Abstract
Background
This phase Ib study was designed to determine the maximum tolerated doses (MTD) and dose limiting toxicities (DLTs) of irinotecan and cetuximab with sorafenib. Secondary objectives included characterizing the pharmacokinetics and pharmacodynamics and evaluating preliminary antitumor activity in patients with advanced colorectal cancer (CRC).
Methods
Patients with metastatic, pretreated CRC were treated at five dose levels.
Results
Eighteen patients were recruited with median age 56.5 years. In the first five patients treated, 2 irinotecan related DLTs were observed. With reduced dose intensity irinotecan, there were no further DLTs. The most common toxicities were diarrhea, nausea/vomiting, fatigue, anorexia and rash. DLTs included neutropenia and thrombocytopenia. Two patients had partial responses (one with a KRAS mutation) and 8 had stable disease (8–36 weeks). The median progression free survival (PFS) and overall survival (OS) were 2.5 and 4.7 months respectively. Pharmacokinetic analyses suggest sorafenib and metabolite exposure correlate with OS and DLTs.
Conclusions
The recommended phase II dose (RP2D) is irinotecan 100mg/m2 i.v. days 1, 8; cetuximab 400mg/m2 i.v. days 1 and 250mg/m2 i.v. weekly; and sorafenib 400mg orally twice daily in advanced, pretreated CRC. The combination resulted in a modest response rate.
Keywords: sorafenib, cetuximab, irinotecan, pharmacokinetics, colorectal cancer
Introduction
Colorectal cancer (CRC) carcinogenesis and progression are characterized by aberrant activation and interaction of multiple pathways including the mitogen activated protein kinase (MAPK) and vascular endothelial growth factor (VEGF) pathways [1-3]. Cetuximab, a chimeric mouse-human monoclonal antibody directed against the epidermal growth factor receptor (EGFR), has been shown to improve outcomes in patients with KRAS wild type (wt) tumors with or without chemotherapeutic agents such as irinotecan, including those previously refractory to the latter agent [4-7].
However, activating mutations in the KRAS gene coding for a GTPase immediately downstream to EGFR in the MAPK pathway, seen in 30 – 40% of all CRC patients is a negative predictive factor for therapy with EGFR monoclonal antibodies with near 100% predictive value [8-12]. Furthermore, attempts at improving outcomes by combining bevacizumab, a monoclonal antibody against VEGF with cetuximab containing regimens have been unsuccessful [13,14].
Sorafenib, a multi-tyrosine kinase inhibitor, is a potent inhibitor of kinases downstream of Ras in the MAPK pathway such as C-Raf and B-Raf. In addition, it also inhibits multiple other kinases important in carcinogenesis, including VEGF receptor (VEGFR)-2, VEGFR-3 and platelet-derived growth factor (PDGF) receptor beta [15]. Sorafenib has been shown to have activity in preclinical models of colorectal cancer [15,16] and preclinical data shows that it has synergistic activity with cetuximab; emerging clinical evidence suggests promising clinical activity of sorafenib when combined with irinotecan in CRC, including those with KRAS mutated tumors [17,18].
The main objectives of this study were to determine the maximum tolerated dose (MTD) and describe the toxicity spectrum of sorafenib in combination with cetuximab and irinotecan in patients with advanced colorectal cancer. Other objectives were to characterize pharmacokinetics of this combination, to determine the pharmacodynamic effects of the combination in skin and tumor biopsies, and to define preliminary antitumor activity.
Patients and Methods
Eligibility criteria
Eligible patients had histologically confirmed advanced or metastatic CRC with progression after at least first line therapy, evaluable or measurable disease by RECIST (Response Evaluation Criteria in Solid Tumors) [19] and Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Patients also had to be 18 years or older with adequate hematopoietic, hepatic and kidney function. Exclusion criteria included: treatment with radiotherapy or chemotherapy within 4 weeks of study entry; prior therapy with targeted agents against the MAPK pathway; known central nervous system metastases; HIV positive patients on antiretroviral therapy; any medical condition that would impair administration of an oral medication; and clinically significant bleeding diathesis.
The protocol (ClinicalTrials.gov Identifier: NCT00134069) was approved by the institutional review boards of participating institutions, and written informed consent was obtained for all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Drug administration and dose-escalation procedures
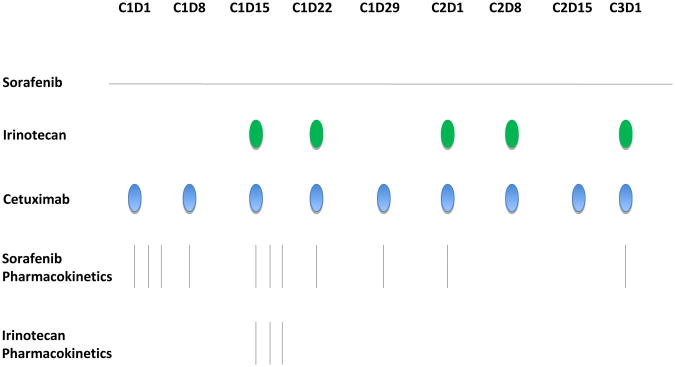
Cetuximab and irinotecan are FDA approved for metastatic CRC and are commercially available while sorafenib was provided by the Cancer Therapy Evaluation Program (CTEP). Initially, patients were treated with oral sorafenib in escalating doses up to a maximum of 400 mg po bid, cetuximab 250 mg/m2 intravenously weekly over 60 minutes (following a loading dose of 400 mg/m2 on day 1 of week 1 over 120 minutes) and irinotecan 120 mg/m2 i.v. over 90 minutes on days 1, 8, 15, 22 of a 42 day cycle based on prior studies (figure 1). After the first 5 patients, the study was amended to a 3-week cycle with irinotecan 100 mg/m2 i.v. over 90 minutes days 1 and 8 without changing the other agents. In both portions of the study, there was a lead-in with cetuximab and sorafenib for 2 weeks in cycle 1.
