Abstract
Background and Purpose
Memory impairment is both a predictor and a consequence of stroke, but memory decline is common even in healthy elderly. We compared the long-term trajectory of memory functioning before and after stroke to memory change in stroke-free elderly.
Methods
Health and Retirement Study participants age 50+ (n=17,340) with no stroke history at baseline were interviewed biennially up to 10 years for first self- or proxy-reported stroke (n=1,574). Age-, sex-, and race- adjusted segmented linear regression models were used to compare annual rates of change in a composite memory score before and after stroke among three groups: 1,189 stroke survivors; 385 stroke decedents; and 15,766 cohort members who remained stroke-free.
Results
Before stroke onset, individuals who later survived stroke had significantly (p<0.001) faster average annual rate of memory decline (-0.143 points/year) than those who remained stroke-free throughout follow-up (-0.101 points/year). Stroke decedents had even faster pre-stroke memory decline (-0.212 points/year). At stroke onset, memory declined an average of -0.369 points among stroke survivors, comparable to 3.7 years of age-related decline in stroke-free cohort members. Following stroke, memory in stroke survivors continued to decline at -0.142 points/year, similar to their pre-stroke rate (p=0.93). Approximately 50% of the memory difference between stroke survivors shortly after stroke and age-matched stroke-free individuals was attributable to pre-stroke memory.
Conclusions
Although stroke onset induced large decrements in memory, memory differences were apparent years before stroke. Memory declines before stroke, especially among those who did not survive the stroke, were faster than declines among stroke-free adults.
Keywords: Memory functioning change, memory impairment, stroke
Introduction
Several cross-sectional and longitudinal studies report that prevalence of dementia and cognitive impairment is higher among stroke survivors than age-matched stroke-free adults1-4. Because cognitive impairment is an established predictor of stroke incidence5-7, the high dementia rates among stroke survivors likely partially reflect pre-stroke cognitive differences8. Memory functioning typically declines with age even among elderly with no clinical stroke, and little is known about differential long-term rates of change in memory score among stroke patients. Few cohorts have appropriate prospective data with the large number of events needed to examine longitudinal changes in memory prior to and after stroke. Accumulating evidence indicates that acute stroke follows a long period of accumulating cerebrovascular injury, possibly associated with subtle ischemic injury, silent strokes9, and stroke symptoms1, 10-12. Our analysis builds on prior work by examining the trajectory of memory functioning prior to stroke and after stroke over a long-term follow-up. Carefully describing these trajectories is critical not only to show the natural development of memory in stroke patients but also to help identify pre-stroke risk factors that remediate or exacerbate the consequences of stroke.
In the current study, we used 10 years of data from a nationally representative cohort, the Health and Retirement Study (HRS)13, to describe the long-term pre- and post-stroke trajectory of memory functioning among stroke survivors. We compared memory changes in stroke survivors to memory changes in individuals who died shortly after stroke and to memory changes among cohort participants who remained stroke-free.
Methods
Study population
HRS is a nationally representative cohort study initiated in 1992 with additional enrollments in 1993, 1998 and biennial interviews.14, 15 HRS is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. Our analyses used the 1998 survey as baseline and included follow-up data through 2008. Analyses were weighted to represent non-institutionalized Americans aged 50+ in 1998.
From 20,567 HRS participants aged 50+ in 1998, we restricted to 18,987 (92.3%) who were stroke-free at baseline; we additionally excluded respondents with missing cognitive scores (n=1538) or missing risk factor information (n=109), for a final analytic sample of 17,340 (91.3%).
Dependent variable
Memory was assessed by immediate and (approximately 5-minute) delayed recall tests of a 10-word list of common nouns read aloud. Additionally, for individuals who were too impaired to directly participate in memory assessments, proxy informants, typically spouses, were asked to assess the participants' memory on a 5-item Likert scale and completed a 16-item version of the Informant Questionnaire for Cognitive Decline (IQCODE)16, 17. The validity and reliability of these measures have been documented elsewhere18. We previously developed a composite memory score combining proxy and direct memory assessments for longitudinal analyses (Wu et al., under review). The composite score algorithm was developed in a subsample of 856 HRS cohort members who participated in a comprehensive neuropsychological battery as part of the Aging, Demographics, and Memory Study (ADAMS)19, 20. Briefly, this approach involved deriving scores for all ADAMS participants, and then applying regression models to calibrate the brief assessments available in the full cohort to predict the ADAMS outcomes. This calibration was then applied to derive a composite memory score for all HRS respondents at each wave. Further details of development and validation of the memory score are available from the authors. The composite memory score was standardized by dividing each score by the baseline (1998) standard deviation so that every unit change in composite memory score corresponds to a change of one standard deviation at baseline. The standardized score ranged from -5.5 to 5.9 and each decade of age was associated with approximately a 1-point lower score at baseline.
