I. EXECUTIVE SUMMARY
This guideline supersedes the 1994 U.S. Public Health Service (PHS) Guidelines for Preventing Transmission of Human Immunodeficiency Virus through Transplantation of Human Tissue and Organs, hereafter referred to as the 1994 PHS guidelines.1 The most significant changes are:
Expanding the guideline to include hepatitis B virus (HBV) and hepatitis C virus (HCV), in addition to human immunodeficiency virus (HIV);
Using factors known to be associated with an increased likelihood of recent HIV, HBV, or HCV infection to identify potential donors who may be at increased risk for transmitting infection; and
Limiting the focus to organs and blood vessel conduits recovered for organ transplantation because the Food and Drug Administration (FDA) implemented more comprehensive regulations for human cell and tissue products.2
As with the 1994 PHS guidelines, the recommendations relate to adult and pediatric donors who are living or deceased, as well as transplant candidates and recipients. This guideline is not intended to assess infectious risks beyond HIV, HBV, and HCV.
This document provides guidance to organ procurement organization (OPO) personnel; transplant center personnel, including physicians, nurses, administrators, and clinical coordinators; laboratory personnel responsible for testing and storing donor and recipient specimens; and individuals responsible for developing, implementing, and evaluating infection prevention and control programs for OPOs and transplant centers.
The Health Resources and Services Administration (HRSA), a PHS agency, oversees organ procurement and transplantation in the United States through its oversight of the Organ Procurement and Transplantation Network (OPTN). The United Network for Organ Sharing (UNOS) is the entity that currently serves as the contractor to operate the OPTN. In writing this guideline, the PHS sought assistance from public and private health professionals and representatives of transplantation, organ recovery, public health, and other organizations.
Unexpected transmission of HIV, HBV, and HCV through organ transplantation is a patient safety and public health issue. Such events, although rare, can result in serious illness and death in organ recipients who are immunosuppressed, particularly when transmission is unexpected. Notification of state public health authorities is required when donors or recipients are identified as newly infected with HIV, HBV, or HCV. When an organ recipient is newly infected and the infection is suspected of being donor-derived, immediate notification of institutions that recovered or transplanted organs and tissues from the same donor is important. Such notification may not only allow for early treatment of newly infected recipients to minimize the impact of the disease, but also prevent further distribution or implantation of potentially infected tissues. Unexpected transmission of HIV, HBV, and HCV from infected donors has been reported in heart, liver, kidney, and pancreas recipients.3–13
The objective of this guideline is to improve organ transplant recipient outcomes by reducing the risk of HIV, HBV, and HCV transmission, keeping in mind that transplantation can never be free of this risk. Given the large discrepancy between the number of candidates on the transplant list and the number of organs available, recommendations in this document may differ from policies or regulations in the setting of blood or tissue donation, due to different risk and benefit considerations for organ transplantation. Even though attempts should be made to ensure the highest level of safety, organ donor and recipient selection practices and policies should not be restrictive, considering the clinical need. Therefore, informed decision-making is an important part of this process for transplant clinicians and their patients.
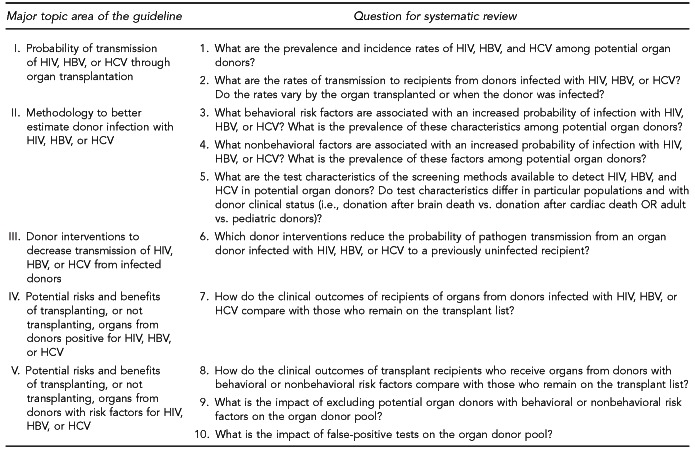
To evaluate the evidence on reducing transmission of HIV, HBV, and HCV, we examined data addressing 10 key questions within five major topic areas (Figure 1). A sixth topic area includes questions addressed by expert opinion (Figure 2). We drew upon subject-matter experts to draft summaries related to these questions, as a preliminary scan of the literature showed that a systematic review would likely yield insufficient data.
Figure 1.
Major topic areas and key questions for the systematic literature review concerning HIV, HBV, and HCV transmission through organ transplantation
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
Figure 2.
Major topic area and questions addressed by expert opinion relevant to HIV, HBV, and HCV transmission through organ transplantation

HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
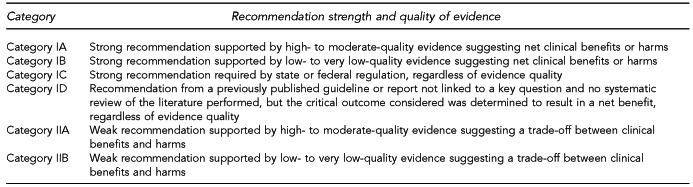
Recommendations related to the 10 key questions were based on a targeted systematic review of the best available evidence, with explicit links between the evidence and recommendations. To accomplish this review, we used a modified Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for evaluating quality of evidence and determining strength of recommendations.14–18 If weighing the critical outcomes for a key question resulted in a net benefit or a net harm, then a Category I recommendation was formulated to recommend strongly for or against the given intervention, respectively. If weighing the critical outcomes for a key question resulted in a trade-off between benefits and harms, then a Category II recommendation was formulated to recommend that providers or institutions consider the intervention when deemed appropriate.
In addition to a category rating, recommendations were also assigned a level rating (A through D) to reflect the quality of the evidence base underlying the recommendations. Level A represents high- to moderate-quality evidence and Level B represents low- to very low-quality evidence. No recommendations were assigned a Level A rating. Level C represents required practices by state or federal regulations, regardless of evidence quality. Level D represents recommendations from previously published guidelines or reports for topics not directly addressed by the systematic review of the evidence, but deemed critical to the target user; in this level, critical outcomes were determined to result in net benefits, regardless of evidence quality.
It is important to note that the strength of a Category IA recommendation is equivalent to that of a Category IB, IC, or ID recommendation; it is only the quality of the evidence underlying the Category IA recommendation that makes it different.
Recommendations related to the three expert opinion questions were based on the expert opinion summaries and are designated either as IB if they represent a strong recommendation or IIB if they represent a weak recommendation.
Areas in need of further research identified during the evidence review, from public comment and during review following public comment, are outlined in the Recommendations for Further Study section. This section addresses gaps that affected the ability to adequately address many of the key questions; therefore, specific recommendations either could not be supported because of the absence of available evidence or were supported by low-quality evidence. These recommendations provide guidance for new research or methodologic approaches that should be used in future studies.
To examine the primary evidence underlying the recommendations related to the 10 key questions, please refer to the Evidence Review section on page 272 and the GRADE ratings in the appendices on pages 305–43 from “Solid Organ Transplantation and the Probability of -Transmitting HIV, HBV, or HCV: A Systematic Review to Support an Evidence-Based Guideline,”19 hereafter referred to as the Evidence Report. In the Evidence Report, the Evidence tables include all study-level data and the GRADE tables assess the overall quality of evidence for each question. The Evidence Report is accessible at http://stacks.cdc.gov/view/cdc/12164/.
The Evidence Review section of the guideline includes narrative summaries of the data presented in the Evidence Report.19 A more detailed description of the approach used to develop the guideline appears in the Methods section.
II. RECOMMENDATIONS
There are 12 criteria listed in this section to assess donor risk for HIV, HBV, and HCV infection. Eleven of the criteria are to evaluate infection risk for all three pathogens collectively; one criterion is to evaluate infection risk for HCV only. The 34 recommendations are numbered and grouped into sections as follows: risk assessment (screening) of living and deceased donors (recommendations 1–5); testing of living and deceased donors (recommendations 6–9); informed consent discussion with transplant candidates (recommendations 10–15); testing of recipients pre- and posttransplant (recommendations 16–20); collection and/or storage of donor and recipient specimens (recommendations 21–25); and tracking and reporting of HIV, HBV, and HCV (recommendations 26–34).
If a recommendation was based on evidence for one of the 10 key questions, the key question is referenced (e.g., Key Question 3). If a recommendation was based on evidence for one of the three expert opinion questions, the expert opinion question is referenced (e.g., Expert Opinion—Question 1). Additional information on categorizing the recommendations can be found in Figure 3 and under the Methods section starting on page 262.
Figure 3.
Categorization scheme applied to the 34 recommendations concerning HIV, HBV, and HCV transmission through organ transplantation
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
Throughout the recommendations, the following conditions and definitions of terms are applicable:
As blood vessel conduits are classified as organs, recommendations relating to recovered or transplanted organs also apply to these vessel conduits.
A presumed HBV-infected donor is defined as being positive for hepatitis B surface antigen (HBsAg), antibody to hepatitis B core antigen (anti-HBc), and/or HBV by nucleic acid testing (NAT). A presumed HBV-infected transplant candidate is defined as being positive for HBsAg, immunoglobulin M antibodies to HBc (IgM anti-HBc), and/or HBV by NAT. (A transplant candidate who is positive only for immunoglobulin G antibodies to HBc [IgG anti-HBc] could be a chronic carrier with HBsAg at an undetectable level or could have cleared the virus.)
A presumed HCV-infected donor or transplant candidate is defined as being positive for antibodies to hepatitis C virus (anti-HCV) and/or HCV by NAT.
The term “increased risk” applies to donors at higher-than-average risk for HIV, HBV, and HCV infection, or for only one of the pathogens when specifically identified.
The recommendations in this guideline that cite increased risk donors are referring to donors with one or more of the risk factors for HIV, HBV, or HCV infection listed in the next section.
Risk factors for recent HIV, HBV, or HCV infection
This section lists the risk factors associated with an increased likelihood of recent HIV, HBV, or HCV infection. The initial list of risk factors identified from the literature review was modified by subject-matter experts on HIV and hepatitis due to the paucity of evidence for recent (i.e., incident) infection from the studies that met inclusion criteria. Development of this list took into consideration that (1) certain risk factors are probably markers for other factors identified in the systematic review; (2) scientific evidence associating certain factors with the pathogens exists, but may not have met the inclusion criteria of the systematic review; and (3) certain studies were of insufficient quality to draw conclusions.
Donors who meet one or more of the following 11 criteria should be identified as being at increased risk for recent HIV, HBV, and HCV infection. Each factor listed reflects increased risk of all three pathogens as an aggregate, as there is overlap of associated risk, even though each factor does not convey risk from all pathogens equally. The first six risk factors address sexual contact; the definition of “had sex” refers to any method of sexual contact, including vaginal, anal, and oral contact:
People who have had sex with a person known or suspected to have HIV, HBV, or HCV infection in the preceding 12 months
Men who have had sex with men (MSM) in the preceding 12 months
Women who have had sex with a man with a history of MSM behavior in the preceding 12 months
People who have had sex in exchange for money or drugs in the preceding 12 months
People who have had sex with a person who had sex in exchange for money or drugs in the preceding 12 months
People who have had sex with a person who injected drugs by intravenous, intramuscular, or subcutaneous route for nonmedical reasons in the preceding 12 months
A child who is ≤18 months of age and born to a mother known to be infected with, or at increased risk for, HIV, HBV, or HCV infection
A child who has been breastfed within the preceding 12 months and the mother is known to be infected with, or at increased risk for, HIV infection
People who have injected drugs by intravenous, intramuscular, or subcutaneous route for nonmedical reasons in the preceding 12 months
People who have been in lockup, jail, prison, or a juvenile correctional facility for more than 72 consecutive hours in the preceding 12 months
People who have been newly diagnosed with, or have been treated for, syphilis, gonorrhea, Chlamydia, or genital ulcers in the preceding 12 months
Donors who meet the following criterion should be identified as being at increased risk for recent HCV infection only:
People who have been on hemodialysis in the preceding 12 months
Risk assessment (screening) of living and deceased donors
1. All living potential donors and individuals interviewed about deceased potential organ donors (e.g., next of kin, life partner, cohabitant, caretaker, friend, or primary treating physician) should be informed of the donor evaluation process, including the review of medical and behavioral history, physical examination, and laboratory tests to identify the presence of infectious agents or medical conditions that could be transmitted by organ transplantation. (Category ID)
2. To ascertain whether potential organ donors are at increased risk for HIV, HBV, or HCV infection, living donors, or individuals contacted about deceased donors, should be interviewed in a confidential manner about behaviors that may have increased the potential donor's probability of having HIV, HBV, or HCV infection. (Category IB) (Key Questions 3 and 4)
3. Living potential donors with behaviors associated with an increased risk of acquiring HIV, HBV, or HCV identified during evaluation should receive individualized counseling on specific strategies to prevent exposure to these viruses during the time period prior to surgery. (Category ID)
4. If a potential donor is ≤18 months of age or has been breastfed within the preceding 12 months, the birth mother, if available, should be interviewed about behaviors that may have placed her at risk for HIV, HBV, or HCV infection. (Category IB) (Expert Opinion—Question 3)
5a. When a deceased potential organ donor's medical/behavioral history cannot be obtained or risk factors cannot be determined, the donor should be considered at increased risk for HIV, HBV, and HCV infection because the donor's risk for infection is unknown. (Category ID)
5b. When a deceased potential organ donor's blood specimen is hemodiluted, the donor should be considered at increased risk for HIV, HBV, and HCV infection because the donor's risk for infection is unknown. (Category IB) (Expert Opinion—Question 3)
Testing of living and deceased donors
For deceased potential organ donors, the recommended tests and time when results should be available are listed in Figure 4. For living potential organ donors, the recommended tests and timing of tests are listed in Figure 5.
Figure 4.
Deceased potential organ donor test recommendations based on risk status for HIV, HBV, and HCV infection
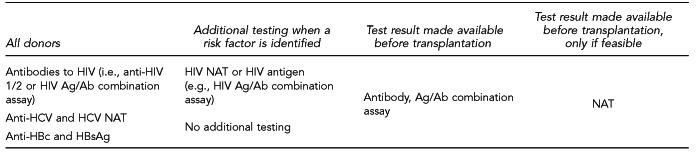
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
Anti-HIV = antibodies to HIV
Ag/Ab = antigen/antibody
NAT = nucleic acid test
Anti-HCV = antibody to hepatitis C virus
Anti-HBc = antibody to hepatitis B core antigen
HBsAg = hepatitis B surface antigen
Figure 5.
Living potential organ donor test recommendations based on risk status for HIV, HBV, and HCV infection

HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
Anti-HIV = antibodies to HIV
Ag/Ab = antigen/antibody
NAT = nucleic acid test
Anti-HCV = antibody to hepatitis C virus
Anti-HBc = antibody to hepatitis B core antigen
HBsAg = hepatitis B surface antigen
6. All living potential donors should be tested for HIV, HBV, and HCV as close as possible to the date of the organ recovery operation, but at least within the 28-day time period prior to surgery. (Category ID)
7. All potential organ donors (living or deceased) should be tested for antibodies to HIV (i.e., anti-HIV 1/2 or HIV antigen/antibody [Ag/Ab] combination assay). All potential organ donors identified as being at increased risk for HIV infection should also be tested for HIV ribonucleic acid (RNA) by NAT or HIV antigen (e.g., HIV Ag/Ab combination assay). Donor blood specimens should be obtained before procurement. Ab or Ag/Ab test results should be made available before transplantation. (Category IB) (Key Question 5) (Note: Optimally, all NAT results for deceased donors should be available before the transplant occurs; however, if having NAT results before transplantation is not feasible, test results can be useful to guide recipient treatment.)
8. All potential organ donors (living or deceased) should be tested for both anti-HCV and for HCV RNA by NAT. Donor blood specimens should be obtained before procurement. Ab test results should be made available before transplantation. (Category IB) (Key Question 5) (Note: Optimally, all NAT results for deceased donors should be available before the transplant occurs; however, if having NAT results before transplantation is not feasible, test results can be useful to guide recipient treatment.)
9. All potential organ donors (living or deceased) should be tested for anti-HBc and for HBsAg. Donor blood specimens should be obtained before procurement. Ab/Ag test results should be made available before transplantation. (Category IB) (Key Question 5)
Informed consent discussion with transplant candidates
10. An informed consent process discussion between the transplant candidate, or medical decision maker, and the listing clinician should start before the patient is placed on the transplant wait list. Patients should be counseled to consider potential risks of both accepting and rejecting organs from donors known to be infected with HBV or HCV, or donors at increased risk for HBV, HCV, or HIV infection. (Category IB) (Expert Opinion—Question 1)
11. The transplant candidate, or medical decision maker, should have opportunities to discuss with clinicians issues related to the associated risk of HIV, HBV, or HCV transmission with organ acceptance while the patient is on the transplant wait list. (Category IB) (Expert Opinion—Question 1)
12. At the time of the organ offer, if a donor is identified as being at increased risk for HIV, HBV, or HCV infection, the transplant center team primarily responsible for the patient's care should include this risk information in the informed consent discussion with the transplant candidate or medical decision maker. (Category IB) (Expert Opinion—Question 1)
13. If prior to transplantation or repair of a transplanted organ it is known or anticipated that stored blood vessel conduits (from a donor who is different from the donor of the primary organ being transplanted or repaired) may be used, and the donor is identified as being at increased risk for HIV, HBV, or HCV infection, then the transplant center team should include this risk information in the informed consent discussion. (Category IB) (Expert Opinion—Question 1)
14. When organs from HBV- or HCV-infected donors will be used, the transplant center team primarily responsible for the patient's care should have an informed consent discussion with the transplant candidate, or medical decision maker, prior to transplantation regarding the risks related to disease transmission. (Category IB) (Key Question 7)
15. Transplant candidates should be informed that although all donors are screened for HIV, HBV, and HCV, donor screening has limitations and no screening question or laboratory test can completely eliminate the risk for transmitting these infections (or any other infection). (Category IB) (Expert Opinion—Question 1)
Testing of recipients pre- and posttransplant
For transplant candidates and recipients, the recommended tests and timing of tests are listed in Figure 6.
Figure 6.
Pre- and posttransplant recipient test recommendations when a donor is at increased risk for HIV, HBV, or HCV infection; the donor's risk for HIV, HBV, and HCV infection is unknown; or the donor is infected with HCV or HBVa

aUnless transplant patient infection was documented pre-transplant
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
NAT = nucleic acid test
Ag/Ab = antigen/antibody
HBsAg = hepatitis B surface antigen
Anti-HBs = antibody to hepatitis B surface antigen
Anti-HBc = antibody to hepatitis B core antigen
16. Pre-transplant testing of transplant candidates for HIV, HBV, and HCV should be conducted when the donor (living or deceased) meets any of the following conditions: (1) identified as being at increased risk for HIV, HBV, and HCV infection (Note: If the donor is only identified as being at risk for HCV infection due to hemodialysis in the preceding 12 months, then testing for HCV only is recommended); (2) screening specimens are hemodiluted; or (3) the medical/behavioral history is unavailable. When the donor meets any of the three conditions, transplant candidate testing should occur during hospital admission for the organ transplant but prior to implantation of the organ, unless the transplant candidate is known through prior testing to be infected. (Category IB) (Expert Opinion—Question 2)
17. Pre-transplant testing of transplant candidates for HBV or HCV should be conducted when the donor (living or deceased) is known to be infected with HBV or HCV. Transplant candidate testing should occur during hospital admission for the organ transplant but prior to organ implantation, unless the transplant candidate is known through prior testing to be infected. (Category IB) (Expert Opinion—Question 2)
18. Posttransplant HBV testing of recipients should be conducted when the donor (living or deceased) meets any of the following conditions: (1) identified as being at increased risk for HBV infection, (2) screening specimens are hemodiluted, (3) the medical/behavioral history is unavailable, or (4) the donor is infected with HBV. Recipient testing should be performed sometime between one and three months posttransplant to include HBV NAT and HBsAg, and at 12 months posttransplant to include antibody to hepatitis B surface antigen (anti-HBs), anti-HBc, and either HBV NAT or HBsAg (unless infection was documented pre-transplant). (Category IB) (Expert Opinion—Question 2)
19. Posttransplant HIV testing of recipients should be conducted when the donor (living or deceased) meets any of the following conditions: (1) identified as being at increased risk for HIV infection, (2) screening specimens are hemodiluted, or (3) the medical/behavioral history is unavailable. Recipient testing should be performed sometime between one and three months posttransplant to include HIV NAT or an HIV Ag/Ab combination assay (unless infection was documented pre-transplant). NAT or an Ag/Ab combination assay for HIV detection is important as infected recipients may remain Ab-negative due to immunosuppression. (Category IB) (Expert Opinion—Question 2)
20. Posttransplant HCV testing of recipients should be conducted when the donor (living or deceased) meets any of the following conditions: (1) identified as being at increased risk for HCV infection, (2) screening specimens are hemodiluted, (3) the medical/behavioral history is unavailable, or (4) the donor is infected with HCV. Recipient testing should be performed sometime between one and three months posttransplant to include HCV NAT (unless infection was documented pre-transplant). NAT is important for HCV detection as infected recipients may remain Ab-negative due to immunosuppression. (Category IB) (Expert Opinion—Question 2)
Collection and/or storage of donor and recipient specimens
21. For deceased donors, the OPO should consider collecting two blood specimens, when possible, for HIV, HBV, and HCV real-time testing (i.e., prior to organ recovery)—an ethylenediaminetetraacetic acid (EDTA) plasma specimen or serum specimen for serologic assays and a separate EDTA plasma specimen for NAT. Additionally, the OPO should consider collecting two blood specimens for archiving, when possible. If it is only feasible to collect one specimen, a plasma specimen collected in EDTA, rather than a serum specimen, is optimal. (Category IIB) (Expert Opinion—Question 3)
22. The OPO should consider archiving blood specimens from deceased donors for at least 10 years. (Category IIB) (Expert Opinion—Question 3)
23. For living donors, transplant candidates, and recipients, two blood specimens should be collected when HIV, HBV, or HCV testing is planned—an EDTA plasma specimen or serum specimen for serologic assays and a separate EDTA plasma specimen for NAT. (Category IB) (Expert Opinion—Question 3)
24. Infusion of crystalloid and colloid solutions and transfusion of blood products can cause hemodilution and produce false-negative results for HIV, HBV, and HCV testing. Therefore, the OPO should make an effort to collect a qualified (non-hemodiluted) specimen—that is, a specimen that is deemed acceptable for testing according to an appropriate hemodilution algorithm and calculation method, such as provided by the FDA.2 Furthermore, a hemodilution calculation should be performed on archived specimens of deceased donors to facilitate interpretation of test results. (Category IB) (Expert Opinion—Question 3)
25. All stored blood vessel conduits from a donor found to be infected with HIV, HBV, or HCV should be quarantined immediately and not released for clinical use unless the HBV- or HCV-infected vessel conduits are needed for the initial transplant procedure in the recipient. After completing the initial transplant procedure, any remaining vessel conduits should be disposed of in accordance with hospital policy to prevent inadvertent release from quarantine and unintentional use in other patients. (Category ID)
Tracking and reporting of HIV, HBV, and HCV
26a. When an OPO receives information before organ recovery that a deceased potential donor is at increased risk for or is infected with HIV, HBV, or HCV, the OPO should notify (1) the OPTN, (2) the transplant centers receiving organ offers, and (3) any institutions considering tissue and eye recovery. (Category IB)(Expert Opinion—Question 2)
26b. The OPO should also notify the public health authorities where the potential donor is admitted, in accordance with state requirements for reporting notifiable infections, if the deceased potential donor is infected. (Category IC)
27a. When an OPO receives information after organ recovery that a deceased donor was infected with HIV, HBV, or HCV, or that an organ recipient infection with HIV, HBV, or HCV is suspected of being donor-derived, the OPO should notify (1) the OPTN, (2) the transplant centers that received organs and/or blood vessel conduits from the deceased donor, and (3) any institutions that recovered tissues and eyes from the donor. (Category IB) (Expert Opinion—Question 2)
27b. The OPO should also notify public health authorities where the organ recovery took place, in accordance with state requirements for reporting notifiable infectious diseases, if the deceased donor was infected. (Category IC)
28a. When a transplant center receives information that a recipient of an organ or blood vessel conduit from any deceased donor is newly infected with HIV, HBV, or HCV posttransplant and the infection is suspected of being donor-derived, the transplant center should notify (1) the OPTN and (2) the OPO that procured the organs and any blood vessel conduits. (Category IB) (Expert Opinion—Question 2)
28b. In accordance with state requirements for reporting notifiable infectious diseases, the transplant center where the transplant took place should also notify public health authorities of the recipient infection. (Category IC)
29a. When a living donor recovery center receives information before organ recovery that a living potential donor is infected with HIV, HBV, or HCV, the living donor recovery center should notify the transplant center intended to receive the organ. If the organ from an HBV- or HCV-infected donor is used for transplantation, the living donor recovery center should also notify the OPTN. (Category IB) (Expert Opinion—Question 2)
29b. In accordance with state requirements for reporting notifiable infectious diseases, the living donor recovery center should also notify public health authorities where the potential donor lives of the potential living donor's infection. (Category IC)
30a. When a living donor recovery center receives information after organ recovery that a living donor is infected with HIV, HBV, or HCV, the living donor recovery center should notify (1) the OPTN and (2) the transplant center that received an organ from the living donor. Disclosure to the OPTN and transplant center should be in accordance with state requirements. (Category IB) (Expert Opinion—Question 2)
30b. In accordance with state requirements for reporting notifiable infectious diseases, the living donor recovery center should also notify public health authorities where the organ recovery took place of the living donor's infection. (Category IC)
31. When a living donor recovery center receives information after organ recovery that an organ recipient infection with HIV, HBV, or HCV is suspected of being donor-derived, the living donor recovery center should notify the OPTN. (Category IB) (Expert Opinion—Question 2)
32a. When a transplant center receives information that a recipient of an organ from a living donor is newly infected with HIV, HBV, or HCV posttransplant and the infection is suspected of being donor-derived, the transplant center should notify (1) the OPTN and (2) the living donor recovery center that procured the organ. (Category IB) (Expert Opinion—Question 2)
32b. In accordance with state requirements for reporting notifiable infectious diseases, the transplant center should also notify public health authorities where the transplant took place of the recipient's infection. (Category IC)
33. A living donor whose blood specimen is positive for HIV, HBV, or HCV when tested by the living donor recovery center should be notified by the living donor recovery center of his or her infectious disease status. (Category ID)
34. OPOs should have a system in place allowing tracking between a common deceased donor and (1) recovered organs, (2) recovered associated blood vessel conduits, and (3) recovered tissues and eyes to facilitate notification when a donor-derived disease transmission is suspected. This system should include accurate records of the distribution and disposition of each organ and initial distribution of associated blood vessel conduits, along with procedures to facilitate the timely notification of transplant centers and tissue and eye recovery establishments when a donor-derived disease transmission is suspected. To facilitate notification by the OPO, transplant centers should keep accurate records of all organs and associated blood vessel conduits received and the disposition of each. (Category ID)
III. RECOMMENDATIONS FOR FURTHER STUDY
The systematic review for this guideline revealed numerous gaps in the evidence that affected the guideline's ability to adequately address many of the key questions reviewed. Additional gaps in evidence were identified from other sources, such as comments submitted during the public comment period or in review following public comment.
The following are 20 specific areas recommended for further study. These recommendations are arranged to correspond to the order of the 10 key questions followed by the three expert opinion questions; they are not listed in priority order.
Estimate the incidence and prevalence of HIV, HBV, and HCV among deceased potential organ donors in the U.S. (Key Question 1)
Collect, analyze, and report national data on HIV, HBV, and HCV infection transmission rates annually based on donor and recipient testing to inform policy decisions and future screening recommendations. (Key Question 2)
For transplant candidates who are HBV-uninfected and receive a non-hepatic organ from an HBV-infected donor who is anti-HBc positive only, evaluate transmission rates where IgM and IgG testing is performed and where various prophylaxis measures, including vaccination, are used as a way to improve knowledge of best practices to minimize transmission risk. (Key Question 2)
Conduct a cost-benefit and risk-benefit analysis of archiving blood specimens that are collected from transplant candidates who are not tested for HIV, HBV, and HCV just before organ transplantation. Analysis should include the feasibility of maintaining specimens at –70°C or colder (the storage temperature recommended by NAT test manufacturers) and patient safety issues associated with delays in determining whether an infection is donor-derived when a recipient is newly infected posttransplant with no pre-transplant blood specimen. (Key Question 2)
Identify behavioral and nonbehavioral risk factors associated with increased incidence and prevalence of HIV, HBV, and HCV infection specifically among the potential organ donor population, including pediatric donors. Such data could then be used to evaluate the utility of the 12-month risk period used in the donor behavioral risk assessment for indication of recent HIV, HBV, and HCV infection, and whether a shorter time interval may be an equally effective indicator. (Key Questions 3 and 4)
Develop and implement a validated uniform donor infection risk assessment questionnaire. To determine feasibility for possible inclusion in a future questionnaire, include the number of sexual partners in the preceding 12 months and intranasal use of an illicit drug (e.g., cocaine or heroin) in the preceding 12 months as survey questions only during the validation phase of the questionnaire. (Key Questions 3 and 4)
Prospectively study the performance of assays for HIV, HBV, and HCV in organ donors and transplant recipients (e.g., HCV NAT). Such data also could be used to enable the calculation of useful statistics including predictive values, likelihood ratios, and posttest probabilities of these tests among potential organ donors. (Key Question 5)
Evaluate the performance of tests, such as Ag/Ab combination assays, to be used for testing living and deceased donors and transplant recipients. (Key Question 5)
Develop standardized algorithms for real-time discrimination of initially reactive organ donor test results (e.g., immunoassay and NAT) to distinguish between true- and false-positive results. Retesting reactive specimens can better inform the utility of assays; the prevalence of infectious disease in the potential organ donor population; and decisions by OPOs, transplant centers, and transplant candidates on organ suitability and pre- and posttransplant recipient testing. (Key Question 5)
Assess interventions (e.g., pathogen reduction methods) to reduce or eliminate the viral burden of HIV, HBV, and HCV in donors or donor organs before or after recovery, but prior to transplantation. (Key Question 6)
Evaluate the risk-benefit of transplanting organs from HIV-infected donors into HIV-infected transplant candidates, given the need for transplants in HIV-infected patients and improved outcomes with the availability of highly active antiretroviral therapy. However, prior to any studies, legal analysis of the National Organ Transplant Act of 1984 (NOTA)20 and the U.S. Department of Health and Human Services (HHS) Final Rule21 may be required, which obligates the OPTN to adopt standards that prevent the recovery of organs from HIV-positive donors. (Key Question 7)
Evaluate outcomes of patients receiving HBV- or HCV-positive organs vs. patients who remain on the transplant wait list, with statistical adjustment for relevant baseline characteristics, consideration of posttransplant prophylaxis, and consideration of patient race/ethnicity. More comprehensive analyses of competing risks would help inform critical decision-making. (Key Question 7)
Evaluate transplant candidate outcomes if organ donors with behavioral and nonbehavioral risk factors for HIV, HBV, and HCV were declined. This process may also require comparing incidence of infection among population subsets within risk factors. If these donor organs are subsequently transplanted into other transplant candidates, include recipient outcome data. (Key Questions 8 and 9)
Evaluate the rate of false-positive test results (e.g., immunoassay and NAT) for HIV, HBV, and HCV among potential organ donors and recipients, including analysis of the results of confirmatory tests performed for any reactive test result and the percentage of cases in which donors are declined or organs are discarded due to false-positive results, stratified by organ type. (Key Question 10)
Identify the limits of acceptable hemodilution. Hemodilution algorithms and calculation methods are not standardized for organ donors, and the limits of acceptable hemodilution have not been validated across HIV, HBV, and HCV serologic assays used for organ donors. In addition, evaluate the effect of analyte movement from the vascular compartment during and immediately following the introduction of crystalloids or colloids to the vascular system. (Expert Opinion 3)
To better quantify risk based on behavior in a given donor, develop and evaluate a relative or comparative risk-based quantitative process, such as a donor risk index, to allow the transplant center and patient to assess a donor based on the donor's level of risk for transmitting HIV, HBV, or HCV. Because data are lacking to calculate precise quantitative values, risk assessments in this guideline are qualitative (i.e., a donor is categorized either as being at increased risk or not at increased risk). (From public comment)
Conduct a risk-benefit analysis of storing blood vessel conduits from HBV- and HCV-infected donors where the vessels would be used in transplant recipients who received an organ from the infected donor. Analysis should include the number of these recipients who are in need of subsequent vascular repair, the time frame between transplant and subsequent repair, and the availability of vessels from uninfected donors and other sources. (From public comment)
Study the effectiveness of systems for traceability, such as electronic bar coding, to ensure blood vessel conduits are transplanted into the intended candidates or recipients, which may allow for the safe storage of hepatitis-infected grafts for later potential use. (During review following public comment)
Evaluate transplantation, infection, and hepatic graft outcomes for transplant candidates (both HBV-positive and HBV-negative) who receive organs from HBV-positive donors. (During review following public comment)
Evaluate transplantation, infection, and hepatic graft outcomes for transplant candidates (both HCV-positive and HCV-negative) who receive organs from HCV-positive donors. (During review following public comment)
IV. BACKGROUND
Federal oversight of organ recovery and transplantation
Federal agencies within HHS regulate or oversee the procurement and transplantation of organs. HRSA, a PHS agency, provides oversight of organ procurement and transplantation through the OPTN. As amended, NOTA requires that the OPTN be administrated by a private, nonprofit entity through a contract overseen by HRSA. UNOS is the entity that currently serves as the contractor to operate the OPTN.
