 2005).
In this study, we used PP2A inhibitors OA and SV40-small t antigen, in combination with a specific siRNA-targeting individual member of PP2A-like phosphatases, to investigate the potential role of Ser/Thr protein phosphatases in the regulation of IR-induced G2/M DNA-damage checkpoint response. Results show that PP2A specific activity is required for IR-induced activation of G2/M checkpoint signaling and ultimately G2/M cell cycle arrest.
Results
PP2A activity is required for IR-induced G2/M arrest of MCF-7 breast cancer cells
To study the possible role of Ser/Thr phosphatase PP1 and PP2A in IR-induced DNA-damage checkpoint response, we examined the effect of phosphatase inhibitor OA on the activities of PP2A and PP1 in MCF-7 breast cancer cells.
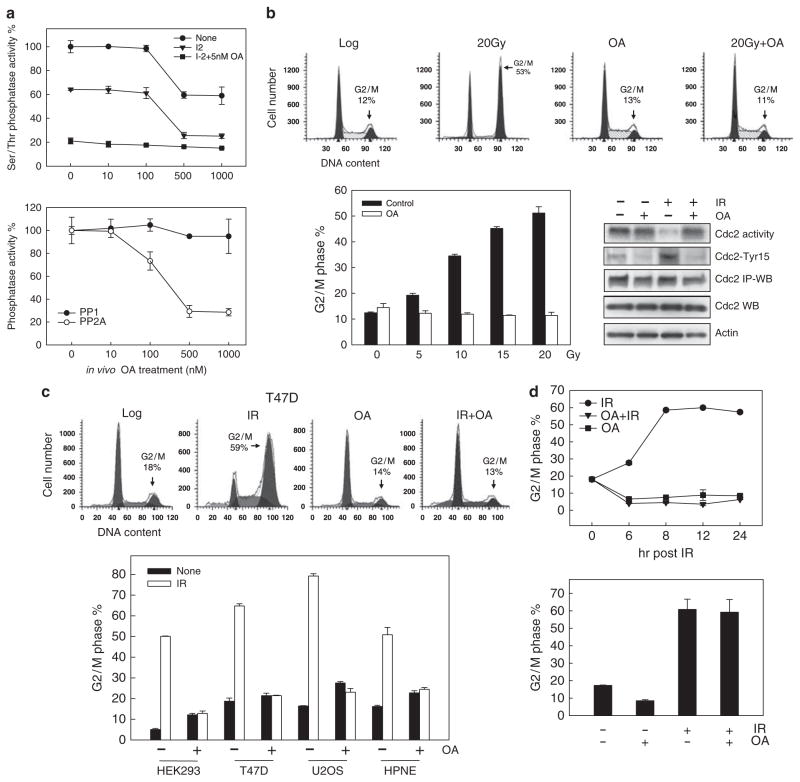
Exponentially growing MCF-7 cells were incubated in the presence of increasing concentrations of OA for 1 h and then PP2A and PP1 activities in soluble cell extracts were determined using a Ser/Thr phosphatase assay kit as described in Materials and methods. In brief, previously characterized methods were used to quantitate the relative activities of PP1 and PP2A (Cohen et al., 1989; Cohen, 1991; Yan and Mumby, 1999). Accordingly, phosphatase assays were carried out in the absence of added inhibitor, in the presence of 0.2 μM inhibitor-2 (I-2), or in the presence of 0.2 μM I-2 and 5 nM OA. Under these conditions, the proportion of Ser/Thr phosphatase activity in cell extracts attributable to PP1 corresponds to the activity inhibited by 0.2 μM I-2, whereas the proportion attributable to PP2A corresponds to the activity resistant to 0.2 μM I-2 but inhibited by 5 nM OA. As shown in Figure 1a, incubation of MCF-7 cells with OA resulted in a dose-dependent reduction in total Ser/Thr phosphatase activity (upper panel: circle). A maximum 40% inhibition in total Ser/Thr phosphatase activity was observed in cells incubated with 0.5 μM OA (Figure 1a, upper panel: circle). Addition of 0.2 μM I-2 (inhibitor of PP1) to extracts derived from untreated cells resulted in a 30–35% reduction in total Ser/Thr phosphatase activity (Figure 1a, upper panel: triangle). As shown in Figure 1a (lower panel: solid circle), the phosphatase activity inhibited by the incubation of extracts with I-2, which represents PP1 activity (Cohen, 1991), remained nearly unchanged after incubation of cells with increasing doses of OA. Mean PP1 activity in cells treated with 1 μM OA was only 5–10% lower than the PP1 activity in control untreated cells and the differences were not statistically significant (Figure 1a, lower panel: solid circle). In contrast, PP2A activity in untreated cells, represented by the proportion of Ser/Thr phosphatase activity that was not affected by the incubation of extracts with 0.2 μM I-2 but sensitive to 5 nM OA addition, was inhibited by in vivo incubation of cells in the presence of OA in a dose-dependent manner (Figure 1a, lower panel: open circle). As shown in Figure 1a (lower panel: open circle), incubation of cells with 0.5 μM OA resulted in an 80% inhibition of PP2A activity.
Figure 1.
PP2A inhibition by OA abrogates IR-induced G2/M cell-cycle arrest. (a) After incubation of cells with OA at the indicated doses for 1 h at 37 °C, PP1 and PP2A activity in cell lysates was determined as described in Materials and methods. Upper panel: Assays were performed in the presence of (1) no inhibitors (circle), (2) 0.2 μM inhibitor-2 (triangle) or (3) 0.2 μM inhibitor-2+5nM OA (square). Lower panel: PP1 activity is represented as the phosphatase activity inhibited by in vitro incubation with inhibitor-2 (solid circle) and PP2A activity is represented as the phosphatase activity inhibited by in vitro incubation with 5 nM OA (open circle). Data represent the mean±s.d. of quadruplicate assays. (b) Upper panels: MCF-7 cells were incubated in the presence or absence of 0.75 μM OA for 1 h at 37 °C, exposed to 20-Gy IR and then analyzed for DNA content by FACS following additional 24 h incubation at 37 °C. Lower left panel:MCF-7 cells were incubated in the presence or absence of 0.75 μM OA for 1 h, exposed to IR at the doses indicated, incubated for an additional 24 h at 37 °C and analyzed for DNA content. Results depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of two sets of experiments with duplicate samples. Lower right panel: Cells were treated as described in upper panels and incubated for 2 h after IR. Cdc2 was immunoprecipitated from lysates and analyzed for kinase activity using histone-H1 as substrate (Cdc2 activity), and Cdc2-Tyr15 phosphorylation by immunoblotting (Cdc2-Tyr15). Cdc2 in immunoprecipitates (Cdc2 IP-WB) and in cell lysates (Cdc2 WB) were determined by immunoblotting. (c) Upper panels: T47D cells were incubated in the presence or absence of 0.5 μM OA for 1 h, exposed to 15-Gy IR, incubated for an additional 24 h and then analyzed for DNA content. Lower panel: HEK293, T47D, U2OS and HPNE cells were incubated in the presence or absence of 0.5 μM OA for 1 h, exposed to 10-Gy (HEK293 and HPNE cells) or 15-Gy IR (T47D and U2OS cells), incubated for an additional 24 h at 37 °C and analyzed for DNA content. The results depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of two sets of experiments with duplicate samples. (d) Upper panel: MCF-7 cells were incubated with 0.5 μM OA for 1 h and exposed to 20-Gy IR. The cells were then incubated for the indicated hours and analyzed for DNA content. Lower panel: Cells were exposed to 20-Gy IR or left unirradiated and incubated for 8 h at 37 °C. The cells were then incubated in the presence or, as a control, absence of 0.5 μM OA for an additional 16 h and analyzed for DNA content. The results depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of triplicate samples.