Independent variable
Incidence of stroke was defined as self-reported doctor's diagnosis of stroke drawn from the question “Has a doctor ever told you that you had a stroke?” When patients were deceased or unavailable for direct interview, HRS used proxy informants (usually patients' spouses) to report on stroke status. Participants or proxies reporting stroke (n=1,574) also reported the month and year of event. A small number of participants did not report the precise month (n=131, 8.3%) or year (n=166, 10.5%) of event between the biennial interview waves. To retain these people in analyses, we used the midpoint of the last known stroke-free date and the date when the stroke was first reported, based on all available biennial interviews and any date information provided. Because our study focused on the effect of first stroke onset, only the first stroke during follow-up was identified; many stroke survivors may have had subsequent strokes but we consider all memory reports after the date of their first stroke as “post-stroke”.
We categorized participants into three groups: 1) stroke survivors, who reported a first stroke during the follow-up period and survived to provide at least one subsequent memory assessment score (n=1,189); 2) stroke decedents, who experienced (per proxy-report) a first stroke during the follow-up period and died prior to their next interview (n=385); 3) stroke-free subjects (n=15,766), for whom no stroke was reported during follow-up. For all respondents, follow-up continued through the last interview wave in 2008, the respondent's death, or respondent's attrition from the sample. We included individuals in the category of “stroke decedents” regardless of whether the primary cause of death was stroke. However, we consider that for most individuals who died within two years after stroke, the stroke would have been an important contributor to cause of death.
The primary exposures were the timing of memory assessment with respect to stroke, i.e., number of years before and number of years after stroke. We defined the year of stroke onset as time zero and, for each memory assessment, calculated the years until stroke (to describe the pre-stroke memory trajectory) or the years since stroke (to describe the post-stroke memory trajectory).
Other Covariates
All models were adjusted for age at each interview (centered at 75 years) and other covariates defined at the baseline interview, including demographic variables: race (white vs other), gender, marital status (married/partnered, separated/divorced/widowed, never married), birth place (born in the Northeast, Midwest, South, West, or outside the US); and socioeconomic status indicators, including height21, years of education (0-17), mother's years of education (0-17), and natural log of household wealth. Continuous variables were centered at the mean and the reference categories for categorical variables were chosen as the most common category (married/partnered white women born in the Midwest). The missing indicator method was used for 10.4% of participants missing information on “mother's education.”
Statistical analysis
We used segmented linear regression models to describe the longitudinal trajectory of memory score. We estimated memory change over time with both linear and curvilinear trends. To facilitate interpretation of results, we report parameter estimates from linear forms throughout. However, we depict the higher-order time trends visually in figures and describe these patterns. We present results based on three models: the first model included only stroke survivors, while the second and third model sequentially added in stroke decedents and stroke-free subjects.
To describe memory function change of stroke survivors, we specified: a slope for annual rate of memory change prior to non-fatal stroke; a discontinuity term for change in memory function at the time of stroke; and a slope for rate of memory change in the years following non-fatal stroke. This model was adjusted for baseline covariates and age at stroke onset (centered at 75 years), following the analytic model of Mendes de Leon22. In the second model, we included individuals who did not survive stroke and estimated the rate of annual memory change prior to fatal stroke. We compared the pre-stroke slopes for stroke survivors and stroke decedents using an interaction between survivor status and the time prior to stroke variable. In the third model, we included cohort members who remained stroke-free throughout follow-up. We estimated rate of memory change for each additional year of age, centering at age 75. The intercept for this model thus compared predicted memory score in a 75-year-old who never had a stroke to memory in a 75-year-old immediately prior to stroke (full model parameterization is shown in Appendix 1).