NOTA contains two requirements concerning the procurement and transplantation of organs from donors regarding HIV infection status. First, the OPTN is required to “adopt and use standards of quality for the acquisition and transportation of donated organs, including standards for preventing the acquisition of organs that are infected with the etiologic agent for acquired immune deficiency syndrome.”22 Second, each OPO is required to “arrange for the acquisition and preservation of donated organs and provide quality standards for the acquisition of organs which are consistent with the standards adopted by the [OPTN] under [42 U.S.C. 274(b)(2)(E)], including arranging for testing with respect to preventing the acquisition of organs that are infected with the etiologic agent for acquired immune deficiency syndrome.”23
HHS's implementing regulations governing the operation of the OPTN (the OPTN Final Rule), codified at 42 C.F.R. Part 121, provide that “[t]he OPTN shall adopt and use standards for preventing the acquisition of organs from individuals known to be infected with human immunodeficiency virus.” The OPTN Final Rule also provides that “[a]n OPTN member procuring an organ shall assure that laboratory tests and clinical examinations of potential organ donors are performed to determine any contraindications for donor acceptance, in accordance with policies established by the OPTN.”24 Finally, the OPTN is responsible for developing policies “consistent with recommendations of the Centers for Disease Control and Prevention [CDC] for the testing of organ donors and follow-up of transplant recipients to prevent the spread of infectious diseases.”25 Thus, with regard to the screening and testing of organs to prevent the spread of infectious diseases, the OPTN is charged with developing policies that are consistent with PHS guidelines while also ensuring that the OPTN adopts and uses standards for preventing the acquisition, within the OPTN system, of organs known to be infected with HIV. The OPTN Final Rule describes the process for developing OPTN policies, which includes an opportunity for OPTN members and members of the public to comment on proposed policies.25 OPTN policies are not subject to sanctions by the HHS Secretary unless and until such policies are approved by the HHS Secretary in accordance with the OPTN Final Rule.
HIV, HBV, HCV, and organ transplantation
Transplantation of organs, including kidney, heart, liver, lung, pancreas, and intestine, to patients with end-stage organ disease is performed to improve recipient survival and functional capacity. Organs can be donated by living or deceased donors, with a majority of organs recovered from deceased heart-beating donors (i.e., donors after brain death, as diagnosed by means of neurological criteria). Transplantation rates differ by organ. Kidney transplantation occurs most often followed by liver, heart, lung, kidney-pancreas, intestine, and heart-lung transplants. On average, three organs are recovered from each deceased donor. In 2011, a total of 27,698 patients on the OPTN transplant wait list received organ transplants from 8,126 deceased donors and 6,022 living donors in the U.S. (Unpublished data, UNOS Research Department, OPTN, July 2012).
A large discrepancy exists between the number of candidates on the transplant list and the number of organs available, with thousands of patients on the wait list dying annually. To narrow this gap and respond to the urgency of organ transplantation, the OPTN has attempted to increase the organ donor pool by facilitating placement of expanded criteria donors (i.e., donors meeting certain criteria such as ≥60 years of age where transplanted kidneys typically have a decreased rate of graft survival)26 and recovering organs from donors who may be at increased risk of harboring transmissible infections, including HIV, HBV, or HCV.
OPTN policy was revised in 2005 to require OPOs and transplant centers to report to the OPTN any unexpected potential transmission of an infection from an organ donor (e.g., HIV, Mycobacterium tuberculosis, Strongyloides, and West Nile virus).27 When an organ recipient is suspected of having a donor-derived infection, the OPTN and all institutions that recovered organs or tissues or that transplanted organs from the donor are notified. Notification should occur immediately so that recipient evaluation for infection can be initiated and further distribution or use of potentially infected tissues can be prevented. Notification of state public health authorities should also occur when organ donors or recipients are identified as newly infected with HIV, HBV, or HCV. For public health purposes, when a deceased potential or actual donor is found to be infected, it is important that next of kin be notified, in accordance with state law, because of possible transmission between the donor and close contacts, depending on the pathogen.
In most instances, an investigation ensues with testing of donor and recipient blood specimens to determine the infection source and provide information to facilitate medical treatment decisions. OPTN policy28 and standards established by the Association of Organ Procurement Organizations require deceased donor blood specimens to be retained for 10 years following procurement of organs. HIV, HBV, and HCV are nationally notifiable diseases, and confirmed infections require notification to local or state health agencies, as stipulated. All cases are reviewed by the OPTN Ad Hoc Disease Transmission Advisory Committee (DTAC) to determine the likelihood of the donor being the source of infection.29
From January 1, 2005, to December 31, 2007, 30 recipients were confirmed (identified as proven, probable, or possible by DTAC) to have a donor-derived infectious disease transmission, including one co-transmission of HIV and HCV from a donor to four recipients.29 From January 1, 2008, to December 31, 2011, 104 recipients were confirmed (identified as proven or probable) to have a donor-derived infectious disease transmission from 74 donors. Of these 104 recipients, HCV transmission occurred in 10 recipients involving six donors, HBV transmission occurred in four recipients involving two donors, and HIV transmission occurred in one recipient involving one donor (Unpublished data, UNOS Research Department, OPTN, October 2012).
Expected vs. unexpected donor-derived infection
Donor-derived infections can be divided into expected and unexpected transmissions. Expected transmissions occur when organs are transplanted from donors who are known to be infected with specific diseases. Unexpected transmissions occur when donor infections are not detected prior to transplantation.
Federal regulations exclude donation from potential blood and tissue donors who have certain risk factors for bloodborne pathogen infections, such as HIV. However, organs from donors at increased risk for disease transmission are not excluded, but can be accepted by transplant programs if potential recipients are informed of the risks involved and consent to receive the organ.27 This guideline provides a definition of organ donors at increased risk of HIV, HBV, and HCV infection to better inform clinical practice, including informed consent discussions. These discussions would likely reflect the risk of disease transmission in the overall context of other risks associated with transplantation.
Expected transmissions are a common occurrence in organ transplantation. Given the large disparity between the need for and availability of solid organs and that certain organ transplants are lifesaving, it has become acceptable medical practice to transplant organs from donors with certain infections. A large number of donors with recognized infections—such as cytomegalovirus and Epstein-Barr virus, and less frequently HBV and HCV—are used as part of routine practice. When routine laboratory blood tests detect infection in the donor, OPTN policy requires transplant programs to obtain informed consent prior to organ transplantation.27 In addition, preventive interventions or monitoring and testing must be offered to the recipient posttransplant, as appropriate.
Expected HBV and HCV donor-derived infection.
Transplantation of organs from HBV- and HCV-infected donors is accepted medical practice. These organs are typically offered to recipients who are known to be infected with the same pathogen or, in rare circumstances, to uninfected recipients in cases of urgent medical need where the benefit is deemed to outweigh the risks.
In 2011, at least 1,210 organ transplants were reported in the U.S. from donors who tested positive for HBV, HCV, or both HBV and HCV. Of these donor organs, 539 were transplanted into recipients who were known to be infected with the virus, 121 into recipients who were known to be uninfected with the virus, and 550 into recipients whose infectious status for HBV or HCV was not reported. Donors and recipients reported as positive for HBsAg or anti-HBc were defined as HBV-positive. Donors and recipients reported as positive for anti-HCV were defined as HCV-positive (Unpublished data, UNOS Research Department, OPTN, June 2012).
In these situations, prophylaxis or treatment with immunizations, antivirals, and/or immunoglobulin is offered, if appropriate, to prevent virus transmission or development of hepatitis disease, or to reduce the disease severity. Therefore, early posttransplant recipient testing for HBV or HCV is critical, unless the recipient is known to be infected prior to transplantation. Early posttransplant recipient testing is also important in circumstances where the donor test results are negative for HIV, HBV, or HCV, but the donor is identified as being at increased risk for infection or risk for infection cannot be determined (e.g., hemodiluted blood specimen).
Unexpected HIV, HBV, and HCV donor-derived infection.
Reports of unexpected HIV, HBV, and HCV transmission have occurred when laboratory blood tests could not detect donor infection. Most commonly, this lack of detection happens when a donor becomes infected close to the time of organ donation. There is an initial period of time after exposure during which the virus replicates in target cells.30,31 The primary site for HIV is the CD4+ lymphocytes.32 Hepatitis virus replication is mainly confined to hepatocytes in the liver. During this phase, the virus is not detectable in blood nor thought to be transmitted through blood transfusion; however, the virus is present in infected organs. At the end of this phase, low concentrations of virus begin to circulate in the blood, followed by an exponential increase enabling detection by NAT.31 Viral production eventually plateaus and subsequently declines due to the formation of antibodies, which may be days or weeks after detectable virus appears.33
The window period between the onset of viremia (i.e., the presence of virus in the blood) and detection of viral material or antibodies can vary depending on the particular virus, the sensitivity of the test used, and the initial viral load at the time of inoculation. For deceased donors, the availability of only a hemodiluted specimen for testing also contributes to the inability to detect infection. With individual donor NAT, HIV RNA detection is estimated to be five to six days after the onset of viremia;30,33,34 HCV RNA has a shorter window period of three to five days.33,34 Several HIV Ag/Ab combination assays have demonstrated detection of HIV infection soon after a positive NAT with average detection times of two to nine days after NAT,35–37 for an estimated window period of seven to 15 days after the onset of viremia. Ab detection is estimated to be 19 to 20 days after the onset of viremia for HIV,30,33,34 with a longer window period of 58 to 65 days for HCV.33,34 The window period for HBV deoxyribonucleic acid (DNA) detection is much longer compared with HIV and HCV; estimates range from 20 to 25 days after the onset of viremia with individual donor NAT and 36 to 44 days to detection of HBsAg after the onset of viremia.33,38
Infections transmitted through transplantation have occurred despite improved donor screening. The following are published transmission cases since 2000:
In 2000, HCV was transmitted to three organ and five tissue recipients from a common donor who was negative for anti-HCV at the time of organ and tissue procurement. Two years following the donation, after a recently transplanted tissue recipient was diagnosed with acute HCV infection, an archived donor blood sample was tested for HCV by NAT with virus detected.8
In 2007, simultaneous transmission of HIV and HCV occurred in four organ recipients from the same donor who was identified as high risk due to a reported history of engaging in MSM behavior. Routine donor blood test results were negative for HIV and HCV antibodies. Ten months after transplantation, a kidney recipient tested positive for HIV and HCV. Subsequently, the heart, liver, and other kidney recipients tested positive for HIV and HCV, and an archived donor specimen tested positive for HIV and HCV by NAT.9
In 2009, a liver transplant recipient who was negative for anti-HCV prior to surgery inadvertently received a stored vessel conduit from an anti-HCV-positive donor and tested HCV-positive posttransplant. The transplanted liver and vessel conduit were from two different donors. At that time, transplant centers were allowed to store recovered vessel conduits from HBV- and HCV-positive donors for use in transplant patients experiencing surgical complications.10
In 2009, a kidney recipient acquired HIV from a living donor transplant. The donor had a history of engaging in MSM behavior, but was negative for anti-HIV approximately two months before organ recovery. One year post-recovery, the donor tested HIV-positive. Stored recipient specimens collected 11 days pre-transplant and 12 days posttransplant were tested by HIV NAT, with the posttransplant specimen confirmed positive. The stored donor specimen collected 11 days pre-transplant was also HIV NAT-positive.11
In 2010, three of five organ transplant recipients were infected with HBV from a donor who was identified as not being at increased risk for HBV infection and was HBV-negative using serologic testing. HBV transmission through transplantation was suspected approximately one year later when a recipient tested positive for HBV infection. In retesting a stored donor specimen, the results were HBV serology-negative, but HBV NAT-positive, which is indicative of a recent infection.12
In 2011, two kidney recipients tested HCV-positive approximately six months after transplantation. Donor blood specimens, tested by the OPO and tissue bank, were negative for anti-HCV. However, it was discovered during the investigation that an HCV NAT result had been incorrectly read as negative at the time of donation. Retesting of a stored donor specimen confirmed that the donor had been HCV NAT-positive. Although a recall of distributed donor tissue was initiated, a cardiopulmonary patch already had been implanted in one recipient, who was infected with HCV from the donor graft.13
Prevalence and incidence estimates of HIV and HCV among deceased potential organ donors
In a study of 13,667 potential organ donors evaluated from January 2004 through mid-2008 by 17 OPOs recovering organs from more than half of the deceased donors in the U.S., the prevalence of HIV and HCV in normal-risk potential donors was 0.10% and 3.45%, respectively; the prevalence of HIV and HCV among potential donors identified by OPOs as high risk was 0.50% and 18.20%, respectively. Test results that are NAT-positive but Ab-negative serologically are indicative of recent, or incident, infections. Applying a model based on known prevalence, Ellingson et al. estimated the incidence of undetected viremia for normal-risk potential donors to be one in 60,000 for HIV and one in 5,000 for HCV. For high-risk potential donors, the incidence of undetected viremia was estimated to be one in 12,000 for HIV and one in 1,000 for HCV. For the study, potential organ donors were those who had authorization for donation, and organs may or may not have been recovered. Of the 64 potential donors positive for anti-HIV, six donors had organs recovered from them, but none of the organs was transplanted. Of the 924 potential organ donors positive for anti-HCV, 332 of them did not have organs recovered.39
Donor screening and testing
OPTN policy lists the minimum standards for an OPO in evaluating deceased potential donors. This evaluation includes screening the donor for risk factors for communicable diseases by obtaining a donor medical and behavioral history, reviewing the medical chart, obtaining vital signs, performing a physical examination, and testing the donor for specific communicable infections, including HIV, HBV, and HCV.29 Living potential kidney donors also are required to be evaluated for exposure to HIV, HBV, and HCV through a social history evaluation and testing.40
OPTN policy defines a potential donor as being at “increased risk”28 if he or she meets any of the exclusionary criteria in the 1994 PHS guidelines.1 The definition of an increased risk donor has been expanded by many OPOs to include criteria associated with other infections, such as HBV and HCV. A 2008 survey of OPOs found that the percentage of recovered organ donors who were reported as having behaviors that the OPO classified as “high risk” varied among these organizations from 2.3% to 26.1% of annual donor volume.41
Transplant candidate and recipient testing
According to OPTN policy, prior to being placed on the transplant wait list, transplant candidates must be tested for HIV, HBV, and HCV “except in cases where such testing would violate applicable federal laws or regulations.”27 Transplant centers may choose to evaluate candidates for additional infections to determine suitability for placement on the transplant wait list. Candidates who test positive for HIV may be placed on the wait list if judged to be medically appropriate by the transplant center.
Because donor-derived infections can result in substantial morbidity and mortality, particularly when there is a delay in diagnosis, the 1994 PHS guidelines recommended that recipients be tested for HIV immediately prior to transplantation and at three months posttransplant, until the risk of HIV transmission from organ donors was clarified by future studies. However, testing recipients who receive organs from increased-risk donors is not required by OPTN policy, and data have shown it is not standard practice.42 Benefits of routine testing in these situations include early identification of infection and treatment before signs and symptoms develop, as well as early notification of recipients of organs from the same donor should a donor-derived infection be suspected. Because testing recipients posttransplant by serology alone could miss infection, direct testing for the virus, by quantitative viral load or NAT, is recommended.9
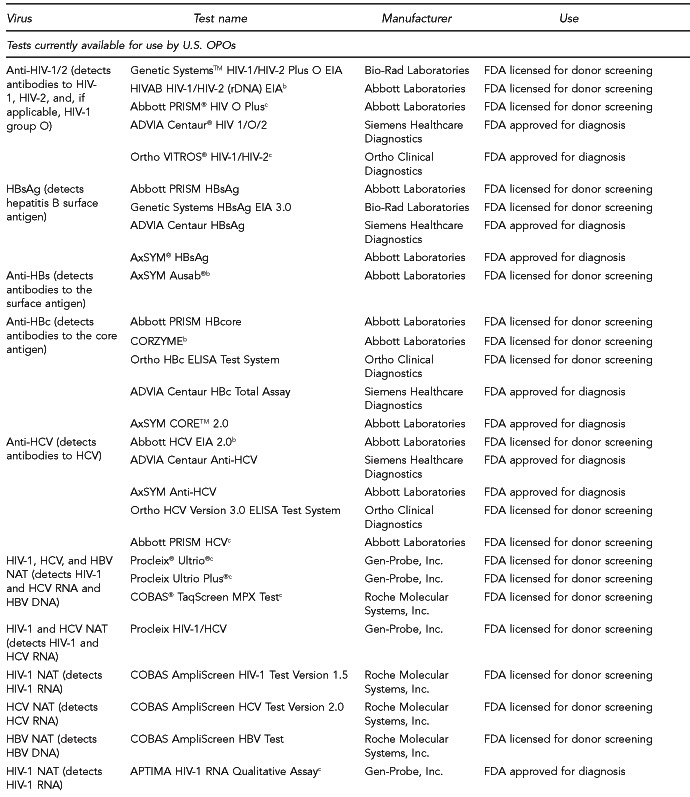
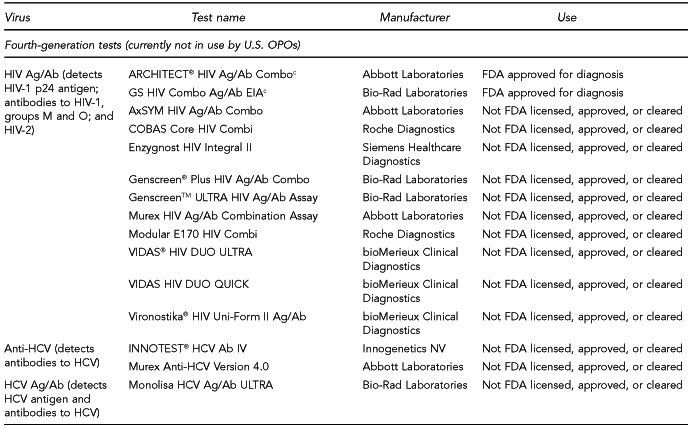
Available assays for HIV, HBV, and HCV detection
FDA has licensed several HIV, HBV, and HCV tests for donor screening or diagnostic uses, including assays that detect antibodies to the virus, viral Ag, and viral genetic material (Figure 7). Fourth-generation tests, which are combined Ag/Ab tests, have recently been developed for HIV and HCV. Each generation of serologic test has decreased the time period for detecting initial infection. Based on data submitted by the manufacturer, FDA determines if these tests can be labeled for use with particular specimen types (e.g., fresh or frozen samples, serum, or plasma) or in donor screening or diagnosis of disease. If the test is intended for donor screening, the data submitted would determine for which donors (e.g., living donors, deceased [pre-asystole] organ donors, or cadaveric [post-asystole] donors) the assay may be labeled for use. Currently, there are fourth-generation HIV tests approved by FDA for diagnostic use, but there is no fourth-generation HCV test licensed or approved by FDA for use in the U.S.
Figure 7.
HIV, HBV, and HCV tests used for organ donor screeninga
aFor the systematic review of the literature, 35 tests of interest were included: (1) FDA-licensed or -approved immunoassays and NAT assays routinely used by U.S. OPOs at the time the literature review began and (2) fourth-generation HIV and HCV Ag/Ab tests in use outside of the U.S.
bNo longer available in the U.S.
cFDA-licensed or -approved assays not routinely used by U.S. OPOs at the time of the literature review, and not included in the review
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
OPO = organ procurement organization
EIA = enzyme immunoassay
FDA = Food and Drug Administration
rDNA = recombinant deoxyribonucleic acid
HBsAg = hepatitis B surface antigen
Anti-HBs = antibody to hepatitis B surface antigen
Anti-HBc = antibody to hepatitis B core antigen
Anti-HCV = antibody to HCV
ELISA = enzyme-linked immunosorbent assay
NAT = nucleic acid test
RNA = ribonucleic acid
DNA = deoxyribonucleic acid
Ag/Ab = antigen/antibody
OPTN policy requires deceased donor testing for anti-HIV 1/2, HBsAg, anti-HBc, and anti-HCV. Anti-HIV testing must be performed using an FDA-licensed screening test. FDA-licensed, approved, or cleared serologic screening tests are required for HBV and HCV; however, an FDA-licensed, approved, or cleared diagnostic test is permitted when an FDA-licensed screening test is unavailable.28
Although there are minimum standards for organ donor blood-specimen testing, actual testing protocols vary. The serologic tests used differ based on the OPO. In addition, the use of NAT for HIV, HBV, and HCV testing varies significantly. NAT has received increased attention given that in instances of recent exposure to HIV, HBV, or HCV, NAT can detect virus days to months before antibodies develop, depending on the virus. However, there are also concerns with NAT, including the lack of standard algorithms to confirm an initial positive result, the potential for false-positive results if testing is performed in a laboratory where staff lack proficiency or testing volume is low,43 the feasibility of performing NAT, and the duration of time to perform the test. In 2008, approximately 50% of OPOs reported performing HIV and HCV NAT on all potential donors compared with 25% for HBV NAT. Of the OPOs that used NAT, 45% sent the specimens to a different state where 24/7 NAT screening was available. Other OPOs reported using on-site locations, transplant center laboratories, and outside laboratories within the same state.41
V. METHODS
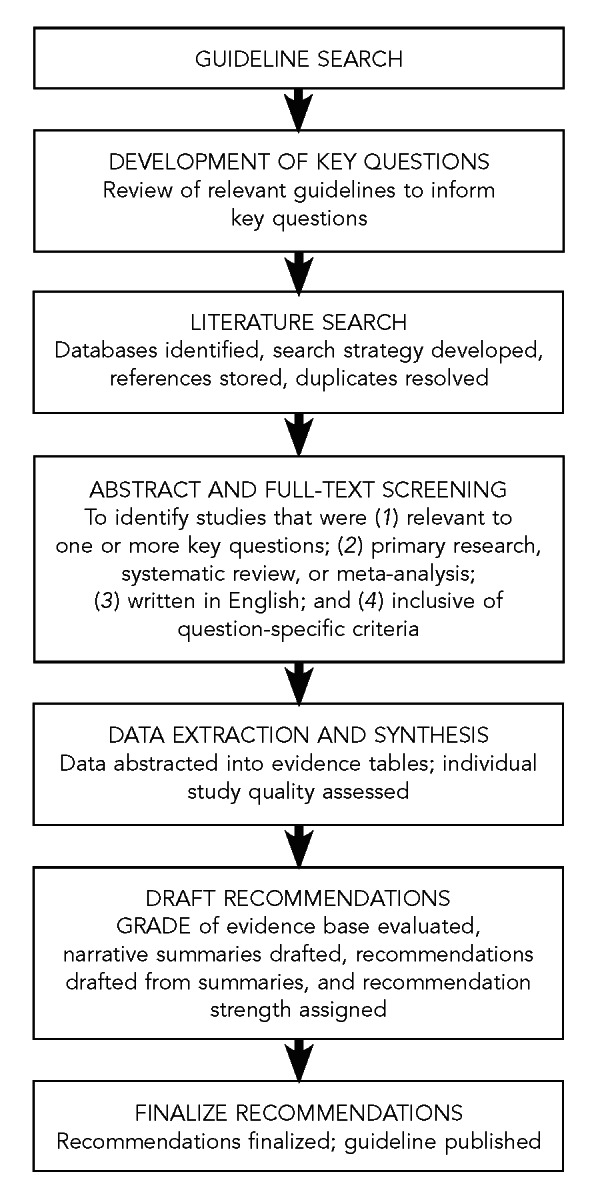
As described previously, recommendations related to the 10 key questions were based on a targeted, systematic review of the best available evidence on reducing HIV, HBV, and HCV infection transmitted through organ transplantation. We used a modified GRADE approach14–18 to provide explicit links between the available evidence and the resulting recommendations. The guideline development process is outlined in Figure 8.
Figure 8.
Evidence-based process used to develop guideline recommendations for reducing HIV, HBV, and HCV transmission through organ transplantation
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
GRADE = Grading of Recommendations Assessment, Development, and Evaluation
Development of the guideline involved participation by multiple groups. The Methodology Working Group included staff from the CDC Office of Blood, Organ, and other Tissue Safety in the Division of Healthcare Quality Promotion; the Center for Evidence-Based Practice at the University of Pennsylvania Health System; and the ECRI Institute. This group was accountable for all phases of guideline methodology, including the development of key questions and the Evidence Report,19 as well as providing the Expert Panel and Review Committee with progress updates. The Expert Panel comprised individuals with subject-matter expertise; assistance was sought from various members of the Expert Panel to address specific issues throughout development of the guideline. The Review Committee was formed to provide stakeholder input from a public health, regulatory, and transplantation perspective for the topics addressed in the guideline, as well as contribution from manufacturers of infectious disease tests. Both the Expert Panel and Review Committee participated in regular updates via conference calls at key steps and provided review and feedback on the key questions, the bibliography resulting from the literature review, the Evidence Report,19 and the guideline content. The PHS Guideline Revision Working Group performed an in-depth review of public comment submitted regarding the draft guideline recommendations and participated in revision of the full document. The PHS Guideline Revision Working Group comprised representatives from the Office of the Assistant Secretary for Health and PHS agencies.
Development of key questions
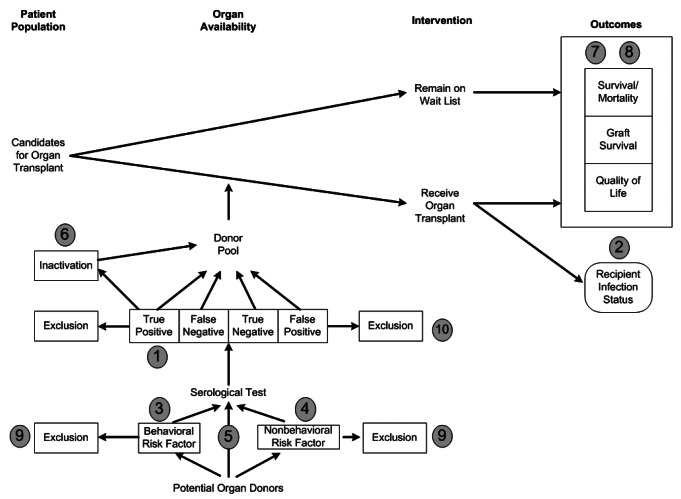
We first conducted an electronic search of the Agency for Healthcare Research and Quality's National Guideline Clearinghouse®, the National Library of Medicine's MEDLINE® database, EMBASE®, and the Cochrane® Health Technology Assessment Database. We then contacted experts to identify existing national and international guidelines and reviews relevant to HIV, HBV, and HCV transmission in organ transplantation. A preliminary list of key questions was developed from a review of the relevant guidelines and reviews were identified in the search. Key questions were put in final form after vetting them with the Expert Panel and Review Committee. An analytical framework depicting the relationship among the key questions is included in Figure 9.
Figure 9.
Analytical framework depicting the relationships among donor characteristics, organ availability, patient interventions, and subsequent outcomesa
aNumbers represent the 10 key questions about organ donation and transplantation used to guide the literature review on HIV, HBV, and HCV transmission through organ transplantation.
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
Literature search
Following the development of the key questions, we developed search terms to identify the literature that was most relevant to those questions. For quality assurance purposes, we compared these terms with those used in relevant seminal studies and reviews. These search terms were then incorporated into search strategies for the relevant electronic databases. Searches were performed in EMBASE, The Cochrane Library Databases, the National Library of Medicine's PREMEDLINE® and MEDLINE, the Agency for Healthcare Research and Quality's National Guideline Clearinghouse, and the ECRI Institute Healthcare Standards Directory. Resulting references were imported into a citation management database where duplicates were resolved; the database was last updated on June 30, 2009. Mechanisms used to retrieve additional relevant information included review of bibliographies/reference lists from peer-reviewed and grey literature (i.e., reports, studies, articles, and monographs that do not appear in the peer-reviewed journal literature and are produced by federal and local government agencies, private organizations, educational facilities, consulting firms, and corporations). The detailed search strategy used to identify primary literature can be found in the Evidence Report.19
Study selection
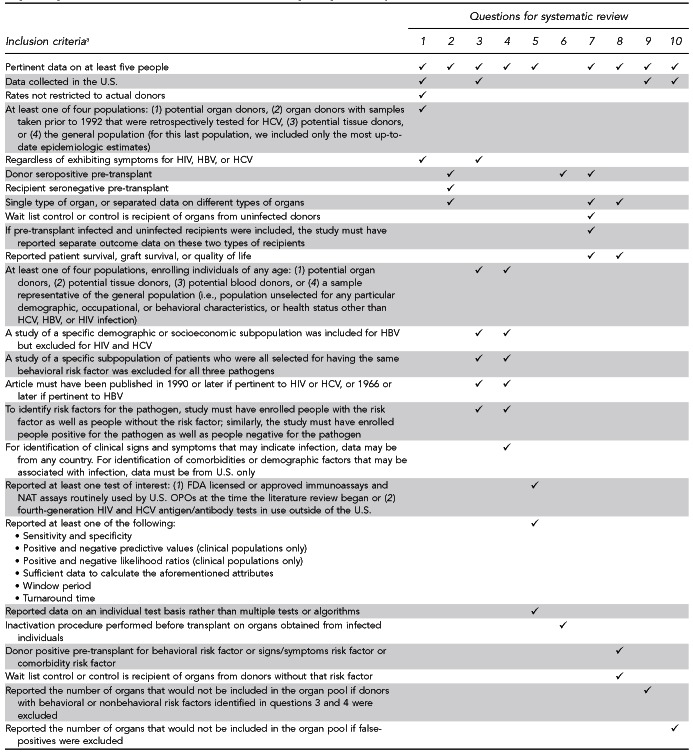
Titles and abstracts from references were screened by a single reviewer from ECRI. Full-text articles were retrieved if they were relevant to one or more key questions and met inclusion criteria (i.e., universal as well as question-specific criteria). Universal criteria included studies that were written in English; were peer-reviewed, full-length publications with original data; and included HIV, HBV, or HCV with determination of the infection based on laboratory test(s) rather than subjective estimates, physician interviews, or patient interviews. Additional criteria applied on a per-question basis are depicted in Figure 10.