We next examined the effect of PP2A inhibition by OA on IR-induced G2/M cell cycle arrest. For these studies, MCF-7 cells were incubated in the presence or absence of 0.75 μM OA for 1 h and then exposed to increasing doses of IR. As shown in Figure 1b, although IR exposure alone in the absence of treatment with the inhibitor resulted in a marked increase in the proportion of cells in G2/M arrest (upper panel: 20Gy, lower panel: solid bars), incubation of cells with OA completely abolished IR-induced G2/M arrest, as determined by DNA content analysis (upper panel: 20Gy+OA, lower panel: open bars). These data suggest that PP2A or PP2A-like phosphatase activity is essential for IR-induced G2/M arrest in MCF-7 cells.
As previous studies have shown that G2/M cell-cycle arrest induced by DNA damage requires inhibition of Cdc2/Cycin B complex activity (Atherton-Fessler et al., 1993), we examined the effect of PP2A on Cdc2 activity following irradiation of MCF-7 cells. As shown in Figure 1b (lower right panel), although IR exposure resulted in marked inhibition of Cdc2 activity in MCF-7 cells within 2 h following IR, this effect was abrogated by incubation of cells with OA before IR treatment. To verify this effect of OA on Cdc2 activity in irradiated MCF-7 cells, we also assessed the effect of OA on IR-induced Cdc2-Tyr15 phosphorylation, which has previously been shown to be essential for Cdc2 inhibition following IR exposure (Atherton-Fessler et al., 1993; Jin et al., 1996). Consistent with the OA effect on Cdc2 activity, treatment with OA completely abolished IR-induced Cdc2–Tyr15 phosphorylation in irradiated MCF-7 cells (Figure 1b, lower right panel). As previous studies indicated that the activity of Cdc2/Cyclin B controls the G2/M transition of the cell cycle, we also examined the effect of PP2A inhibition on the proportion of cells in mitosis following IR exposure of MCF-7 cells using histone H3 phosphorylation as a marker of cells in mitosis (Xu et al., 2001). The results of these studies indicate that IR exposure of MCF-7 cells resulted in a 78% decrease in the proportion of mitotic cells, which contain both 4N-DNA content and phospho-histone H3 (Xu et al., 2001), relative to control nonirradiated cells (Supplementary Figure 1). However, incubation of cells with OA abolished the reduction of cells in mitosis following irradiation (Supplementary Figure 1).
We also examined the effect of OA on IR-induced cell cycle response in other cell types, including HEK293 human embryonic kidney cells, T47D human breast cancer cells, U2OS human osteosarcoma cells and HPNE primary human pancreatic ductal cells. As shown in Figure 1c, IR-induced G2/M cell cycle arrest was completely abrogated by the presence of 0.5 μM OA in all four cell types.
We next examined the effect of OA on the induction of G2/M arrest following IR exposure using a time course experiment. For these studies, MCF-7 cells were exposed to 20-Gy IR in the presence or absence of 0.5 μM OA and analyzed for DNA content by fluorescence-activated cell sorting (FACS) at the indicated times following IR exposure. As shown in Figure 1d, IR exposure resulted in a time-dependent increase in the amount of G2/M DNA content cells, with a maximum detected at 8 h after IR treatment (upper panel: circle). In contrast, cells exposed to IR and incubated in the presence of OA showed no increase in the amount of G2/M DNA content cells at all time points examined (upper panel: triangle). Furthermore, cell samples treated with OA alone in the absence of IR treatment also showed no detectable change in the amount of G2/M DNA content cells (upper panel: square). These results suggest a requirement for PP2A activity in the induction of G2/M arrest by IR.
To explore the possible role of PP2A in the maintenance of G2/M arrest following irradiation, MCF-7 cells were first irradiated and incubated at 37 °C for 8 h in the absence of OA. As described in Materials and methods, these irradiated cells were then incubated at 37 °C in the presence of OA for an additional 16 h and harvested for DNA content analysis. As shown in Figure 1d (lower panel), cells incubated in the presence of OA beginning 8 h after irradiation continued to have the same level of G2/M arrest as the irradiated cells that were incubated in the absence of OA. These results indicate that once the signaling cascade leading to G2/M arrest is activated following irradiation, inhibition of PP2A or PP2A-like phosphatases is not necessary for the maintenance of G2/M checkpoint activation in MCF-7 cells following IR treatment.
The effect of PP2A inhibition on IR-induced ATM signaling activation
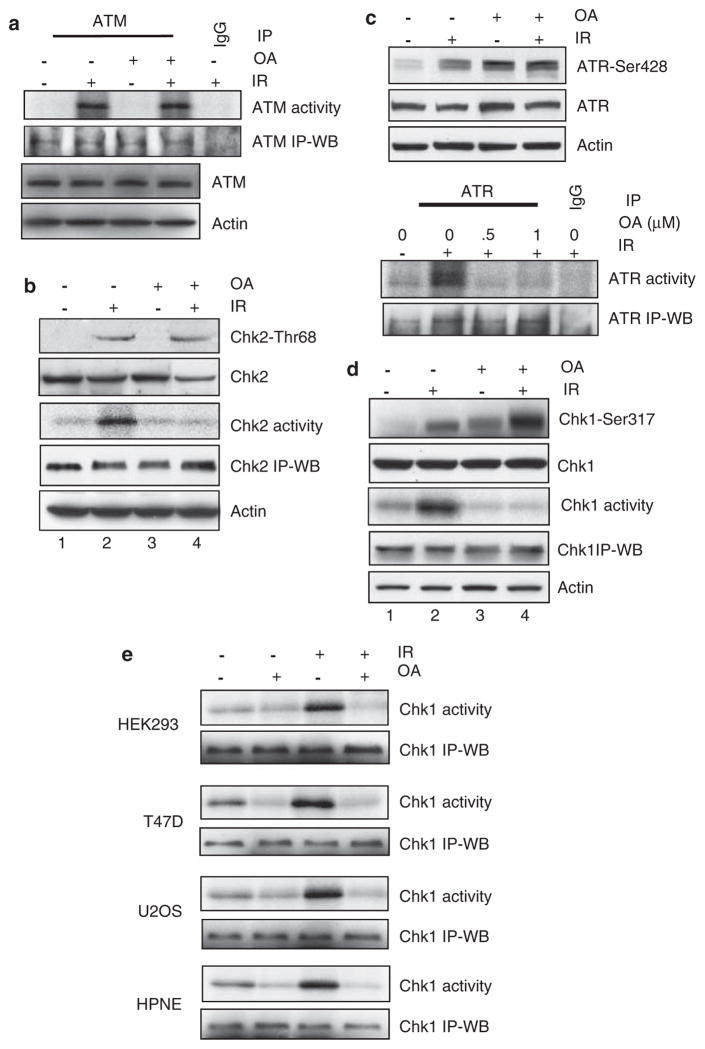
As ATM is activated in response to IR, the effect of PP2A inhibition on ATM signaling was determined. For these studies, MCF-7 cells were exposed to 15-Gy IR in the presence or absence of 0.75 μM OA and incubated for an additional 1 h before analysis. As shown in Figure 2a, incubation of cells with OA had no effect on ATM kinase activation following IR exposure, suggesting that PP2A or PP2A-like phosphatase is not required for IR-induced ATM kinase activation.
Figure 2.