For all models, we used generalized linear regression with robust variance estimates to account for repeated measures on the same individual. Models were estimated in SAS 9.2. The HRS sample was drawn from a complex sample design, the design effect estimated from cross-sectional models accounting for the complex design was modest (<20%), therefore sample clustering was ignored in these analyses. Formally incorporating a design effect correction did not affect the statistical significance of any coefficients describing linear rate of memory change, though some quadratic terms (with estimates close to zero) were only marginally statistically significant after design effect correction. All analyses were weighted according to the 1998 sample weights to be representative of the 1998 community-dwelling US population born 1947 or earlier.
In sensitivity analysis, because drop-out after non-fatal stroke onset could lead to underestimation of the post-stroke decline rate for memory score, the third model was iteratively estimated with restriction to stroke survivors who remained in the cohort at least 2 years, 3 years, or 4 years after stroke onset (Appendix 2). Quantile regression models at 25th, 50th and 75th percentiles were conducted to assess sensitivity to outliers or non-interval scaling of the composite memory score (Appendix 3). Finally, we estimated the full model excluding all respondents for whom stroke date was imputed (Appendix 4).
Results
Respondents who remained stroke-free were, on average, younger, more likely to be white, married, with higher own and maternal education and higher wealth compared to respondents who experienced a stroke (whether fatal or non-fatal) (Table 1). Over 10 years of follow-up on 17,340 eligible participants (139,457 person-years): 1189 (6.9%) participants survived a stroke; 385 (2.2%) suffered a stroke and did not survive; and 15,766 (90.9%) experienced no stroke during follow-up (Table 2).
Table 1. Baseline characteristics of Health and Retirement Study participants who were stroke-free in 1998 (n=17,340).
| Participants with 1st incident stroke during follow-up (1998 to 2008) | P-value comparing stroke survivors to decedents1 | Participants with no incident stroke during follow-up (1998-2008) | P-value comparing stroke-free participants to those with stroke2 | ||
|---|---|---|---|---|---|
|
|
|
||||
| Stroke Survivors Number (%) | Stroke Decedents Number (%) | Number (%) | |||
|
|
|||||
| 1189(100%) | 385(100%) | 15766(100%) | |||
| Baseline memory score(SD) | 0.83(0.60) | 0.55(0.72) | 1.20(0.38) | ||
| Gender | 0.023 | 0.537 | |||
| Male | 517(43.5%) | 142(36.9%) | 6728(42.7%) | ||
| Female | 672(56.5%) | 243(63.1%) | 9038(57.3%) | ||
| Race | 0.385 | <0.001 | |||
| White | 948(79.7%) | 299(77.7%) | 13192(83.7%) | ||
| Non-white | 241(20.3%) | 86(22.3%) | 2574(16.3%) | ||
| Age (SD) | 69.8 (9.8) | 75.6(9.8) | <0.001 | 65.9(10.2) | <0.001 |
| Marital status | |||||
| Married/partnered | 721(60.6%) | 161(41.8%) | <0.001 | 10895(69.1%) | <0.001 |
| Widowed/sep/divorced | 431(36.3%) | 205(53.3%) | <0.001 | 4419(28.0%) | <0.001 |
| Never married | 37(3.1%) | 19(4.9%) | 0.093 | 452(2.9%) | 0.121 |
| Years of education (SD) | 11.8(3.1) | 11.2(3.3) | 0.002 | 12.4(3.0) | <0.001 |
| Mother's education years (SD) | 8.8(2.7) | 8.5(2.1) | 0.019 | 9.4(3.0) | <0.001 |
| Wealth in $1000 (25th, 75th) | 257.5(29.0, 282.2) | 211.4(10.5, 212.4) | <0.001 | 343.1(50.2, 353) | <0.001 |
| Height in meters (SD) | 1.68(0.1) | 1.67(0.1) | 0.005 | 1.69(0.1) | <0.001 |
| Place of birth | |||||
| Northeast | 222(18.7%) | 75(19.5%) | 0.724 | 3321(21.1%) | 0.041 |
| Midwest | 308(25.9%) | 112(29.1%) | 0.219 | 4749(30.1%) | 0.005 |
| South | 523(44.0%) | 155(40.3%) | 0.199 | 5636(35.7%) | <0.001 |
| West | 69(5.8%) | 21(5.4%) | 0.798 | 1176(7.5%) | 0.011 |
| Non US | 67(5.6%) | 22(5.7%) | 0.953 | 884(5.6%) | 0.938 |
Descriptive data are not weighted.
For individuals who were aged 50+ and stroke free at baseline in 1998. Values in the table are counts (percentage) for categorical variables and means (SD) for continuous variables. SD is standard deviation. 25th and 75th are the first and the third quartiles.