Figure 1. Dosing and pharmacokinetic sampling schedule of sorafenib, irinotecan, and cetuximab schedule.
The combination was explored in successive cohorts of 3-6 patients each with a standard 3+3 design. Intra-patient dose escalation was not permitted. DLTs were determined during the first cycle of each dose level and were defined as any treatment-related ≥ grade 3 non-hematological toxicity, ≥ grade 4 hematological toxicity, or persistent nausea, vomiting and diarrhea in spite of adequate supportive care.
Accrual was held twice during the study: after the first five patients to amend to a reduced dose of irinotecan, and then as a mandatory hold from CTEP in 2008 due to emerging KRAS data with anti-EGFR antibodies.
Clinical evaluation and safety assessment
Patients underwent history and physical examination, performance status assessment and vital signs, complete blood count (CBC), chemistries, coagulation parameters, urinalysis, tumor markers, and EKG at baseline. Serum pregnancy test and baseline tumor measurements (within 4 weeks of initiation of therapy) were also obtained. On study, patients underwent weekly evaluations. Adverse events were classified/graded weekly according to the Common Terminology Criteria of Adverse Events, version 3.0. Response was assessed after the first cycle (including the 2-week lead-in period) and every 2 cycles thereafter.
Pharmacokinetic and pharmacodynamic studies
Sorafenib pharmacokinetics were evaluated during cycle 1 when administered in combination with cetuximab or in combination with cetuximab and irinotecan (Figure 1). Total sorafenib and sorafenib N-oxide concentrations in plasma were quantitated using a validated analytical assay consisting of a high-performance liquid chromatographic with tandem mass spectrometric detection [20,21]. The sorafenib fraction unbound (Fu) in patient plasma was determined using the method for the equilibrium dialysis[22]. The unbound sorafenib concentration was calculated as Fu × total sorafenib concentration. Unbound sorafenib N-oxide was not determined. Irinotecan pharmacokinetics were evaluated during cycle 1 in combination with sorafenib (day 15). Plasma samples were analyzed for irinotecan and its metabolites using a modified HPLC technique with fluorescence detection as previously described [23]. Pharmacokinetic variables were calculated by standard noncompartmental methods using WinNonlin professional (version 5.3) as previously described for sorafenib [24] and irinotecan [23].
Pharmacodynamic analysis was performed on optional, paired radiologic guided fine needle aspiration core tumor and/or skin punch biopsies. Full methods are included in supplementary materials.
Statistical analysis
Patients who were compliant and with complete pharmacokinetic sampling were considered evaluable for pharmacokinetic analysis and were included in the descriptive statistics. After testing for normality in parameter value distribution, univariate linear-regression analysis was used to assess the relation between age, body-size indices, human α1-acid glycoprotein, albumin, or total bilirubin and sorafenib exposure. Pearson's correlation coefficient or Mann–Whitney U-tests were used to assess correlations between exposure (Cmax, Cmin,ss, or AUC) and toxicity and exploratory PD end points. These tests were performed using JMP Statistical Discovery software (version 7.0.1; SAS Institute, Cary, NC, USA). All P-values were two-sided, not adjusted for multiple comparisons, and were considered significant at a P>0.05.
Results
Patient Demographics and Treatment
Eighteen patients with previously treated metastatic colorectal cancer (16 colon cancer: 2 rectal cancer) were enrolled from August 2006 to September 2010 (tables 1 and 2). Of the 18 patients, 12 were men and 6 were women with a median age of 56.5 years (range 38-75 years). All patients received a median of 2.5 (range 1-6) prior systemic chemotherapy regimens. Of these, 12 patients had received prior irinotecan while 14 had received bevacizumab. KRAS status was unknown in the first 6 patients enrolled, prior to the establishment of predictive significance of this marker; in the remaining 12 patients, 6 had KRAS WT and another 6 had KRAS-mutated tumors per testing done on fresh or archival tumor tissue evaluated for mutations in codons 12 and 13 of exon 2 of this gene. A total of 60 cycles were administered and the median number of cycles per patient was 3 (range, 1-14). Reasons for study discontinuation were progressive disease in 78% (14 patients), DLT with progression while recovering from toxicity in 5% (1) and withdrawal of consent in 17% (3). All three patients who withdrew consent had stable disease and two patients had fatigue and the third requested a treatment break citing cumulative toxicities.