Figure 10.
Question-specific inclusion criteria applied during the systematic review of the literature regarding HIV, HBV, and HCV transmission through organ transplantation
aFor each question for systematic review, universal criteria were also applied. A checkmark in a given column means that a study must have met that criterion to be included for the numbered key question for systematic review.
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
FDA = Food and Drug Administration
NAT = nucleic acid testing
OPO = organ procurement organization
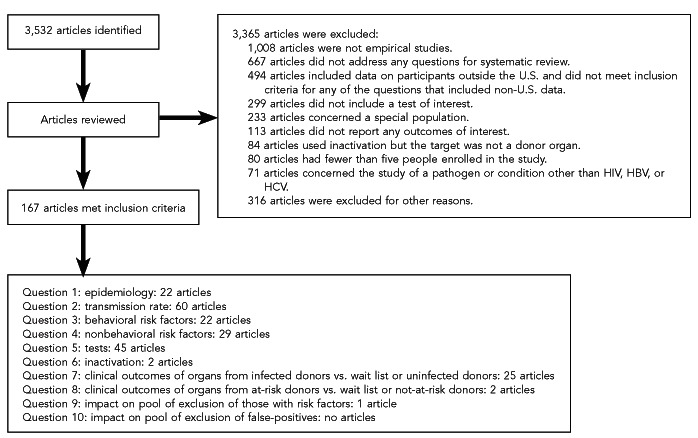
We treated multiple publications of the same study as a single study rather than as multiple studies to avoid double-counting patients. Two independent reviewers from ECRI screened full-text articles and resolved disagreements through discussion. The results of this process are shown in Figure 11. To ensure that all relevant studies were captured in the search, the Expert Panel and Review Committee vetted the bibliography.
Figure 11.
Results of the study selection process to identify articles meeting inclusion criteria for the 10 key questions about HIV, HBV, and HCV transmission through donated organsa
aSelected articles provided the evidence base to develop guideline recommendations for reducing HIV, HBV, and HCV transmission through organ transplantation.
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
The specific tests of interest for Key Question 5 (What are the test characteristics of the screening methods available to detect HIV, HBV, and HCV in potential organ donors? Do test characteristics differ in particular populations and with donor clinical status [i.e., heart-beating vs. non-heart-beating donors or adult vs. pediatric donors]?) are listed in Figure 7.
Data extraction and synthesis
For those studies meeting inclusion criteria, a single reviewer from ECRI extracted the data into evidence tables. The remaining Methodology Working Group members resolved any disagreements regarding inclusion. Data and analyses were extracted as originally presented in the included studies and displayed in evidence tables for each question. For the purposes of our review, we defined statistical significance as p≤0.05.
Grading of evidence
First, the quality of each study included was assessed using the quality assessment criteria (adapted from existing instruments for quality assessment) listed in Figure 12. Next, the Methodology Working Group assessed the evidence bases described in the evidence tables for each key question using methods adapted from GRADE. GRADE tables were developed for each of the key questions and included any outcomes listed in the evidence tables that were judged to be clinically important, the quantity and type of evidence for each outcome, the relevant findings, and the GRADE of evidence for each outcome.
Figure 12.
Criteria used to assess data quality of each selected study for key questions regarding HIV, HBV, and HCV transmission through organ transplantationa
aThe quality rating is one of several criteria that determine the GRADE of an evidence base for an outcome of interest.
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
GRADE = Grading of Recommendations Assessment, Development, and Evaluation
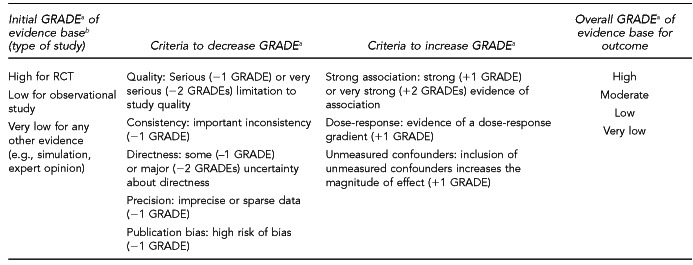
The initial GRADE of evidence for each outcome was deemed high if the evidence base included a randomized controlled trial (RCT) or a systematic review of RCTs, low if the evidence base included only observational studies, or very low if the evidence base consisted only of expert opinion or uncontrolled studies. The initial GRADE could then be modified by as many as nine criteria. Criteria that could decrease the GRADE of an evidence base included shortcomings in quality (Figure 12), consistency, directness, precision, and publication bias. Criteria that could increase the GRADE included a large magnitude of effect, a dose-response gradient, or inclusion of unmeasured confounders that would increase the magnitude of effect (Figure 13). For questions regarding prevalence, incidence, or rates of transmission from donors to recipients (Key Questions 1, 2, 3b, and 4b), no RCTs were necessary to address the questions. Therefore, the starting evidence GRADE was high, and we applied the other components of the GRADE system as appropriate. GRADE definitions are as follows:14
High—further research is very unlikely to change confidence in the estimate of effect.
Moderate—further research is likely to affect confidence in the estimate of effect and may change the estimate.
Low—further research is very likely to affect confidence in the estimate of effect and is likely to change the estimate.
Very low—any estimate of effect is very uncertain.
Figure 13.
Process for rating the quality of evidence for each outcome of interest concerning HIV, HBV, and HCV transmission through organ transplantation
aThe GRADE approach establishes an overall quality rating of the evidence base for each outcome of interest.
bThe initial GRADE of the evidence base for each outcome of interest depends on the type of study (or types of studies) evaluated for that outcome. The final GRADE category of the evidence base could be higher or lower than the initial GRADE category based on the criteria noted in the figure.
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
GRADE = Grading of Recommendations Assessment, Development, and Evaluation
RCT = randomized controlled trial
After determining the GRADE of the evidence base for each outcome of a given key question, we calculated an overall GRADE of the evidence base for any sets of outcomes within the GRADE figure for the key question. The overall GRADE was based on the GRADE category occurring most often for the outcomes deemed critical to making a -recommendation; if more than one GRADE category occurred at the same count, the overall GRADE was based on the lowest GRADE. For questions that had outcomes that were not deemed critical by the Methodology Working Group, no overall GRADE was assigned to the evidence.
Formulating recommendations
Narrative evidence summaries were then drafted by the guideline authors using the evidence and GRADE tables. One summary was written for each key question. The guideline authors used the narrative evidence summaries to develop guideline recommendations. In some instances, multiple recommendations emerged from a single narrative evidence summary.
Factors determining the strength of a recommendation included the following: (1) the values and preferences used to determine which outcomes were critical, (2) the harms and benefits that emerged by weighing the critical outcomes, and (3) the overall GRADE of the evidence base. A fourth factor, resource use, was not systematically considered.16 The categorization scheme for recommendations is shown in Figure 3.
If weighing the critical outcomes for a given key question resulted in a net benefit or a net harm, then a Category I recommendation was formulated to recommend strongly for or against the given intervention, respectively. If weighing the critical outcomes for a given key question resulted in a trade-off between benefits and harms, then a Category II recommendation was formulated to recommend that providers or institutions consider the intervention when deemed appropriate.
Category I recommendations are defined as strong recommendations with the following implications:16
For patients: Most people in the patient's situation would want the recommended course of action and only a small proportion would not; the patient should request discussion if the intervention is not offered.
For clinicians: Most patients should receive the recommended course of action.
For policy makers: The recommendation may be adopted as policy or is currently part of federal and/or state statutes, regulations, or standards.
Category II recommendations are defined as weak recommendations with the following implications:16
For patients: Many people in the patient's situation would want the recommended course of action, but many would not.
For clinicians: Different choices will be appropriate for different patients, and clinicians must help each patient to arrive at a management decision consistent with her or his values and preferences.
For policy makers: Policy-making will require substantial debate and involve many stakeholders.
Levels A and B represent the quality of the evidence underlying the recommendation, with A representing high- to moderate-quality evidence and B representing low- to very low-quality evidence. Level C represents required practices by state or federal regulations, regardless of evidence quality.
We compared evidence-based recommendations with those from guidelines identified in our original systematic search and identified four recommendations from the 1994 PHS guidelines1 for topics not directly addressed by our systematic review of the evidence. These recommendations are included in the Recommendations section, as they were deemed critical to the target users of this guideline. We revised the recommendations to make them applicable to current expected or actual practice. Two recommendations, in response to a 2009 HIV transmission from a living organ donor,11 were deemed critical to the target users and included in the Recommendations section for the same reason. One recommendation, in response to inadvertent use of an infected blood vessel conduit, was also deemed critical to target users of this guideline.10 Unlike recommendations informed by our literature search, these recommendations are not linked to a key question and were listed as Level D.
The strength of a Category IA recommendation is equivalent to that of a Category IB, IC, or ID recommendation; it is only the quality of the evidence that makes each category different. Recommendations related to the three expert opinion questions were designated either IB if they represent a strong recommendation or IIB if they represent a weak recommendation because they were based on expert opinion only. Recommendations included from previously published guidelines or reports were designated ID, as the theoretical benefits for each recommendation were clear, regardless of evidence quality.
The wording of each recommendation was carefully selected to reflect the recommendation's strength. When writing Category I recommendations (strong recommendations), we used phrases such as “should” or “should not” and verbs without conditionals to convey certainty. When writing Category II recommendations (weak recommendations), we chose words such as “consider” and phrases such as “may be considered” or “should be considered” to reflect the lesser certainty of the Category II recommendations. Rather than a simple statement of fact, each recommendation is actionable, describing precisely a proposed action to take. All recommendations focus only on efficacy, effectiveness, and safety. Yet, the optimal use of this guideline should include a consideration of the costs relevant to the local setting of guideline users.
Figures from the Evidence Report
The figures in this guideline are from the Evidence Report,19 except for Figures 2–6, 8, 13, and 14.
Figure 14.
Behavioral and nonbehavioral characteristics associated with HIV, HBV, or HCV identified by low- to high-quality evidence from a systematic review of the literature regarding the risks of transmitting HIV, HBV, and HCV through organ transplantation
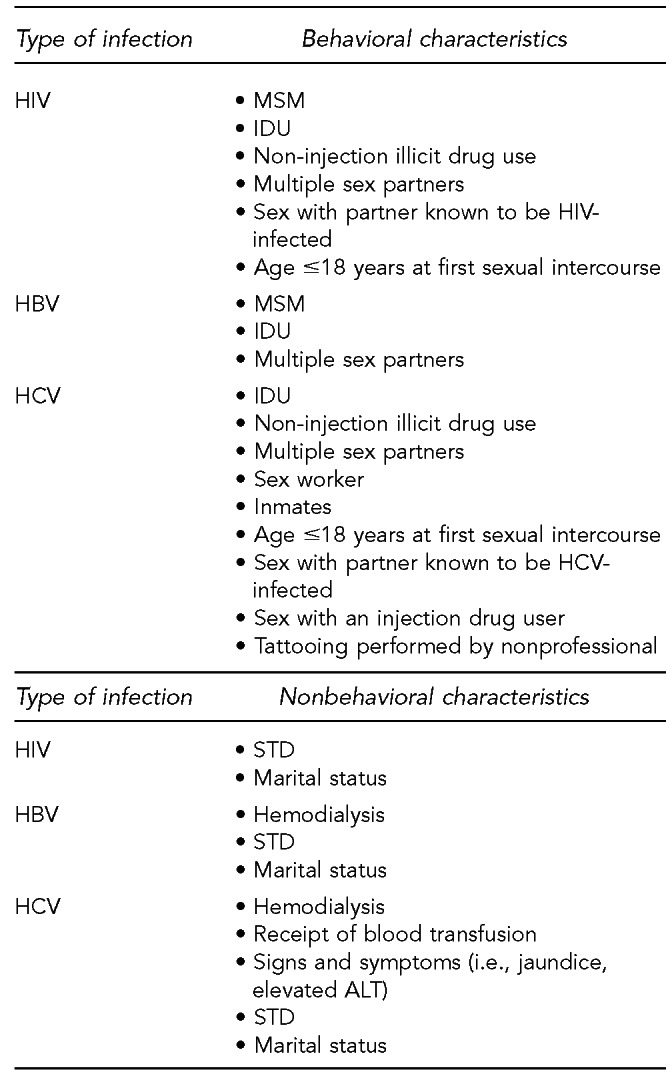
HIV = human immunodeficiency virus
HBV = hepatitis B virus
HCV = hepatitis C virus
MSM = men who have sex with men
IDU = injection drug use
STD = sexually transmitted disease
ALT = alanine aminotransferase
Finalizing the guideline
After a draft of the tables, narrative summaries, and recommendations was completed, the guideline authors shared the draft guideline with the Expert Panel and Review Committee and made revisions to the guideline based in part on their feedback. The draft guideline was then posted on the Federal Register for public comment. The PHS Guideline Revision Working Group participated in the revision of the guideline recommendations in consideration of public comment and provided feedback on the full document. The draft guideline was then shared with the Expert Panel and Review Committee for technical considerations. Finally, the Office of the Assistant Secretary for Health (OASH) submitted the guideline for review and approval by HHS. The opinions of individual members of the Expert Panel or Review Committee might not be fully reflected in this document, as the guideline represents the position of the PHS agencies and is not a consensus document.
Updating the guideline
Future revisions to this guideline will be dictated by new research and technological advancements for preventing the transmission of HIV, HBV, and HCV through organ transplantation.
VI. EVIDENCE REVIEW
The Evidence Report19 comprises the primary evidence underlying the recommendations. This section provides a summary of the primary evidence by key questions.
Topic I: Probability of transmission of HIV, HBV, or HCV through organ transplantation (Key Questions 1 and 2)
Key Question 1: What are the prevalence and incidence rates of HIV, HBV, and HCV pathogens among potential organ donors?
For the three listed pathogens, the quality of evidence for U.S. incidence (the percentage of potential organ donors who newly acquire the pathogen) and prevalence (the percentage of potential organ donors at a given time who test positive for the pathogen) rates were reviewed. Due to the small amount of evidence on living and deceased potential organ donors, the scope was expanded to include other possibly relevant populations. Ultimately, the search comprised potential deceased and living organ donors,44–56 potential tissue donors,57 and the U.S. general population.58–63 The U.S. general population studies did not indicate if cases included living organ or tissue donors. Testing methods and criteria varied among the included studies. Some studies reported hepatitis prevalence but did not differentiate HBV from HCV. Additionally, it is not clear if Ab tests were confirmed by a more specific method such as Western blot, recombinant immunoblot assay, or NAT in most of these studies. These differences in donor populations and methods used to diagnose and report infection probably contributed to the range in reported incidence and prevalence rates.
Q1.A. HIV prevalence and incidence. The review found low-quality evidence from four studies that examined HIV prevalence and incidence among donors and the general U.S. population and no studies providing data from 2000 or later for potential deceased or living organ donors (Appendix A). Of the four studies that provided prevalence data, two studies related to organ donation. In a 1995 study of potential living-related donors (n=22), none were HIV-positive.45 In a 1993 study of deceased potential organ donors, 2% of 94 were positive for HIV or syphilis; it was unclear if all potential donors had been tested for HIV.47 Among 10,910 potential tissue donors, the prevalence of confirmed HIV was 0.093%.57 HIV prevalence in a U.S. general population study was approximately 0.37%.64 The U.S. general population studies did not indicate if cases included living organ or tissue donors. Our search did not identify studies estimating incidence of HIV in organ donors. However, two U.S. studies examining HIV incidence in the tissue donor and general population found estimated incidence to be 30.11 per 100,000 person-years among potential tissue donors57 and 18.8 per 100,000 person-years among the U.S. general population.58,59
Q1.B. HBV prevalence and incidence. A review of the available studies found low-quality evidence of HBV prevalence and incidence in donors and the U.S. general population (Appendix A). Five studies provided prevalence data.45–47,57,62 Among 446 potential organ donors, 4.9% were positive for HBV.46 The rate of viral hepatitis, type unspecified, was 5.3% in a study of 94 deceased potential donors; it was unclear if all potential donors had been tested for HBV in this study.47 A second study of hepatitis, type unspecified, reported a prevalence of 18.2% among potential living-related organ donors.45 Among 10,901 potential tissue donors, the prevalence of confirmed HBV was 0.229%.57 The prevalence of HBV chronic infection in the U.S. general population, including incarcerated individuals, was 0.36%.62 Our search did not identify studies estimating the incidence of HBV in organ donors. However, two U.S. studies examined HBV incidence in tissue donors and the general population. The incidence of HBV was 18.3 per 100,000 person-years for potential tissue donors51 and 14.4 per 100,000 person-years for the general population.60,61
Q1.C. HCV prevalence and incidence. This review found low-quality evidence from nine U.S. studies that estimated HCV prevalence and incidence in donors and the general population (Appendix A). Most of the prevalence data for organ donors were derived from donations that occurred before 1992. Among 55 living-related potential donors (i.e., people who are related genetically, such as a parent or sibling), the prevalence of HCV was 3.6%.44 A 1993 study of 94 deceased potential donors identified 5.3% as having active hepatitis, type unspecified; it was not clear if all 94 potential donors had been tested for hepatitis.47 Another study reported the prevalence of unspecified hepatitis at 18.2% among potential living-related donors.45 Of four retrospective studies of organ donation that occurred from 1986 to 1992, the combined estimate of HCV prevalence was 4.0%;48,49,51–56 three of the studies comprised deceased donors and the fourth did not report whether donors were deceased or living. The prevalence of HCV was 1.091% among 10,915 potential tissue donors.57 The prevalence of HCV infection in the U.S. general population, excluding incarcerated individuals, was 1.6%.63 Our search did not identify studies estimating HCV incidence in organ donors. However, two U.S. studies reviewed HCV incidence in tissue donors and the general population. The incidence of HCV was 12.4 per 100,000 person-years for potential tissue donors57 and 5.7 per 100,000 person-years for the general population.60,63
Key Question 2. What are the rates of transmission to recipients from donors infected with HIV, HBV, or HCV? Do the rates vary by the organ transplanted or when the donor was infected?
This question concerns the specific situation when the donor tests positive for a pathogen but the transplant candidate does not. We did not identify any studies that reported on HIV transmission results, likely due to federal regulations that prohibit transplantation of organs from individuals known to be HIV-infected. However, a number of studies addressed HBV and HCV, where we observed considerable variation in transmission rates. This variation could have been influenced by numerous factors, including:
The organ transplanted;
Whether HBV prophylaxis was used in the case of an HBV-positive donor;
Type of serologic testing used for detection;
The specific antibodies or antigens for which the donor was positive;
When the studies were performed, as earlier generations of HCV serological assays were less sensitive than the current generation;
Whether NAT, which would detect recent infection, was performed; and
The frequency and length of follow-up testing after transplantation.
Q2.A. HBV transmission from liver transplantation. Evidence of low quality was found on the transmission rate of HBV from infected donors to uninfected recipients (Appendix B). Of 22 publications, there were 17 unique studies.65–86 Studies measured virus transmission in 14 different ways (i.e., assays used to detect HBV) with results ranging from 0% to 94%. This difference may be due to whether HBV prophylaxis was used, with the lowest rates found primarily in studies in which all recipients received prophylaxis. There was also significant variation in the frequency of posttransplant screening and liver biopsy.83 Eight of these studies reported de novo HBV infection occurring in 72 recipients from three to 48 months posttransplant.66,67,69–75,85,87
Q2.B. HBV transmission from kidney transplantation. This review found low-quality evidence from observational studies regarding estimated transmission rates of HBV from infected donors to uninfected recipients (Appendix B). Of 10 publications, there were nine unique studies.75,77,78,84,87–92 Studies measured virus transmission in 13 different ways (i.e., assays used to detect HBV) with results ranging from 0% to 55%. Transmission rates were likely underreported in some of the studies, as the use of prophylaxis can result in negative recipient testing despite transmission. Of the reported transmission rates whereby the donor was anti-HBc positive with HBsAg status unknown, HBV was not detected; the study did not report whether prophylaxis was used.75 De novo HBV infection occurred in no recipients three to 12 months posttransplant.87
Q2.C. HBV transmission from heart transplantation. We found very low-quality evidence from observational studies that examined transmission rates of HBV from infected donors to uninfected recipients (Appendix B). Of seven publications, there were six unique studies.75,77,78,93–96 Studies measured virus transmission in nine different ways (i.e., assays used to detect HBV) with results ranging from 0% to 65%. Transmission rates are likely underreported in some of the studies, as the use of prophylaxis can result in negative recipient testing despite transmission. Of the reported transmission rates whereby the donor was anti-HBc positive with HBsAg status unknown, the range was 18%–65% when recipients were tested for anti-HBc75 and 0%–4% when recipients were tested for HBsAg;75,93 none of these studies reported whether prophylaxis was used. Frequency of posttransplant screening varied in three studies that reported the use of this screening.93–95 One study reported de novo HBV infection occurring in one recipient 10 months posttransplant.93
Q2.D. HBV transmission from lung transplantation. A review of the one study that met inclusion criteria revealed low-quality evidence estimating HBV transmission from infected donors to uninfected recipients (Appendix B). The study measured virus transmission applying two variations in donor and recipient testing; no recipients were reported as having a positive test.97 All recipients received prophylaxis, which may result in negative recipient testing despite transmission. The study did not report on the frequency of posttransplant screening, and no de novo HBV posttransplant infections were reported.
Q2.E. HCV transmission from liver transplantation. This review found low-quality evidence from studies48,56,98 that examined transmission rates of HCV from infected donors to uninfected recipients (Appendix B). Studies measured virus transmission in three different ways (i.e., assays used to detect HCV) with results ranging from 24% to 100%. HCV transmission was detected in 67% of recipients who were tested for anti-HCV and 100% of recipients who were tested for HCV RNA in one study in which the donor was positive for HCV RNA.98 The frequency of performing posttransplant immunoassay testing varied considerably.48,56,98 One study reported de novo HCV infection occurring in 48 recipients within 24 months.48,56
Q2.F. HCV transmission from kidney transplantation. This review found very low-quality evidence from studies that examined transmission rates of HCV from infected donors to uninfected recipients (Appendix B). Of 17 publications, there were 10 unique studies.49–55,99–108 Studies measured virus transmission applying eight variations in donor and recipient testing, with results ranging from 0% to 100%. Of the three studies in which donors tested positive for HCV RNA, the transmission rate was 0%–35% when recipients were tested for anti-HCV,49,106,107 0% when tested for radioimmunoassay,49 and 0%–57% when tested for HCV RNA.49,106,107 Seven studies reported the frequency of posttransplant immunoassay testing that varied such that the data were not useful.50–53,102,103,108 Eight studies reported de novo HCV infection occurring in 41 recipients 10–60 months posttransplant.54,55,102–104,106–108
Q2.G. HCV transmission from heart transplantation. We found very low-quality evidence from observational studies regarding transmission rates of HCV from infected donors to uninfected recipients (Appendix B). Of six publications, there were four unique studies.109–114 Studies measured virus transmission in six different ways (i.e., assays used to detect HCV), with results ranging from 11% to 100%. One study reporting transmission rates detected HCV transmission in 44% of recipients when tested for anti-HCV and 100% of recipients when tested for HCV RNA. This study also reported regular monitoring without stating a frequency of posttransplant serology testing.68 One study reported de novo HCV infection occurring in three recipients 19–55 months posttransplant.113
Topic II: Methodology to better estimate donor infection with HIV, HBV, or HCV (Key Questions 3, 4, and 5)
Regarding Key Questions 3 and 4, the 1994 PHS guidelines stated that “regardless of their HIV antibody test results, persons who meet any of the criteria listed should be excluded from donation of organs or tissues unless the risk to the recipient of not performing the transplant is deemed to be greater than the risk of HIV transmission and disease:”1
MSM in the preceding five years;
People who report nonmedical intravenous, intramuscular, or subcutaneous injections of drugs in the preceding five years;
People with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates;
Men and women who have engaged in sex in exchange for money or drugs in the preceding five years;
People who have had sex in the preceding 12 months with any person described in the aforementioned items or with a person known or suspected to have HIV infection;
People who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane;
Inmates of correctional systems;
Children meeting any of the exclusionary criteria previously listed for adults;
Children >18 months of age born to mothers with HIV infection or mothers who meet the behavioral exclusionary for adult donors, or who have been breastfed within the past 12 months, unless HIV Ab tests, physical examination, and review of medical records do not indicate evidence of HIV infection; and
Children ≤18 months of age born to mothers with or at risk for HIV infection, or children who have been breastfed within the past 12 months.
Given the paucity of data evaluating behavioral and nonbehavioral risk factors for HIV, HBV, and HCV in potential organ donors, we also searched for studies meeting inclusion criteria in the following populations:
tissue donors
blood donors
general population (For the literature search, general population was defined as a population unselected for any particular demographic, occupational, or behavioral characteristics, or health status other than HCV, HBV, or HIV infection.)
After broadening the search, only a few articles met the inclusion criteria that identified behavioral and nonbehavioral risk for HBV. Therefore, we included studies of demographic or socioeconomic subpopulations, such as college students or veterans admitted to a psychiatric inpatient ward, for HBV. In addition, there were no data for evaluating nonbehavioral risk factors in organ donors.
Based on the quality of evidence from the literature review, the evaluation of behavioral and nonbehavioral risk factors for HIV, HBV, or HCV infection was categorized as indicated in the Evidence Review. Behavioral and nonbehavioral characteristics that were associated with an increased likelihood of HIV, HBV, or HCV infection and identified by “low,” “moderate,” or “high” quality evidence in the systematic review are reported in Figure 14.
Given the paucity of literature identifying risk factors for incident infection, the potential risk factors in Figure 14 were reviewed with subject-matter experts on HIV and hepatitis to create a revised list of risk factors (see Risk Factors for Recent HIV, HBV, or HCV Infection on pages 250–1) that would facilitate the identification of recent (i.e., incident) infections in potential organ donors. The revised list takes into consideration that (1) certain risk factors are probably markers for other factors identified in the systematic review; (2) scientific evidence associating certain factors with the pathogens exists, but may not have met the inclusion criteria of the systematic review; or (3) certain studies were of insufficient quality to draw conclusions.
Key Question 3. What behavioral risk factors are associated with an increased probability of infection with HIV, HBV, or HCV? What is the prevalence of these characteristics among potential organ donors?
The first half of the question attempts to identify behaviors that may put individuals at increased risk for recent HIV, HBV, or HCV infection, while the second half of the question attempts to address the frequency of those behaviors among potential organ donors. The following sections present all behaviors identified as exclusion criteria from the 1994 PHS guidelines, as well as additional behaviors identified from the literature but not included as exclusion criteria from the 1994 PHS guidelines.
Q3.A. HIV and behavioral risk factors.
MSM. This review found moderate-quality evidence associating MSM with HIV infection (Appendix C). The 1994 PHS guidelines identified “men who have had sex with another man in the preceding 5 years” as a risk factor for HIV. We identified no studies that addressed this particular time frame. Two studies of the general population, one using National Health and Nutrition Examination Survey (NHANES) data115 and the other using New York City HANES data,116 found a significantly higher rate of HIV for MSM in univariate analyses.115, 116 Among infected males, 52.0%115 and 53.8%116 reported a history of MSM behavior. The evidence of prevalence of MSM, reported as 3.7%115 and 9.3%116 of men in the general population studies, respectively, was rated as low quality.
Injection drug use (IDU). This review found low-quality evidence suggesting an association between IDU and HIV (Appendix C). The 1994 PHS guidelines identified “persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years” as a risk factor for HIV. We identified no studies that investigated the risk of IDU within this particular time frame; most studies evaluated lifetime IDU. Two studies of the general population, using NHANES and HANES data, found significant associations between IDU and HIV with large effect sizes in univariate analyses.115,116 A third study of patients at an urban medical care center did not find an association in univariate analysis.115 The evidence of prevalence of IDU, reported as 1.4%116 and 7.9%,117 was rated as low quality.
Sex worker. This review found very low-quality evidence from a single study that did not find an association between sex workers and HIV (Appendix C). The 1994 PHS guidelines identified “men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years” as a risk factor for HIV. We did not find any literature that studied the association between this factor and HIV within this particular time frame. One corneal donor study involving a next-of-kin interview did not find any association between sex work and infection with HIV.118 One study of patients at an urban medical care center reported a 2.3% prevalence of exchanging sex for drugs or money117 (Appendix C), and this evidence was rated low quality.
- High-risk sex partner. The 1994 PHS guidelines identified people who have had sex in the preceding 12 months with any of the following people—MSM, injection drug users, sex workers, or people known or suspected to have HIV infection—as a risk factor for HIV. This review did not identify any literature on infection risk in people with high-risk sex partners during the listed time frame.
- Sex with an injection drug user. This review did not identify studies that examined the association between sex with an injection drug user and acquiring HIV.
- Sex with a sex worker. We identified no studies that examined the association between HIV and sex with a sex worker. One study in an urban medical care center reported that 7.4% of patients had had sex with a sex worker,117 and this evidence was rated low quality.
- Sex with people known to be HIV-infected. This review found low-quality evidence associated with having sex with someone who is HIV-positive and having HIV (Appendix C). One study of a general population found that the relationship between having a sex partner with HIV and acquiring HIV was significant with a large magnitude of effect in a univariate analysis.117 The study reported that 3.6% of participants indicated having sex with someone known to be infected with HIV,117 and this evidence was rated low quality.
- Other high-risk sex partners. No studies were identified that met the inclusion criteria.
Inmates. This review found very low-quality evidence from a single study to suggest an association between being incarcerated and having HIV (Appendix C). The 1994 PHS guidelines identified being an inmate of a correctional system as a risk factor for HIV. No studies that examined the association between “present” incarceration and infection were identified. However, one study examined and did not identify an association between lifetime history of incarceration as reported by next of kin with HIV infection in potential corneal donors.118
Risk factors in children. No studies were identified on behavioral risk factors for HIV in children, or on the risk of infection from mothers who engage in those risk behaviors. While a body of literature exists on vertical transmission as a mode of hepatitis or HIV transmission, this body of literature lacks the evidence to assess the 1994 PHS guidelines criteria as risk factors.
Multiple sex partners. A review of the available studies revealed low-quality evidence to suggest an association between having multiple sex partners and being at increased risk of HIV (Appendix C). Studies defined multiple partners using different thresholds. Having multiple partners, including heterosexual partners, was associated with HIV infection in one general population study of hospital inpatients and outpatients.115 HANES data of New York City adults found that having multiple sex partners in the past year was not associated with HIV,116 nor was having at least 10 lifetime sex partners in the patient study;117 however, having 50 or more lifetime sex partners was associated with HIV in an NHANES study.115 All of these analyses were univariate. In the studies, 3.5%115 and 6.6%116 of patients reported having at least 50 lifetime sex partners; 74% reported having sex with at least two partners115 and with 2–49 partners during their lifetime,116 whereas 22% reported having sex with multiple partners during the previous year.116 This review found low-quality evidence of the prevalence of individuals having at least 50 partners, whereas we found moderate-quality evidence regarding prevalence estimates of having multiple sex partners, at least two sex partners, and 2–49 sex partners.
Same-sex partners. This review found low-quality evidence to suggest an association between having a same-sex partner and acquiring HIV (Appendix C). A univariate analysis with a large magnitude of effect between having a same-sex partner and HIV, including both men and women, was detected in an urban medical care study.117 The evidence of prevalence of same-sex partners, reported as 8.2%,117 was rated low quality.
Age at first sexual intercourse. This review found low-quality evidence to support younger age at first sexual intercourse as a risk factor for HIV infection (Appendix C). An NHANES study reported that first sexual intercourse at ≤18 years of age was associated with HIV in a univariate analysis with a large effect size.115 The proportion of adults who reported having sex at ≤18 years of age was 59%,115 and this evidence was rated high quality.