Null hypothesis for P-value1: Baseline characteristics are the same for stroke survivors as for stroke decedents.
Null hypothesis for P-value2: Baseline characteristics are the same for participants who experienced first incident stroke as for stroke-free cohort members.
Table 2. Description of crude incidence and follow-up time by stroke status.
| All participants included in this study N=17,340 | |
|---|---|
|
|
|
| Crude Incidence | |
| All Strokes | |
| Number of individuals with incident first strokes | 1574 |
| Crude incidence rate, any first stroke (/1000 PY) | 11.29 |
| Stroke Survivors | |
| Number of stroke survivors | 1189 |
| Crude incidence rate, survived first strokes (/1000 PY) | 8.53 |
| Stroke Decedents | |
| Number of stroke decedents | 385 |
| Crude incidence rate, first strokes not survived (/1000 PY) | 2.76 |
| Follow-up Time | |
| Total number of person-years | 139,457 |
| Stroke Survivors | |
| Number of person-years before stroke onset | 5614 |
| Number of person-years after stroke onset | 4754 |
| Stroke Decedents | |
| Number of person-years before stroke onset | 1788 |
| Stroke Free Members | |
| Number of person-years | 127,156 |
The trajectory of change in memory functioning
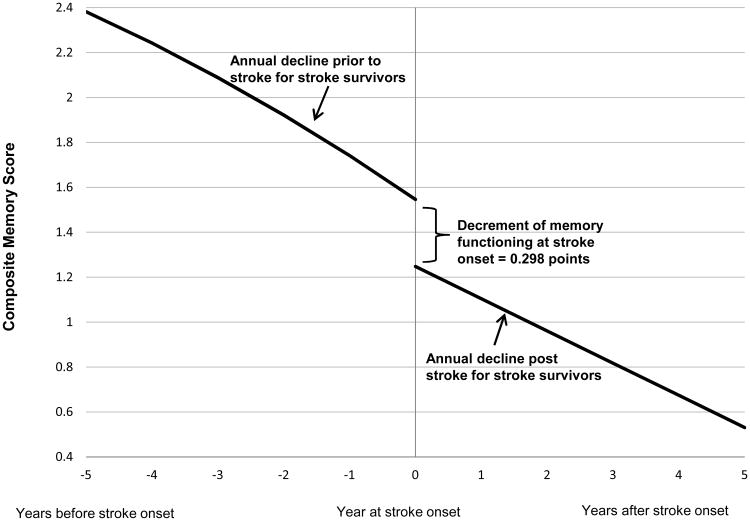
Among the 1189 individuals who survived a stroke, memory function significantly declined with each additional year of age both before (-0.143 points/year; 95% CI: -0.156, -0.130) and after stroke (-0.142 points/year; 95% CI: -0.162, -0.122), with a large decrement at the time of stroke onset averaging -0.369 points (95% CI: -0.450, -0.288) (Figure 1).
Figure 1.
Trajectory of memory score for stroke survivors (n=1189). Time 0 indicates time of stroke onset. Decline at stroke onset indicates the decrement of memory score at stroke onset. The curve to the left of stroke onset indicates change in memory score before stroke onset while the curve to the right indicates change in after stroke onset. Predicted values are for an individual who experienced stroke at age 75, corresponding to the reference category in the regression models.
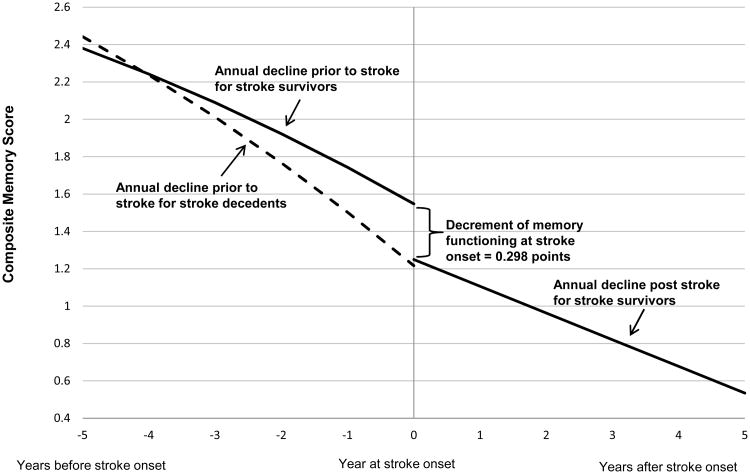
The average annual pre-stroke memory decline for the 385 individuals who did not survive their stroke was -0.212 points/year (95% CI: -0.238, -0.186), significantly faster than annual pre-stroke rate of decline among stroke survivors (p<0.001). After modeling time pre and post stroke with linear and quadratic trends, memory change showed small but statistically significant curvilinear trends among both stroke survivors and stroke decedents. As the time of stroke onset approached, the decline of memory functioning accelerated (Figure 2).