Table 1. Baseline demographics and patient characteristics.
| Characteristic | n | % |
|---|---|---|
|
| ||
| Sex | ||
| Male | 12 | 67 |
| Female | 6 | 33 |
|
| ||
| Age, years | ||
| Median | 56.5 | |
| Range | 38 - 75 | |
|
| ||
| ECOG performance status | ||
| 0 | 6 | 33 |
| 1 | 12 | 67 |
|
| ||
| KRAS Status | ||
| Wild type | 6 | 33 |
| Mutated | 6 | 33 |
| Unknown | 6 | 33 |
|
| ||
| Previous lines of systemic therapies | ||
| Median | 2.5 | |
| Range | 1 - 6 | |
|
| ||
| Previous therapy | ||
| Chemotherapy | 18 | 100 |
| Radiotherapy | 7 | 39 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group;
Table 2. Patient dosing and DLT assessment.
| Pre-amendment at original planned dose of I; cycle = 6 weeks* | ||||||
|---|---|---|---|---|---|---|
| Dose cohort | Irinotecan (mg/m2 on d1,8,15,22) | Sorafenib (mg) | Cetuximab (mg/m2 weekly)** | N | Median no of completed cycles (range) | DLT |
| 1 | 120 | 200 QD | 400 LD/ 250MD | 4 | 2 (1-3) | 2 |
| -1 | 120 | 200 QOD | 400 LD/ 250MD | 1 | 2 (2) | 0 |
| Post-amendment at reduced dose of I; cycle = 3 weeks* | ||||||
| Dose cohort | Irinotecan (mg/m2 on d1,8) | Sorafenib (mg) | Cetuximab (mg/m2 weekly) | N | Median no of completed cycles (range) | DLT |
| 1A | 100 | 200 QD | 400 LD/ 250MD | 3 | 2 (1-2) | 0 |
| 2 | 100 | 200 BID | 400 LD/ 250MD | 3 | 1-14 | 0 |
| 3*** | 100 | 400 BID | 400 LD/ 250MD | 7 | 1,4 (1-12) | 0 |
1st cycle with an additional 2 week lead-in with S, C only
LD = Loading Dose; MD = Maintenance Dose
Recommended Phase II Dose
Dose escalation and determination of recommended phase II dose
Five patients were treated per the original protocol dosing schedule (table 2). One patient experienced a DLT of severe grade 3 fatigue after 6 weeks and was hospitalized and eventually had progression of disease prior to recovery from the fatigue (table 3). In addition, another patient on that dose level experienced a drug-related DLT of febrile neutropenia after 8 weeks of treatment. Since he was the second DLT out of 3 subjects, dose level -1 was explored. Another patient started treatment at dose level 1 prior to the 2nd DLT occurrence and developed grade 3 diarrhea during cycle 2 requiring treatment with multiple anti-diarrheal agents and dose reductions. Thus, three of four subjects experienced grade ≥3 toxicities including 2 DLTs at dose level 1, all of which were likely related to the standard drug irinotecan since the diarrhea occurred only after irinotecan was started. Therefore, the protocol was amended to reduce irinotecan dose intensity, and additional dose levels with escalating doses of sorafenib (up to 400 mg bid) were examined without any further DLTs (table 2). Further dose escalation beyond this level was halted as pre-specified in the protocol and irinotecan 100 mg/m2 iv days 1, 8, cetuximab 400 mg/m2 iv day 1 followed by 250 mg/m2 weekly and sorafenib 400 mg po bid was deemed RP2D. At this dose, an expanded cohort of patients (n=7) was treated to further characterize its toxicities.
Table 3. All treatment-related toxicities with ≥ 15% incidence according to cohort.