Additional various (sexual) associations. This review found additional reported associations between sexual practices and infection identified in the literature, but did not assign a quality-of-evidence rating because these factors were reported by so few studies. Not using condoms was not associated with HIV infection in two general population studies.116,117 Anal-insertive sex occurring at least six weeks ago among men and anal-receptive sex occurring at least six weeks ago among women and men was associated with HIV in a general population.117 Having vaginal sex was associated with a reduced risk in HIV compared with people who did not have vaginal sex (but may have been having anal sex).111
- Non-injection substance.
- Other illicit drugs. Low-quality evidence was found associating the use of cocaine or street drugs and HIV infection (Appendix C). HIV was associated with any prior cocaine or street drug use in a univariate analysis using NHANES data115 and in a multivariate analysis among patients in an inner-city emergency department.119 The proportion of respondents who reported using street drugs/cocaine was 21%,115 and this evidence was rated moderate quality due to indirectness.
- Alcohol. This review found very low-quality evidence associating alcohol with an increased risk of HIV (Appendix C). HIV was associated with having an alcohol and/or (unspecified) drug problem among health maintenance organization (HMO) enrollees,120 but not with alcohol use among potential corneal donors.118 Both of these analyses were univariate.
- Tobacco. We found very low-quality evidence in one study that did not find an association between cigarette use and HIV (Appendix C) among corneal donors.118
Tattoos and piercing. Very low-quality evidence was found in one study that did not find an association among tattoos, piercings, and acupuncture (as collectively analyzed as one outcome and reported by next of kin) in potential corneal donors (Appendix C).118
International travel. Evidence of very low -quality was found in one study that did not find an association between international travel and HIV (Appendix C) among potential corneal donors.118
Q3.B. HBV and behavioral risk factors.
MSM. We found moderate-quality evidence to support an association between MSM behavior and HBV (Appendix C). The 1994 PHS guidelines identified “men who have had sex with another man in the preceding 5 years” as a risk factor for HIV. We identified no studies that addressed this particular time frame. Two studies found associations with HBV. One study compared the prevalence of HBV among MSM in a sample drawn from the general population (4.7% HBV-positive) with those who self-reported as not MSM (age-adjusted prevalence of HBV = 5.7%) and found a significantly increased prevalence of HBV (age-adjusted prevalence = 26.8%) among MSM.121 In a population of college students, HBV infection rates among MSM were compared with men who have never had sex and showed that the rate of HBV was higher among MSM in both univariate and multivariate analyses.122 For both studies, the magnitude of the effect was large.
IDU. Low-quality evidence was found to support IDU as a risk factor for HBV infection. Although all but one study found an association, not all of the studies found a large magnitude of effect (Appendix C). The 1994 PHS guidelines identified “persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years” as a risk factor for HIV. We identified no studies that investigated the risk of IDU within the listed time frame; most studies associated lifetime IDU with infection. HBV was significantly associated with IDU in four studies,122–125 and three of these studies had large effect sizes,122–124 with a point estimate odds ratio of ≥2.0. One study comprised volunteers from the general population,123 two comprised IDU veterans in inpatient psychiatric hospitals,124,125 and one involved college students.122 Only two studies123,125 performed multivariate analyses. The fifth study did not find an elevated risk in an obstetric population.126 One study also considered steroid injection but did not find a significant relationship between injection steroid use and HBV infection.122 The evidence of prevalence of IDU, reported in the general population study as 3.5%,123 was rated low quality.
Sex worker. This review found low-quality evidence associating sex work and HBV infection (Appendix C). The 1994 PHS guidelines identified “men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years” as a risk factor for HIV. We identified no literature that studied the association between this factor and the given time frame. None of the three studies found an association between sex work (including sex bartering or sex for drugs) and HBV.118,124,125 A study of corneal donors did not find any association between sex work and infection with HBV in univariate analysis.118 Two studies of psychiatric inpatient veterans did not detect an association between sex bartering124 or unprotected sex for drugs125 with HBV infection in multivariate analyses.
- High-risk sex partner. The 1994 PHS guidelines identified people who have had sex in the preceding 12 months with any of the following people—MSM, injection drug users, sex workers, or people known or suspected to have HBV infection—as a risk factor for HIV. We did not identify any literature on infection risk in people with high-risk sex partners during the listed time frame. However, having a high-risk or infected sex partner was associated with HBV in five of six studies.118,122–124,126,127
- Sex with an injection drug user. We found very low-quality evidence supporting an association between HBV infection and having sex with an injection drug user (Appendix C). Four studies examined sex with an injection drug user.122–124,127 Sex with an injection drug user was significantly associated with HBV in the general population in a multivariate analysis123 and in a univariate analysis among college students122 but not among veterans admitted to the inpatient psychiatric ward.124 Sex or household contact with an injection drug user was associated with HBV infection in a univariate but not a multivariate analysis.127 In one study, 5% of the general population participants reported having sex with an injection drug user,123 and this evidence was rated low quality.
- Sex with a sex worker. We found very low-quality evidence in a single study of psychiatric inpatient veterans that did not find an association between HBV and having sex with a sex worker (Appendix C) in a univariate analysis.124
- Sex with people known to be HBV infected. This review found very low-quality evidence associating having a sex partner with a known HBV infection as a risk factor for HBV (Appendix C). Sex with a partner with hepatitis was found to be a significant risk factor for HBV in college students122 but not in an obstetric population.126 Both of these analyses were univariate.
- Other sex partners. HBV infection was not associated with sex with a blood transfusion recipient, with a health-care worker, or with a person with a foreign birth in an endemic area.127 This study was reported because it is germane to the larger issue of having high-risk sex partners. However, the quality of the evidence was not rated because this factor was reported by one study only.
Inmates. This review found very low-quality evidence to suggest an association between recent or past incarceration and having HBV (Appendix C). The 1994 PHS guidelines identified being an inmate of correctional systems as a risk factor for HIV. No studies examined the association between present incarceration and HBV infection. The search did identify studies that examined the association between recent or lifetime history of incarceration. A history of incarceration was associated with HBV in three of four studies.118,122,124,127 In the general population, imprisonment within the last six months was associated with recent HBV infection in univariate but not multivariate analysis.127 Incarceration was also significantly associated with HBV in a univariate analysis among psychiatric inpatient veterans124 and among college students incarcerated for at least 24 hours.122 A history of incarceration as reported by next of kin was not associated with HBV in potential corneal donors.118
Risk factors in children. We identified no literature on any behavioral risk factors in children, or on the risk of infection from mothers who engage in those risk behaviors. While a body of literature exists on vertical transmission as a mode of hepatitis or HIV transmission, this body of literature lacks the evidence to assess the 1994 PHS guidelines criteria as risk factors.
Multiple sex partners. This review found moderate-quality evidence to support having multiple sex partners as a risk factor for HBV infection. The strength of this association was due to a positive dose-response association (Appendix C). Having multiple partners, including heterosexual partners, was associated with an increased risk of HBV infection in five studies.121–123,125,127 Studies defined multiple partners using different thresholds. In multivariate analyses, HBV infection was associated with sex with multiple partners in a general population123 and among psychiatric inpatient veterans,125 and with multiple partners within the last six months in a general population.127 In a sample of people representative of the general population, having ≥2–9, ≥10–14, or ≥50 lifetime sex partners vs. 0–1 lifetime sex partners was associated with HBV in a multivariate analysis. However, the prevalence of HBV in the general population was 4.7% compared with an age-adjusted prevalence of 4.4% of individuals having 2–9 lifetime sex partners.121 Among college students, having >50 lifetime heterosexual sex partners and >5 heterosexual sex partners in the preceding four months were both associated with HBV infection in univariate analyses.122 In one study, 26% of respondents reported having sex with multiple partners,123 and this evidence was rated moderate quality.
Same-sex partners. The literature review did not identify any studies that examined an association between same-sex partners and HBV infection.
Age at first sexual intercourse. This review found low-quality evidence suggesting an association between age at first sexual intercourse and HBV infection (Appendix C). Being ≥18 years of age was not associated with HBV in a multivariate analysis of general population participants in an NHANES study.121 Age ≤15 years at first intercourse was associated with HBV infection among college students.122
Additional various (sexual) associations. The literature review did not identify any studies regarding the association between other sexual behaviors and an increased risk for HBV.
- Non-injection substance.
- Other illicit drugs. This review found very low-quality evidence associating non-injection illicit drug use with HBV infection (Appendix C). Two of five studies found an association with HBV and non-injection illicit drugs.118,121,122,124,125 An NHANES study of the general population found any prior cocaine use was associated with HBV in a multivariate analysis, but this study did not control for IDU, a major confounding variable.121 HBV infection was associated with intranasal drug use among college students.122 However, HBV infection was not associated with inhaled or snorted drugs in psychiatric inpatient veterans.124,125 Illicit drug use was not associated with HBV in a corneal donor study.118
- Alcohol. This review found very low-quality evidence to suggest an association between alcohol use and HBV infection (Appendix C). Alcohol use, as reported by next of kin, was not associated with HBV infection among potential corneal donors in a univariate analysis.118 HBV was not associated with alcohol use disorder among psychiatric inpatient veterans in a multivariate analysis.125
- Tobacco. We found very low-quality evidence in a single study suggesting an association between tobacco use and HBV (Appendix C). No association was found between cigarette smoking and HBV among corneal donors.118
Tattoos and piercing. This review found low-quality evidence that did not support tattoos and piercings as risk factors for HBV (Appendix C). Tattoos, piercings, and acupuncture (collectively analyzed as one outcome and reported by next of kin) were not associated with HBV in corneal donors.118 Tattoos were not associated with HBV infection among psychiatric inpatient veterans,124 women receiving prenatal care,126 or college students,122 unless the college students were tattooed with reused non-autoclaved needles. Having a tattoo in the last six months was not associated with acute HBV infection.127 Piercings were not associated with HBV among psychiatric inpatient veterans,124 and body piercings were not associated with HBV among college students.122 Piercings within the last six months were also not associated with acute HBV in the general population.127
International travel. This review found very low-quality evidence that did not determine an association between international travel and having HBV (Appendix C) among potential corneal donors.118
Q3.C. HCV and behavioral risk factors.
MSM. Low-quality evidence was found to support MSM behavior as a risk factor for HCV infection (Appendix C). The 1994 PHS guidelines identified “men who have had sex with another man in the preceding 5 years” as a risk factor for HIV. We identified no studies that addressed this particular time frame. One study comprising blood donors found a significant association between HCV and MSM in a univariate analysis; however, an increased prevalence was not found adjusting for confounding by IDU.128 A general population study found no association in a univariate analysis.129
IDU. Moderate-quality evidence was found to support IDU as a risk factor for HCV. There were consistently large effect sizes found in all studies except one study on steroid use (Appendix C). The 1994 PHS guidelines identified “persons who report nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years” as a risk factor for HIV. We identified no studies that investigated the risk of IDU within this time frame; most studies associated lifetime IDU with infection. Three blood donor studies128,130,131 and four general population studies63,120,123,129 detected associations between IDU and HCV. Three of the general population studies63,123,129 performed multivariate analyses and determined that IDU was an independent risk factor. One blood donor study found use within the last six months to be associated with infection.130 This study also considered past steroid injection reported by three donors and did not find an increased risk; however, a trend toward higher infection rate in those who injected steroids longer than six months ago was found.125 Additionally, reporting living with an injection drug user was associated with HCV among blood donors, even when adjusted for confounding by IDU,128 as was living with an injection drug user in the last six months.130 A general population study reported that both being at a social gathering with injection drugs and witnessing the IDU were associated with HCV.132 Prevalence of IDU reported in the general population studies was 1.7% from NHANES data63 and 3.5% from patients and volunteers in urban areas,123 and this evidence was rated low quality.
Sex worker. Low-quality evidence was found to suggest an association between sex work and HCV (Appendix C). The 1994 PHS guidelines identified “men and women who have engaged in sex in exchange for money or drugs in the preceding 5 years” as a risk factor for HIV. We did not find any literature that studied the association between this factor and the given time frame. Sex work was significantly associated with HCV in a multivariate analysis in a blood donor study128 and in univariate analyses in two general population studies.129,132 However, in one study, all women who reported sex work also reported IDU.129 One study of corneal donors did not find any association between sex work and infection with HCV.118 One urban medical care center study reported that 2.3% of participants had exchanged sex for money or drugs,117 and this evidence was rated low quality.
- High-risk sex partner. The 1994 PHS guidelines identified people who have had sex in the preceding 12 months with any of the following people—MSM, injection drug users, sex workers, or people known or suspected to have HCV infection—as a risk factor for HIV. The literature review did not identify any studies on infection risk in people with high-risk sex partners during the listed time frame.
- Sex with an injection drug user. A review of the outlined studies found moderate-quality evidence supporting having sex with an injection drug user as a risk factor for HCV. Studies consistently found an association with large magnitude of effect (Appendix C). Sex with an injection drug user or intravenous drug user was significantly associated with HCV in blood donors in univariate128 and multivariate analyses,130 and in the general population in univariate132 and multivariate analyses.123 In one study, 5% of patients reported having sex with an injection drug user,123 and this evidence was rated low quality.
- Sex with a sex worker. This review found very low-quality evidence to suggest an association between having sex with a sex worker and having HCV (Appendix C). Sex with a sex worker was associated with HCV among blood donors in univariate and multivariate analyses.128 Sex with a sex worker also was associated with HCV in general population studies in univariate analyses;129,132 however, the relationship was no longer significant in a multivariate analysis.129
- Sex with people known to be HCV infected. We found low-quality evidence from two studies of blood donors to support having sex with people known to be HCV infected as a risk factor for HCV infection (Appendix C), one with a multivariate analysis128 and one with a univariate analysis.130 One study limited the behavior to the previous six months.130
- Other sex partners. Some miscellaneous types of sex partners not mentioned in the original guideline were also reported, such as sexual promiscuity (defined as a history of a sexually transmitted disease [STD] or at least five sex partners per year)131 and sex with a transfusion recipient128 in studies of blood donors. We report these studies because they are germane to the larger issue of having high-risk sex partners. However, no quality-of-evidence rating was given because these two studies reported on different factors.
Inmates. This review found low-quality evidence to support incarceration as a risk factor for HCV (Appendix C). The 1994 PHS guidelines identified being an inmate of a correctional system as a risk factor for HIV. We identified no studies that examined the association between present incarceration and HCV infection. The searches did, however, identify studies that examined the association between recent or lifetime history of incarceration. A history of incarceration was associated with HCV in four128,130–132 of five118 studies. One blood donor study found that incarceration for more than three days was an independent risk factor,128 while another study found no association once adjusting for confounding by IDU.131 In addition, having been arrested was associated with HCV infection in a univariate analysis of a general population sample.132 A history of incarceration, as reported by next of kin, was not associated with HCV infection in potential corneal donors.118
Risk factors in children. The literature review did not identify any behavioral risk factors in children or the risk of infection from mothers who engage in those risky behaviors. While a body of literature exists on vertical transmission as a mode of hepatitis or HIV transmission, this body of literature lacks the evidence to assess the 1994 PHS guidelines criteria as risk factors.
Multiple sex partners. We found moderate-quality evidence associating HCV infection with having multiple sex partners. Studies demonstrated a positive dose-response association (Appendix C). Having multiple sex partners (defined using different thresholds) was associated with an increased risk of infection in six studies.63,120,123,128,130,132 Having at least 11 male sexual partners (compared with having no sexual partners) was associated with HCV infection in a multivariate analysis in female blood donors,128 whereas having the same number of lifetime female partners was not associated with HCV in men. Having two or more sexual partners in the last six months, whether same sex or not, was associated with an increased rate of HCV infection overall in a univariate analysis.130 In general population studies, HIV was associated with having frequent sex partners,120 multiple sex partners,123 and at least 20 sexual partners in a univariate anlaysis.63 Another general population study also reported an association between having a greater numbers of sex partners and HCV infection in a univariate analysis.132 One study noted the strong association between having a greater numbers of sex partners and IDU. Of the 18.5% of respondents who reported having at least 10 sex partners, 84% also reported IDU.129 In a study of the U.S. general population, 29% of survey respondents indicated having had at least 10 sex partners,63 and 26% of volunteers from an urban area reported sex with multiple partners.123
Same-sex partners. This review found very low-quality evidence to suggest an association between same-sex partners and HCV (Appendix C). A study of female blood donors found an increased risk of HCV in females with same-sex partners in a multivariate analysis,128 but this risk was no longer significant when adjusted for confounding by IDU. Among outpatients, no association was found between having sex with a person of the same sex and HCV infection in a univariate analysis.132
Age at first sexual intercourse. This review found low-quality evidence associating younger age at first intercourse with HCV infection. This single study identified a dose-dependent relationship (Appendix C). In the general population, first sexual intercourse at age ≤17 years was associated with HCV in a univariate analysis. The study stratified age at first intercourse (i.e., <11, 12–15, and 16–17 years of age). The groups of people who were younger at the time of their first sexual intercourse had the highest risk of HCV. The proportion of adults who reported having sex at ≤18 years of age was 58%,63 and this evidence was rated moderate quality.
Additional various (sexual) associations. Unprotected sex was associated with HCV infection in a general population.120 However, we did not assign a quality-of-evidence rating because only one study reported this factor.
- Non-injection substance.
- Other illicit drugs. Evidence of low quality was found associating HCV with non-IDU (Appendix C). Seven63,120,128–132 of eight118 studies found an association with HCV and non-injection substances. Intranasal drugs were associated with HCV infection in blood donors,128,130,131 when adjusted for confounding by IDU or other factors, in two studies.128,131 In a general population study, use of snorting or inhaling nonprescription drugs,132 inhaling cocaine,120 using intranasal cocaine,129 and use of non-injection drugs other than marijuana63 were all associated with an increased prevalence of HCV. One of the studies using NHANES data found only a marginal independent association with HCV between non-IDU (except marijuana) compared with those who reported either no illicit drug use or marijuana use.63 Being at a social gathering with cocaine was associated with HCV132 and having friends who use street drugs was associated with an increased risk of HCV among blood donors.130 Illicit drug use was not associated with HCV in a corneal donor study.118 In the general population, 17% reported lifetime use of drugs other than marijuana,63 and this evidence was rated moderate quality.
- Alcohol. Very low-quality evidence was found suggesting a relationship between alcohol use and HCV (Appendix C). HCV was associated with heavy alcohol use in heart donors,133 and with having at least two units of alcohol per day among adults tested for HCV because of clinical suspicion.129 However, in univariate analyses, HCV was not associated with alcohol use among corneal donors,118 having at least five alcoholic drinks per week in patients,132 or alcoholism in HMO enrollees.120
- Tobacco. This review found very low-quality evidence associating tobacco use with HCV (Appendix C) and low-quality evidence estimating the prevalence of tobacco use. In univariate analyses, a history of tobacco use was associated with HCV in heart donors133 and cigarette smoking was associated with HCV in corneal donors.118 Among actual heart donors, 36% had a history of tobacco use,133 and the evidence was rated low quality.
Tattoos and piercing. A review of included studies found low-quality evidence to associate -tattoos and piercings with HCV (Appendix C). Tattoos were consistently associated with HCV in six120,128–132 of seven studies,118 whereas piercings were inconsistently associated with HCV in three studies.128,131,132 Three blood donor studies associated tattoos with HCV in univariate analyses.128,130,131 One study focused on having had a tattoo within the last six months and the risk of acute HCV.130 When multivariate analyses were performed, tattoos were not significantly associated with infection in one study130 but remained an independent predictor in a second study, although the odds of infection were reduced once adjusted for confounding by IDU.128 Three general population studies also detected significant associations between tattoos and HCV.120,129,132 Only one of the three general population studies found that tattoos were an independent predictor of HCV.129 Adults were enrolled based upon clinical suspicion of hepatitis, and most reported that their tattoos had been applied by friends, fellow gang members, or other inmates. Among blood donors, HCV was not associated with body piercing in the last six months130 but was associated with ear piercing among men131 and pierced ears or body parts128 in multivariate analyses. Two general population studies did not find an association between body piercing and HCV in univariate analyses,120,129 but a general population study found ear piercing in adult patients was associated with HCV in a univariate analysis.132 Tattoos, piercing, and acupuncture (collectively analyzed as one outcome and reported by next of kin) were not associated with HCV in potential corneal donors.118
International travel. Low-quality evidence from two studies did not suggest an association between international travel and HCV infection (Appendix C). Among potential corneal donors, international travel was not associated with HCV.118 International travel within the last six months was not associated with acute HCV in a general population study127 or among blood donors.130 Having ever lived outside the U.S. was also not found to be significantly associated with HCV in a blood donor study.128
Key Question 4. What nonbehavioral factors are associated with an increased probability of infection with HIV, HBV, or HCV? What is the prevalence of these factors among potential organ donors?
The primary intent of this question was to identify signs and symptoms of incident infections (i.e., those that have been recently acquired), but also to include data on signs and symptoms of chronic infection, medical comorbidities, socioeconomic information, and demographic factors. The second half of the question addresses the frequency of these nonbehavioral factors. Information regarding risk factors listed in the 1994 PHS guidelines is presented first, and then information regarding additional factors for which at least two studies provided evidence regarding the same factor is presented.
Q4.A. HIV and nonbehavioral risk factors.
People with hemophilia or related clotting disorder who received clotting factor blood products. We identified no studies that met the inclusion criteria. The 1994 PHS guidelines identified “persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates” as a risk factor for HIV.
Exposure to infected or suspected infected blood. We identified no studies that met the inclusion criteria. The 1994 PHS guidelines identified “persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane” as a risk factor for HIV.
Children. This literature review did not identify any studies regarding the nonbehavioral risk factors, listed previously, in relation to children or children of mothers who engage in those nonbehavioral risk factors.
Signs and symptoms. We identified no studies that met the inclusion criteria. An objective of this section was to identify nonbehavioral factors that could be predictive of infection, especially acute infection during the window period before tests could recognize the infection.
Receipt of blood transfusion. We identified no studies that met the inclusion criteria.
- Nonspecific exposure.
- Accidental needlestick injury. Very low-quality evidence from a single study was found that did not determine an association between accidental needlesticks and HIV infection (Appendix D). Data were collected from next of kin for potential corneal donors. The study did not identify if the needlestick injury occurred in an occupational or nonoccupational setting.118
- Hemodialysis. We identified no studies that met the inclusion criteria.
- Surgery. We found very low-quality evidence from a single study that did not find an association between having surgery and acquiring HIV. Data were collected from next of kin for potential corneal donors.118
- Organ and corneal transplantation recipients. No organ donor studies were identified that met the inclusion criteria. Evidence of very low quality from a single study was found that did not determine an association between having a corneal transplant and HIV (Appendix D). Receipt of organ transplantation was not associated with a greater risk of HIV in a corneal donor study.118
- Acupuncture. We identified no studies that met the inclusion criteria.
- Dental work. We identified no studies that met the inclusion criteria.
- Blood draws. This review found very low-quality evidence from a study that did not find an association between having blood drawn and HIV (Appendix D). A corneal donor study did not find an association between blood drawn for HIV testing and HIV, based upon next-of-kin interviews.118
- Other blood exposure. We identified no studies that met the inclusion criteria.
- Household exposure. We identified no studies that met the inclusion criteria.
Other infections. This review found low-quality evidence associating HIV with having another infection (Appendix D). In general population studies, HIV infection was associated with an STD diagnosis in one study,117 herpes simplex virus-2 (HSV-2) in two studies with large effect sizes in univariate analyses,115,116 and syphilis or infections other than HIV in a fourth study in a multivariate analysis.119 Rabies exposure was not associated with HIV in a corneal donor study.118 The prevalence of antibodies to HSV-2 was 28% in a general population study comprising New Yorkers.116 STD diagnoses were reported by 18% of patients in an urban medical care center.117
- Demographic factors.
- Age or year of birth. This review found very low-quality evidence to suggest a relationship between age and having HIV (Appendix D). Studies assessed different age ranges, complicating comparison. One study that conducted a multivariate analysis found adults aged 18–30 years had increased HIV prevalence.134 Another study that conducted a univariate analysis found higher HIV prevalence in adults aged 25–40 years compared with younger people aged 15–24 years with a large effect size.117 A third study found increased prevalence among adults aged 35–44 years.119
- Race/ethnicity and national origin. This review found very low-quality evidence to suggest an association between race/ethnicity and HIV (Appendix D). Two general population studies using univariate analyses found a higher prevalence of HIV in Asian people and those with Hispanic ethnicity compared with white people,116,117 and those who spoke Spanish vs. English in emergency room patients.134 Two of these studies found an increased prevalence of HIV with a large effect size among people of black race;116,117 however, the third study in the general population did not.134
- Occupation. We identified no studies that met the inclusion criteria.
- Economic factors. This review found very low-quality evidence suggesting an association between economic factors and having HIV (Appendix D). Being homeless was independently associated with an increased prevalence of HIV in public hospital emergency room patients,134 while having a poverty index of <1 was not associated with HIV.115
- Marital status. Low-quality evidence was found from a single study to suggest a relationship between marital status and having HIV (Appendix D). Being married or cohabitating was associated with a lower prevalence of HIV in the general population.115
Q4.B. HBV and nonbehavioral risk factors.
People with hemophilia or related clotting disorder who received clotting factor blood products. No studies were identified in the literature regarding an association between clotting factor and prevalence of infection. The 1994 PHS guidelines identified “persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates” as a risk factor for HIV.
Exposure to infected or suspected infected blood. The review found very low-quality evidence supporting exposure to blood that was known or suspected to be HBV infected as a risk factor for HBV (Appendix D). The 1994 PHS guidelines identified “persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane” as a risk factor for HIV. Among embalmers, no association was found between needlestick injury with exposure to HBV during embalming and HBV infection.135
Children. We identified no studies regarding the nonbehavioral risk factors, listed previously, in relation to children or children of mothers who engage in those nonbehavioral risk factors.
Signs and symptoms. We identified no studies that met the inclusion criteria. An objective of this section was to identify nonbehavioral factors that could be predictive of infection, especially acute infection during the window period before tests could recognize the infection.
Receipt of blood transfusion. This review found very low-quality evidence to support receipt of blood transfusions as a risk factor for HBV (Appendix D). Although no studies reported on risk in people with clotting disorders or who have received clotting factor blood products, several studies investigated the risk of infection associated with blood transfusion. Blood transfusion was independently associated with HBV infection in a general population,123 as was blood transfusion before 1991 among college students.122 Blood transfusion was not associated with HBV in a low-prevalence obstetric patients study.125 Among volunteers from an urban area, 20% reported having ever had a blood transfusion.123
- Nonspecific exposure.
- Accidental needlestick. Evidence of very low quality was found on the relationship between accidental needlestick injury and acquiring HBV (Appendix D). In a general population study, needlestick injuries were actually associated with a lower prevalence of HBV infection.123 This finding may be because a substantial proportion of the enrollees were health-care workers, and in that study health-care workers had a lower prevalence of HBV than the group as a whole. According to data collected from next of kin, accidental needlestick injuries were not associated with HBV among potential corneal donors.118
- Hemodialysis. Moderate-quality evidence from two studies was found suggesting hemodialysis as a risk factor for HBV (Appendix D). Studies using univariate analyses found that hemodialysis was associated with HBV infection among participants in a general population study123 and college students122 with large effect sizes.
- Surgery. This review found very low-quality evidence that did not support an association between having had surgery and HBV (Appendix D). In the general population, surgery was associated with a slightly lower HBV prevalence in a univariate analysis,123 and surgery during the last six months was not associated with acute HBV.127 Based upon next-of-kin data for potential corneal donors, no association was found between HBV and surgeries.118
- Organ and corneal transplantation recipients. No organ donor studies were identified that met the inclusion criteria. This review found very low-quality evidence of having had a corneal transplant and HBV (Appendix D). Receipt of organ transplantation was not associated with a greater risk of HBV in a corneal donor study118 or with HBV among psychiatric inpatient veterans.124
- Acupuncture. Low-quality evidence from two studies was found suggesting a relationship between acupuncture and having HBV (Appendix D). Acupuncture during the last six months was not associated with an increased incidence of acute HBV in the general population127 or an increased prevalence of HBV among psychiatric inpatient veterans.124
- Dental work. Very low-quality evidence from a single general population study was found that did not suggest an association between dental work within the last six months and HBV127 (Appendix D).
- Blood draws. We found very low-quality evidence from a single study that did not suggest an association between blood draws and having HBV (Appendix D). A corneal donor study did not find an association between HIV testing and HBV based upon next-of-kin interviews.118
- Household exposure. This review found very low-quality evidence associating household exposure with someone who had HBV and having HBV (Appendix D). Having household contact with someone with hepatitis was associated with HBV among college students in a univariate analysis,122 as was a family history of HBV among Asian Americans in multivariate analyses.136 However, having a household member with HBV was not associated with HBV in an obstetric population,126 nor was being the wife of a man with HBV among Korean American churchgoers.137 Sharing a razor or toothbrush with a household member was not associated with HBV in a general population study.127
Other infections. This review found low-quality evidence to suggest an association between having HBV and exposure to, or having, other types of infections (Appendix D). Among college students, HBV infection was associated with having had an STD in a multivariate analysis.122 Rabies exposure was not associated with HBV in a corneal donor study.118
- Demographic factors.
- Gender. This review found very low-quality evidence to suggest an association between gender and having HBV (Appendix D). Males had higher rates of HBV than females in studies of the general population,123 the Asian American population in multivariate analyses,136 and psychiatric inpatient veterans (of whom nearly all were male),124 but not in studies of Korean American churchgoers.138 Among college students, females had higher rates of HBV in a multivariate analysis.122 Among children who had received a blood transfusion, rates were not significantly different between genders.139
- Age or year of birth. This review found very low-quality evidence to suggest age as a risk factor for HBV (Appendix D). Studies assessed different age ranges, thereby complicating the comparison. One general population study found an increased risk of HBV in individuals >60 years of age in a multivariate analysis.123 The remaining studies were in demographic or socioeconomic subpopulations. One study of college students found an association with increased mean age in a multivariate analysis122 while a study of psychiatric inpatient veterans did not.124 In univariate analyses, increased prevalence was associated with age >35 years among embalmers in high-prevalence urban areas with a large effect size135 and age <20 years among Korean-American churchgoers,137 but not age ≥50 years among psychiatric inpatient veterans in a multivariate analysis.125 In another study of Korean-American churchgoers, lower prevalence was found in people <49 years of age.138 The last study comprising Asian Americans did not find any association between age and HBV.136
- Race/ethnicity and national origin. This review found very low-quality evidence to suggest a relationship between HBV and an individual's race/ethnicity or national origin (Appendix D). Being of non-Hispanic black race compared with non-Hispanic white race was associated with a higher prevalence of HBV in a general population,121 among college students,122 and among psychiatric inpatient veterans in multivariate analyses.125 This association was not found in a study of psychiatric inpatient veterans.124 Being Mexican American was not associated with a different prevalence of HBV than non-Hispanic white race in a multivariate analysis of a general population study.121 And in demographic or socioeconomic subpopulations, Hispanic ethnicity124 and Hispanic or Latino ethnicity122 were not associated with HBV infection. Additionally, white race or Hispanic ethnicity was associated with lower HBV prevalence in a general population study in a multivariate analysis.123 Among college students, Asian students had higher rates of HBV.122 Regarding national origin, HBV was independently associated with being born in Southeast Asia or Africa in one general population study.123 A special population study found that children born in Korea had a higher prevalence of HBV than children born in the U.S.137 Other special population studies did not find significantly different rates of HBV in Asian Americans born in East Asia (excluding China) or Southeast Asia or the Pacific Islands compared with Asian Americans born in China.136 In a general population study, acute HBV was not associated with birth in an area with a high endemic rate of HBV or having a household contact with someone who was born in an endemic area.127 In multivariate analyses, a general population study121 and a study of Asian Americans136 found that being born in the U.S. was associated with a lower prevalence of HBV; the relative odds of HBV comparing other national origins with the U.S. was 3.4 for other national origins when compared with the U.S. Among African American, Caucasian, Asian, and Hispanic children who received blood transfusions, the prevalence of HBV was not significantly different.127
- Occupation. Evidence of low quality was found that examined the association between the type of occupation and having HBV (Appendix D). It is important to note that some of these studies included data on health-care workers before 1992, when HBV vaccination requirements for health-care workers took effect. Being a health-care worker with frequent blood exposure was associated with an increased prevalence of HBV in college students,122 but being a health-care worker was not associated with HBV in general population studies121,123 or having been a health-care worker in psychiatric inpatient veterans wards.124 Another general population study did not associate health-care employment or household contact with someone who is a health-care worker with HBV,127 and a special population study did not associate being a health-care worker or the spouse of one with HBV.126 Being in the military also was not associated with HBV in a general population study.121
- Education. Very low-quality evidence was found suggesting a relationship between education level and HBV status (Appendix D). Compared with patients who had some college education, those with less than a high school education had a higher prevalence of HBV in a multivariate analysis.121 A second study reported that students enrolled in four-year colleges had lower rates of HBV than students enrolled in two-year colleges in a multivariate analysis.122 However, years of education was not associated with HBV among psychiatric inpatient veterans.124
- Economic factors. This review found very low-quality evidence of no association between economic factors and HBV (Appendix D). Being homeless was not associated with an increased risk of HBV among psychiatric inpatient veterans.124 Another special population study did not associate homelessness, institutionalization, or other non-independent living arrangements with HBV.125
- Health insurance. We identified no studies that met the inclusion criteria.