Figure 2.
Trajectory of memory score for stroke survivors (n=1189) vs. stroke decedents (n=385) during follow-up. Solid curve represents memory score change among stroke survivors while dashed curve represents memory score change among stroke decedents. Time 0 indicates time of stroke onset. Decline at stroke onset indicates the decrement of memory score at stroke onset among stroke survivors. The curves to the left of stroke onset indicate change in memory score before stroke onset while the curve to the right indicates change after stroke onset. Predicted values are for individuals who experienced stroke at age 75, corresponding to the reference category in the regression models.
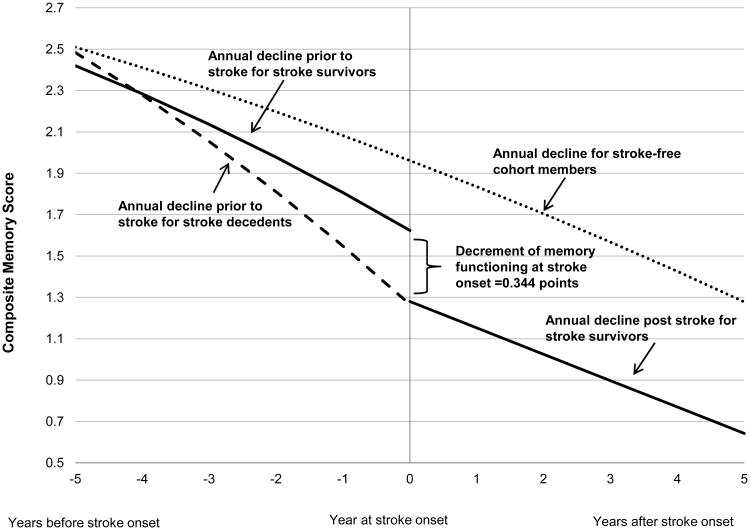
The average annual rate of memory decline with aging among those who remained stroke-free throughout follow-up was -0.101 points/year (95% CI: -0.102, -0.099). Compared with annual memory decline in stroke-free participants, memory decline occurred most rapidly among respondents who experienced a fatal stroke, followed by respondents who survived a stroke (Figure 3). In other words, memory declined nearly twice as fast for those who subsequently experienced a fatal stroke as for subjects with no recorded stroke (p<0.001). Pre-stroke memory decline among those surviving stroke was 42% faster than among subjects with no recorded stroke (p<0.001). Even 4 years prior to stroke, the average memory function of those who would subsequently experience stroke was worse than memory among those who remained stroke-free. For example, memory scores in 2002 for individuals who first reported a stroke in 2006 were an average of 0.35 points lower than the year 2002 memory scores for respondents who did not experience a stroke during follow-up (p<0.01). This model suggests that approximately 50% of the difference in memory between a stroke survivor shortly after stroke and an age-matched stroke-free individual is attributable to pre-stroke memory performance.
Figure 3.
Trajectory of memory score for stroke survivors (n=1189) vs. stroke decedents (n=385) vs. stroke-free cohort members (n=15766) during entire follow-up. Solid curve represents memory score change among stroke survivors, dashed curve represents memory score change among stroke decedents and dotted curve represents memory score change among stroke free cohort members. Time 0 indicates time of stroke onset for participants who experienced first incident stroke at75 years of age (stroke survivors and stroke decedents) while it represents age 75 for stroke-free cohort members. Decline at stroke onset indicates the immediate decrement of memory score at stroke onset among stroke survivors. The curves to the left of stroke onset indicate change in memory score before stroke onset while the curve to the right indicates change after stroke onset.
Patterns were relatively consistent when restricting by duration of post-stroke follow-up (appendix 2); for cognitive performance at the 25th, 50th, or 75th percentiles (appendix 3); or when excluding individuals with imputed stroke dates (appendix 4).