Effects of PP2A inhibition on IR-induced ATM and ATR signaling activation. (a) After preincubation for 1 h at 37 °C in the presence or absence of 0.75 μM OA, MCF-7 cells were exposed to 15-Gy IR or left nonirradiated and then incubated for an additional 1 h at 37 °C. ATM was immunoprecipitated from cell lysates using Ab-3 anti-ATM antibody and assayed for kinase activity using p53 recombinant protein as substrate (ATM activity). As controls, ATM protein levels in the immunoprecipitates (ATM IP-WB) and total ATM and actin protein in cell lysates were assessed by immunoblotting (ATM and actin). (b) Chk2-Thr68 phosphorylation (Chk2-Thr68) and Chk2 protein levels (Chk2) in cell lysates were analyzed by immunoblotting. Chk2 was immunoprecipitated from cell lysates and assayed for kinase activity using Cdc25C recombinant protein as substrate (Chk2 activity). As controls, Chk2 protein in immunoprecipitates (Chk2 IP-WB) and actin (actin) in cell lysates were measured by immunoblotting. (c) Upper panel: Phosphorylation of ATR-Ser428 (ATR-Ser428) and levels of ATR (ATR) and actin (actin) in cell lysates were analyzed by immunoblotting. Lower panel: ATR was immunoprecipitated from cell lysates using N-19 anti-ATR antibody and assayed for kinase activity using p53 recombinant protein as substrate (ATR activity). As controls, ATR protein levels in the immunoprecipitates (ATR IP-WB) were determined by immunoblotting. (d) Levels of Chk1-Ser317 phosphorylation (Chk1-Ser317) and total Chk1 (Chk1) and actin (actin) protein in cell lysates were determined by immunoblotting using specific antibodies. Chk1 was immunoprecipitated from cell lysates using G-4 anti-Chk1 antibody and kinase activity was assayed using Cdc25C recombinant protein as substrate (Chk1 activity). As controls, Chk1 protein in the immunoprecipitates was measured by immunoblotting (Chk1 IP-WB). (e) HEK293, T47D, U2OS and HPNE cells were treated as described in Figure 1c and incubated for 1 h following IR. Chk1 was immunoprecipitated from cell lysates and kinase activity was assayed using Cdc25C recombinant protein as substrate (Chk1 activity). As controls, Chk1 protein in immunoprecipitates (Chk1 IP-WB) was measured by immunoblotting.
Previous studies have indicated that ATM is the primary kinase that phosphorylates and activates Chk2 kinase (Ahn et al., 2000; Matsuoka et al., 2000). We therefore studied the effect of PP2A inhibition on IR-induced Chk2 phosphorylation and activation. For these studies, MCF-7 cells were exposed to 15-Gy in the presence or absence of 0.75 μM OA and then incubated for an additional 1 h at 37 °C. As shown in Figure 2b, although incubation with OA had no influence on IR-induced Chk2-Thr68 phosphorylation in MCF-7 cells (Chk2-Thr68, lane 4 vs 2), the incubation completely abrogated Chk2 kinase activation following IR exposure (Chk2 activity, lane 4 vs 2). Thus, although PP2A or PP2A-like activity is not necessary for the phosphorylation of Chk2-Thr68 by ATM kinase following IR exposure, it is necessary for the activation of Chk2 kinase following IR exposure.
PP2A is essential for IR-induced activation of ATR signaling
Previous studies from our laboratory have shown that activation of ATR signaling is required for the induction of G2/M arrest in MCF-7 cells following IR exposure (Yan et al., 2007); we therefore examined the effect of PP2A on IR-induced ATR signaling. For these studies, MCF-7 cells were treated with IR in the presence or absence of OA and the levels of ATR and Chk1 phosphorylation and activities were assessed. As shown in Figure 2c (upper panel), phosphorylation of ATR-Ser428 was induced in cells exposed to IR and was even higher in cells following incubation with OA. There was little, if any, difference in the magnitude of ATR-Ser428 phosphorylation in cells treated with OA alone in the absence of radiation exposure compared with cells treated with irradiation and incubated in the presence of the inhibitor (Figure 2c, upper panel: lane 4 vs 3).
ATR kinase activity was also examined in cells treated with IR and incubated in the presence or absence of OA. As shown in Figure 2c (lower panel), although IR exposure resulted in a marked activation of ATR kinase activity, incubation with OA completely blocked the activation of ATR following IR treatment. Taken together, the results in Figure 2c indicate that PP2A or PP2A-like activity is required for IR-induced activation of ATR kinase, whereas it is not involved in IR-induced phosphorylation of ATR-ser428.
As previous studies have indicated that ATR regulates G2/M checkpoint control through the activation of Chk1 kinase (Zhao and Piwnica-Worms, 2001), the effects of OA on IR-induced Chk1 activation were also examined in MCF-7 cells. We first compared the effects of OA on Chk1-Ser317 phosphorylation in MCF-7 cells following exposure to 15-Gy IR. As shown in Figure 2d, exposure to IR resulted in a marked induction of Chk1-Ser317 phosphorylation, relative to nonirradiated cells. Although incubation of cells with OA alone resulted in an increase in Chk1-Ser317 phosphorylation (lane 3 vs 1), incubation of irradiated cells in the presence of OA resulted in a further increase in Chk1-Ser317 phosphorylation, relative to cells treated with IR alone or OA alone (Figure 2d, Chk1-Ser317).
We next determined the effect of OA on IR-induced Chk1 kinase activation. As shown in Figure 2d, while Chk1 activity was markedly induced in MCF-7 cells following IR exposure, incubation of cells with OA inhibited the activation of Chk1 following irradiation. Furthermore, although incubation of cells with OA alone in the absence of IR exposure resulted in a noticeable increase in Chk1-Ser317 phosphorylation, this did not lead to Chk1 activation. These results are similar to those described above regarding the effect of OA on IR-induced phosphorylation and activation of ATR kinase. As Chk1 has a key role in G2/M checkpoint activation following DNA damage (Liu et al., 2000), we verified the effect of OA on IR-induced Chk1 using other cell types. The results shown in Figure 2e indicate that OA treatment also abrogated IR-induced Chk1 activation in HEK293, T47D, U2OS and HPNE cells, implicating PP2A or PP2A-like activity in the IR-induced activation of both ATR and Chk1 kinases in multiple cell types.
PP2A inhibition by siRNA and SV40-small t antigen attenuates IR-induced G2/M arrest
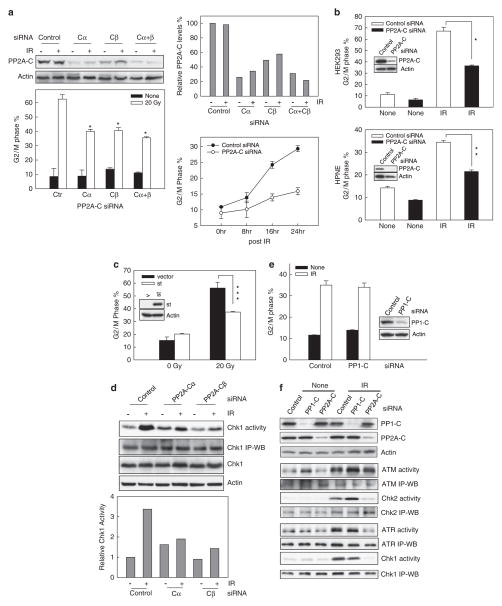
Previous studies indicate that incubation of cells with OA is highly selective for inhibiting PP2A activity in intact cells (Favre et al., 1997; Yan and Mumby, 1999). However, in vitro studies show that OA can inhibit PP1 activity, although the IC50 for PP1 inhibition is 100-fold greater than that for PP2A (IC50=0.1–0.3 nM for PP2A vs 15–30 nM for PP1) (Swingle et al., 2007). To examine the specificity of PP2A on IR-induced G2/M checkpoint response, we transfected cells with siRNA targeting the PP2A catalytic subunit. For these studies, MCF-7 cells were transfected with control nontargeting siRNA or siRNA targeting PP2A-Cα and/or PP2A-Cβ and incubated at 37 °C for 48 h. As shown in Figure 3a (upper panels), PP2A-C subunit protein levels were reduced by 70% in cells transfected with PP2A-Cα siRNA, 55% in cells transfected with PP2A-Cβ siRNA and 75% in cells transfected with both PP2A-Cα and PP2A-Cβ siRNAs, relative to that expressed in control siRNA-transfected cells, as determined by western blot analysis using an antibody that recognizes both PP2ACα and PP2A-Cβ (PP2A-C). In contrast, transfection of MCF-7 cells with nontargeting control siRNA had no effect on PP2A-C subunit protein levels relative to nontransfected cells (data not shown). Furthermore, transfection of MCF-7 cells with PP2A-C-specific siRNAs resulted in a significant attenuation of IR-induced G2/M cell cycle arrest compared with control siRNA-transfected cells (Figure 3a, lower left panel: open bars). Transfection of cells with control siRNA had no noticeable effect on IR-induced G2/M arrest compared with nontransfected cells (data not shown).