| Cohort(n) | 1 (4) | -1 (1) | 1A (3) | 2 (3) | 3 (7) | Total AEs across all | AEs in cycle 1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Grades | G1/2* G3/4* | G1/2* G3/4* | G1/2* G3/4* | G1/2* G3/4* | G1/2* G3/4* | All grade | ≥ G 3 (%) | All grade | ≥ G 3(%) |
| Anemia | 1 | 2 (11) | 1 (6) | 1 (6) | |||||
| Neutropenia | 1 | 1 | 1 | 4 (22) | 2 (11) | 4 (22) | 1 (6) | ||
| Nausea/vomiting | 2 | 1 | 1 | 1 | 4 | 10 (56) | 1 (6) | 7 (39) | |
| Diarrhea | 2 | 1 | 1 | 2 | 5 | 14 (78) | 3 (17) | 11 (61) | 2 (11) |
|
Elevated AST, ALT or Alk phos |
1 1 |
1 | 1 | 1 | 3 (17) | 1 (6) | 3 (17) | 1 (6) | |
| Fistula | 1 (6) | 1 (6) | |||||||
| Obstruction | 1 | 1 (6) | 1 (6) | ||||||
| Mucositis | 2 | 1 | 1 | 1 | 4 (22) | 3 (17) | |||
| Change in Taste | 2 | 1 | 3 (17) | 1 (6) | |||||
| Dehydration | 1 | 1 | 3 (17) | 1 (6) | 2 (11) | ||||
| Fatigue | 1 | 1 | 1 | 2 | 3 | 10 (56) | 2 (11) | 9 (50) | 2 (11) |
| Anorexia / weight loss | 2# 3 | 1 | 2 | 3 | 9 (50) | 6(33) | |||
| Hypophosphatemia | 1 | 2 | 5 (28) | 2 (11) | 3 (17) | ||||
| Hypomagnesemia | 2 | 4 (22) | 2 (11) | 3 (17) | 1 (6) | ||||
| Hypokalemia | 1 | 1 | 1 | 5 (28) | 3 (17) | 4 (22) | 1 (6) | ||
| Rash | 1 | 1 | 3 | 1 2 | 2 6 | 13 (72) | 12(67) | ||
| Tetany | 1 | 1 (6) | 1 (6) | ||||||
| Pain | 1 | 2 | 3 (17) | 3 (17) | |||||
Toxicities with possible, probable or definite relation to study treatment and graded per NCI CTCAE version 3.
Note: Patients with a recurrent AE counted only once at higher grade for final AE analysis but lower grade cycle 1 AE also denoted above.
DLTs
Abbreviations: ALT, alanine amino-transferase; AST, aspartate amino-transferase
Safety
The most common treatment related toxicities across all cohorts were diarrhea in 78% (14 patients), rash in 72% (13 patients), nausea/vomiting in 56% (10 patients) and anorexia in 50% 9 patients; table 3). All grade 3 or 4 toxicities included diarrhea (3), hypokalemia (3), fatigue (2), fever (2), hypomagnesemia (2), hypophosphatemia (2), neutropenia (2), anemia (1), nausea/ vomiting (1), small bowel obstruction (1), small bowel fistula (1), dehydration (1), elevated liver function tests (1), hyponatremia (1) and tetany (1). Grade 4 toxicities included febrile neutropenia (1) and small bowel fistula (1). Overall, a total of seven grade 3 or greater toxicities over 10 cycles were observed in the 5 patients treated (including 2 DLTs) at the higher dose of irinotecan as compared to thirteen grade ≥ 3 toxicities over 50 cycles in 13 patients treated at a reduced dose after protocol amendment. Of these 13 grade ≥ 3 toxicities, six were electrolyte abnormalities including hypophosphatemia and hypokalemia (2 each), hyponatremia and hypomagnesemia (1 each). One patient with hypomagnesemia and hypocalcemia developed symptomatic grade 3 tetany. Twenty-two percent of patients (4 patients) had neutropenia, half being ≥ grade 3, and were all prior to amending the protocol to reduce the dose of irinotecan. No further episodes of neutropenia were observed in the cohorts treated with lower dose of irinotecan.
At dose level 1A, multiple grade ≥ 3 toxicities including diarrhea (1), nausea/vomiting (1), intestinal fistula and bowel obstruction were noted. In subsequent cohorts, there was a higher incidence of grade 1-2 nausea/vomiting and diarrhea along with one patient who had grade 3 diarrhea. A total of 13 patients developed rash with increased frequency in later cohorts that were dosed at a higher initial doses of sorafenib but this did not correlate with overall drug exposure.
Pharmacokinetic and pharmacodynamic studies
All patients were evaluable for pharmacokinetic analysis of both sorafenib and irinotecan (table 4). Consistent with previous reports, total sorafenib exhibited large variability with a plasma concentration-time profile exhibiting slow absorption and long elimination on both days 1 and 15 [25]. The rate of conversion of sorafenib to the N-oxide metabolite was variable ranging from 0-29% and was more variable than previously reported [24,26,27]. Sorafenib was extensively bound to plasma proteins with an unbound fraction (%) ranging from 0.27-0.67%, which was more variable than previously reported [24]. There was no difference in dose-normalized exposure (Cmax, AUC0-8) when sorafenib was administered across dose levels and the accumulation noted on day 15 is consistent with previous reports. There was no alteration in sorafenib N-oxide conversion (p=0.13) or unbound fraction (p=0.83). Age, body-size indices, gender, and pre-treatment AAG, HSA, or total bilirubin concentrations did not correlate with tot al or unbound sorafenib exposure (p>0.05; data not shown). Irinotecan and metabolite exposure was highly variable and consistent with previously reported literature [28]. Correlations were observed with the irinotecan metabolites and side effects including more severe fatigue being correlated with higher SN-38 exposure (AUClast, p = 0.03) and increased nausea/vomiting being correlated with higher APC exposure (Cmax, p=0.03;AUClast, p = 0.04; AUClast ratio, p=0.03). The 2 patients who experience DLTs did have higher maximum irinotecan exposure (Cmax, p=0.05) and higher total sorafenib and sum of total sorafenib and sorafenib N-oxide Day 1 exposure (AUC0-8h, p=0.04 for both AUC0-8h) compared to patients who did not experience a DLT.