Discussion
In a large, nationally representative cohort, we found faster memory decline in the years prior to stroke compared to memory decline in cohort members who remained stroke-free. Rate of decline was especially accelerated among stroke decedents. Among stroke survivors, long-term memory decline after stroke continued at a similar rate as prior to stroke.
This study contributes significantly to existing studies on stroke and memory function; however, limitations should be noted. Most important for the current analyses, we have no medical record verification of stroke and no way to detect silent strokes. Possible measurement error could lead to an underestimate of the differences between stroke survivors and stroke-free elderly, especially if strokes are less likely to be reported for memory-impaired individuals. Also, no distinctions of stroke subtype, severity, or location could be made in this study. Furthermore, any imprecision in the reported date of stroke would lead to an underestimate of the immediate memory decrement associated with stroke. The estimates of post-stroke memory trajectory should be interpreted as long-term patterns, averaged over several years. Additionally, although changes in other cognitive domains are of great interest, verbal memory was the only outcome available at each interview for the full nationally representative HRS cohort age 50+. Further studies using other data sources, with imaging and more comprehensive cognitive assessments, would be ideal. Finally, these data were not collected with this specific investigation in mind; a confirmation of our findings through prospective studies is needed. Key strengths of the study include the large, nationally representative sample, long follow-up, comprehensive socio-demographic assessments, prospectively assessed repeated pre-stroke measurements of memory, and high follow-up rates, even for memory impaired individuals.23
The results are consistent with prior studies indicating impaired cognitive function predicts stroke 5, 6, 24, 25, and previous studies showing stroke survivors experience significant cognitive impairment and dementia after stroke onset 2-4.
In one of the few existing longitudinal studies following patients before and after stroke, Kase et al1 reported a significant change of -3.7 points (comparable to -1.3 standard deviations) in the mean Mini-Mental State Examination scores in 74 stroke patients in Framingham Heart Study (FHS) tested within 6 months of stroke onset; no change occurred in 74 control subjects tested over similar time intervals. Our study yielded a range of effects somewhat smaller than this, with a predicted change of approximately -0.71 SD over a comparable time period including stroke. Including 1115 more non-fatal events than the FHS study, our study extends this earlier work to show that even pre-stroke, future stroke patients experience a long period of memory decline, a decline that continues for years after stroke.
Our findings show that average memory scores are already lower among stroke patients prior to stroke. Pre-stroke memory decline is most likely an early sign of cerebrovascular disease. However, it is also plausible that lower memory performance increases vulnerability to clinically manifest stroke. Individuals with very high memory scores may have better cognitive reserve and be able to sustain an acute ischemic event without severe clinical manifestations. We could not directly investigate this possibility with our current study design. However, the distinction has important clinical implications and therefore merits further exploration.
This study adds to prior research by showing the trajectory of decline in memory function with comparisons of stroke patients and similarly aged stroke-free subjects. Impairment of memory function occurs not only at stroke onset, but also years before and after stroke. The accelerated rate of decline among stroke decedents has two alternative interpretations: these individuals may have particularly severe underlying cerebrovascular disease that causes very rapid cognitive declines culminating in fatal stroke, or the pre-stroke cognitive declines may be attributable to causes other than cerebrovascular disease, but render the patient frail or unusually vulnerable to death as a result of the stroke26, 27. Future studies could address these mechanisms directly.
Summary
Although stroke is associated with large short-term decrements in memory score, memory decline is accelerated before stroke for both stroke survivors and stroke decedents compared to stroke-free elderly. More effective management of risk factors related to memory functioning in individuals experiencing memory decline might be beneficial. We also found that memory declines continue after stroke, with no apparent abatement in rate of decline. This finding may indicate that cerebrovascular risk factors are not adequately controlled following stroke diagnosis, or that acute stroke presages an ongoing, cascading process of neurologic injury.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Qiong Wu for expert technical assistance; NIA and AHA for funding; the HRS funders (the National Institute on Aging NIA U01AG009740) and staff at the University of Michigan.