Figure 3.
PP2A-specific inhibition attenuates IR-induced G2/M checkpoint response. (a) Left panels: MCF-7 cells were transfected with nontargeting control siRNA (control) or specific siRNA targeting PP2A-Cα (Cα) and/or PP2A-Cβ (Cβ). Transfected cells were incubated for 2 days at 37 °C and exposed to 20-Gy or, as a control, left nonirradiated. After additional incubation for 24 h at 37 °C, PP2A-C and actin protein levels were determined by immunoblotting (upper panel) and DNA content was assessed by FACS (lower panel). Data represent the mean±s.d. of 4N-DNA content cell percentage in duplicate cell samples from three separate experiments. *P<0.001 (n=6), significant difference from cells transfected with control siRNA and exposed to IR. Right upper panel: Immunoblot densities of PP2A-C and actin were quantified using imageJ software and relative PP2A-C expression versus actin was determined. Right lower panel: Cells were transfected with either nontargeting siRNA (control siRNA) or siRNA targeting PP2A-Cα/Cβ (PP2A-C siRNA), incubated for 2 days and treated with 10-Gy IR. The cells were incubated for the indicated times and analyzed for 4N-DNA content. Data represent the mean±s.d. of 4N-DNA content cell percentage of triplicate cell samples. **P=0.005 (n=3), significant difference between cells transfected with control siRNA and cells transfected with PP2A-Cα/Cβ siRNA. (b) HEK293 cells (upper panel) and HPNE cells (lower panel) were transfected with either nontargeting siRNA (control siRNA) or siRNA targeting PP2A-Cα/Cβ (PP2A-C siRNA), incubated for 2 days at 37 °C and then exposed to 10-Gy IR. The irradiated cells were incubated for an additional 24 h and analyzed for levels of PP2A-C and actin by western blotting (insets) and DNA content by FACS (bar graphs). The results of FACS analysis depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of triplicate cell samples. *P=<0.001 (n=3); **P=<0.001 (n=3); significant difference from cells transfected with control siRNA. (c) MCF-7 cells were stably transfected with a tetracycline-inducible SV40-small t antigen (st) or control vector (vector) as described in Material and methods. The cells were incubated in the absence of doxcycline for 48 h to induce SV40-small t expression, and then exposed to 20-Gy IR. After 24 h incubation at 37 °C, cells were analyzed for SV40-small t expression by western blotting (inset) and for DNA content by FACS (bar graph). The results depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of duplicate cell samples from two separate experiments. ***P=0.006 (n-4), significant difference between cells transfected with control vector and cells transfected with SV40-small t expressing vector. (d) Upper panel: Cells were transfected with control siRNA or with siRNA specific for PP2A-Cα or PP2A-Cβ, incubated for 2 days and then exposed to 20-Gy IR or left nonirradiated. After incubation for 1 h at 37 °C, Chk1 was immunoprecipitated from cell lysates and assayed for kinase activity using Cdc25C recombinant protein as substrate (Chk1 activity). Levels of Chk1 protein in immunoprecipitates (Chk1 IP-WB) and in lysates (Chk1) were determined by immunoblotting. Lower panel: The relative activity of Chk1 kinase in each sample was determined by densitometry of the autoradiograph of 32p-Cdc25C formed during each Chk1 kinase assay (Chk1 activity), relative to the amount of Chk1 protein in each assay (Chk1 IP-WB), which was determined using image J software analysis of western blot of immunoprecipitates. The bar graph depicts the relative Chk1 activity in each sample. (e) MCF-7 cells were transfected with either nontargeting control siRNA (control) or siRNA targeting PP1-C (PP1-C). Cells were incubated for 2 days, exposed to 15-Gy IR and then incubated at 37 °C for an additional 24 h. The cells were analyzed for levels of PP1-C and actin by western blotting (inset) and for DNA content by FACS (bar graph). The results of FACS analysis shown depict the percentage of cells with 4N-DNA content and represent the mean±s.d. of duplicate cell samples of three independent experiments. (f) MCF-7 cells were transfected with either nontargeting siRNA or siRNA targeting PP1-C or PP2A-Cα/Cβ, incubated for 2 days and treated with 15-Gy IR or nonirradiated. The cells were analyzed for PP1-C and PP2A-C expression by western blotting (PP1-C and PP2A-C) and for ATM, ATR, Chk1 and Chk2 activities by kinase assay (ATM activity, Chk2 activity, ATR activity and Chk1 activity). The levels of ATM, Chk2, ATR and Chk1 protein in immunoprecipitates were determined by western blotting (ATM IP-WB, Chk2 IP-WB ATR IP-WB and Chk1 IP-WB).
We also examined the time course of the effect of PP2A on the induction of IR-induced G2/M arrest in MCF-7 cells. For these studies, MCF-7 cells transfected with siRNA targeting PP2A-Cα/Cβ were incubated for 2 days at 37°C and then exposed to 10-Gy IR. After IR treatment, the cells were harvested at the times indicated and analyzed for 4N-DNA content by FACS analysis. As shown in Figure 3a (lower right panel), cells transfected with PP2A-C siRNA showed a retardation in the induction of G2/M arrest following IR exposure compared with control siRNA-transfected cells. Statistical analysis indicates a significant difference in 4N-DNA content between PP2A-Cα/Cβ siRNA-transfected cells and control siRNA-transfected cells, determined 24 h after IR exposure (P=0.005, n=3).
To determine the effect of PP2A-C knockdown on IR-induced G2/M arrest in other cell types, HEK293 and HNPE cells were transfected with siRNA targeting PP2A-Cα/Cβ and the effect was then examined following irradiation. As shown in Figure 3b (insets), transfection of PP2A-C siRNA markedly decreased endogenous PP2A-C expression in both cell types, as determined by western blotting. Furthermore, this was associated with a significant diminution of IR-induced G2/M cell cycle arrest in both HEK293 and HNPE cells (IR: solid bars vs open bars).
As previous studies have shown that SV40-small t antigen binds to PP2A and suppresses PP2A phosphatase activity (Sontag et al., 1993), we stably transfected MCF-7 cells with an SV40-small t antigen expression vector as described in Materials and methods. As shown in Figure 3c, there was a significant attenuation in IR-induced G2/M arrest in MCF-7 cells expressing SV40-small t compared with control MCF-7 cells (20 Gy: open bar vs solid bar). The expression of SV40-small t in MCF-7 cells was verified by immunoblotting (inset). In contrast, there was no difference in IR-induced G2/M arrest between nontransfected cells and MCF-7 cells transfected with control vector (data not shown).