- Marital status. This review found low-quality evidence to support marital status as a risk factor for HBV (Appendix D). A general population study reported that being divorced or single was associated with a higher prevalence of HBV than any other status in a multivariate analysis.121 Being currently married vs. any other status was not associated with any difference in HBV prevalence among psychiatric inpatient veterans.125
Q4.C. HCV and nonbehavioral risk factors.
People with hemophilia or related clotting disorder who received clotting factor blood products. No studies were identified in the literature regarding an association between clotting factor and prevalence of infection. The 1994 PHS guidelines identified “persons with hemophilia or related clotting disorders who have received human-derived clotting factor concentrates” as a risk factor for HIV.
Exposure to infected or suspected infected blood. No studies were identified that met the inclusion criteria. The 1994 PHS guidelines identified “persons who have been exposed in the preceding 12 months to known or suspected HIV-infected blood through percutaneous inoculation or through contact with an open wound, non-intact skin, or mucous membrane” as a risk factor for HIV.
Children. No studies were identified in the literature regarding the nonbehavioral risk factors, listed previously, in relation to children or children of mothers who engage in those nonbehavioral risk factors.
Signs and symptoms. This review found low-quality evidence associating HCV with jaundice, alanine aminotransferase (ALT) reactivity, and elevated ALT with large effect sizes. There was very low-quality evidence associating HCV with elevated liver enzyme, and the effect size was not large (Appendix D). There was also low-quality evidence regarding the prevalence of signs and symptoms for HCV. An objective of this section was to identify nonbehavioral factors that could be predictive of infection, especially acute infection, during the window period before tests recognize the infection. We identified four studies.63,120,130,132 In blood donors, ALT reactivity was significantly associated with infection with a large magnitude of effect.130 In a general population study, serum ALT of >40 units per liter (U/L) was associated with HCV.63 Elevated liver enzymes were significantly associated with HCV with a large effect size in adults enrolled in an HMO that comprised individuals at risk for HCV and health-care workers.120 In addition, jaundice was associated with HCV in adults in a general medical clinic with a large effect size.132 All four studies were univariate analyses. In one study, 9% of respondents had ALT levels >40 U/L,63 and evidence was rated low quality.
Receipt of blood transfusion. Moderate-quality evidence was found regarding an association between receiving blood transfusions and HCV with a large effect size (Appendix D). Although no studies reported on risk in people with clotting disorders or who have received clotting factor blood products, many did investigate the risk of infection associated with blood transfusion. All eight studies found some association with transfusion and HCV infection. Receiving a blood transfusion was independently associated with HCV in three blood donor studies (data collection occurring during 1991, 1992–1993, and 1994–1996)128,131,140 and three general population studies (data collection occurring during 1992, 1999–2002, and 2000–2002).63,123,129 In a study of blood donors, sex with a blood transfusion recipient was also associated with HCV.128 Additionally, a significant association was found between blood transfusion and HCV among donors who had never injected drugs. Among general population survey respondents, 6% of those aged 20–59 years and 16% of those aged 60 years or older reported having a blood transfusion before 1992.63 Among volunteers from an urban area, 20% reported having ever had a blood transfusion.123 We found low-quality evidence regarding estimates of the prevalence of having blood transfusions.
- Nonspecific exposure.
- Accidental needlestick injury. This review found very low-quality evidence supporting accidental needlestick injury as a risk factor for HCV (Appendix D). In a general population study, needlestick injuries were actually associated with a lower prevalence of HCV infection.123 This finding may be because a substantial proportion of the enrollees were health-care workers, and in that study health-care workers had a lower prevalence of HCV and HBV than did the group as a whole. Needlestick injuries among health-care worker blood donors were also not associated with HCV.131 However, bloody needlestick injuries in a medical setting were independently associated with an increased prevalence of HCV.128 According to data collected from next of kin, accidental needlestick injuries were not associated with HCV among potential corneal donors.118
- Hemodialysis. This review found low-quality evidence supporting the association between hemodialysis and HCV infection (Appendix D). In two general population studies, HCV infection was independently associated with hemodialysis123 and with kidney dialysis in a univariate analysis with a large effect size.132 A third study involving adults with known HCV, risk factors for HCV, or planned hemodialysis did not find an association between hemodialysis and HCV in univariate analysis.129
- Surgery. This review found very low-quality evidence supporting the relationship between having had surgery and HCV infection (Appendix D). In a univariate analysis, being hospitalized was associated with HCV with a large effect size; however, having surgery or a medical procedure in the six months before blood donation was not associated with HCV.130 Having a history of surgery was not associated with HCV in general population studies;120,123 however, lifetime history of surgery or sutures was independently -associated with elevated HCV prevalence in blood donors.128 Based upon next-of-kin data for potential corneal donors, no association was found between HCV and surgeries.120
- Organ and corneal transplantation recipients. We identified no organ donor studies that met the inclusion criteria. Very low-quality evidence from a single study found no association between having a corneal transplant and HCV (Appendix D). Receipt of organ transplantation was not associated with a greater risk of HCV in a corneal donor study.118
- Blood draws. Very low-quality evidence was found on studies that looked at the association between having blood drawn and HCV infection (Appendix D). A general population study reported an association between having had a blood test for HBV and having an HCV infection in a univariate analysis with a large effect size. In the same study, being a blood donor was associated with a reduced risk of HCV, and having been rejected as a blood donor was associated with an increased risk of HCV, also with a large effect size.132 Based upon next-of-kin data for potential corneal donors, no association was found between HIV testing and HCV.118
- Other blood exposure. These factors were reported because of their relevance, but quality-of-evidence ratings were not performed due to lack of replication of the factors. Having been stuck or cut with a bloody object was independently associated with HCV among blood donors.140 Among blood donors, blood exposure during fighting, by biting, at an accident site, or during a manicure in the last six months was associated with HCV in a univariate analysis with a large effect size, but not during a haircut in the last six months.130 Contact with blood was not associated with HCV in members of a general population.129
- Household exposure. This review found very low-quality evidence to suggest a relationship between household exposure to HCV and having HCV (Appendix D). Among blood donors, living with someone with hepatitis or having a relative with hepatitis was not associated with HCV infection, but in a multivariate analysis, living with a transfusion recipient was associated with HCV infection.128 Additionally, sharing a toothbrush or razor with another person was associated with HCV among blood donors.128 In the general population, having at least one family member treated for viral hepatitis was not associated with an increased risk of HCV in one study,120 but having at least one family member with HCV was associated with an increased risk of HCV in another study.132
Other infections. This review found very low-quality evidence to suggest an association between STDs or other infections and having HCV (Appendix D). Among blood donors, HCV infection was significantly associated with a history of STD,128,131 having an STD within six months of donating,130 and seropositivity for other reactive infectious diseases,130,140 with two of the studies demonstrating an independent association.128,140 Among people from general populations, having past treatment for STDs was not associated with HCV,132 but having herpes simplex virus type 2 (HSV-2) infection was associated with HCV.63 HIV infection was not associated with HCV infection in a univariate analysis of a general population,129 and rabies exposure was not associated with HCV in a corneal donor study.118 This review found moderate-quality evidence regarding estimates of the prevalence of HSV-2 infection.
- Demographic factors.
- Gender. This review found very low-quality evidence to suggest an association between gender and having HCV (Appendix D). Being male was independently associated with an increased risk of HCV among heart transplant donors133 and blood donors,140 but was not associated in two blood donor studies.130,131 In the general population, significantly higher proportions of males had HCV infection in four studies.63,124,132,141
- Age or year of birth. This review found very low-quality evidence associating age and HCV (Appendix D). Studies assessed different age ranges, thereby complicating comparison. An organ donor study found that HCV infection was associated with older median age.133 One blood donor study associated HCV with older mean age130 while the other did not.131 In general population studies, HCV was associated with increased mean age141 and decade of birth (with people born from 1940 to 1959 having the highest prevalence),132 but not age <50 years120 or age <60 years.123
- Race/ethnicity and national origin. Very low-quality evidence was found in studies that examined the association between HCV and an individual's race/ethnicity, as well as associating HCV with national origin/birthplace and preferred language (Appendix D). Among heart donors, ethnicity was not associated with HCV.133 Black race was associated with increased rates of HCV compared with white race in two blood donor studies,131,140 of which one was an independent finding.140 In general population studies, black race or Hispanic ethnicity was independently associated with infection in one study,63 but was not associated with infection in two other studies.132,141 Among blood donors, one study found that Hispanic ethnicity led to a higher risk of HCV than white race in a multivariate analysis,140 but another study did not come to this conclusion.133 Being Asian vs. white was associated with having a lower prevalence of HCV among blood donors in a multivariate analysis.140 One blood donor study did not associate foreign birth with HCV in a univariate analysis,130 but another study did in multivariate analyses.140 A general population study found that people born outside of the U.S. had a lower prevalence of HCV in a multivariate analysis.63 Birth in Southeast Asia or Africa was not associated with an increased prevalence of HCV in another general population study.123 Another general population study found no association between HCV and U.S. citizenship.132 The prevalence of HCV was not significantly different among African American, Caucasian, Asian, and Hispanic children who received blood transfusions.123
- Occupation. This review found very low-quality evidence associated with an individual's type of occupation as being a risk factor for HCV (Appendix D). Among blood donors, occupational blood exposure was independently associated with HCV infection,128 but a medical or dental job or a public safety job with frequent blood contact was not associated with HCV infection.130 In the general population, work contact with blood was not associated with increased HCV,120 and being a health-care worker was associated with a lower rate of HCV.123 In two general population studies, having served in the armed forces was not associated with HCV.63,120 A study of health clinic patients did, however, associate having a job at a prison with having HCV.132
- Education. Evidence of very low quality was found associating education level with HCV infection (Appendix D). Lower educational attainment was associated with HCV in blood donors,130,131 with one study reporting a large effect size in a univariate analysis.130 One general population study associated having fewer than 12 years of education with having HCV,63 but two other studies found no association between educational attainment and HCV.132,141
- Economic factors. Evidence of very low quality was found associating economic factors with HCV (Appendix D). Ever having been homeless was associated with an increased risk of HCV in adults attending general medicine or hepatology clinics.132 Neither income level nor living in poverty was associated with HCV.63,132
- Health insurance. Very low-quality evidence from a single study failed to show an association between type of health coverage (e.g., Medicaid, Medicare, private, or self-pay) and having HCV (Appendix D), with Medicaid patients having the highest HCV prevalence.141
- Marital status. Low-quality evidence from a single study did not show an association between marital status and HCV infection (Appendix D). Being married was associated with a lower rate of HCV in one blood donor study.128
Key Question 5. What are the test characteristics of the screening methods available to detect HIV, HBV, and HCV in potential organ donors? Do test characteristics differ in particular populations and with donor clinical status (i.e., donation after brain death vs. donation after cardiac death OR adult vs. pediatric donors)?
Numerous tests exist to detect HIV, HBV, and/or HCV in potential donors, and this question concerns the accuracy, sensitivity, and specificity of those tests, as well as the length of the window period and the turnaround time to perform the tests. For the systematic review of the literature, tests of interest included immunoassay tests and NAT assays currently used in the U.S. by OPOs, as well as fourth-generation HIV and HCV Ab/Ag tests currently in use outside the U.S. Other FDA-licensed assays, such as the Abbott PRISM HCV (Abbott Laboratories, Abbott Park, Illinois), Procleix Ultrio Assay (Gen-Probe, Inc., San Diego, California), and Abbott PRISM HIV O Plus (Abbott Laboratories), were not included for review, as these assays were not routinely used by OPOs when the literature review occurred. The p24 Ag test for HIV was not included because it is no longer used by OPOs. Additionally, an HCV Ag assay used in Europe was not included because the assay was licensed subsequent to the guideline literature search.
Initial searches of bibliographic databases were for test instruments for HBV, HCV, or HIV. Once the list of included tests was generated, additional searches were performed specifically by each test's name. The focus of these searches was to identify peer-reviewed literature regarding window period, turnaround time, and test performance characteristics. Because this strategy did not identify information for all of the listed tests, we also searched other literature sources, including FDA product labeling information, package inserts, manufacturers' websites, and additional sources including the World Health Organization. We also searched for FDA approval information for all tests. We used these sources for information on turnaround time and window period but not other test characteristics (e.g., sensitivity and specificity) because these sources of information generally do not report sufficient information to enable assessment of the study design, quality, and other factors that impact the outcomes and the strength of the evidence. Where data from sources other than clinical literature were used for the other characteristics, the source is clearly noted in the Evidence Report19 extraction tables.
The window period is particularly important in this context because a recently infected potential organ donor who is tested using immunoassays only would not be diagnosed prior to organ donation. The information regarding window periods is derived from testing seroconversion panels (i.e., a series of blood draws from patients who eventually become seropositive). A limitation of the panels is that the samples are not typically collected daily but at irregular intervals. Furthermore, studies generally reported the difference in window period between the two tests (e.g., Test 2 detected infection an average of x number of days later than Test 1). Therefore, information capturing absolute window periods was unavailable. Information on the time required to fully administer tests was sparse. Window period and turnaround time were not assessed using quality-of-evidence ratings because information often came from sources other than peer-reviewed publications. No studies reported on positive predictive values or likelihood ratios. No studies compared test characteristics among different donor populations. Although a large number of peer-reviewed publications and pieces of grey literature were included, little or no data addressed each individual study of interest. None of the studies focused on pediatric use.
Q5.A. HIV.
HIV third-generation immunoassays. We found low-quality evidence in studies addressing the sensitivity and specificity of third-generation immunoassay tests (Appendix E). Test sensitivity and specificity were calculated in two analytic studies with a sensitivity of 99.4%–100.0% and specificity of 97.7%–99.7%.142,143 Seroconversion panels showed positive responses ranging from the same day to about 14 days sooner than Western blot.142–145 We found no data on the duration of time required for the test to be performed (i.e., the turnaround time).
HIV NAT. We found low-quality evidence in studies addressing the sensitivity and specificity of NAT assays (Appendix E). Test sensitivity and specificity were calculated in analytic and clinical studies with a sensitivity range of 92.6%–100.0% and a specificity range of 96.9%–100.0%. These studies included individual and HIV/HCV combined assays.143,146,147 NAT results were positive and ranged from 2–24.5 days before Ab assays.146,148–152 NAT results were reactive from 0–28 days before Ag tests.146,148,149,151,152 One study reported a turnaround time of two hours for HIV testing alone,153 whereas another study reported that an experienced operator required six hours to perform two HIV/HCV combined assays and 6.5 hours to perform three HIV/HCV combined assays.148
HIV fourth-generation immunoassays. We found low- to moderate-quality evidence in studies on the specificity and sensitivity accuracy of fourth-generation EIA tests (Appendix E). Test sensitivity and specificity were calculated in five analytic and three clinical studies, with a sensitivity of 100.0%154–160 and a specificity range of 82.5%–100.0%.36,154–156,158–162 Five studies reported differences in window period time from third-generation Ab and polymerase chain reaction (PCR) assays using seroconversion panels. Fourth-generation assays were reactive and ranged from 0 to 6.15 days before third-generation Ab assays.35,155,158–160,163 However, when compared with NAT, fourth-generation assays were reactive and ranged from two to nine days after reverse-transcription PCR (RT-PCR).35–37 Turnaround time ranged from 26 minutes to four hours depending on the test brand.
Q5.B. HBV.
HBsAg. This review found low- to moderate-quality evidence associated with the sensitivity and specificity accuracy of HBsAg assays (Appendix E). Test sensitivity and specificity were calculated in analytic and clinical studies with a sensitivity of 100.0% and a specificity of 97.9%–99.4%.164,165 HBsAg detected reactive results 0-–7 days earlier than unnamed licensed references.166–168 Two studies reported a turnaround time of 29–30 minutes.75,157
Anti-HBs. This review did not identify any studies examining anti-HBs sensitivity and specificity. In one study of 40 seroconversion panels, the anti-HBs assay was reactive a median of 14–18 days after the NAT method.169 In another study, the window periods for the HBsAg and antiHBs assays were the same for 10 of 21 seroconversion panels, with antiHBs reactivity occurring later for the remaining 11 panels.170 No studies reported on the turnaround time for performing the test.
Anti-HBc. This review did not identify any studies examining anti-HBc sensitivity and specificity accuracy. In one study of seven seroconversion panels, the anti-HBc assay detected infection at the same time as an unnamed reference in six panels and one day sooner in the seventh.171 Per product label, anti-HBc appears in the serum of patients infected with HBV one to four weeks after the appearance of HBsAg, at the onset of symptoms.172 No studies reported on turnaround time for performing the test.
HBV NAT. We found very low-quality evidence in studies that examined the sensitivity and specificity of NAT assays (Appendix E). Test sensitivity and specificity were calculated in one clinical study with a sensitivity of 84.8% and specificity of 100.0%.173 NAT assay were reactive 10–20 days before HBsAg.169,174 No studies reported turnaround time for conducting the test.
Q5.C. HCV.
HCV second- or third-generation immunoassays. We found moderate-quality evidence from six studies concerning the sensitivity and specificity of second- and third-generation immunoassays (Appendix E). Test sensitivity and specificity were calculated in clinical studies with a test sensitivity of 73.2%–100.0% and a specificity range of 92.7%–99.9% in second-generation assays,175–177 and a test sensitivity of 100.0% and a specificity range of 94.4%–99.9% in third-generation assays.178–181 Of 19 blood donors who were RNA-positive but initially negative by a second-generation immunoassay, second-generation assays were positive a median of 34–63 days later.182 A third-generation assay was positive a mean of 26–32 days before second-generation assays.183,184 No studies reported the turnaround time for conducting the test.
HCV NAT. This review found low-quality evidence associated with the sensitivity and specificity of NAT assays (Appendix E). Test sensitivity and specificity were calculated in analytic and clinical studies, with a sensitivity of 99.3%–99.6% and a specificity of 97.4%–99.6%. These studies comprised individual and HIV/HCV combined assays.146,147,153 HCV NAT was reactive a mean of 32–85 days before third-generation Ab assays,148,184,185 a mean of 113 days before a second-generation assay,185 and a mean of five days before a fourth-generation test.186 One study reported a turnaround time of two hours to perform the test.153
HCV fourth-generation immunoassays. This review did not identify any studies regarding the sensitivity and specificity of fourth-generation immunoassays. A fourth-generation assay was reactive a mean of 21.6–26.0 days before third-generation assays.186,187 Fourth-generation assays were reactive a mean of 4.8–30.0 days after NAT.187,188 One study reported a turnaround time of 190 minutes to perform the test.188
Topic III: Donor interventions to decrease the transmission of HIV, HBV, or HCV from infected donors (Key Question 6)
Key Question 6. Which donor interventions reduce the probability of pathogen transmission from an organ donor infected with HIV, HBV, or HCV to a previously uninfected recipient?
Two publications of the same study reported on interventions to diminish the risk of viral transmission from infected donors to uninfected recipients.189,190 The 1994 study described perfusion techniques to potentially inactivate virus in kidneys procured from HCV-positive deceased donors. The study investigated the virus-reducing capacity of four inactivation protocols; viral burden was reduced by 69.0% to as much as 99.7%.189 The response appeared to be dose-dependent (i.e., the longer the inactivation procedure, the greater the viral load reduction) (Appendix F).
Topic IV: Potential risks and benefits of transplanting, or not transplanting, organs from donors positive for HIV, HBV, or HCV (Key Question 7)
Key Question 7. How do the clinical outcomes of recipients of organs from donors infected with HIV, HBV, or HCV compare with those who remain on the transplant list?
One study met the initial inclusion criteria of a wait list control group;191 therefore, we expanded the criteria to (1) studies of recipients who were uninfected pre-transplant that compared clinical outcomes of those receiving organs from infected donors with those receiving organs from uninfected donors and (2) studies of recipients who were infected pre-transplant that compared clinical outcomes of those receiving organs from infected donors with those receiving organs from uninfected donors.
Of 23 publications, there were 17 unique studies. None of the studies were randomized or prospective, but all 17 treated the groups concurrently, and 13 studies enrolled patients consecutively. No studies met the inclusion criteria for HIV, likely due to federal regulations that prohibit using organs from HIV-infected donors.
Q7.A. HBV.
Receiving organs from HBV-positive donors compared with remaining on the wait list. We found no studies that met the inclusion criteria.
Receiving organs from HBV-positive donors compared with organs from negative donors when the recipients were HBV-negative pre-transplant. We found very low-quality evidence from one study89 comparing outcomes of receiving kidneys from HBV-positive vs. HBV-negative donors when recipients were negative pre-transplant. HBV positivity was defined as anti-HBc positive and HBsAg-negative (Appendix G). Both patient and graft survival favored receiving a kidney from a negative donor in a univariate analysis. The study did not find any pre-transplant differences between HBV-positive and HBV-negative donor groups except for substantially higher rates of stroke among HBV-positive donors.
Receiving organs from HBV-positive donors compared with organs from negative donors when the recipients were positive before transplant. This review found very low-quality evidence associated with comparing patient and graft survival outcomes for HBV-positive candidates who received organs from HBV-positive vs. HBV-negative donors (Appendix G). For renal transplant recipients, donor HBV positivity was defined as anti-HBc-positive, HBsAg-negative,89 IgG anti-HBc-positive, IgM anti-HBc-negative, HBsAg-negative,87 and HBsAg-positive.192–194 For liver transplant recipients, donor HBV positivity was defined as anti-HBc-positive.195 One study of kidney transplant recipients found significantly improved graft survival using HBsAg-positive donors if the donor was living, and using HBsAg-negative donors if the donor was deceased.192–194 The liver study found no statistically significant differences in graft survival.195 Of the two kidney studies that used statistical adjustments to control for baseline prognosis,87,89 one study found poorer graft survival in the HBV-positive kidneys,87 and the other study found no significant difference in graft survival.89 Of three studies that reported patient survival,89,192–195 only one reported a statistically significant difference. Patient survival was higher in recipients receiving a kidney from an HBsAg-positive donor if the donor was deceased, but there was no statistical difference if the donor was living.192–195 One study used statistical adjustments to control for baseline prognosis and found no significant difference in patient survival when comparing kidney recipients of HBV-positive vs. -negative donors.89
Q7.B. HCV.
Receiving organs from HCV-positive donors compared with remaining on the wait list. We found very low-quality evidence from one observational study191 comparing survival outcomes of patients receiving an HCV-positive organ vs. remaining on the wait list (Appendix G). This study included patients with end-stage renal disease who had been on the transplant wait list from April 1995 to August 2000, and were followed to August 2001. The adjusted hazard ratio of 0.76 was statistically significant, favoring receipt of a kidney from an HCV-positive donor vs. remaining on the transplant wait list. An analysis comparing receipt of a kidney from any deceased donor (regardless of donor HCV status) and being on the wait list favored transplantation substantially (adjusted hazard ratio = 0.47).
Receiving organs from HCV-positive donors compared with organs from negative donors when the recipients were negative before transplant. This review found low-quality evidence associated with patient and graft survival outcomes from observational studies comparing receiving organs from HCV-positive vs. HCV-negative donors when recipients were HCV-negative pre-transplant (Appendix G). Results for patient survival favored receiving an organ from an HCV-negative donor in one study of heart donors110,112 and one study of kidney donors;196,197 a third study involving liver donors found no statistical difference 24 months posttransplant.48,56 Three of the four studies reporting on liver graft survival found no statistically significant difference 24–60 months posttransplant.48,56,196–199 Only one study reported baseline characteristics to enable comparison. There were more male donors and an older mean recipient age in the positive donor group.110,112 The reported hazard ratio of 2.8 was not indicated as adjusted or unadjusted. Another study, which adjusted for pre-transplant differences,196,197 found a significantly shorter survival in patients who received livers from HCV-positive donors. The difference in baseline characteristics, and the possibility that the pre-transplant prognosis may have been poorer for recipients who received organs from infected donors, makes it difficult to interpret these raw results.
Receiving organs from HCV-positive donors compared with organs from negative donors when the recipients were positive before transplant. This review found very low-quality evidence associated with patient and graft survival outcomes from studies that compared receipt of organs from HCV-positive vs. HCV-negative donors when recipients were HCV-positive pre-transplant (Appendix G). Six observational studies addressed kidney transplantation196,197,200–203 and seven studies addressed liver transplantation.48,56,195,198,199,204–207 For kidney transplants and liver transplants, data suggest a small but consistently better graft survival with HCV-positive donors than HCV-negative donors; however, the studies may not have been powered to detect a statistical difference. Additionally, the baseline characteristics between groups differed; donor/recipient ages were older and the recipients' time on the wait list was shorter in HCV-positive donor groups.201,208 Of the 11 studies that also reported patient survival, one study favored recipients of organs from HCV-negative donors,196,197 and another study favored recipients of organs from HCV-positive donors206 (statistical adjustments were applied to control for baseline differences). The remaining nine studies found no significant difference.48,56,195,200,201,203–208
Topic V: Potential risks and benefits of transplanting, or not transplanting, organs from donors with risk factors for HIV, HBV, or HCV (Key Questions 8, 9, and 10)
Key Question 8. How do the clinical outcomes of transplant recipients who receive organs from donors with behavioral or nonbehavioral risk factors compare with those who remain on the transplant list?
This question differs from Question 7 because the donor is not known to be infected but is identified as having an increased probability of infection due to certain behavioral or nonbehavioral characteristics. Two simulation studies met the inclusion criteria209,210 but made different comparisons; therefore, they were considered separately. Due to the paucity of evidence, we also looked for studies comparing the clinical outcomes of recipients of organs from increased risk donors with recipients of organs from standard donors. We identified no such comparative studies.
Q8.A. Estimated recipient outcomes after renal transplantation comparing “transplant” and “discard” policies. One study estimated clinical outcomes of transplant candidates receiving organs from donors with risk factors for HIV, HBV, or HCV vs. remaining on the transplant list. The study applied a Markov process to address whether kidneys of deceased increased risk donors should be transplanted or discarded.209 Four types of increased risk donors were considered: injection drug users, MSM, commercial sex workers (CSWs), and prison inmates (inmates). The main outcome measures were patient survival, quality-adjusted life-years (QALYs), number of organs transplanted, and cost of care. -Comparisons were made between patients on the wait list who received kidneys from either standard criteria donors or increased risk donors (transplant group) or from standard criteria donors only (discard group). Having a risk of HIV or HCV infection only was considered. Assumptions about the incidence and prevalence of these infections within the general, potential donor, and potential recipient populations were reported. Of note, estimated incidence and prevalence rates of HIV and HCV in the specific behavioral risk population were used as a proxy for potential donors. Key assumptions about the donors, recipients, death rates, costs, and QALYs were applied in the model. The base case simulation assumed that increased risk donors were current injection drug users with negative immunoassay and NAT screening results for HIV and HCV. The “transplant” strategy resulted in lower mortality, more QALYs, lower cost, and slightly more HIV infections. For this case, more HCV infections occurred with the discard strategy. This strategy led to more time on hemodialysis, with an assumption that the incidence of HCV is significantly higher when on dialysis than after kidney transplant. Separate analyses were performed for the three other types of increased risk donors (MSM, CSWs, and inmates) with results very similar to the base case for outcomes. Results of numerous one-way sensitivity analyses found that in most cases, the conclusions of the base case “were not substantially changed” except that HCV infections were strongly influenced by assumptions about incidence rates. Authors concluded that “the ‘discard’ policy would yield fewer HCV infections only in a setting where a recipient's risk of infection on dialysis is very low, while the probability of CDC increased risk donor infection in a donor is high” (Appendix H).209
Q8.B. Estimated mortality from receiving a kidney from an at-risk donor vs. one-year wait list mortalities. One study estimated outcomes of transplant candidates receiving organs from donors with risk factors for HIV, HBV, or HCV vs. remaining on the transplant list.210 This study was a comprehensive risk analysis of considerations pertaining to organ donation. The study emphasized that the risk of recipient death from a donor infection is only one among a set of competing risks, including the risk of dying while on the wait list, the risk of dying after the transplant (regardless of the donor's status), and the risks of dying from medications, employment, transportation, and recreation. Much of the data reported in the article were not relevant to the research question; therefore, we were selective in the data we reported. One-year wait list mortality estimates for a standard criteria donor were based on a Markov model that used 90-day wait list mortality and transplantation probabilities from the OPTN and Scientific Registry of Transplant Recipients 2007 Annual Report.211 Mortality rates were reported separately for 12 different types of recipients. For at-risk donors, the study only addressed HIV. When considering disease transmission to a recipient, an infectious risk of 46 per 100,000 population for HIV was provided based on OPTN high-risk donor data. In a conservative analysis, the authors assumed that HIV is 100% fatal, which corresponds to a 0.046% mortality rate. This estimate is much lower than all of the one-year wait list mortality rates ranging from 2.7% to 21.8%. They concluded that the wait list mortality risk far outweighed the risk of HIV-related mortality associated with receiving an organ from a serologically negative donor with a behavioral risk factor. They did not attempt to make mortality estimates for either HBV or HCV due to insufficient documentation in the literature (Appendix H).
Key Question 9. What is the impact of excluding potential organ donors with behavioral or nonbehavioral risk factors on the organ donor pool?
One study estimated the number of donors that would be excluded from organ recovery based on having risk factors for HIV, HBV, or HCV.209 During a 20-year period, the study estimated there would be a 25.3% reduction (250 fewer kidneys per 1,000 patients available for transplantation) if increased risk donors were excluded. The study only considered HIV and HCV and four types of behavioral exclusions (injection drug users, MSM, CSWs, and inmates). Exclusions for other reasons (e.g., HBV risk or nonbehavioral risk factors for HIV or HCV) would likely result in a larger reduction in the organ donor pool.
Key Question 10. What is the impact of false-positive tests on the organ donor pool?
This review did not identify any studies that estimated the number of donors or organs excluded from recovery due to false-positive results for HIV, HBV, or HCV.
VII: EXPERT OPINION SUMMARIES
Topic VI: Approaches as to how recipients can be informed about the risk of HIV, HBV, and HCV transmission and be evaluated for possible exposure posttransplantation (Expert Opinions 1, 2, and 3)
Expert Opinion 1. How and when should informed consent be obtained from potential recipients to help them consider the risks of donor-derived HIV, HBV, and HCV?