Sources of Funding: National Institute on Aging AG03438501, American Heart Association grant 09PRE2080078
Footnotes
Disclosures: Dr. Glymour receives grant support from the NIH, US Army Medical Research, and the American Heart Association.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D'Agostino RB. Intellectual decline after stroke: The framingham study. Stroke. 1998;29:805–812. doi: 10.1161/01.str.29.4.805. [DOI] [PubMed] [Google Scholar]
- 2.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 3.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, et al. Dementia after stroke: The framingham study. Stroke. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham stroke risk profile and poor cognitive function: A population-based study. BMC Neurol. 2008;8:12. doi: 10.1186/1471-2377-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFries T, Avendano M, Glymour MM. Level and change in cognitive test scores predict risk of first stroke. J Am Geriatr Soc. 2009;57:499–505. doi: 10.1111/j.1532-5415.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Elkins JS, Knopman DS, Yaffe K, Johnston SC. Cognitive function predicts first-time stroke and heart disease. Neurology. 2005;64:1750–1755. doi: 10.1212/01.WNL.0000161850.01792.77. [DOI] [PubMed] [Google Scholar]
- 7.de Moraes SA, Szklo M, Tilling K, Sato R, Knopman D. Cognitive functioning as a predictor of ischemic stroke incidence. Epidemiology. 2003;14:673–679. doi: 10.1097/01.ede.0000083262.58396.a3. [DOI] [PubMed] [Google Scholar]
- 8.Henon H, Pasquier F, Durieu I, Godefroy O, Lucas C, Lebert F, et al. Preexisting dementia in stroke patients. Baseline frequency, associated factors, and outcome. Stroke. 1997;28:2429–2436. doi: 10.1161/01.str.28.12.2429. [DOI] [PubMed] [Google Scholar]
- 9.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 10.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent mri infarcts and the risk of future stroke: The cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 11.Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the reasons for geographic and racial differences in stroke (regards) study. Stroke. 2007;38:1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- 12.Kleindorfer D, Judd S, Howard VJ, McClure L, Safford MM, Cushman M, et al. Self-reported stroke symptoms without a prior diagnosis of stroke or transient ischemic attack. Stroke. 2011;42:3122–3126. doi: 10.1161/STROKEAHA.110.612937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health and Retirement Study. Health and Retirement Study Documentation Web Site. Ann Arbor, Michigan: University of Michigan; 2012. [Accessed: june 5, 2012]. http://hrsonline.isr.umich.edu/index.php. [Google Scholar]
- 14.Juster FT., SR An overview of the health and retirement study. J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 15.Herringa SG., CJ . Technical description of the health and retirement study sample design. Ann Arbor, MI: Survey Reserach Center, University of Michigan; 1995. [Google Scholar]
- 16.Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (iqcode): Development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 17.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Informant ratings of cognitive decline in old age: Validation against change on cognitive tests over 7 to 8 years. Psychol Med. 2000;30:981–985. doi: 10.1017/s0033291799002299. [DOI] [PubMed] [Google Scholar]
- 18.Ofstedal MB, Fisher GF, Herzog AR. Documentation of cognitive functioning measures in the health and retirement study. HRS Documentation Report. 2005:DR–006. [Google Scholar]
- 19.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, et al. Incidence of dementia and cognitive impairment, not dementia in the united states. Annals of neurology. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glymour MM, Avendano M, Haas S, Berkman LF. Lifecourse social conditions and racial disparities in incidence of first stroke. Ann Epidemiol. 2008;18:904–912. doi: 10.1016/j.annepidem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes de Leon CF, Bang W, Bienias JL, Glass TA, Vaccarino V, Kasl SV. Changes in disability before and after myocardial infarction in older adults. Arch Intern Med. 2005;165:763–768. doi: 10.1001/archinte.165.7.763. [DOI] [PubMed] [Google Scholar]
- 23.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58:13–19. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Ferrucci L, Guralnik JM, Salive ME, Pahor M, Corti MC, Baroni A, et al. Cognitive impairment and risk of stroke in the older population. J Am Geriatr Soc. 1996;44:237–241. doi: 10.1111/j.1532-5415.1996.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Fratiglioni L, Guo Z, Winblad B, Viitanen M. Incidence of stroke in relation to cognitive function and dementia in the kungsholmen project. Neurology. 2000;54:2103–2107. doi: 10.1212/wnl.54.11.2103. [DOI] [PubMed] [Google Scholar]
- 26.Korten AE, Jorm AF, Jiao Z, Letenneur L, Jacomb PA, Henderson AS, et al. Health, cognitive, and psychosocial factors as predictors of mortality in an elderly community sample. J Epidemiol Community Health. 1999;53:83–88. doi: 10.1136/jech.53.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.