Consistent with the effect of OA on IR-induced G2/M arrest in MCF-7 cells, MCF-7 cells transfected with PP2A-C siRNA also exhibited a marked diminution in Chk1 activation following IR exposure, relative to control siRNA-transfected cells (Figure 3d, Chk1 activity). Transfection of MCF-7 cells with control siRNA had no effect on IR-induced Chk1 activation compared with nontransfected control cells (data not shown). These studies support the data obtained from PP2A inhibitor studies described above (see Figures 1 and 2), indicating that PP2A activity is necessary for Chk1 activation and G2/M arrest in MCF-7 cells following IR treatment.
Inhibition of PP1 has no effect on IR-induced G2/M arrest
We also examined the effect of PP1, another major Ser/Thr phosphatase (Cohen, 2002), on IR-induced G2/M checkpoint response in MCF-7 cells. For these studies, MCF-7 cells were transfected with siRNA targeting PP1 catalytic (C) subunit or control nontargeting siRNA, and their effects on IR-induced G2/M arrest were examined. As shown in Figure 3e (inset), transfection of MCF-7 cells with siRNA targeting the PP1-C subunit (PP1-C), the primary component of the PP1 holo-enzyme, resulted in a decrease in endogenous PP1-C protein levels by 80%, as determined by immunoblotting at 48 h after transfection (PP1-C). As shown in Figure 3e (bar graph), there was essentially no difference in IR-induced G2/M arrest in PP1-C siRNA-transfected cells compared with that in control siRNA-transfected cells. These results suggest that PP1 has little, if any, effect on IR-induced G2/M checkpoint response.
We also compared the effects of PP2A and PP1 on ATM signaling activation following IR exposure. For these studies, MCF-7 cells were transfected with siRNA targeting either PP1-C or PP2A-Cα/Cβ, or with control nontargeting siRNA, and the activation of ATM and Chk2 in cells following IR treatment was examined. As shown in Figure 3f, there was no difference in IR-induced ATM kinase activation in MCF-7 cells transfected with PP2A-Cα/Cβ siRNA, relative to that in control siRNA-transfected cells. In contrast, decreased PP1-C expression in MCF-7 cells following siRNA transfection actually resulted in a slight increase in IR-induced ATM activation (ATM activity). Furthermore, although decreased PP1-C expression in siRNA-transfected cells resulted in an increase in IR-induced Chk2 kinase activity relative to control siRNA-transfected cells, transfection with PP2A-Cα/Cβ siRNA abrogated IR-induced Chk2 kinase activation in MCF-7 cells (Chk2 activity). These results are consistent with the observation described above in cells treated with OA and indicate that PP2A is not required for IR-induced ATM activation but is necessary for Chk2 kinase activation following IR exposure.
As shown in Figure 3f, decreased PP1-C expression following siRNA transfection of MCF-7 cells produced no effect on IR-induced activation of ATR and Chk1 kinases (ATR activity and Chk1 activity). In contrast, decreased PP2A-C expression following siRNA transfection abrogated the activation of both ATR and Chk1 kinases following IR exposure (ATR activity and Chk1 activity). These results support the studies discussed above following treatment of cells with OA, and indicate an essential role of PP2A in the activation of ATR signaling following IR exposure.
Inhibition of PP4, PP5 and PP6 has no effect on IR-induced G2/M cell cycle arrest
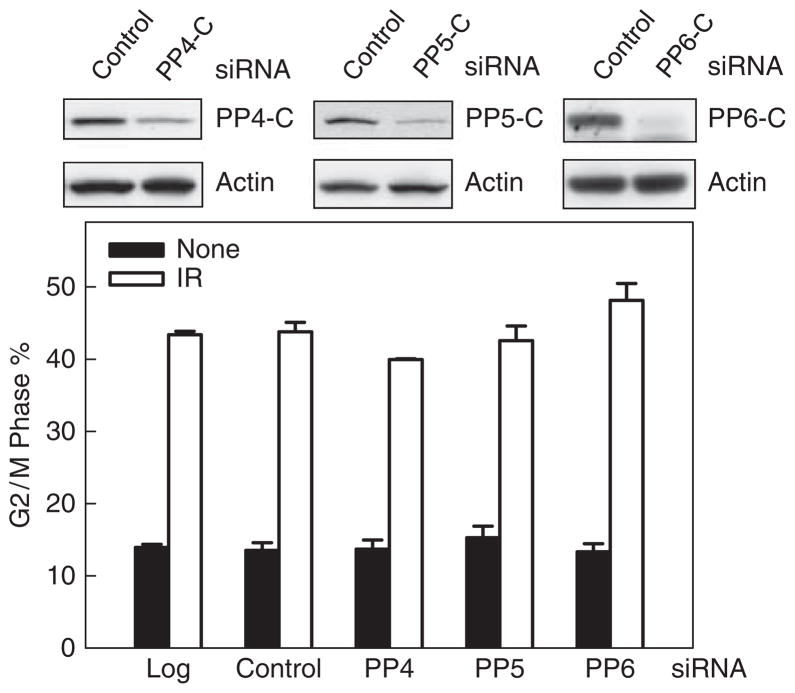
As previous in vitro studies indicate that OA not only inhibits PP1 and PP2A activity but also inhibits PP4, PP5 and PP6 activity with differential selectivities (Swingle et al., 2007), we therefore examined the potential effect of PP4, PP5 and PP6 on IR-induced G2/M cell cycle arrest. As shown in Figure 4, transfection of MCF-7 cells with specific siRNAs targeting the catalytic subunits of PP4, PP5 or PP6 resulted in a decreased expression of the relative target gene 48 h after transfection (upper panel: PP4-C, PP5-C and PP6-C). However, as shown in Figure 4, decreased expression of PP4-C, PP5-C or PP6-C in MCF-7 cells had no effect on IR-induced G2/M cell cycle arrest, relative to control siRNA-transfected cells (lower panel). These results provide additional evidence supporting the specific regulation of PP2A on IR-induced G2/M checkpoint activation.
Figure 4.
Cells were transfected with nontargeting siRNA or siRNA targeting the catalytic subunit of PP4, PP5 or PP6, and incubated for 3 days at 37 °C. The cells were treated with 15-Gy IR or nonirradiated, incubated for an additional 24 h and analyzed for levels of the catalytic subunit of PP4, PP5 and PP6 by western blotting (upper panels) and for DNA content by FACS (lower panel). The percentage of cells with 4N-DNA content (G2/M phase) is depicted (mean±s.d. of three separate experiments with duplicate cell samples).
Discussion
While PP2A Ser/Thr phosphatase has been found to have a critical role in the regulation of cellular signaling that controls cell cycle progression, accumulating evidence also implicates PP2A in the regulation of DNA-damage checkpoint response (Goodarzi et al., 2004; Chowdhury et al., 2005; Li et al., 2007a b). In this report, we have examined in detail the effect of PP2A on IR-induced G2/M DNA-damage checkpoint response. Using specific inhibitors to inhibit PP2A and the siRNA targeting PP2A catalytic subunit (Pallas et al., 1990; Cho et al., 2007), we observed a predominant regulation by PP2A on IR-induced G2/M DNA-damage checkpoint response (see Figures 1–3).