Organ transplantation always carries a risk of donor-derived disease transmission.212,213 Thus, donors without identified risk factors are not presumed to be risk-free; rather, they are differentiated from donors with risk factors in that the former possess no known serological or historical characteristics that indicate elevated risk. Due to the scarcity of transplantable organs and that the loss of donated organs results in increased mortality of patients remaining on the transplant wait list, donors with increased risk for infections are not barred from contributing to the organ supply, as they generally are from contributing to the blood or tissue supply. They are instead evaluated on a case-by-case basis by transplant centers that weigh the risks/benefits for each transplant candidate. Informed consent is an important part of this process. Current recommendations for obtaining informed consent from a potential recipient include the OPTN policy and recommendations from the 2009 consensus conference on NAT screening of potential donors.43
The 1994 PHS exclusionary criteria for HIV have been used to assess risk for HIV, HBV, and HCV infection in potential donors. OPTN policy requires that the host OPO communicate to transplant centers information of donors meeting any of these criteria, which it further defines as high risk.28 Transplant centers must then inform the potential recipient of this information and maintain documentation of informed consent. If a potential recipient is unable to provide informed consent, the legal next of kin or other appropriate surrogate is required to do so. However, there is currently no uniform consent process resulting in variability among transplant centers.
The 2009 consensus conference on NAT screening of potential donors, therefore, recommended establishing uniform consent standards across transplant centers and made recommendations as to what specifics should be addressed at the time of listing and at the time of an organ offer.43 At the time of listing, they recommended discussing the following with potential recipients: (1) that although transplantation carries the risk for potentially donor-derived transmission of infection, not performing the transplant often carries a higher risk of death than the risk attributable to donor-transmitted infection; (2) the risks of donor transmission of infections including HIV, HBV, and HCV; (3) the limitations of available testing and the potential for both false-positive and false-negative test results; (4) the risk of transmission of infections placed in the broader context of risk, including risks associated with the use of expanded criteria donors as well as everyday occurrences to make their magnitude understandable to the potential recipient; and (5) that risk assessment using donor histories may be limited by the knowledge of the person providing the information. At the time of an organ offer, they recommended disclosing specific donor history and testing results to enable the potential recipient to understand the risk while protecting donor identity and emphasizing that the transplant team has assessed the risk of the donor with the risk of not performing the transplant.
Informed consent at the time of an organ offer has been controversial, with some experts concerned that patients may be less able to rationally weigh the risks of accepting or declining a particular organ at the time an organ is offered (i.e., choices made in such hurried circumstances do not reflect patients' underlying values or well-considered preferences).214,215 Alternatively, others have advocated for obtaining specific informed consent and providing full disclosure of the donors' risk behaviors to patients at the time of the organ offer to allow patients to make an informed decision based on the donor's specific characteristics.216 Obtaining specific informed consent on multiple occasions would help to reinforce patient understanding of complex risk information critical to this decision and to promote an informed treatment decision.
Expert Opinion 2. When should testing of a transplant recipient be conducted to detect HIV, HBV, and HCV transmission from the donor?
Unexpected transmission of donor-derived disease to uninfected organ transplant recipients appears to be rare.29 The exact incidence of disease transmission is unknown because disease transmission is not actively or uniformly assessed across transplant centers in the U.S.; this finding is particularly true for bloodborne viruses, such as HIV, HBV, and HCV.42 Furthermore, the OPTN does not have formal policies requiring posttransplant assessment of recipients; therefore, it does not collect results of the testing when performed.
There are two major goals in recommending testing of transplant recipients: (1) to identify donor-derived disease transmission and implement interventions (i.e., antiviral therapy) early posttransplant to try and minimize the impact of the disease on the recipient and (2) to provide insight into the true incidence of donor-derived disease transmission.
As such, testing needs to be conducted to allow recognition of infection early enough to permit timely intervention while at the same time providing sufficient follow-up to prevent missing disease transmission. Several guidelines provide a template for the timing and frequency of screening individuals who have been exposed, through occupational and nonoccupational means, to HIV, HBV, and HCV.217–220 Because HIV, HBV, and/or HCV exposure via organ transplantation may be associated with a larger amount of virus transmission compared with occupational or nonoccupational exposures and more rapid progression of disease -secondary to the use of immunosuppressive medications, a modified recipient testing schedule of one, three, and 12 months posttransplant has been recommended by a 2009 consensus conference on NAT testing of potential donors.43,221 Baseline testing, which should be conducted at the time the recipient is admitted for transplantation but before organ placement, should be done on any recipient for whom follow-up testing will be recommended to rule out preexisting disease in the recipient prior to transplantation.
Seroconversion of transplant recipients may be impeded by the immunosuppressive medications the patients receive.29,222,223 In most cases of known donor-derived HCV transmission, recipients failed to seroconvert to HCV seropositive status despite having high viral titers by NAT.29,221,223 Likewise, in the confirmed HIV/HCV donor-derived transmission, one in four patients had an indeterminate Western blot.223 As such, any posttransplant testing of recipients should include a direct measure of the virus itself through Ag detection (i.e., HBsAg for HBV) or NAT (i.e., HIV and HCV). Recipient baseline testing, conducted at the time the recipient is admitted for transplantation but before organ placement, should also be done to rule out preexisting disease prior to transplantation.
Given that unexpected transmission of HIV, HBV, and HCV has occurred through organ transplantation, OPTN policy requires prompt notification of the OPTN and all institutions that recovered organs or tissues or transplanted organs from the donor when (1) an organ recipient is suspected of having a donor-derived infection and (2) the OPO or living donor recovery center received information after organ recovery that the donor was infected.27
Expert Opinion 3. How should donor and recipient specimens be collected and stored for potential investigation of donor-derived HIV, HBV, and HCV infection?
The availability of both donor and recipient blood specimens is critical for investigations to determine if a new infection with HIV, HBV, or HCV in a recipient is donor-derived. Appropriate specimen collection, labeling, transportation, handling, and storage facilitate the accuracy of reported laboratory test results. Whether for real-time testing or archiving for possible future testing, collecting two separate blood specimens for immunoassay and NAT reduces the possibility of specimen cross-contamination or deterioration of nucleic acids through specimen handling and storage. All serologic assays are FDA-approved for both plasma and serum specimens. All currently available qualitative NAT assays are FDA-approved for EDTA plasma only to ensure optimal sample integrity. If it is only possible to collect and store one specimen, storing plasma generally will allow for testing with either NAT or serologic assays. Labeling each specimen with a minimum of two unique identifiers ensures a confidential and unbroken chain of traceability to the identity of the donor and recipient.
For archived blood specimens, viral nucleic acid may deteriorate over time depending on storage conditions. For example, repeated freeze-thaw cycles can cause a moderate reduction in viral nucleic acid levels.224–226 Procedures to maximize sample quality include separating specimens that might be used for NAT into multiple aliquots prior to long-term storage, with storage temperature maintained at –70°C or colder. Furthermore, avoiding temperature extremes when archived specimens are shipped for testing inhibits specimen hemolysis, which can result in both false-positive serologic results and false-negative NAT results.227 Therefore, transporting archived specimens to a testing laboratory on dry ice is a common practice, as well as documenting the specimen quality and condition, with respect to both temperature and hemolysis, upon receipt in the testing laboratory. OPTN policy requires that deceased donor blood specimens be retained for a minimum of 10 years after transplant.28
Massive blood loss and intravascular volume replacement by transfusion of crystalloid and colloid solutions and blood products can cause hemodilution and result in unreliable test results for transmissible infections.4,228 A qualified (non-hemodiluted) specimen is one that is deemed acceptable for testing according to an appropriate hemodilution algorithm and calculation method, such as provided by the FDA. Test results from assays that used hemodiluted samples and the hemodilution calculation are to be reported to the accepting transplant programs.28 Calculations of dilution effects should take into account blood products and colloid administered. It should also be noted that hemodilution calculation algorithms are not standardized and the limits of acceptable hemodilution have not been validated across all current versions of serologic tests.229 The impact and limits of hemodilution on NAT have not been extensively studied but, from a theoretical perspective based on viral loads documented in acutely infected blood donors and the results of minipool testing in blood donors, may have a significantly greater impact on the detection of some pathogens (e.g., HBV)230,231 compared with others (e.g., HCV and HIV).30,31
Administration of blood products (plasma, red blood cells, and platelets) and intravenous or intramuscular immunoglobulin may result in the transfer of passive Ab and result in false-positive test results.232 Although blood products are universally screened, many of the infectious disease markers of concern, for organ donors and recipients who receive blood product, are significantly more likely to have false-positive results for highly prevalent analytes that do not preclude blood donation (e.g., anti-HBs) but are important as part of the pre-transplant donor or recipient screen. Receipt of blood and immunoglobulin products by donors and recipients in the three months prior to screening should be recorded, if known. -Passive maternal Ab may also be detectable in children <18 months of age; serostatus and infection status may be difficult to resolve. Recent HBV immunization may also result in false-positive HBsAg testing results, as HBsAg reactivity has been found in individuals up to five days after HBV immunization.233 When screening is urgent, efforts should be made to retrieve available pre-transfusion samples on the donor and recipients as close as possible to the time of transplant.
Appendix A.
GRADE rating of evidence for outcomes of interest and overall GRADE of evidence bases concerning HIV, HBV, and HCV transmission through organ transplantation for key question 1: What are the prevalence and incidence rates of HIV, HBV, and HCV among potential organ donors?
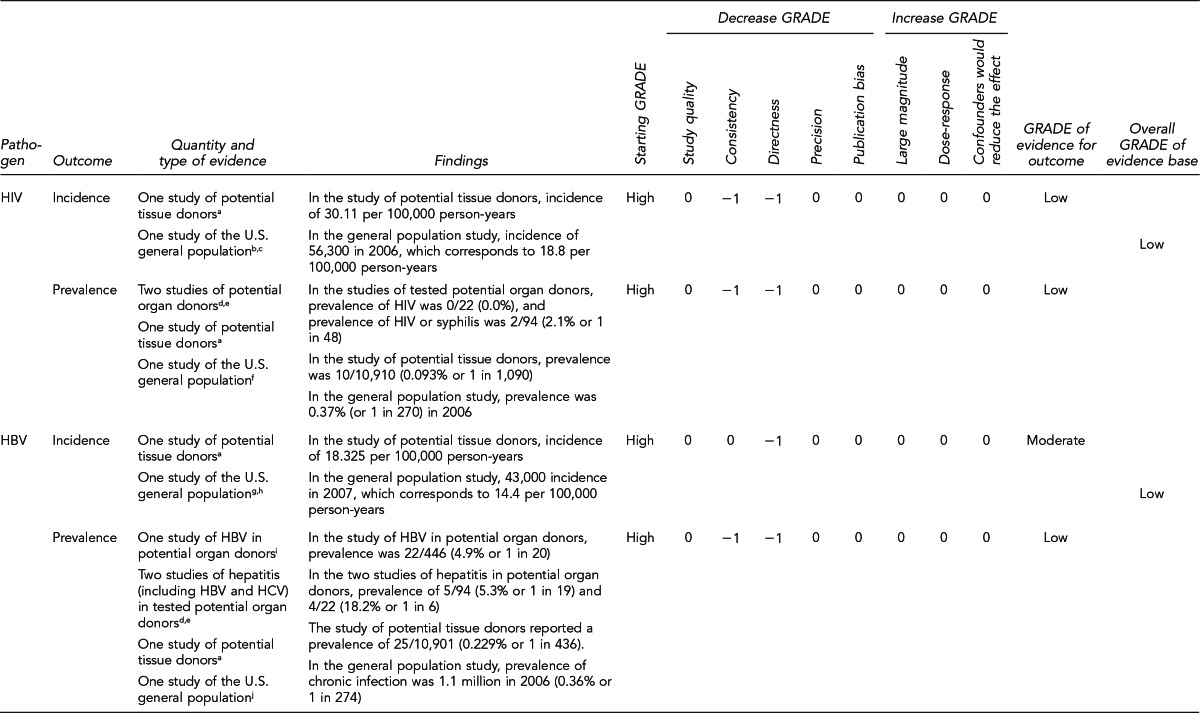
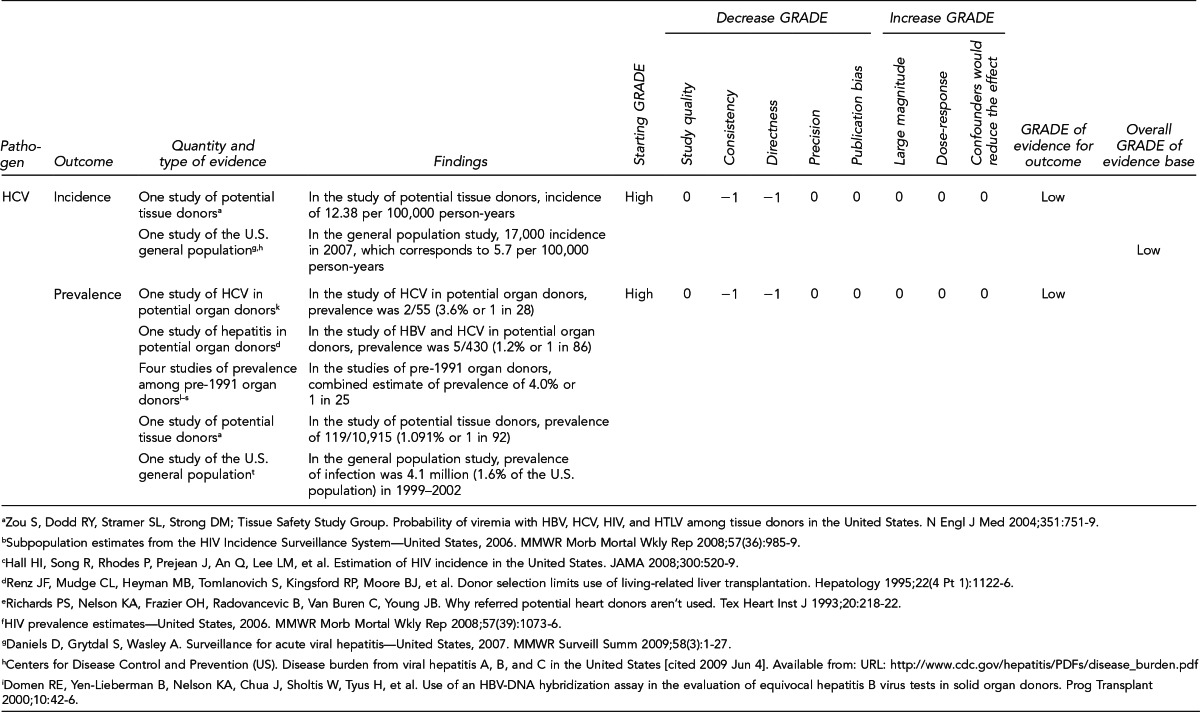
Appendix B.
GRADE rating of evidence for outcomes of interest and overall GRADE of evidence bases concerning HIV, HBV, and HCV transmission through organ transplantation for key question 2: What are the rates of transmission to recipients from donors infected with HIV, HBV, or HCV? Do the rates vary by the organ transplanted or when the donor was infected?
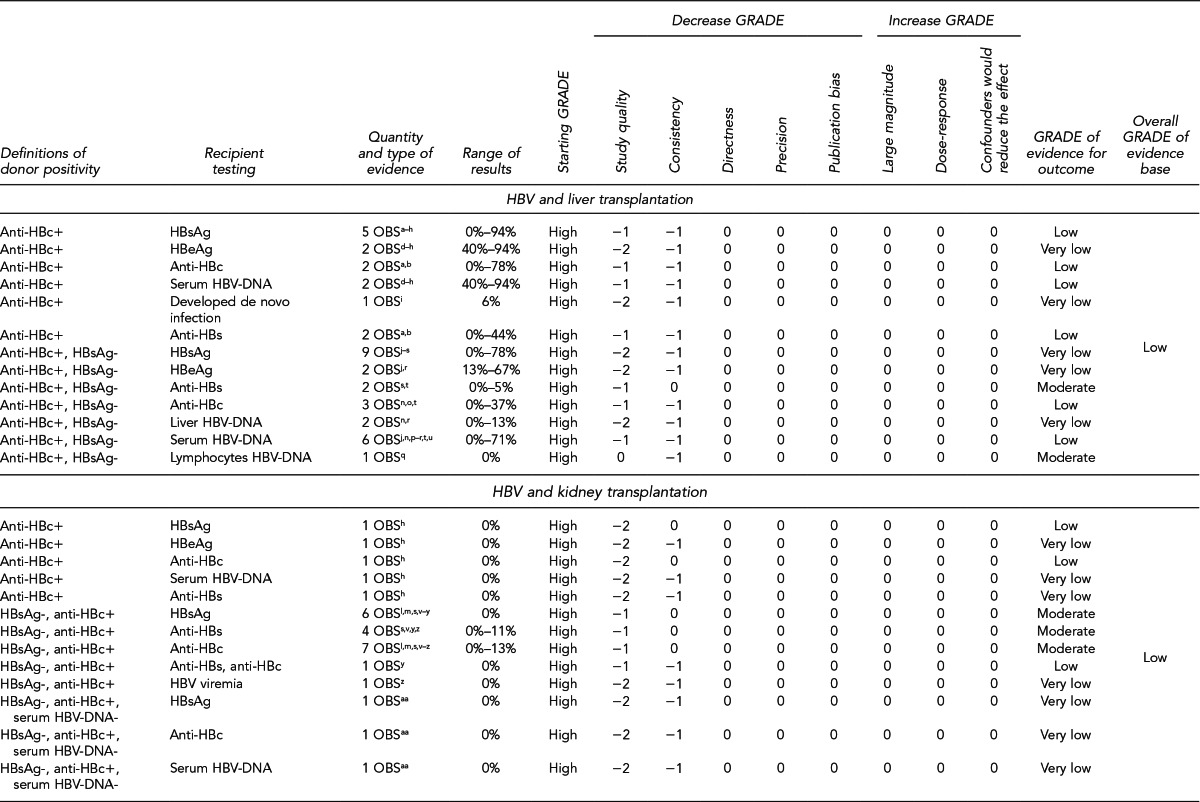
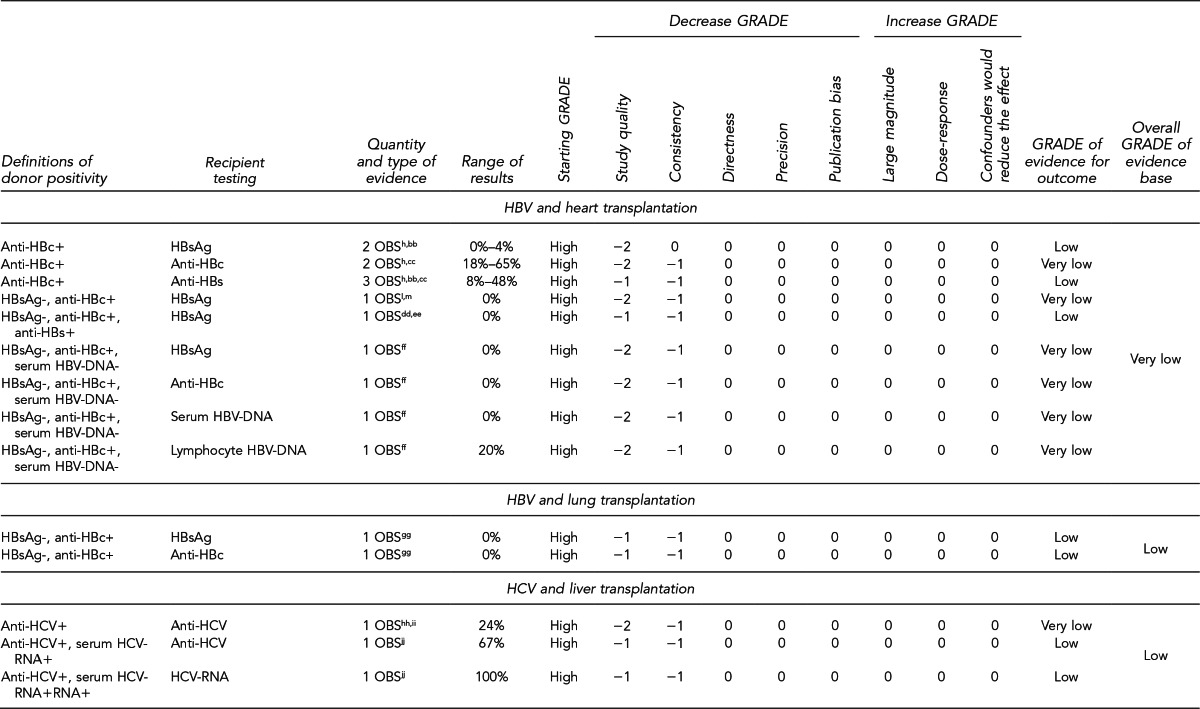
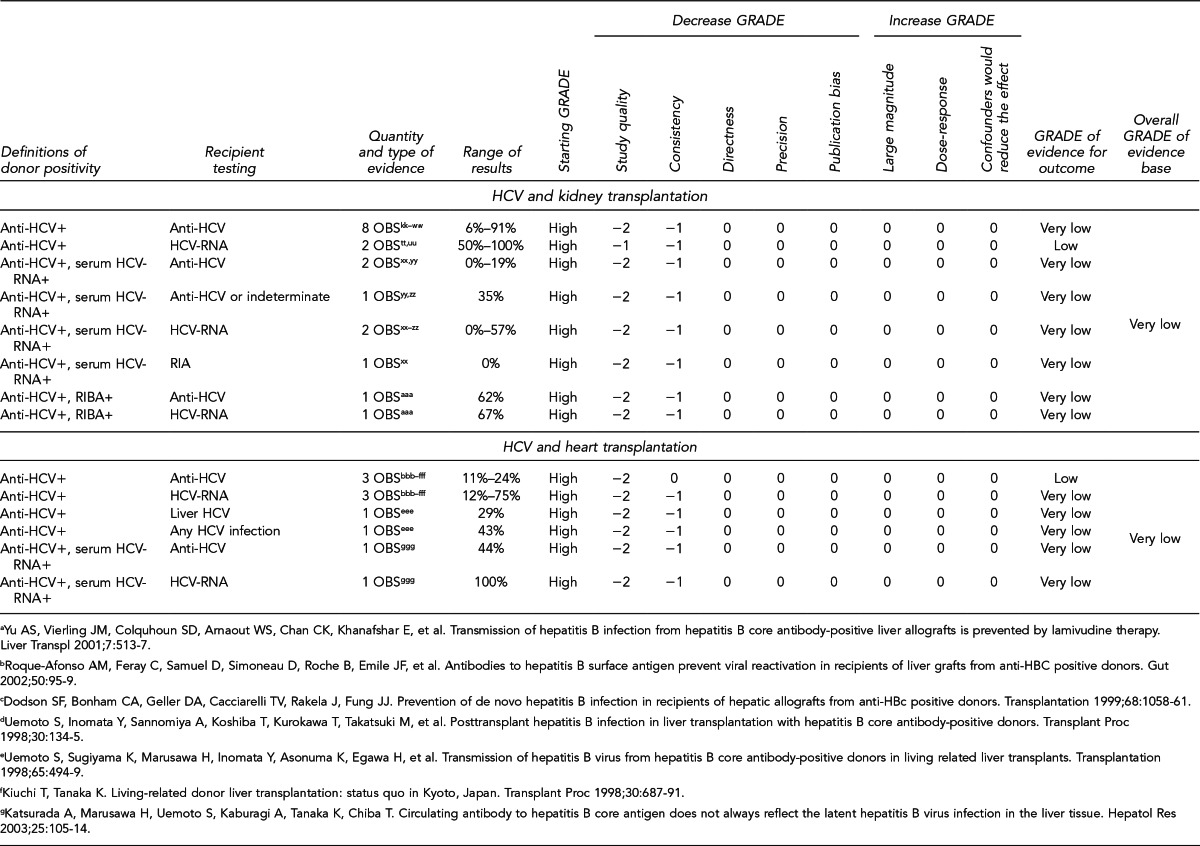



Appendix C.
GRADE rating of evidence for outcomes of interest concerning HIV, HBV, and HCV transmission through organ transplantation for key question 3: What behavioral risk factors are associated with an increased probability of infection with HIV, HBV, or HCV? What is the prevalence of these characteristics among potential organ donors?