Table 4. Plasma pharmacokinetic parameters of sorafenib and irinotecan PK Parameter1.
| Cohort (n) | Cmax (μg/mL) | Tmax (h) | AUC0-8h (μg*h/mL) | AUC0-8h ratio (%) (metabolite/parent) | Css,min (μg/mL) | Accumulation Factor2 |
|---|---|---|---|---|---|---|
| Total Sorafenib – Day 1 | ||||||
| 1 & 1A (7) | 2.17±0.85 | 4.0 (2.0-23.7) | 10.0±5.7 | N.A. | - | - |
| -1 (1) | 2.10 | 24.1 | 7.4 | N.A. | - | - |
| 2 (3) | 1.53±1.46 | 7.0 (2.0-7.0) | 4.4±3.5 | N.A. | - | - |
| 3 (7) | 1.19±0.54 | 4.0 (2.0-7.0) | 4.9±2.3 | N.A. | - | - |
| Total Sorafenib – Day 15 | ||||||
| 1 & 1A (7) | 4.67±3.17 | 4.2 (3.9-9.0) | 23.9±17.6 | N.A. | 1.27±0.87 | 1.0±0.5 |
| -1 (1) | 2.20 | 9.0 | 14.4 | N.A. | 2.23 | 1.1 |
| 2 (3) | 4.77±2.53 | 6.0 (0.8-6.1) | 29.7±19.2 | N.A. | 3.48±2.12 | 5.2±4.8 |
| 3 (7) | 4.71±3.52 | 4.1 (0.3-9.0) | 26.1±24.4 | N.A. | 2.89±1.67 | 4.8±2.2 |
|
| ||||||
| Unbound Sorafenib – Day 1 | ||||||
| 1 & 1A (7) | 0.007±0.003 | 4.0 (2.0-6.0) | 0.033±0.014 | 0.33±0.04 | - | - |
| -1 (1) | 0.007 | 48.1 | 0.030 | 0.34 | - | - |
| 2 (3) | 0.005±0.005 | 7.0 (2.0-7.0) | 0.014±0.010 | 0.31±0.02 | - | - |
| 3 (7) | 0.004±0.002 | 4.0 (2.0-7.0) | 0.016±0.008 | 0.32±0.02 | - | - |
| Unbound Sorafenib – Day 15 | ||||||
| 1 & 1A (7) | 0.014±0.009 | 4.2 (3.9-9.0) | 0.080±0.056 | 0.32±0.04 | 0.004±0.003 | 1.0±0.6 |
| -1 (1) | 0.007 | 9.0 | 0.050 | 0.35 | 0.008 | 1.1 |
| 2 (3) | 0.014±0.008 | 6.0 (0.3-6.1) | 0.087±0.047 | 0.29±0.02 | 0.010±0.006 | 5.2±4.9 |
| 3 (7) | 0.015±0.010 | 4.1 (0.3-9.0) | 0.087±0.069 | 0.38±0.13 | 0.009±0.005 | 4.7±2.3 |
|
| ||||||
| Sorafenib N-oxide – Day 1 | ||||||
| 1 & 1A (7) | 0.32±0.22 | 4.0 (2.0-7.0) | 1.44±0.94 | 14.6±8.9 | - | - |
| -1 (1) | 0.24 | 24.1 | 1.06 | 14.2 | - | - |
| 2 (3) | 3 0.11. 0.33 | 2.0, 7.0 | 0.48, 0.54 | 3 6.4, 10.4 | - | - |
| 3 (7) | 0.012±0.010 | 5.0 (2.0-7.0)4 | 0.52±0.43 | 4 9.1±5.8 | - | - |
| Sorafenib N-oxide – Day 15 | ||||||
| 1 & 1A (7) | 0.68±0.62 | 6.0 (3.9-9.1) | 3.66±3.42 | 11.9±6.4 | 0.16±0.13 | 1.0±0.6 |
| -1 (1) | 0.47 | 9.0 | 2.92 | 20.3 | 0.34 | 1.6 |
| 2 (3) | 1.20±1.00 | 6.0 (0.8-6.1) | 7.17±6.66 | 22.1±7.7 | 0.70±0.64 | 2.7, 4.43 |
| 3 (7) | 0.54±0.45 | 6.0 (0.3-9.0) | 3.15±3.42 | 11.4±4.0 | 0.31±0.21 | 6.6±4.0 |
|
| ||||||
| Irinotecan | ||||||
| 1 & -1 (5) | 2617.6±549.8 | 1.4 (0.5-1.8) | 12737±6396 | N.A. | ||
| 1A, 2, & 3 (13) | 1800.1±501.3 | 1.4 (1.0-2.6) | 9084±3326 | N.A. | ||
|
| ||||||
| APC | ||||||
| 1 & -1 (5) | 246.6±191.5 | 2.5 (1.5-4.5) | 2398±2011 | 18.2±8.9 | ||
| 1A, 2, & 3 (13) | 181.0±118.7 | 3.3 (2.0-5.4) | 2120±2030 | 23.3±16.7 | ||
|
| ||||||
| SN-38 | ||||||
| 1 & -1 (5) | 36.8±12.7 | 2.5 (2.0-5.5) | 300±102 | 2.9±2.0 | ||
| 1A, 2, & 3 (13) | 26.8±18.3 | 2.6 (1.6-4.7) | 232±135 | 2.7±1.9 | ||
Data are presented in the table as mean±SD. T max is presented as median(range). If n<3, the actual values are reported.