G2/M transition of the cell cycle is controlled by the activity of the Cdc2/Cyclin B complex, which is required for cell entry into mitosis. It has been previously shown that DNA damage induces phosphorylation of the Tyr15 residue of Cdc2, resulting in inhibition of Cdc2/Cyclin B activity and G2/M arrest (Smits and Medema, 2001). The cellular response to DNA damage involves the activation of Wee1 kinase, which directly phosphorylates Cdc2-Tyr15, as well as activation of Chk1 kinase, which phosphorylates and inhibits Cdc25A/Cdc25C phosphatases that dephosphorylate Cdc2-Tyr15 (Chen and Sanchez, 2004; Sancar et al., 2004). Consistent with the effect of PP2A inhibition on IR-induced Chk1 activation and G2/M arrest, inhibition of PP2A also resulted in diminution of IR-induced phosphorylation of Cdc-Tyr15 (see Figures 1 and 2) and an increase in the entry of cells from G2 into M phase in MCF-7 cells exposed to IR (see Supplementary Figure 1). These results indicate that PP2A activity is necessary for IR-induced G2/M checkpoint activation and for the regulation of the Cdc2/Cyclin B complex.
Although previous studies indicate that IR exposure can also induce arrest in G1 in some cell types (Iliakis et al., 2003), we observed no increase in the proportion of cells in G1 following IR treatment of MCF-7 cells (data not shown). In fact, other studies have reported the absence of IR-induced G1 arrest in MCF-7 cells and suggested that these cells may have a defect in G1 checkpoint, despite having a wild-type p53 expression (Nagasawa et al., 1998). In contrast, several studies have shown that IR exposure induces a significant G2/M arrest in MCF-7 cells (Nagasawa et al., 1998; Essmann et al., 2004; Yan et al., 2005).
To examine whether PP2A is essential for the maintenance of IR-induced G2/M checkpoint activation, irradiated MCF-7 cells were incubated for 8 h in the absence of OA and then treated with the inhibitor. Under these conditions, inhibition of PP2A by OA several hours after irradiation produced no diminution of IR-induced G2/M arrest (see Figure 1d, lower panel). These results indicate that once the signaling cascade leading to G2/M arrest is activated following irradiation, PP2A activity is not necessary for the maintenance of G2/M checkpoint activation in MCF-7 cells following IR treatment.
Both ATM and ATR have important roles in mediating the DNA-damage checkpoint response (Iliakis et al., 2003; O’Connell and Cimprich, 2005). Recent reports identified an interaction between PP2A and ATM in unperturbed cells (Goodarzi et al., 2004) and an involvement of PP2A in the regulation of both ATM and ATR protein function (Leung-Pineda et al., 2006). It is interesting that the studies presented in this report show that, although PP2A is necessary for IR-induced ATR activation, it is not required for IR-induced activation of ATM (see Figures 2 and 3). Furthermore, although inhibition of PP2A in MCF-7 cells treated with OA results in an increase in ATR-Ser428 phosphorylation in both nonirradiated and irradiated cells, kinase assays show that this phosphorylation of ATR does not correlate with the activation of ATR kinase in irradiated cells (see Figure 2c). Similarly, additional studies indicate that OA treatment also results in ATM-Ser1981 phosphorylation in unirradiated MCF-7 cells, which is also not associated with the activation of ATM kinase (data not shown). These results are consistent with previous observations using human lymphoblastoid cells, which showed that OA treatment of nonirradiated cells induces ATM-Ser1981 phosphorylation in the absence of induction of ATM kinase activity (Goodarzi et al., 2004).
Chk2 activation by DNA damage is a multistep process (Reinhardt and Yaffe, 2009). In response to DNA damage, ATM activation triggers phosphorylation of Chk2-Thr68, which in turn results in autophosphorylation of Chk2 at multisites and subsequent activation of Chk2 kinase. Furthermore, although ATM has been proposed to be the primary activator of Chk2 kinase, some studies suggest that Chk2 can also be phosphorylated by other phosphatidylinositol-3 kinase-related kinases, including ATR and DNA–PKs (Reinhardt and Yaffe, 2009). Studies presented in this report show that PP2A inhibition had no effect on either IR-induced ATM activation or phosphorylation of Chk2-Thr68. However, PP2A inhibition completely abolished IR-induced Chk2 kinase activation (Figure 2b). These results indicate that the phosphorylation of Chk2-Thr68 by ATM following IR exposure apparently does not require PP2A. However, PP2A is necessary for the activation of Chk2 kinase and this effect of PP2A occurs downstream of ATM kinase.
Consistent with the effect of PP2A on IR-induced ATR activation, studies presented in this report indicate that PP2A is also required for Chk1 activation following IR (see Figures 2 and 3). Furthermore, incubation of cells with OA also resulted in an increase in Chk1- Ser317 phosphorylation with no apparent activation of Chk1 kinase activity (see Figure 2d). These results are consistent with previous studies using HeLa human cervical cancer cells, which showed that OA treatment of HeLa cells in the absence of DNA damage results in the accumulation of Ser317 and Ser345 phosphorylation of Chk1 without activation of Chk1 kinase activity (Leung- Pineda et al., 2006).
A previous study showed that decrease in PP2A-Aα level in PC12 rat adrenal medulla pheochromocytoma cells by siRNA inhibits PP2A holoenzyme activity and results in apoptosis induction (Strack et al., 2004). We therefore examined the possible effect of PP2A-C-specific siRNA transfection on apoptosis induction in MCF-7 cells. For these studies, cells were transfected with PP2A-C-specific siRNA and incubated for up to 3 days. The cells were then analyzed for sub-G1-DNA content cell population and for cleavage of poly(ADP-ribose) polymerase precursor, which are hallmarks of apoptosis (Liu et al., 1996). As shown in Supplementary Figure 2, FACS analysis of PP2A-C siRNA-transfected MCF-7 cells showed no increase in the proportion of sub-G1-DNA content cell population within the time frames tested. Consistently, western blot analysis also indicated no evidence of poly(ADP-ribose) polymerase precursor cleavage in PP2A-C siRNA-transfected cells, whereas the transfection with PP2A-C siRNA markedly decreased the PP2A-C protein expression in MCF-7 cells. These data indicate that decreased PP2A-C level in MCF-7 cells for up to 72 h is apparently not sufficient to induce apoptosis. Thus, the effect of PP2A inhibition on cell survival is apparently cell type specific.
In summary, the results presented in this report suggest an important role of PP2A in IR-induced G2/M DNA-damage checkpoint response, which involves its predominant effect on the activation of ATR, Chk1 and Chk2 kinases following IR exposure. Additional studies are needed to further define the detailed mechanism through which PP2A elicits its regulation on DNA damage-induced cell cycle checkpoint response.
Materials and methods
Cell culture and drug treatment
The MCF-7 human breast cancer cell line, the HEK293 human embryonic kidney cell line, the T47D human breast cancer cell line and the U2OS human osterosarcoma cell line were obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s medium containing 10% fetal bovine serum. HPNE cells are primary human pancreatic ductal cells immortalized by human telomerase hTERT (kindly provided by Dr Michel Ouellette (University of Nebraska Medical Center)). HPNE cells were maintained in 5% CO2 in Medium D growth medium (four parts high-glucose Dulbecco’s Modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA) to one part M3F (INCELL, San Antonio, TX, USA) supplemented with 5% FCS) (Lee et al., 2005).
For studies involving treatment with phosphatase inhibitor, log-phase-growing cells were incubated in medium containing OA (Alexis, San Diego, CA, USA), which was dissolved in dimethyl sulfoxide, as described previously (Yan and Mumby, 1999). Control cells were incubated in medium containing the same amounts of vehicle alone (0.05% dimethyl sulfoxide). For experiments involving IR exposure, exponentially growing cells were treated with IR and then incubated at 37 °C for the indicated times before analysis. For experiments involving treatment with both phosphatase inhibitor and IR, cells were incubated with the inhibitor for 1 h before IR exposure.