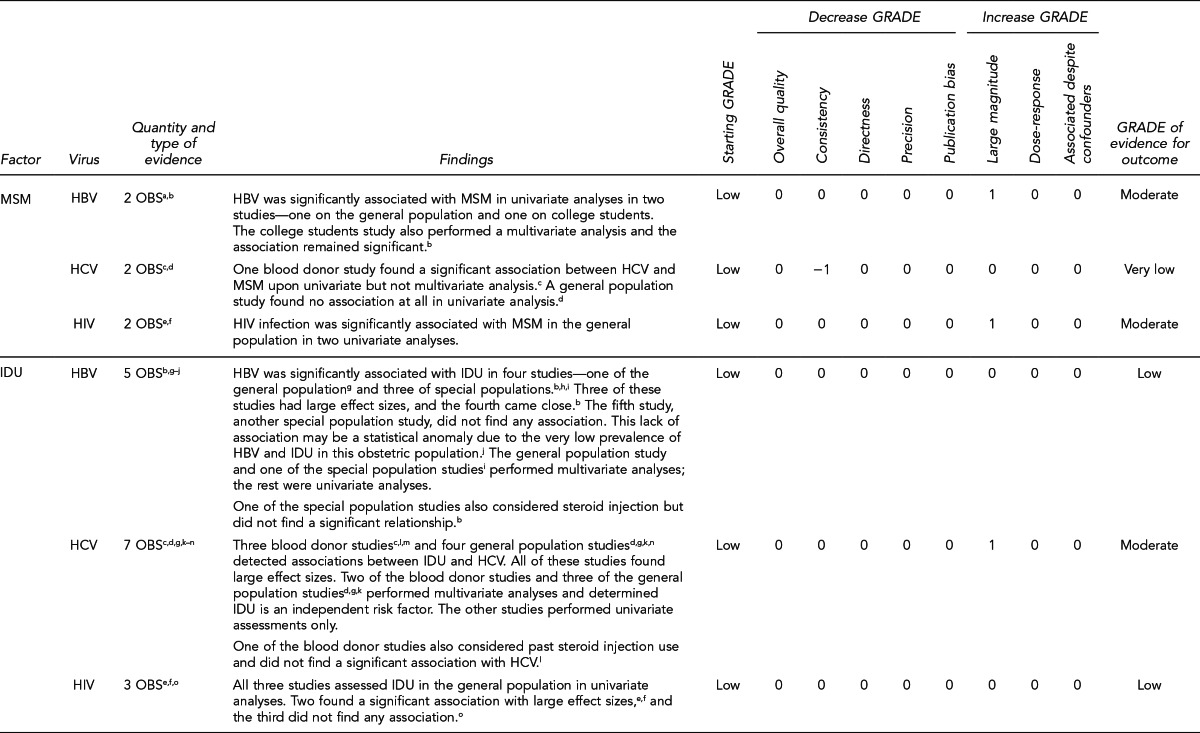
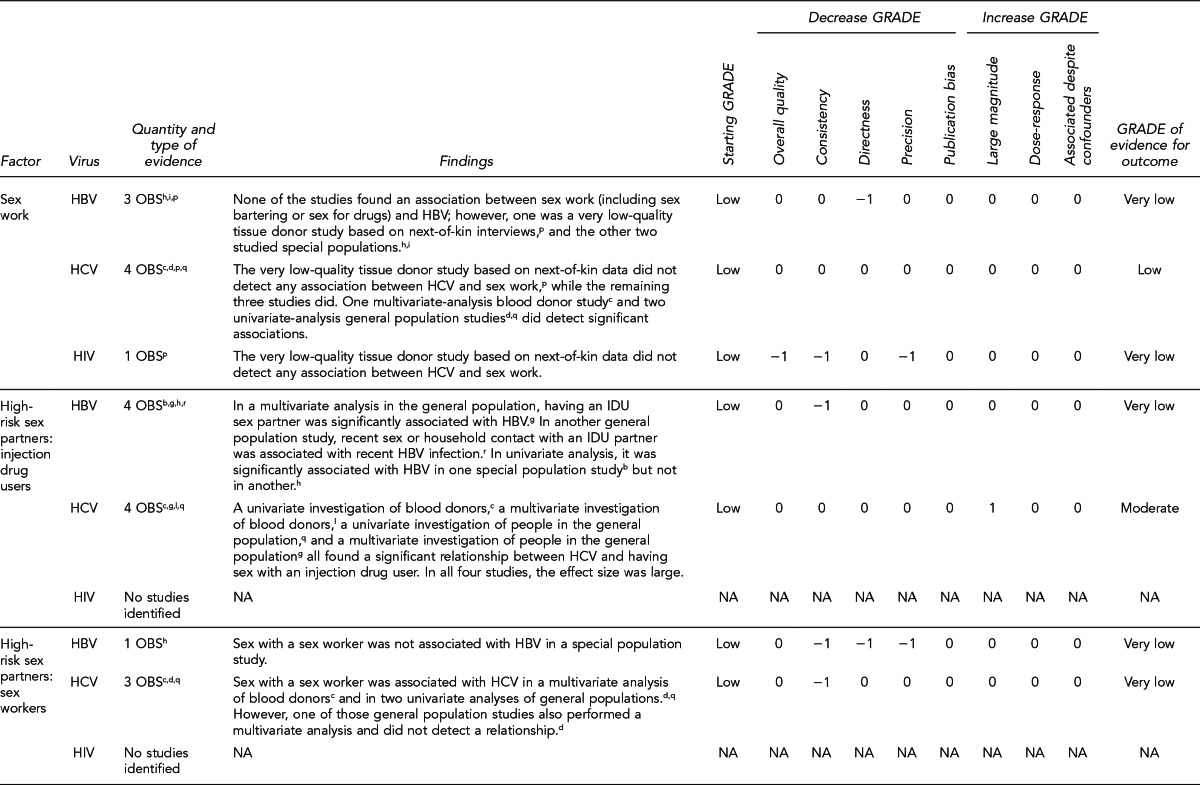
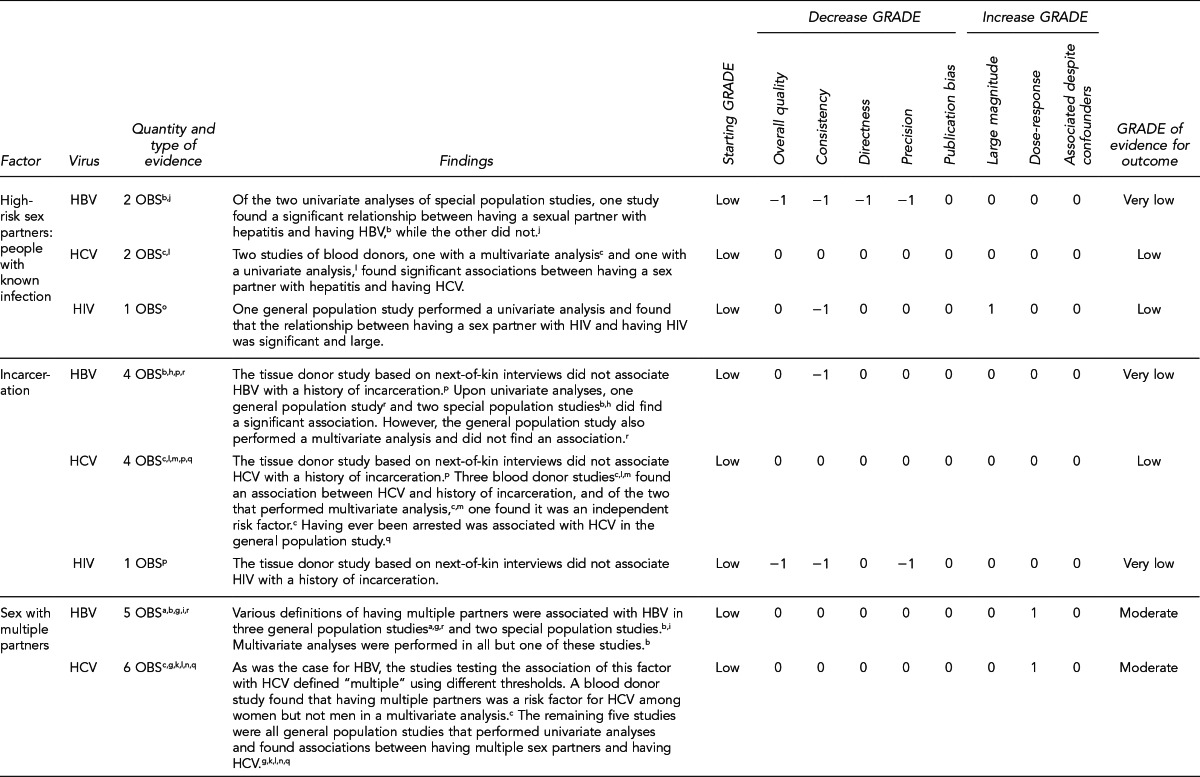
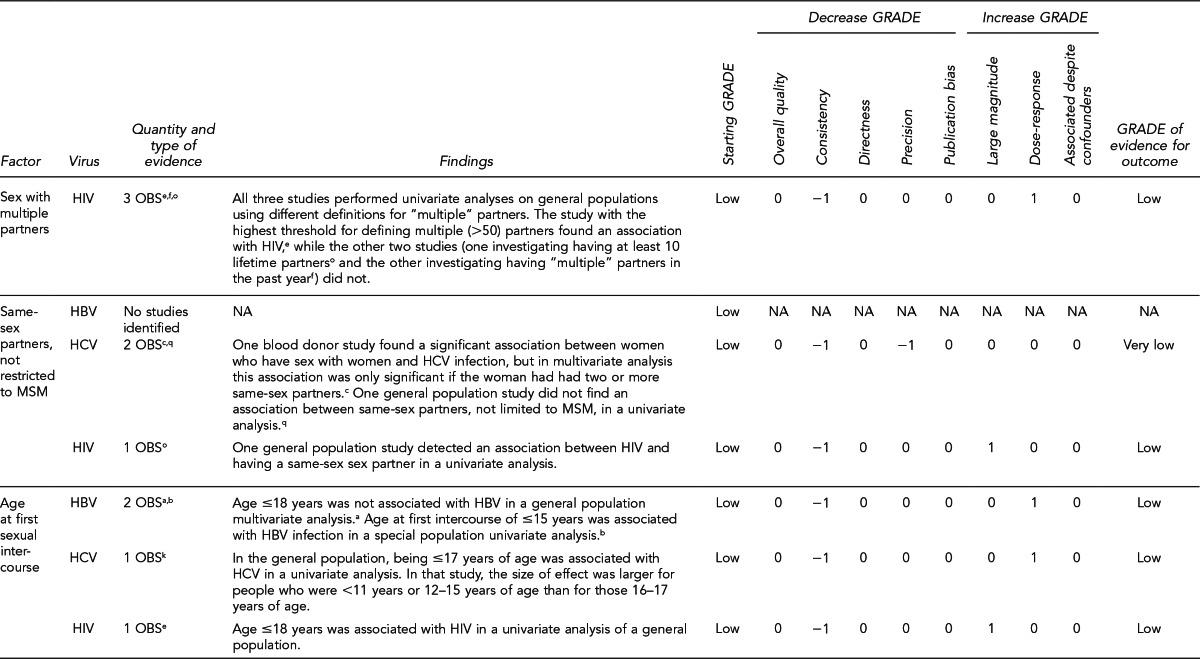
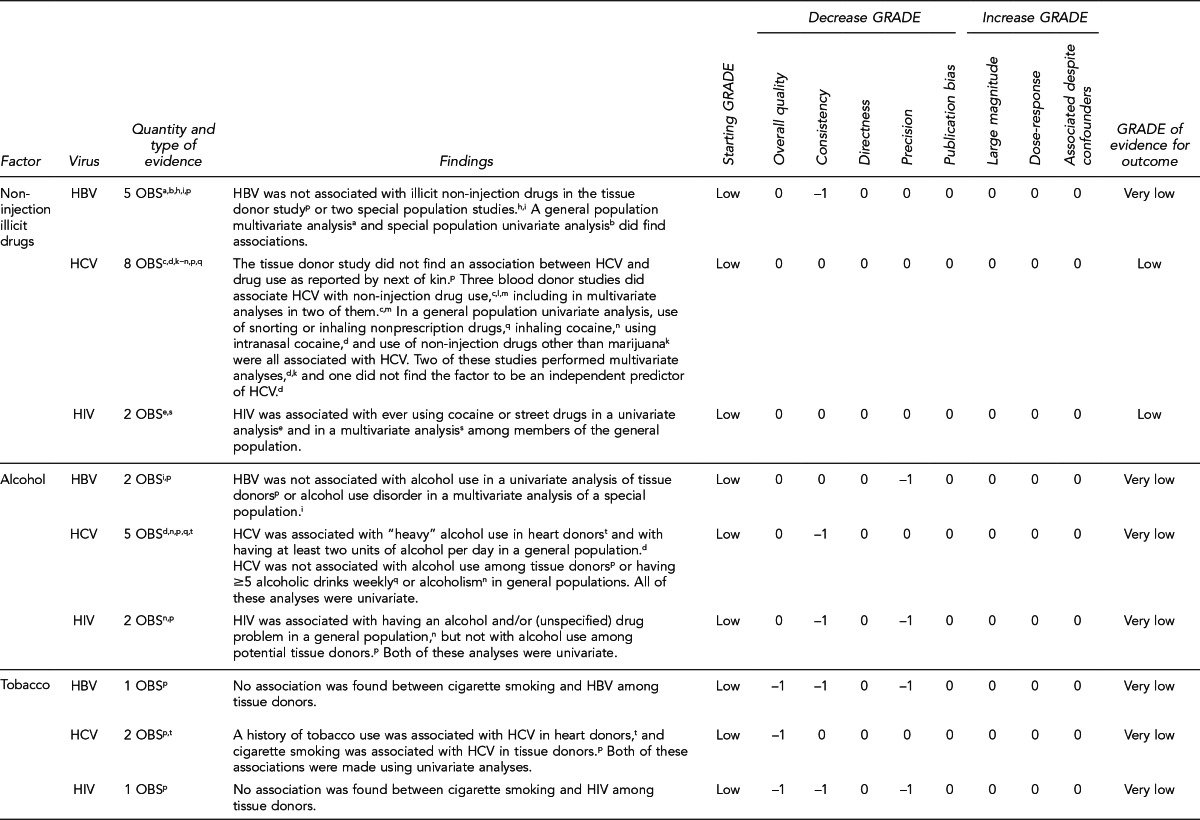
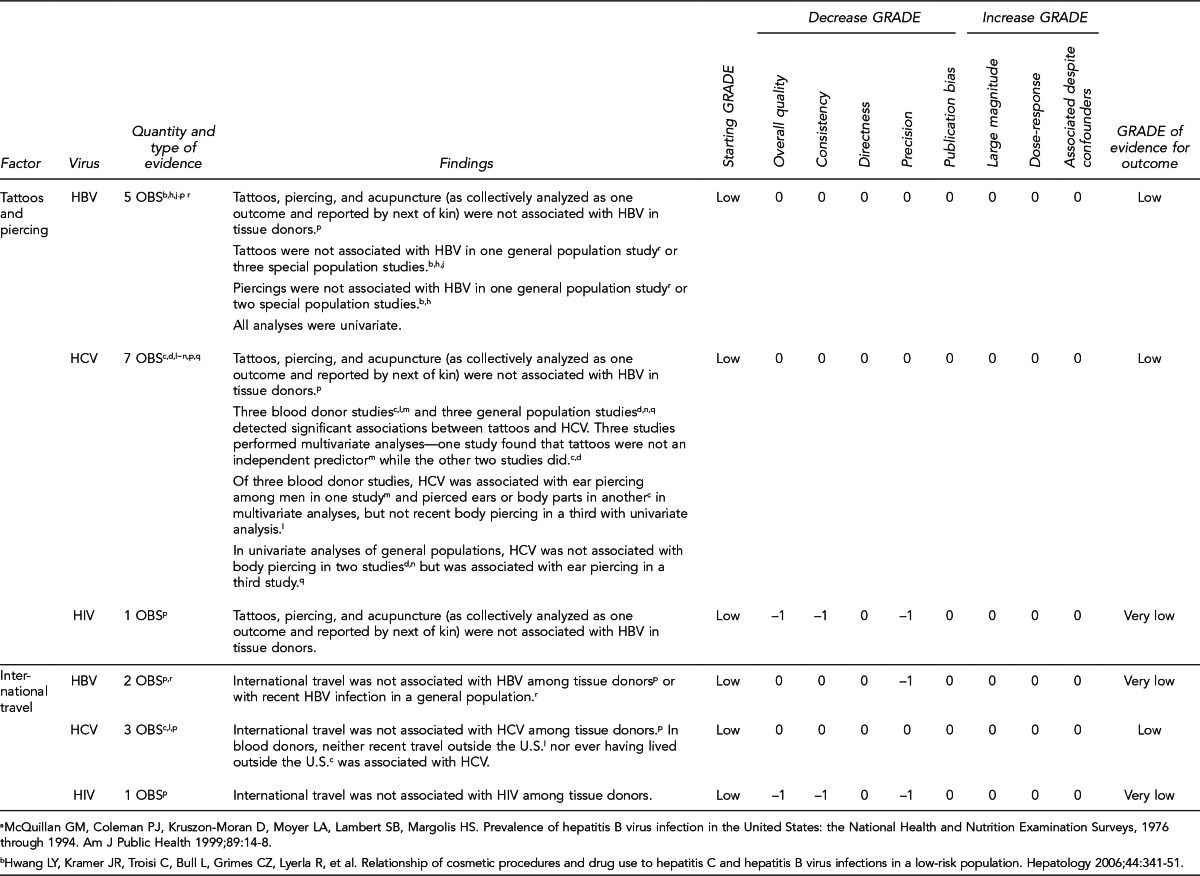

Appendix D.
GRADE rating of evidence for outcomes of interest concerning HIV, HBV, and HCV transmission through organ transplantation for key question 4: What nonbehavioral factors are associated with an increased probability of infection with HIV, HBV, or HCV? What is the prevalence of these factors among potential organ donors?
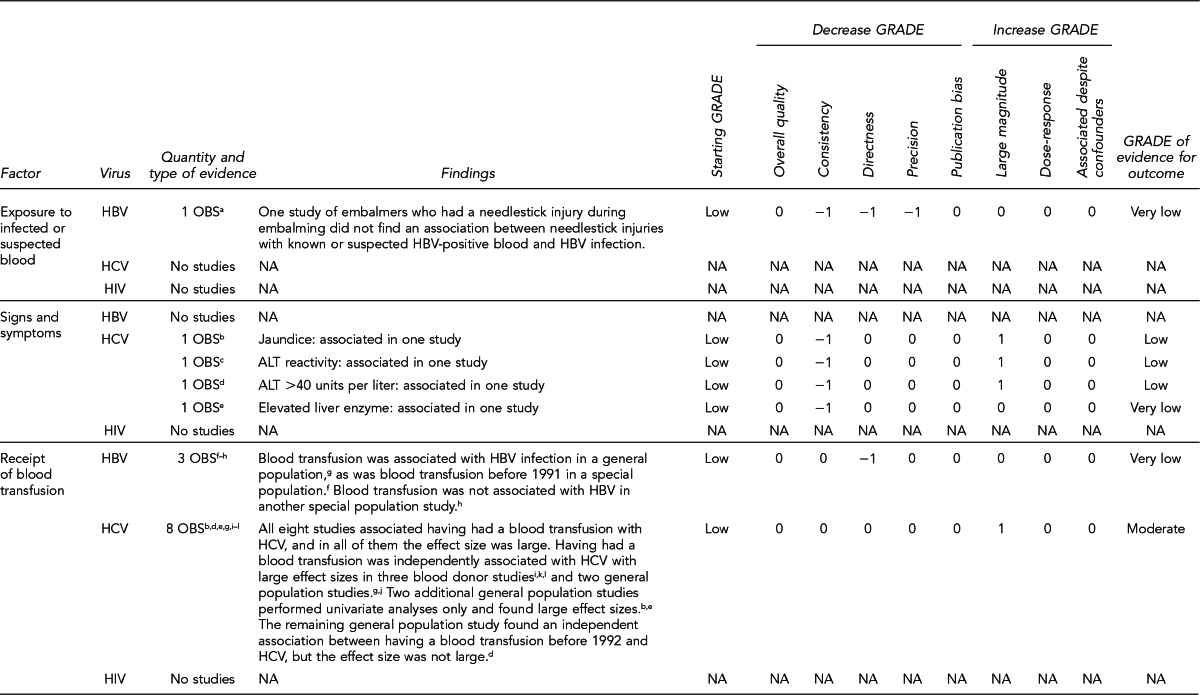
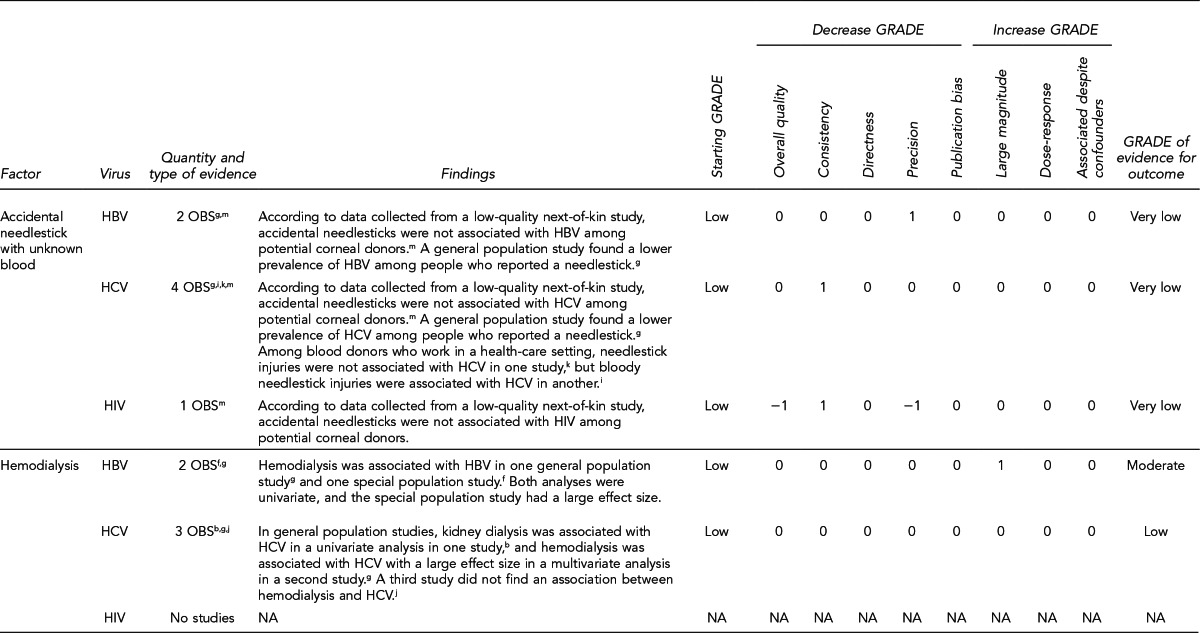
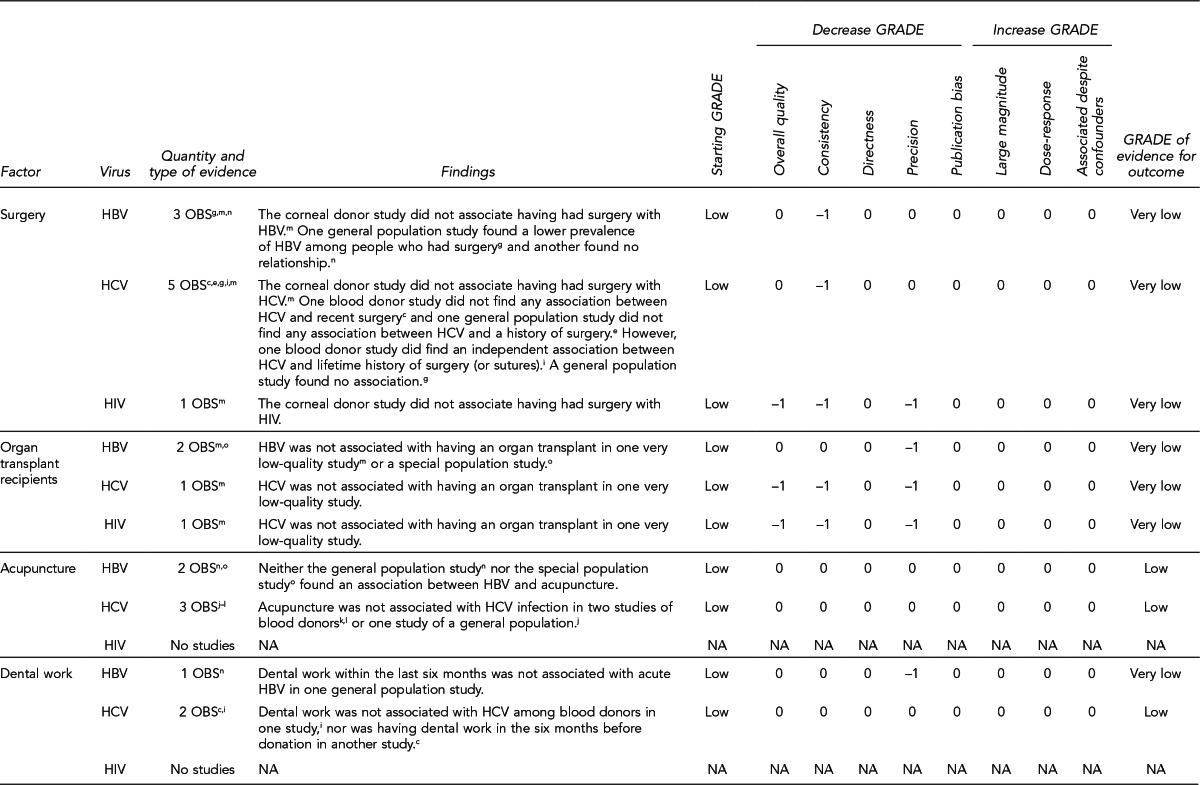
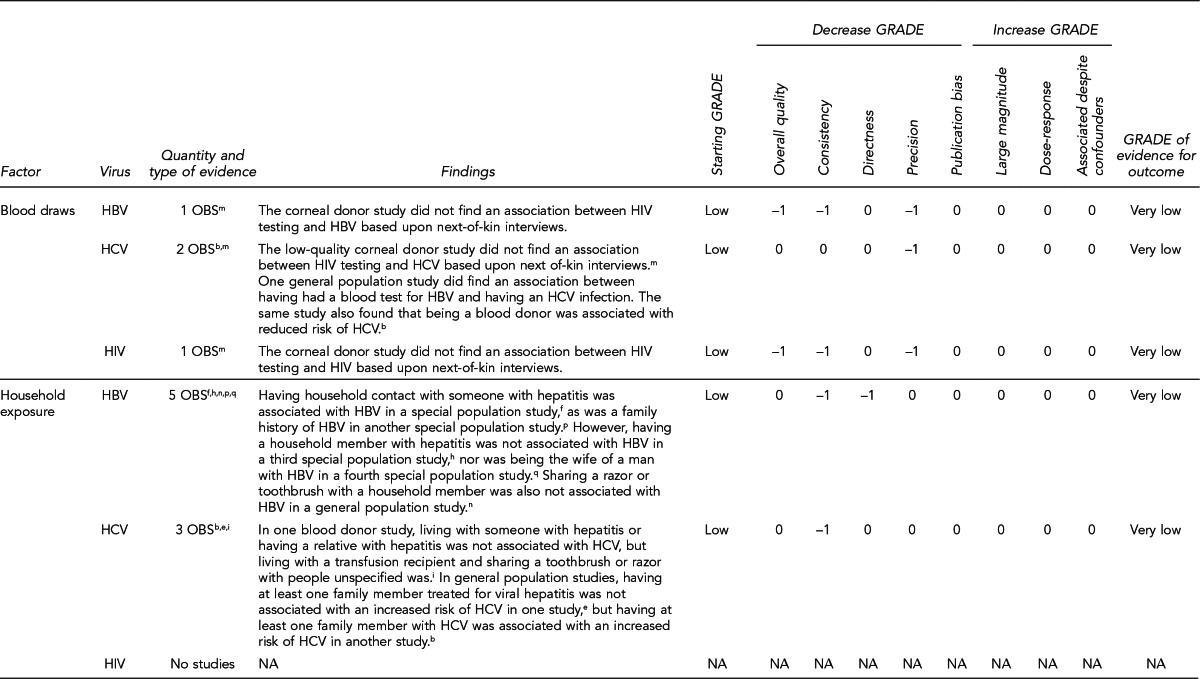
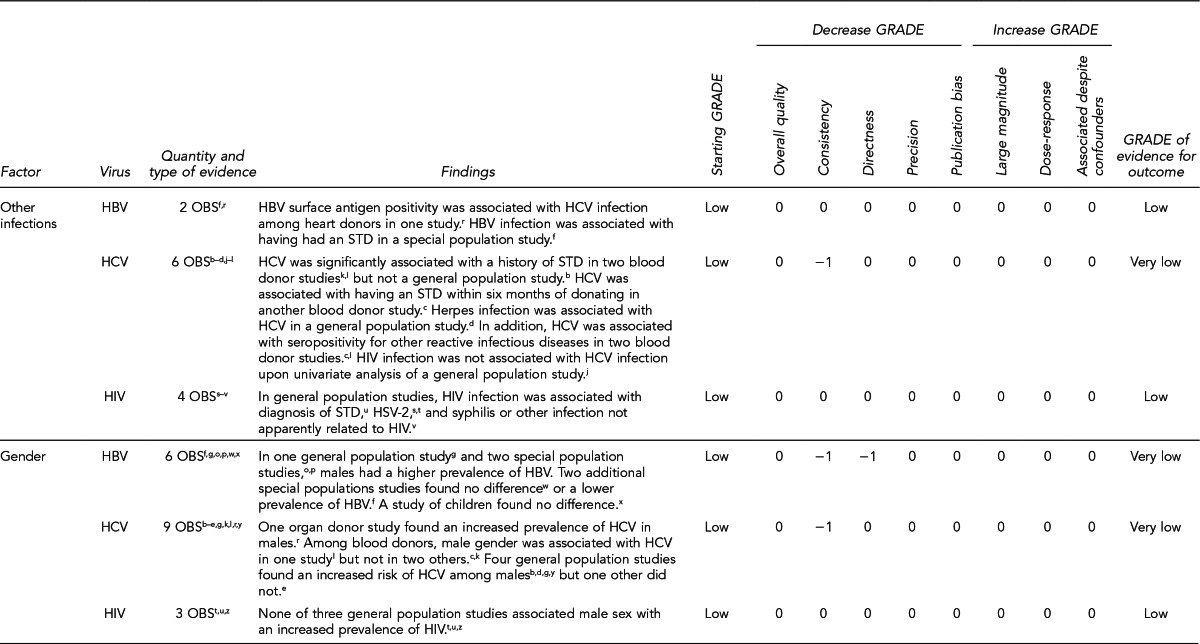
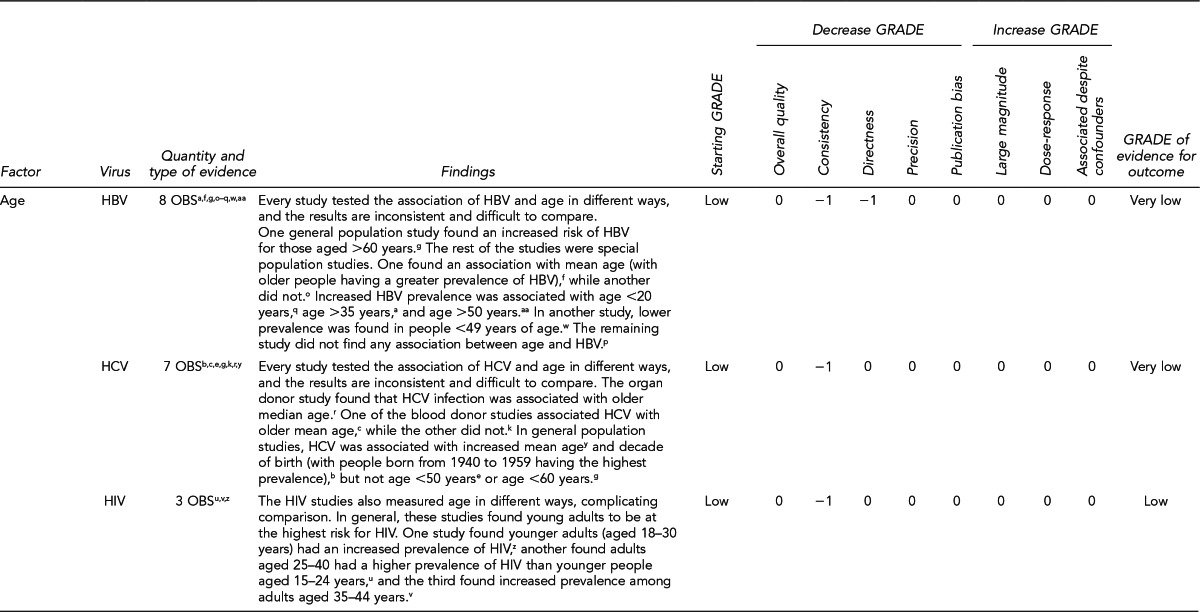
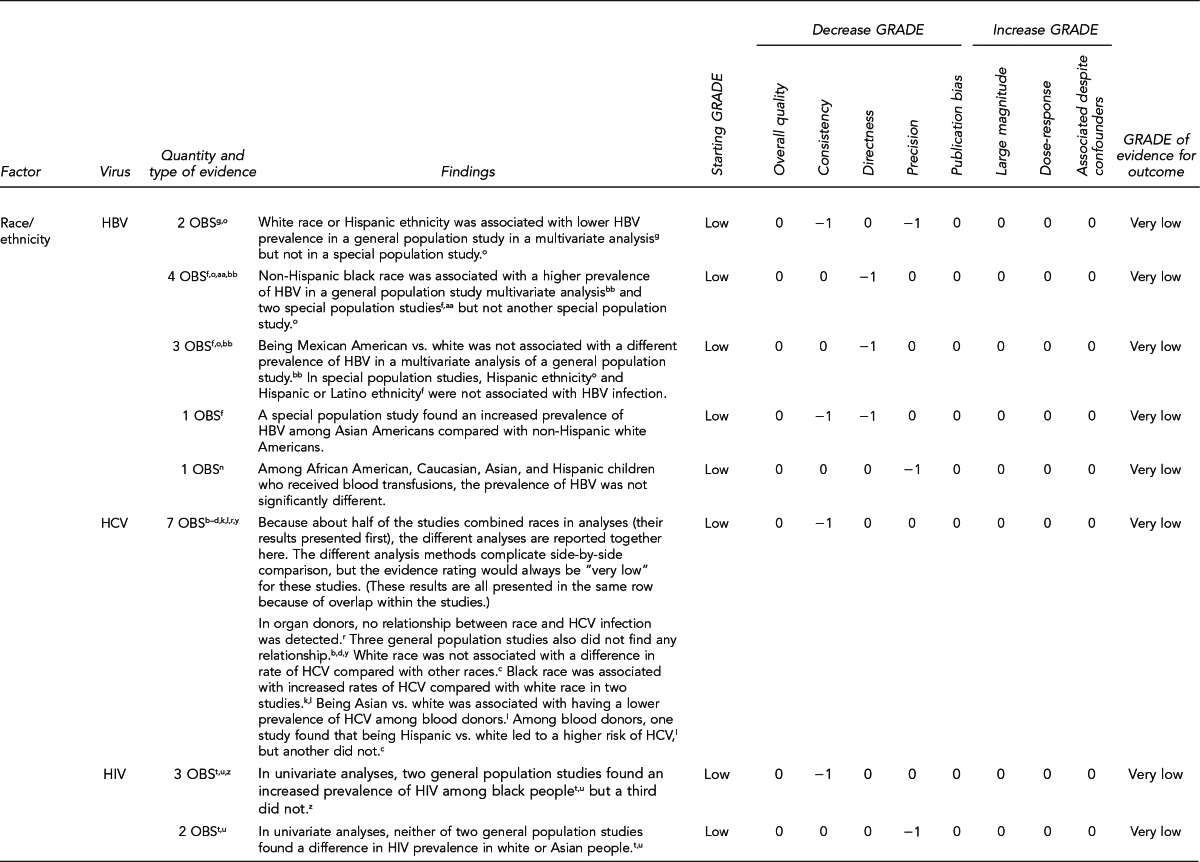
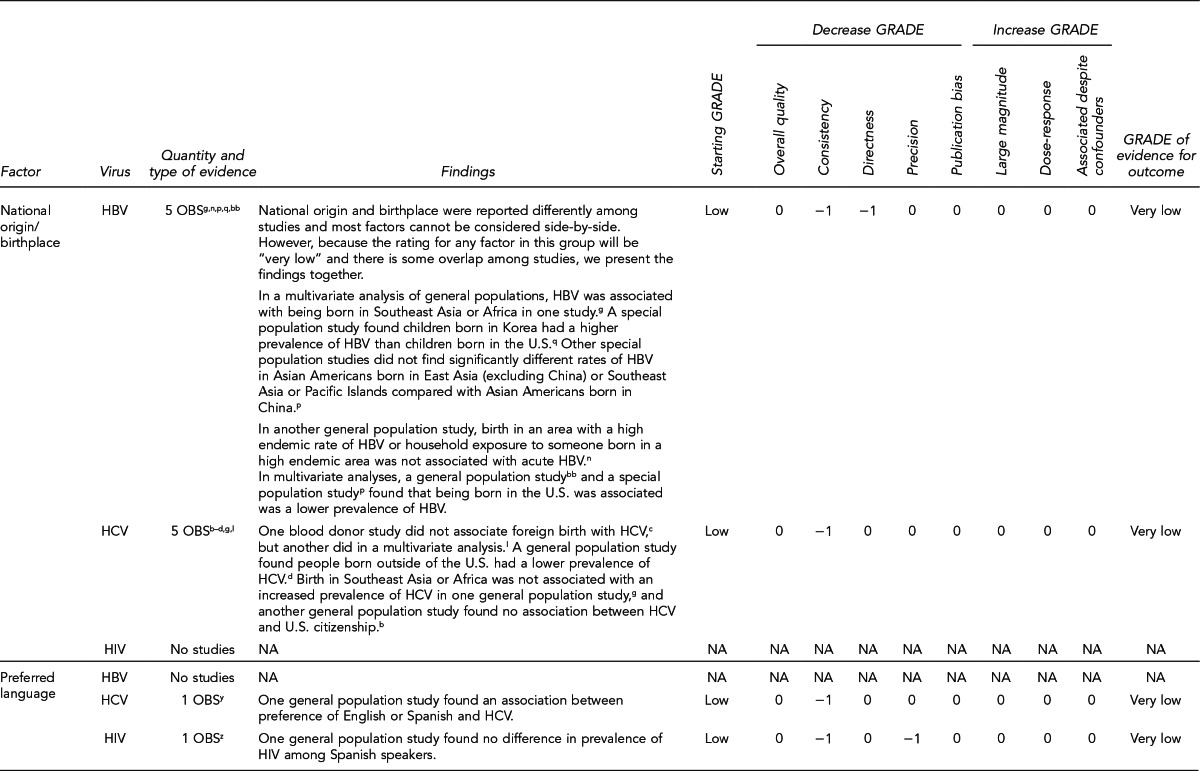
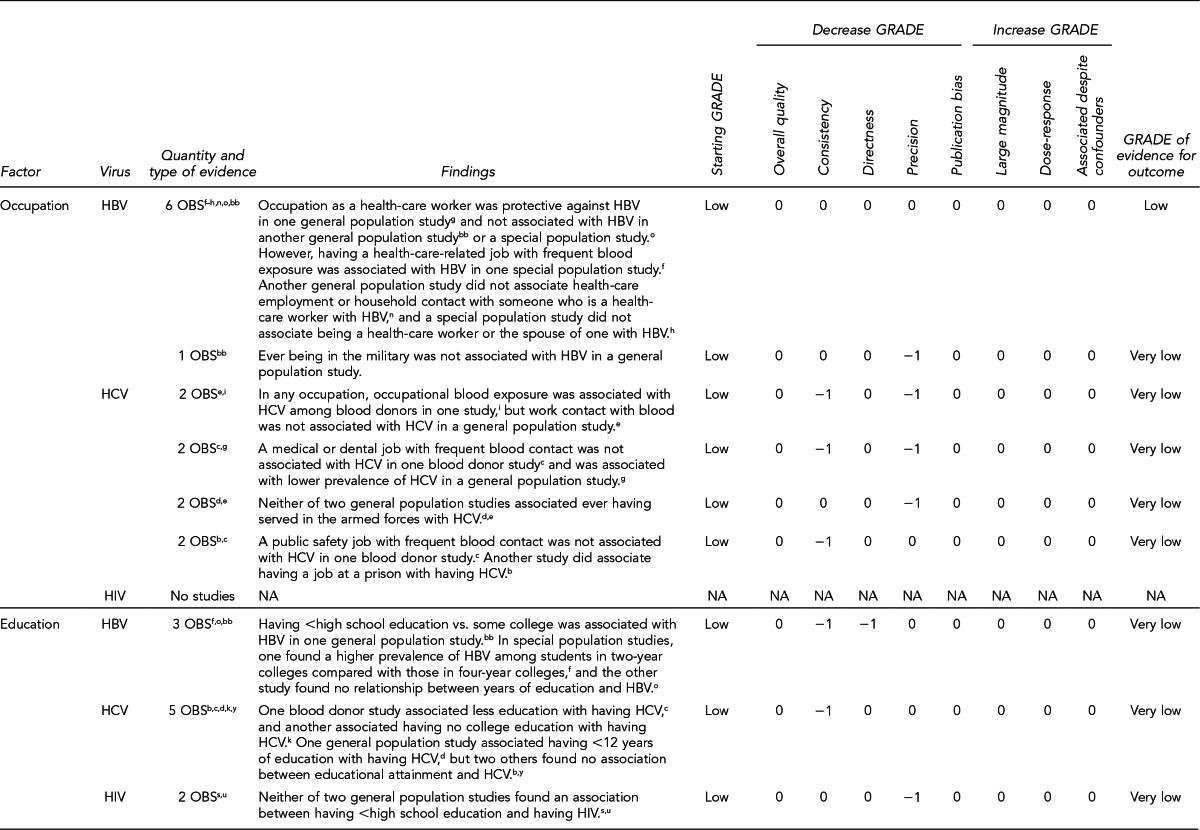
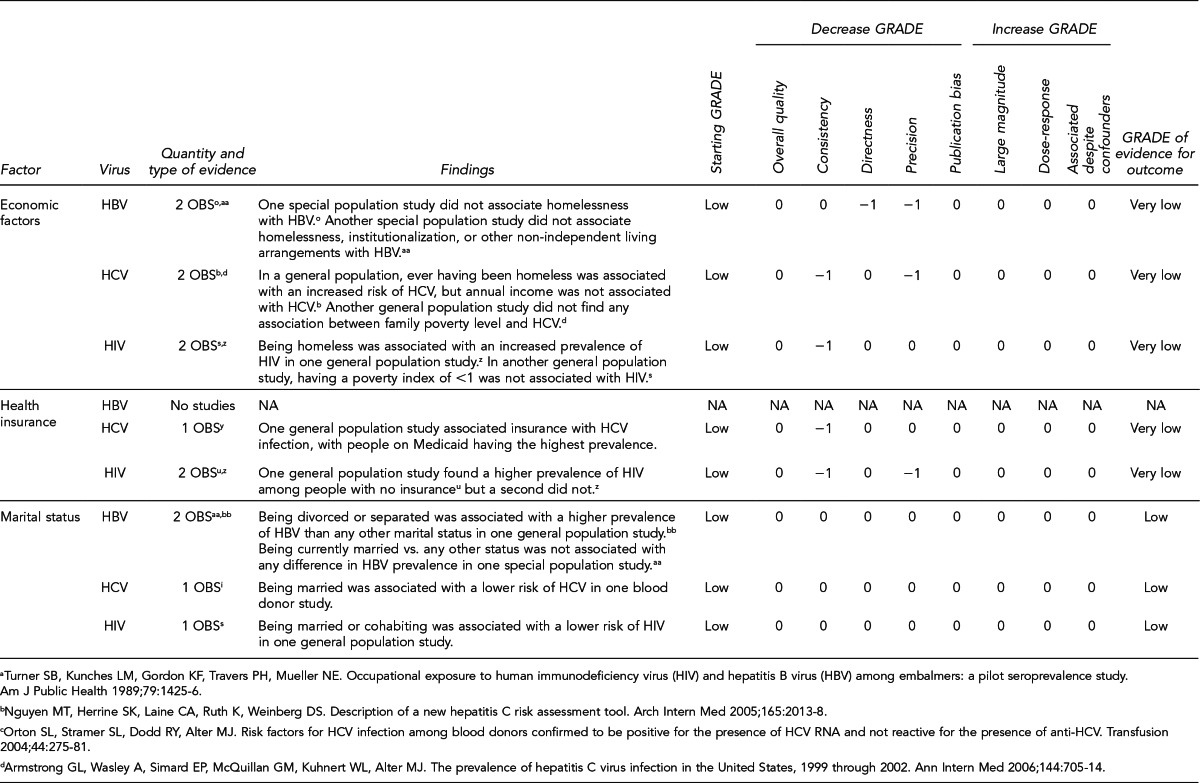

Appendix E.