Accumulation factor was calculated from the ratio of Cmin,ss/Cmin, first dose
One patient had undetectable sorafenib N-oxide concentrations
Abbreviations: AUC0-8h area under the plasma concentration-time curve to 8 hours; AUClast AUC until the last detectable concentration; Cmax peak plasma concentration; Css,min minimal plasma concentration at steady state (average of >day 8 Cmin); N.A. not applicable; Tmax time to peak concentration.
Pharmacodynamic analysis was performed only in a limited number of samples due to technical and size limitations. Results from pharmacodynamic studies are detailed in Supplementary Materials.
Antitumor Activity
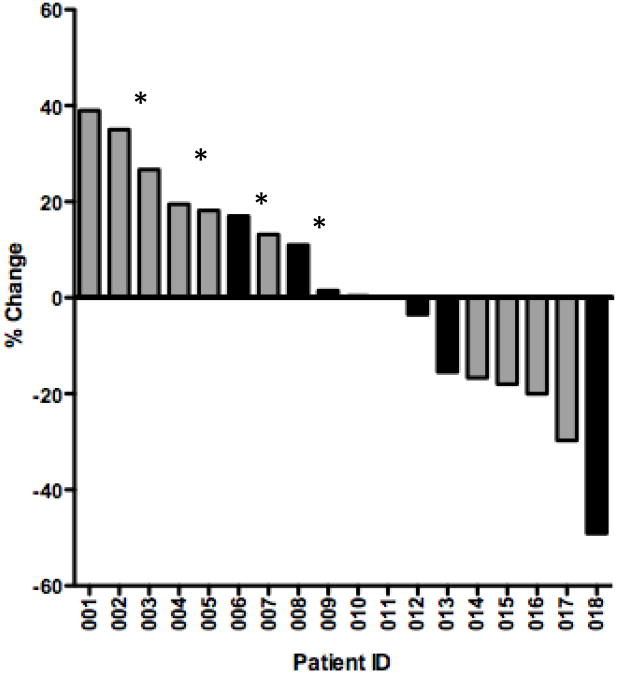
All 18 patients in this trial were included for efficacy analysis (Supplemental Figure 1). Two patients at dose level 2 had partial responses (PR) one of which was KRAS WT and another was KRAS mutation positive. However, the patient with the KRAS WT tumor was previously untreated with either an irinotecan or an EGFR-targeting monoclonal antibody and also had a cavitary lesion that rendered accurate tumor measurements difficult; this patient was on study for 14 cycles and had a confirmed PR. The patient with the KRAS mutated tumor and a PR was previously treated with two different regimens including one with irinotecan before starting the trial. He was treated for a total of four cycles before coming off the study for PD.
Another 8 patients (50%) had stable disease (SD) ranging from 2 – 12 cycles (median 2.5 cycles). Of these 8 patients, 5 stopped treatment due to PD while 3 patients came off study per their preference. One KRAS mutated patient had prolonged stable disease for 12 cycles before he chose to come off study due to fatigue and a desire to take a prolonged break from chemotherapy. The median overall survival (OS) rate was 4.7 months ranging from 3.1 months to NR (not reached; 3 patients are still alive). Overall survival did correlate with day 1 exposure (AUC0-8h) for total sorafenib (p=0.05) and the sum of total sorafenib and sorafenib N-oxide (p= 0.04) but not for sorafenib N-oxide alone (p=0.07) or unbound sorafenib (p=0.06).
Discussion
The objectives of this phase Ib trial were to establish the MTD, toxicity profile of cetuximab and irinotecan in combination with sorafenib and to define the pharmacokinetic and pharmacodynamic parameters of this regimen. The initial starting dose of irinotecan at 120 mg/m2 i.v. over 90 minutes on days 1, 8, 15, 22 of a 42 was associated with intolerable toxicities. After protocol amendment, this regimen was better tolerated achieving a recommended phase II dose: irinotecan 100 mg/m2 i.v. days 1, 8; cetuximab 400 mg/m2 i.v. days 1 and 250 mg/m2 weekly; and sorafenib 400 mg orally bid of a 3 week cycle. Although there was no difference in exposure to sorafenib between dose levels 2 and 3 (which could be explained by the large variability in sorafenib pharmacokinetics as discussed below), this higher dose of sorafenib is consistent with findings of other sorafenib combination studies in CRC including one with oxaliplatin [29] and two with irinotecan [18,30]. Data from these studies also suggests increased activity of sorafenib at 400 mg po bid, further supporting the current study's RP2D. However, it should be noted that in both the studies combining sorafenib with irinotecan, dose intensity of irinotecan was higher: 180 mg/m2 every 2 weeks or 125-140 mg/m2 days 1, 8, 15 and 22 of a 6 week cycle.