Antibodies and recombinant proteins
Antibodies obtained from cell signaling technology (Danvers, MA) include rabbit IgG for PP2A-A (81G5), PP2A-C (52F8), p-ATR (Ser428), p-Chk1 (Ser317) and p-Chk2 (Thr68). Antibodies obtained from EMD Biosciences (San Jose, CA, USA) include mouse IgG for poly(ADP-ribose) polymerase precursor (Ab-2) and rabbit IgG for ATM (Ab-3). Antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) include mouse IgG for Cdc2 (17), Chk1 (G-4) and Chk2 (B-4); goat IgG for ATR (N-19), actin (I-19), p-Cdc2 (Tyr15), PP4-C (C-18) and PP5-C (C-20); rabbit IgG for Cdc2 (C-19) and Chk1 (FL-476). Antibodies obtained from upstate biotechnology (Lake Placid, NY, USA) include mouse IgG for PP2A-B (2G9) and rabbit IgG for PP1-C and PP6-C.
Recombinant p53 protein for ATM and ATR kinase assays was a glutathione S-transferase fusion protein containing fulllength human p53 (Addgene, Cambridge, MA, USA). Cdc25C protein, the substrate for Chk1/Chk2 kinase assay, was purified as a glutathione S-transferase fusion protein containing residues 200–256 of human Cdc25C (kindly provided by Dr Helen Piwnica–Worms (Washington University School of Medicine)). Glutathione S-transferase was used as a control substrate in all kinase assays and was prepared according to standard procedures (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
Immunoblotting, immunoprecipitation and kinase assay
Immunoblotting, immunoprecipitation and kinase assays were performed as described previously (Hall-Jackson et al., 1999; Sarkaria et al., 1999; Yan et al., 2007). Specific protein signals on western blots were visualized by chemiluminescence exposed to X-ray film, scanned using an EPSON Perfection 4490PHOTO scanner and analyzed using the ImageJ analytical program (NIH, Bethesda, MD, USA).
Ser/Thr phosphatase assay
Cell extracts for phosphatase assays were prepared using procedures described previously (Yan and Mumby, 1999). Briefly, cells were harvested by scraping and were washed with phosphate-buffered saline. The cell pellets were lysed for 10 min on ice in three volumes (v/v) of extraction buffer (50mM Tris–HCl, pH 7.0, 0.1mM EDTA, 0.1mM EGTA, 0.5% Triton X-100, 1mM DTT, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 1mM phenylmethylsulfonyl fluoride and 10% glycerol). The crude extract was passed through a 21-gauge needle several times to facilitate lysis and insoluble material was removed by centrifugation for 5 min at 2,500 g. The soluble fraction was then passed through a Sephadex G-50 spin column (Roche Molecular Biochemicals, Indianapolis, IN, USA) equilibrated with storage buffer (50mM Tris–HCl, pH 7.0, 0.1mM EDTA, 0.1mM EGTA, 1mM DTT, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 1mM phenylmethylsulfonyl fluoride and 20% glycerol) to remove low-molecular-weight substances that may interfere with the protein phosphatase assays. The cell extracts were aliquoted and stored at −80 °C. Each aliquot was only thawed once for phosphatase assay. Phosphatase assays were performed using a malachite green phosphate detection system (Upstate Biotechnology, Lake Placid, NY, USA) in accordance with the manufacturer’s directions. In brief, cell lysates containing Ser/Thr phosphatases were incubated at 30 °C for 10 min with a phosphopeptide specific for PP1 and PP2A (KRpTIRR) and phosphatase activities were determined by measuring the release of Pi from the phosphopeptide (KRpTIRR) using malachite green phosphate detection solution using absorbance spectrophotometry at 650 nM (Ambach et al., 2000; Pankov et al., 2003). All assays were carried out under conditions in which the release of Pi was linear with time (less than 20% of the substrate consumed) and were directly dependent on the amount of extract protein.
To determine PP1 and PP2A activities in cell extracts, we used previously described methods (Cohen et al., 1989; Cohen, 1991; Yan and Mumby, 1999). Accordingly, phosphatase assays were performed on cell extracts using three separate conditions as described: (1) in the absence of any inhibitors to determine the total level of Ser/Thr phosphatase activity in cell extracts; (2) in the presence of 0.2 μM inhibitor-2 (I-2) to inhibit PP1 activity; and (3) in the presence of both 0.2 μM I-2 and 5 nM OA to inhibit both PP1 and PP2A activity in cell extracts (Cohen et al., 1989). Under these conditions, the proportion Ser/Thr phosphatase activity attributable to PP1 corresponds to the activity inhibited by 0.2 μM I-2, whereas the proportion attributable to PP2A corresponds to the activity resistant to 0.2 μM I-2 but inhibited by 5 nM OA. As PP2B and PP2C are not inhibited by either 0.2 μM I-2 or 5 nM OA in vitro (Cohen, 1991), their activities are not assessed in these assays.
siRNA transfection
Short interfering RNA (siRNA) duplexes were obtained from Dharmacon Research (Chicago, IL, USA). Nontargeting control siRNA contains at least four mismatches to any human, mouse or rat gene, as previously determined by the manufacturer. The sequence for control siRNA is 5′-UAAGGCUAUGAAGAGAUAC-3′. SMARTpool siRNA targeting PP2A-Cα consists of four siRNA targeting multiple sites on PP2A-Cα. The siRNA sequences for PP2A-Cα are 5′-UAACCAAGCUGCAAUCAUG-3′, 5′-UAACCAAGCU GCAAUCAUG-3′, 5′-GAACUUGACGAUACUCUAA-3′ and 5′-CGAGAAGGCUAAAGAAAUC-3′. SMARTpool siRNA targeting PP2A-Cβ consists of four siRNA targeting multiple sites on PP2A-Cβ. The siRNA sequences for PP2A-Cβ are 5′-GGAAUUAGAUGACACUUUAUU-3′, 5′-GUAAGCAGCUGAACGAGAAUU-3′, 5′-CACGAAAG CCGACAAAUUAUU-3′ and 5′-AAAGGUGCGUUAUCC AGAAUU-3′. SMARTpool siRNA targeting PP1-C consists of four siRNA targeting multiple sites on PP1-C. The siRNA sequences for PP1-C are 5′-CAAGAUCUGCGGUGACA UAUU-3′, 5′-CAAGAGACGCUACAACAUCUU-3′, 5′-GAA CGACCGUGGCGUCUCUUU-3′ and 5′-CCAAGUUCCU CCACAAGCAUU-3′. SMARTpool siRNA targeting PP4-C consists of four siRNA targeting multiple sites on PP4-C. The siRNA sequences for PP4-C are 5′-GCACUGAGAUCU UUGACUA-3′, 5′-GGAGCCGGCUACCUAUUUG-3′, 5′-G ACAAUCGACCGAAAGCAA-3′ and 5′-GCACUUAAGG UUCGCUAUC-3′. SMARTpool siRNA targeting PP5-C consists of four siRNA targeting multiple sites on PP5-C. The siRNA sequences for PP5-C are 5′-GAACAAAGCCU CCUACAUC-3′, 5′-AGAAGUACAUCAAGGGUUA-3′, 5-G AAUGUGAAUACCAGAUU-3′ and 5-CAGAGGAGCUCA AGACUCA-3′. SMARTpool siRNA targeting PP6-C consists of four siRNA targeting multiple sites on PP6-C. The siRNA sequences for PP6-C are 5′-GCAAGUACCUGCCAG AGAA-3′, 5′-GAACGACAACGCCAUAUUU-3′, 5′-CACGA AGGCUAUAAAUUUA-3′ and 5′-CUAAAUGGCCUGAUC GUAU-3′.