The reasons for decreased tolerability to irinotecan in the current study are unclear. One reason may be increased exposure due to a drug interaction from the CYP3A4 or glucuronidation pathways involved in the elimination of both irinotecan and sorafenib. Increased exposure to irinotecan and SN38 was noted at sorafenib doses greater than 400 mg BID that could be related to UGT1A1 inhibition by sorafenib [18]. However, this increased exposure to irinotecan and its metabolite was not associated with increased toxicity [18]. Our study did not reproduce these findings, although this may be related to the fact that the dose intensity of irinotecan was less. Total sorafenib exposure was consistent with previously reported literature. However, there was large variability in active N-oxide metabolite and unbound drug exposure, which could contribute to the intolerance. Indeed, the 2 patients who experienced DLTs did have the highest sorafenib and the sum of total sorafenib and sorafenib N-oxide day 1 exposure in addition to higher irinotecan exposure. Genetic polymorphisms in UGT1A1 were not assessed as a potential contributing factor in toxicity to irinotecan [31] and sorafenib [32].
In this previously treated patient population, we observed two PRs, a median PFS of 2.4 months, and median overall survival (OS) of 4.7 months. This is the first report to correlate both sorafenib and the sum of total sorafenib and sorafenib N-oxide exposure to a clinical endpoint, in this case OS, though our study did have small numbers and the results were not corrected for multiple correlations. A recently concluded phase II trial of sorafenib in combination with irinotecan in 54 patients with KRAS mutated, irinotecan refractory, metastatic CRC showed a disease control rate of 64.9% with median progression free survival and overall survival of 3.5 and 7.7 months respectively [30]. Another phase I trial of 34 patients (16 irinotecan refractory) with 23 CRC patients, disease control rate was 60% during the initial dose escalation phase (20 patients) and 77% in the CRC expansion cohort (14 patients) [18]. In the current trial, of the 10 patients with either SD or PR, 7 had received prior treatment with irinotecan. The results of these trials suggest a potential role for multi-tyrosine kinase inhibitors such as sorafenib in reversal of irinotecan resistance including those with KRAS mutated tumors.
In spite of such suggestions of activity of sorafenib in CRC, convincing evidence of benefit, especially from randomized trials is lacking. In contrast, results from a planned interim analysis of the randomized, double-blind, placebo controlled, phase III trial evaluating regorafenib in metastatic, chemo-refractory CRC patients show promising activity for this agent with significant improvement in OS [33]. Regorafenib is a novel, multi-tyrosine kinase inhibitor that differs from sorafenib only by a single fluorine atom which is thought to confer additional anti-angiogenic activity over the latter [34]. Thus, regorafenib may well replace sorafenib as the oral multi-tyrosine kinase inhibitor of choice in future CRC trials.
In summary, the dose of irinotecan in this phase Ib trial of sorafenib, cetuximab and irinotecan was lower than in other combination trials due to unclear reasons. Severe irinotecan related toxicity necessitated a protocol amendment for dose reduction. There was suggestion of clinical efficacy but our trial did not outperform other reported studies of irinotecan and sorafenib, and further advancement of this combination is not planned.
Supplementary Material
Supplementary Figure 1: Immunohistochemical pharmacodynamic analyses of paired pre-treatment and day 15 skin biopsies for EGFR (1A), p-Akt/t-Akt (1B) and p-ERK/t-ERK (1C). EGF/PDGF pathways gene array on paired pre-treatment and day 15 tumor biopsies are shown in figure 1D.
Supplemental Figure 2: EGF/PDGF pathways gene array on 3 individual patients' paired pre-treatment and day 15 tumor biopsies (figures 2A, 2B, 2C)
Figure 2.
Waterfall plot (2A) showing response with patients who underwent prior irinotecan therapy denoted in grey. Patients with progressive disease due to development of new lesions are marked with an asterisk. PFS (days) is shown in figure 2B.
Acknowledgments
The authors would like to thank Sharyn Baker for helpful scientific discussions. The authors would also like to thank the patients and their families for participating in the study.
Funding: This research was supported by NIH/NCI grant 1K23CA115500 (WM), 1R21CA117125 (WM), U01 CA070095 (MC), and the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005). This publication was made possible by Grant Number UL1RR025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Note: Presented in part at the American Society of Clinical Oncology (ASCO) Annual Meeting, June 2008, Chicago, IL and ASCO Gastrointestinal Oncology Meeting, January 2011, San Francisco, CA
Disclosure of Potential Conflicts of Interest: Dr. Messersmith has received commercial clinical research grant support from Bayer (major) via University of Colorado Cancer Center.
References
- 1.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6(5):322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55(18):3964–3968. [PubMed] [Google Scholar]
- 3.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281(2):951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 4.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 5.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(14):2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, Roh JK, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 8.Brink M, de Goeij AF, Weijenberg MP, Roemen GM, Lentjes MH, Pachen MM, Smits KM, de Bruine AP, Goldbohm RA, van den Brandt PA. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003;24(4):703–710. doi: 10.1093/carcin/bgg009. [DOI] [PubMed] [Google Scholar]
- 9.Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1193–1197. [PubMed] [Google Scholar]
- 10.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 11.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 12.Dasari A, Messersmith WA. New strategies in colorectal cancer: biomarkers of response to epidermal growth factor receptor monoclonal antibodies and potential therapeutic targets in phosphoinositide 3-kinase and mitogen-activated protein kinase pathways. Clin Cancer Res. 2010;16(15):3811–3818. doi: 10.1158/1078-0432.CCR-09-2283. [DOI] [PubMed] [Google Scholar]
- 13.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 14.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, Richel DJ, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm S, Housley T, Rong H, et al. The novel Raf inhibitor BAY 43-9006 blocks signaling and proliferation in BRAF mutant and wildtype melanoma and colorectal tumor cell lines. 44:106609. [Google Scholar]
- 17.Martinelli E, Troiani T, Morgillo F, Rodolico G, Vitagliano D, Morelli MP, Tuccillo C, Vecchione L, Capasso A, Orditura M, De Vita F, et al. Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin Cancer Res. 2010;16(20):4990–5001. doi: 10.1158/1078-0432.CCR-10-0923. [DOI] [PubMed] [Google Scholar]
- 18.Mross K, Steinbild S, Baas F, Gmehling D, Radtke M, Voliotis D, Brendel E, Christensen O, Unger C. Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer. 2007;43(1):55–63. doi: 10.1016/j.ejca.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zhao M, Navid F, Pratz K, Smith BD, Rudek MA, Baker SD. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(29):3033–3038. doi: 10.1016/j.jchromb.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, Smith BD, Messersmith WA, Hidalgo M, Baker SD. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846(1-2):1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Villarroel MC, Pratz KW, Xu L, Wright JJ, Smith BD, Rudek MA. Plasma protein binding of sorafenib, a multi kinase inhibitor: in vitro and in cancer patients. Invest New Drugs. 2011 doi: 10.1007/s10637-011-9767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimeno A, Rudek MA, Purcell T, Laheru DA, Messersmith WA, Dancey J, Carducci MA, Baker SD, Hidalgo M, Donehower RC. Phase I and pharmacokinetic study of UCN-01 in combination with irinotecan in patients with solid tumors. Cancer Chemother Pharmacol. 2008;61(3):423–433. doi: 10.1007/s00280-007-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, Stine A, Zhao M, Baker SD, Carducci MA, Wright JJ, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 26.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, Heym KM, Christensen R, Onciu M, Shurtleff SA, Pounds SB, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29(24):3293–3300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57(5):685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 28.van Erp NP, Baker SD, Zhao M, Rudek MA, Guchelaar HJ, Nortier JW, Sparreboom A, Gelderblom H. Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin Cancer Res. 2005;11(21):7800–7806. doi: 10.1158/1078-0432.CCR-05-1288. [DOI] [PubMed] [Google Scholar]
- 29.Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, Scheulen ME, Christensen O, Brendel E, Schwartz B, Hofstra E, et al. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer. 2005;5(3):188–196. doi: 10.3816/ccc.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 30.Ychou M, Bouche O, Thezenas S, Francois E, Adenis A, Bennouna J, Taieb J, Desseigne F, Seitz J, Conroy T, Galais M, et al. Final results of a multicenter phase II trial assessing sorafenib (S) in combination with irinotecan (i) as second- or later-line treatment in metastatic colorectal cancer (mCRC) patients (pts) with KRAS-mutated tumors (mt; NEXIRI) ASCO Meeting Abstracts. 2011;29(15_suppl):e14002. [Google Scholar]
- 31.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(8):1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 32.Peer CJ, Sissung TM, Kim A, Jain L, Woo S, Gardner ER, Kirkland CT, Troutman SM, English BC, Richardson ED, Federspiel J, et al. Sorafenib Is an Inhibitor of UGT1A1 but Is Metabolized by UGT1A9: Implications of Genetic Variants on Pharmacokinetics and Hyperbilirubinemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-11-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Lenz HJ, Yoshino T, Cihon F, Wagner A, Van Cutsem E on behalf of the CORRECT Study Team. Results of a phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. ASCO Meeting Abstracts. 2012;30(4_suppl):LBA385. [Google Scholar]
- 34.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Immunohistochemical pharmacodynamic analyses of paired pre-treatment and day 15 skin biopsies for EGFR (1A), p-Akt/t-Akt (1B) and p-ERK/t-ERK (1C). EGF/PDGF pathways gene array on paired pre-treatment and day 15 tumor biopsies are shown in figure 1D.
Supplemental Figure 2: EGF/PDGF pathways gene array on 3 individual patients' paired pre-treatment and day 15 tumor biopsies (figures 2A, 2B, 2C)