Cells were transfected with siRNA at 100 nM using DharmaFECT1 siRNA transfection reagent (Dharmacon Research, Chicago, IL, USA) according to the manufacturer’s instruction. For experiments involving both siRNA transfection and IR exposure, transfected cells were first incubated at 37 °C for the indicated times and then exposed to IR.
Cell cycle analysis
Fluorescence-activated cell sorting (FACS) analysis was performed on 20 000 cells using a FACS calibur instrument (Beckon Dickinson, Mansfield, MA, USA), as described previously (Yan et al., 2007).
Analysis for mitotic cells
MCF-7 cells were exposed to IR in the presence or absence of 0.5 μM OA and harvested at 2 h following IR exposure. The mitotic cells in the obtained cell samples were analyzed as described previously (Xu and Kastan, 2004). Briefly, samples were fixed in 70% ethanol and stained with 30 μg/ml phosphatidylinositol-3 and anti-phospho-histone H3 antibody (Upstate Biotechnology, Lake Placid, NY, USA). Mitotic cells in the cell samples, which contain both 4N-DNA content and phospho-histone H3, were determined using a FACS calibur instrument (Beckon Dickinson) as recommended by the manufacturer and analyzed using Cellquest software. Each analysis was performed using 20 000 cells.
Supplementary Material
Acknowledgments
We thank Dr Michel Ouellette for providing HPNE cells, Dr Helen Piwnica Worms for GST-Cdc25C construct, Dr Charles Kuzynski, Victoria Smith and Megan Michalak for assistance on the flow cytometry analysis, and Dr Janina Baranowska-Kortylewicz for assistance on the operation of Mark I 68A Cesium-137 Irradiator. This work was supported by Nebraska DHHS-LB506 grant 2007-45 to YY, NCI Training Grant (NCI T32 CA009476) to RK and NCI Cancer Center Support Grant (P30CA036727) to KC.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol. 2000;30:3422–3431. doi: 10.1002/1521-4141(2000012)30:12<3422::AID-IMMU3422>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Atherton-Fessler S, Parker LL, Geahlen RL, Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol Cell Biol. 1993;13:1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sanchez Y. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair (Amst) 2004;3:1025–1032. doi: 10.1016/j.dnarep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 2007;5:e202. doi: 10.1371/journal.pbio.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D, Keogh M-C, Ishii H, Peterson CL, Buratowski S, Lieberman J. [gamma]-H2AX Dephosphorylation by protein phosphatase 2A facilitates DNA Double-strand break repair. Molecular Cell. 2005;20:801. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992;17:408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Cohen P, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989;250:596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cohen PTW. Protein phosphatase 1—targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Essmann F, Engels IH, Totzke G, Schulze-Osthoff K, Janicke RU. Apoptosis resistance of MCF-7 breast carcinoma cells to ionizing radiation is independent of p53 and cell cycle control but caused by the lack of caspase-3 and a caffeine-inhibitable event. Cancer Res. 2004;64:7065–7072. doi: 10.1158/0008-5472.CAN-04-1082. [DOI] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Jonnalagadda JC, Douglas P, Young D, Ye R, Moorhead GB, et al. Autophosphorylation of ataxiatelangiectasia mutated is regulated by protein phosphatase 2A. Embo J. 2004;23:4451–4461. doi: 10.1038/sj.emboj.7600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Jackson CA, Cross DA, Morrice N, Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–5847. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- Jang Y-J, Ji J-H, Choi Y-C, Ryu CJ, Ko S-Y. Regulation of polo-like kinase 1 by DNA damage in mitosis: inhibition of mitotic plk-1 by protein phosphatase 2A. J Biol Chem‘. 2007;282:2473–2482. doi: 10.1074/jbc.M605480200. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Current Opinion in Genetics & Development. 2005;15:34. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Lee KM, Yasuda H, Hollingsworth MA, Ouellette MM. Notch 2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest. 2005;85:1003–1012. doi: 10.1038/labinvest.3700298. [DOI] [PubMed] [Google Scholar]
- Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR Is Antagonized by a Chk1-Regulated Protein Phosphatase 2A Circuit. Mol Cell Biol. 2006;26:7529–7538. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Elder RT, Qin K, Park HU, Liang D, Zhao RY. Phosphatase Type 2A-dependent and -independent Pathways for ATR Phosphorylation of Chk1. J Biol Chem. 2007a;282:7287–7298. doi: 10.1074/jbc.M607951200. [DOI] [PubMed] [Google Scholar]
- Li HH, Cai X, Shouse GP, Piluso LG, Liu X. A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. Embo J. 2007b;26:402–411. doi: 10.1038/sj.emboj.7601519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HH, Li AG, Sheppard HM, Liu X. Phosphorylation on Thr- 55 by TAF1 mediates degradation of p53: a role for TAF1 in cell G1 progression. Mol Cell. 2004;13:867–878. doi: 10.1016/s1097-2765(04)00123-6. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Jaekel RE, Hemmings BA. Protein phosphatase 2A— a ‘menage a trois’. Trends Cell Biol. 1994;4:287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- McConnell JL, Wadzinski BE. Targeting protein serine/threonine phosphatases for drug development. Mol Pharmacol. 2009;75:1249–1261. doi: 10.1124/mol.108.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Keng P, Maki C, Yu Y, Little JB. Absence of a radiation-induced first-cycle G1-S arrest in p53+ human tumor cells synchronized by mitotic selection. Cancer Res. 1998;58:2036–2041. [PubMed] [Google Scholar]
- O’Connell MJ, Cimprich KA. G2 damage checkpoints: what is the turn-on? J Cell Sci. 2005;118:1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, et al. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Clark K, Matsumoto K, Hahn C, Poulin B, et al. Specific beta1 integrin site selectively regulates Akt/protein kinase B signaling via local activation of protein phosphatase 2A. J Biol Chem. 2003;278:18671–18681. doi: 10.1074/jbc.M300879200. [DOI] [PubMed] [Google Scholar]
- Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Current Opinion in Cell Biology. 2009;21:245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Strack S, Cribbs JT, Gomez L. Critical Role for Protein Phosphatase 2A Heterotrimers in Mammalian Cell Survival. Journal of Biological Chemistry. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A, Sasaki K, Nagai H, Mieskes G, Isobe M, Isono K, et al. Inhibition of specific binding of okadaic acid to protein phosphatase 2A by microcystin-LR, calyculin-A and tautomycin: method of analysis of interactions of tight-binding ligands with target protein. Biochem J. 1995;306(Pt 3):657–665. doi: 10.1042/bj3060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Kastan MB. Analyzing cell cycle checkpoints after ionizing radiation. Methods Mol Biol. 2004;281:283–292. doi: 10.1385/1-59259-811-0:283. [DOI] [PubMed] [Google Scholar]
- Xu B, Kim S, Kastan MB. Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol. 2001;21:3445–3450. doi: 10.1128/MCB.21.10.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Black CP, Cowan KH. Irradiation-induced G2/M checkpoint response requires ERK1/2 activation. Oncogene. 2007;26:4689–4698. doi: 10.1038/sj.onc.1210268. [DOI] [PubMed] [Google Scholar]
- Yan Y, Mumby MC. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G(1) cyclin-dependent kinases. J Biol Chem. 1999;274:31917–31924. doi: 10.1074/jbc.274.45.31917. [DOI] [PubMed] [Google Scholar]
- Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH. BRCA1- mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene. 2005;24:3285–3296. doi: 10.1038/sj.onc.1208492. [DOI] [PubMed] [Google Scholar]
- Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.