GRADE rating of evidence for outcomes of interest concerning HIV, HBV, and HCV transmission through organ transplantation for key question 5: What are the test characteristics of the screening methods available to detect HIV, HBV, and HCV in potential organ donors? Do test characteristics differ in particular populations and with donor clinical status (i.e., donation after brain death vs. donation after cardiac arrest OR adult vs. pediatric donors)?
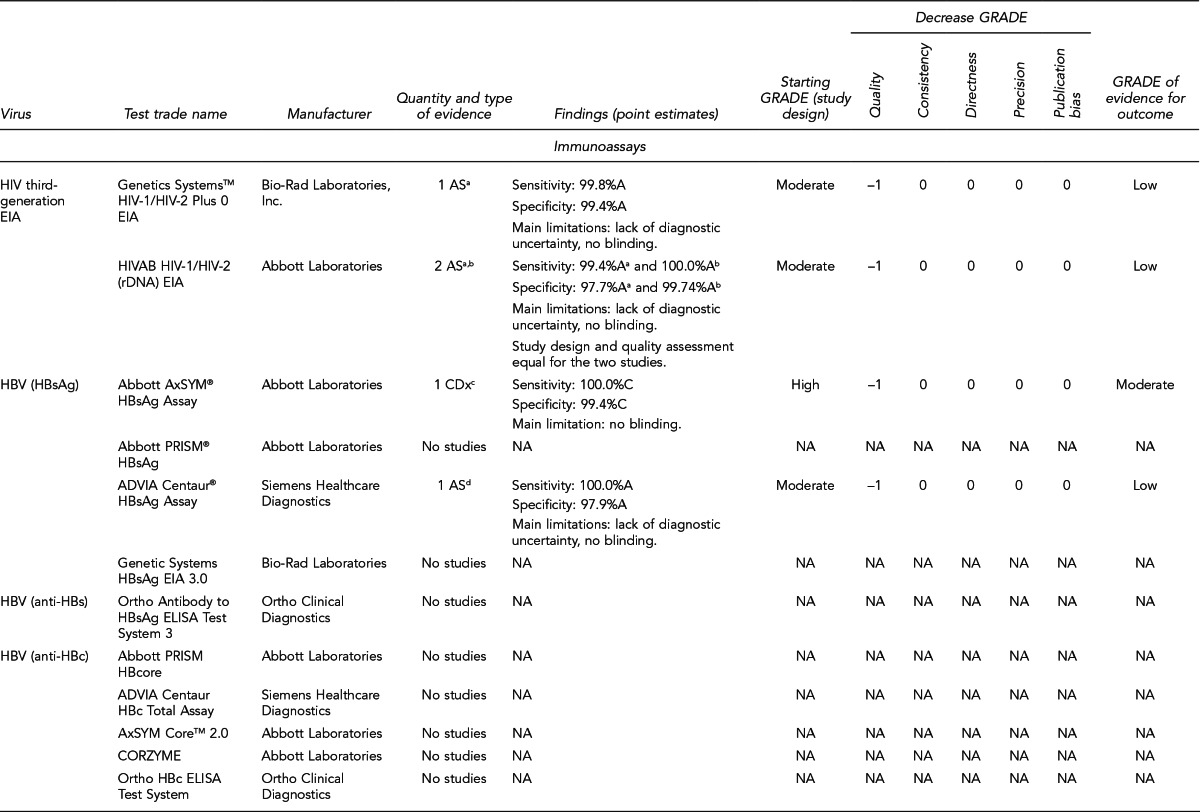
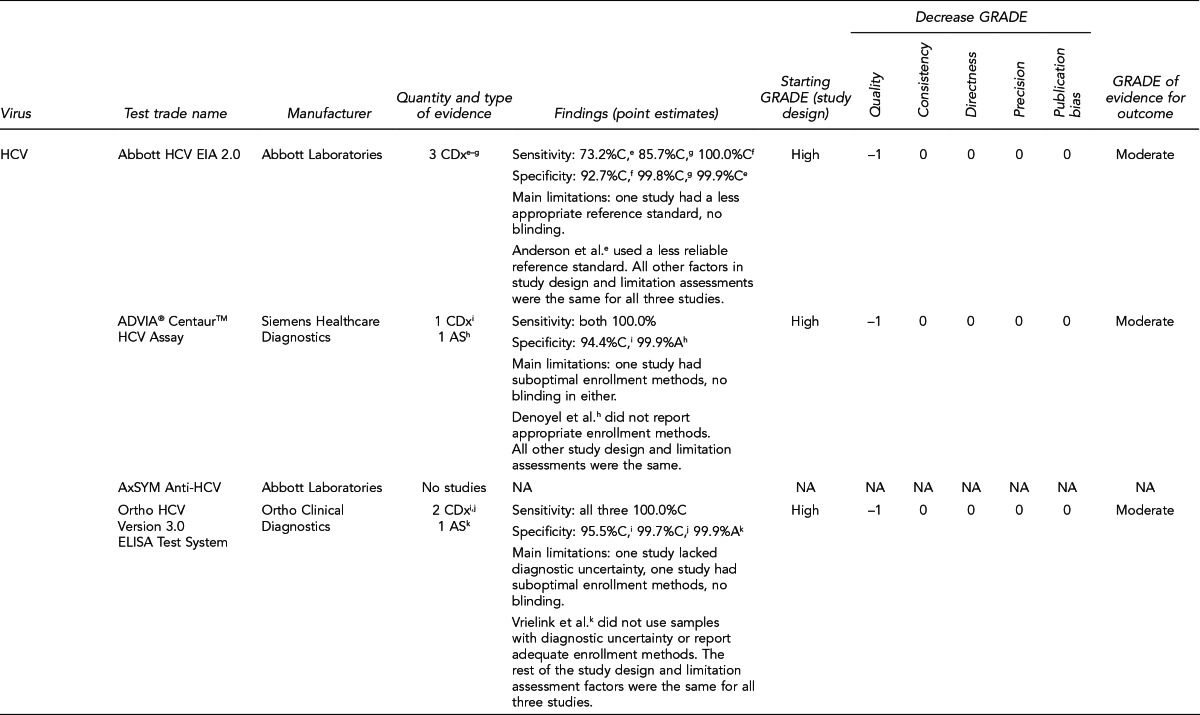
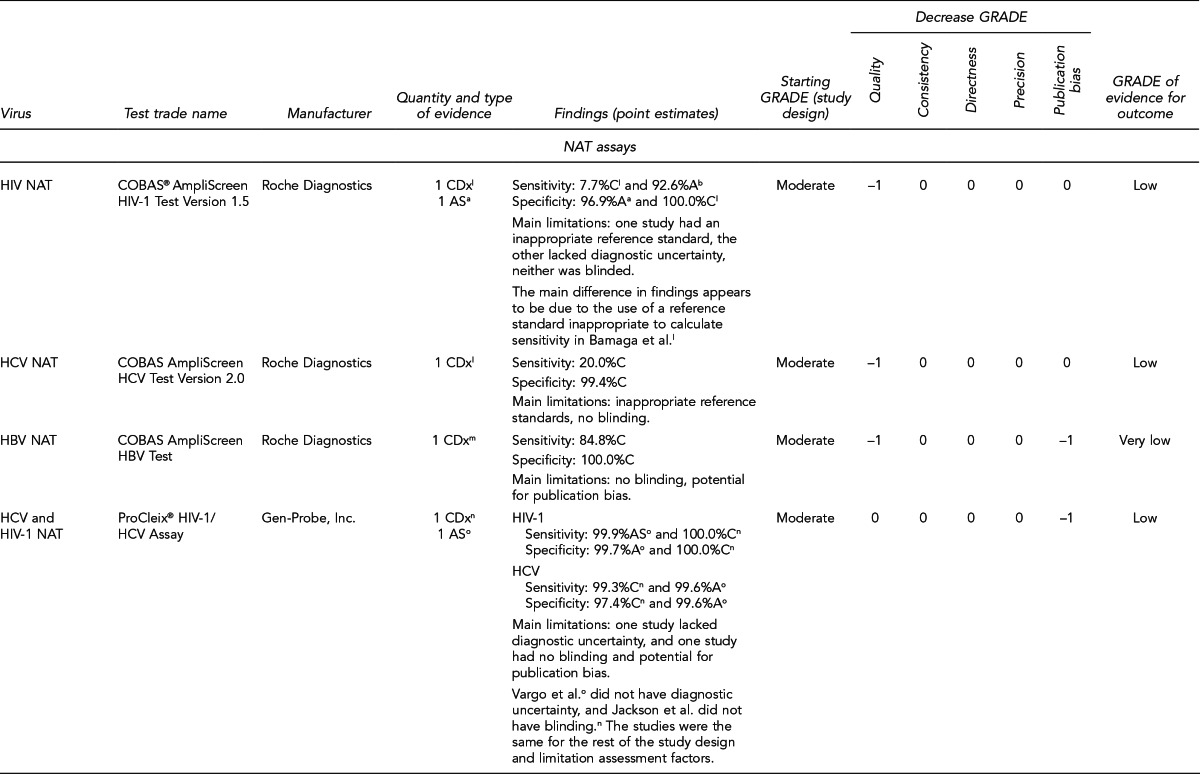
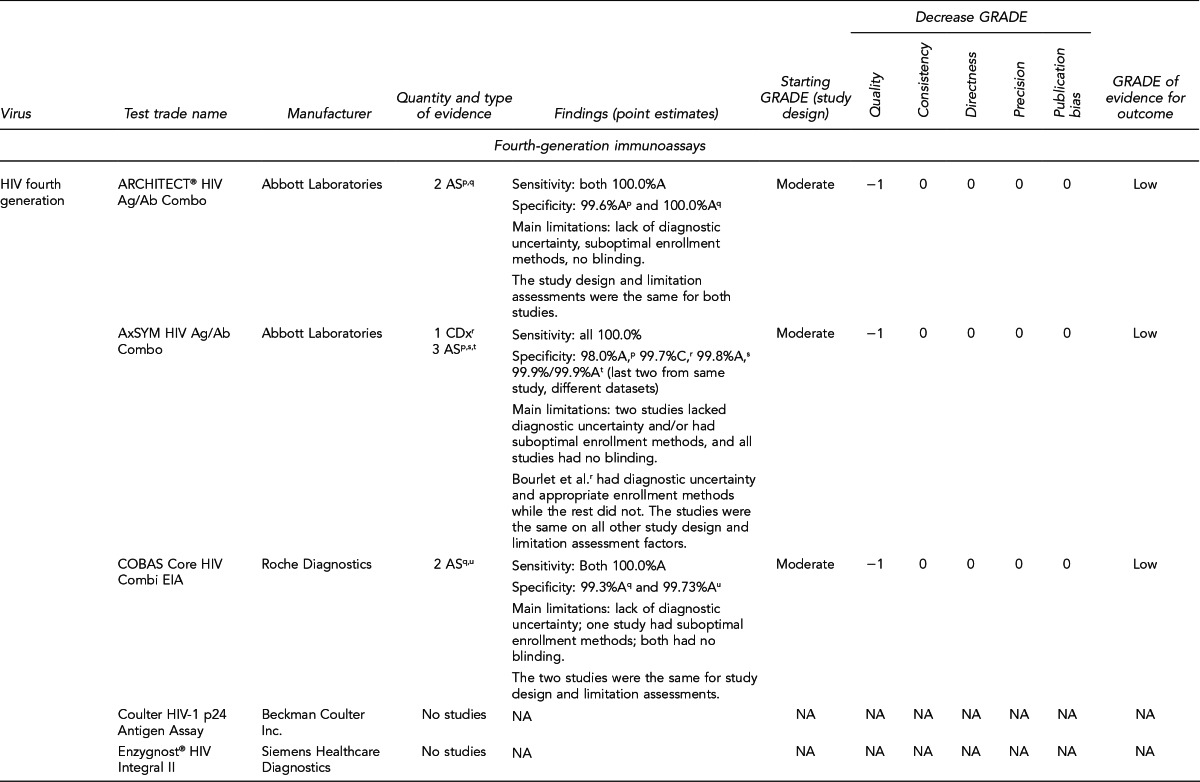
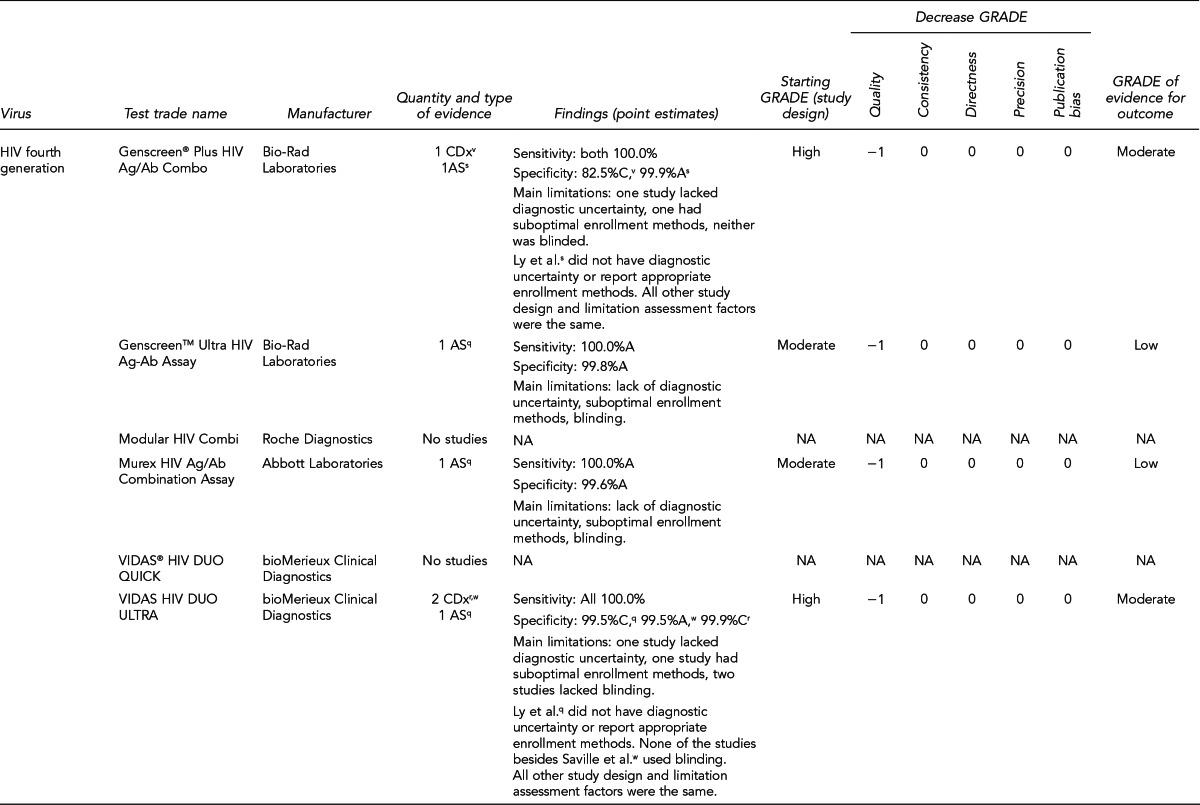
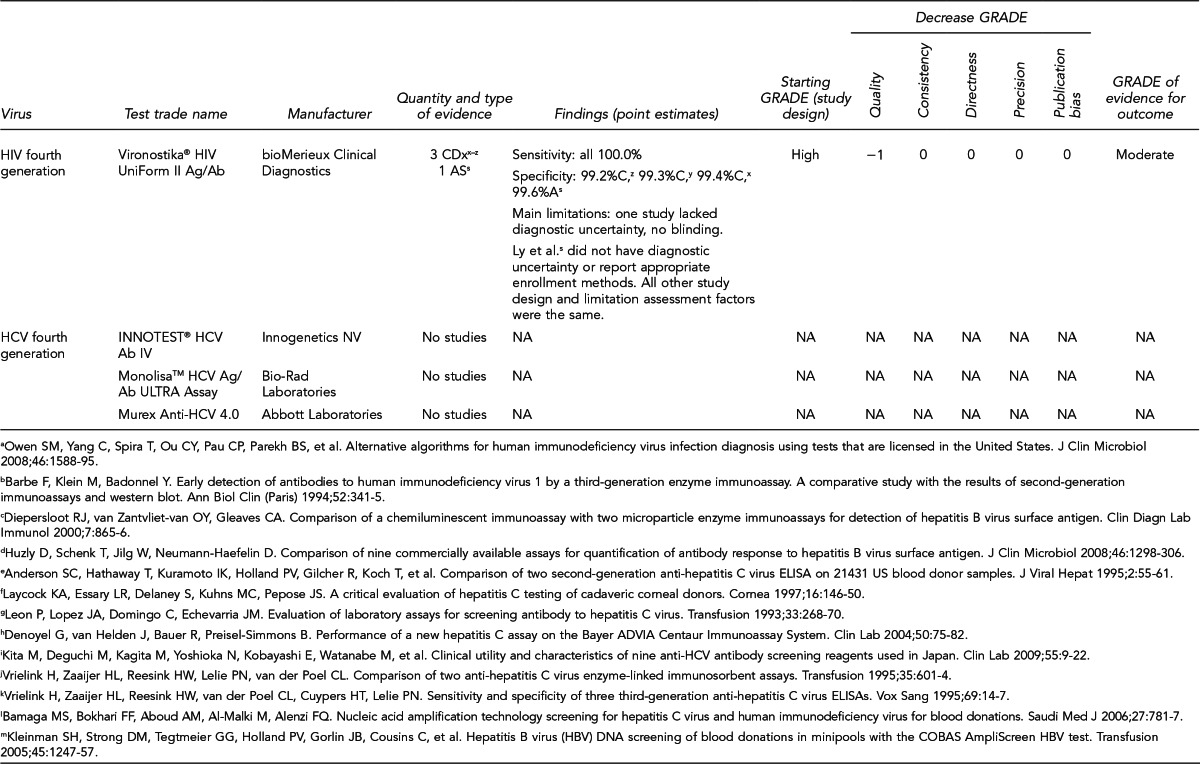

Appendix F.
GRADE rating of evidence for outcomes of interest concerning HIV, HBV, and HCV transmission through organ transplantation for key question 6: Which donor interventions reduce the probability of pathogen transmission from an organ donor infected with HIV, HBV, or HCV to a previously uninfected recipient?
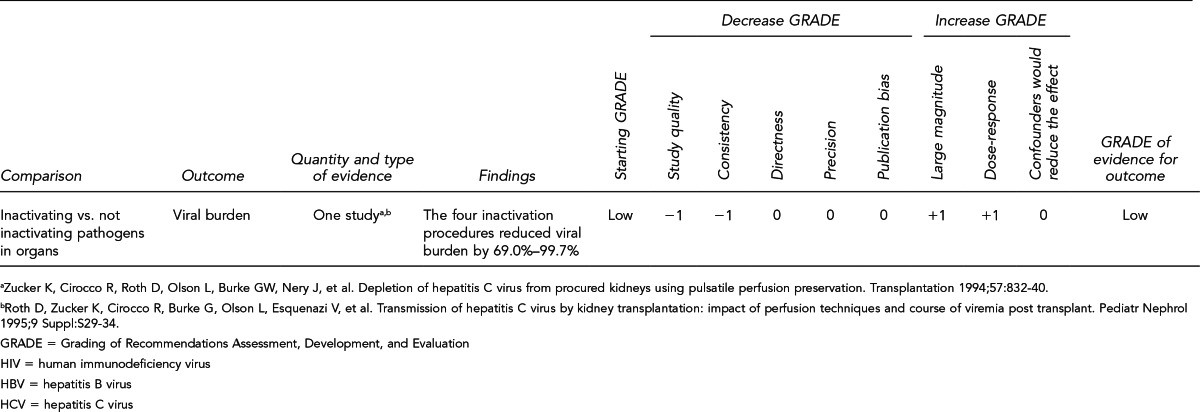
Appendix G.
GRADE rating of evidence for outcomes of interest and overall GRADE of evidence bases concerning HIV, HBV, and HCV transmission through organ transplantation for key question 7: How do the clinical outcomes of recipients of organs from donors infected with HIV, HBV, or HCV compare with those who remain on the transplant list?
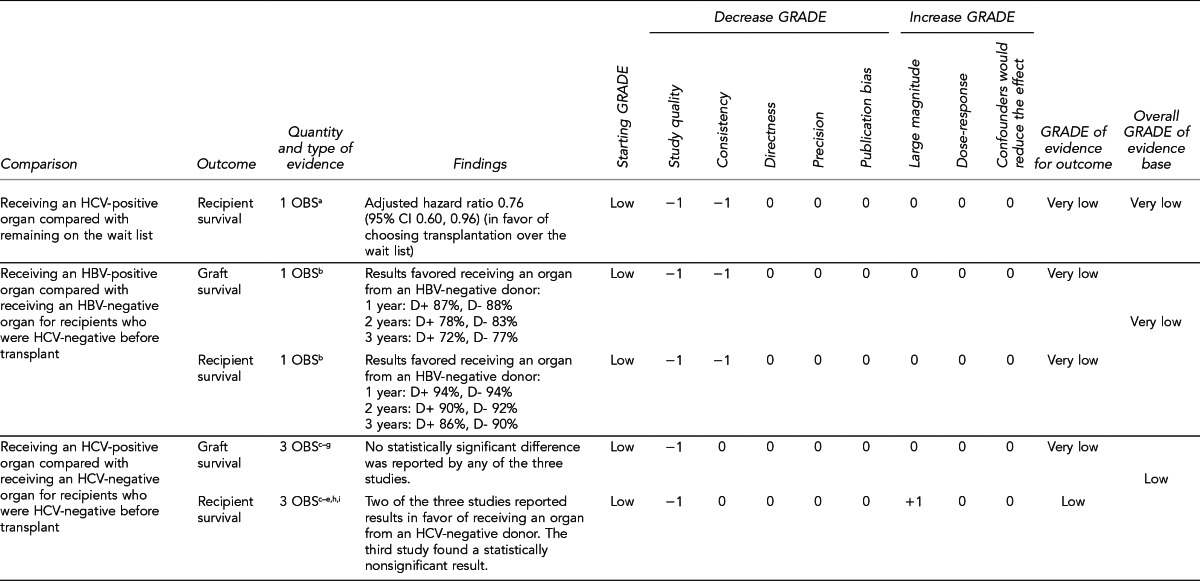
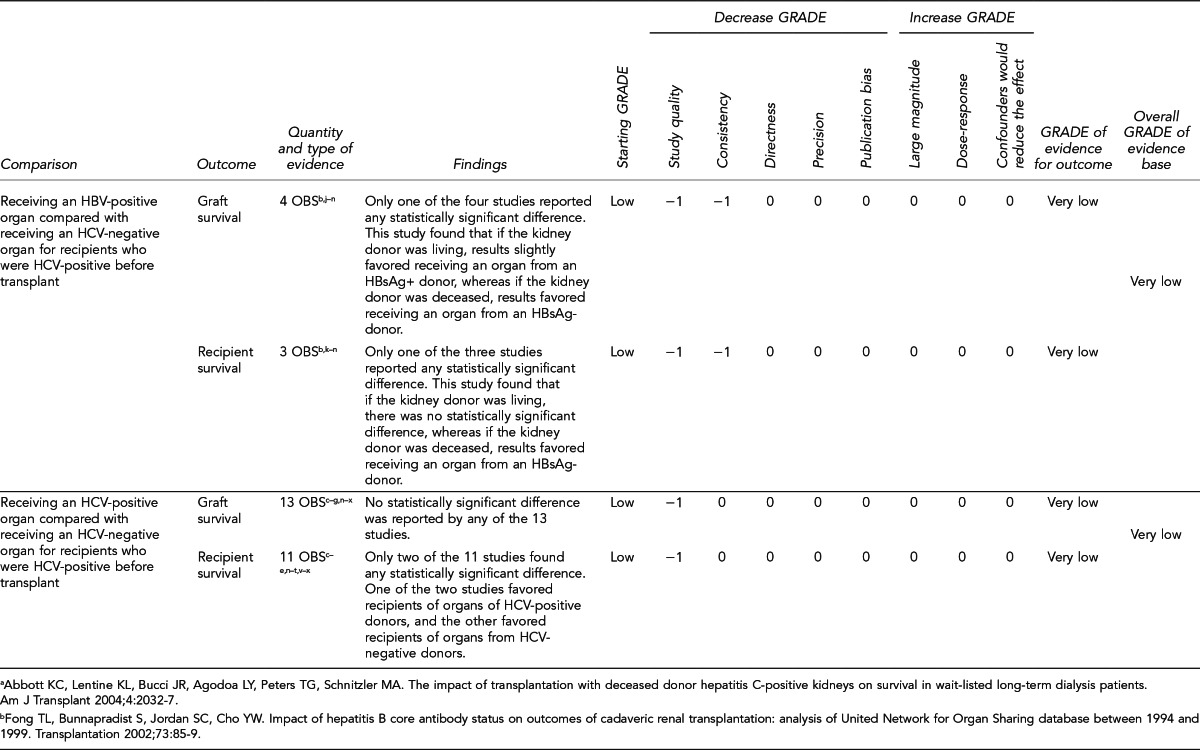

Appendix H.
GRADE rating of evidence for outcomes of interest and overall GRADE of evidence bases concerning HIV, HBV, and HCV transmission through organ transplantation for key question 8: How do the clinical outcomes of transplant recipients who receive organs from donors with behavioral or nonbehavioral risk factors compare with those who remain on the transplant list?
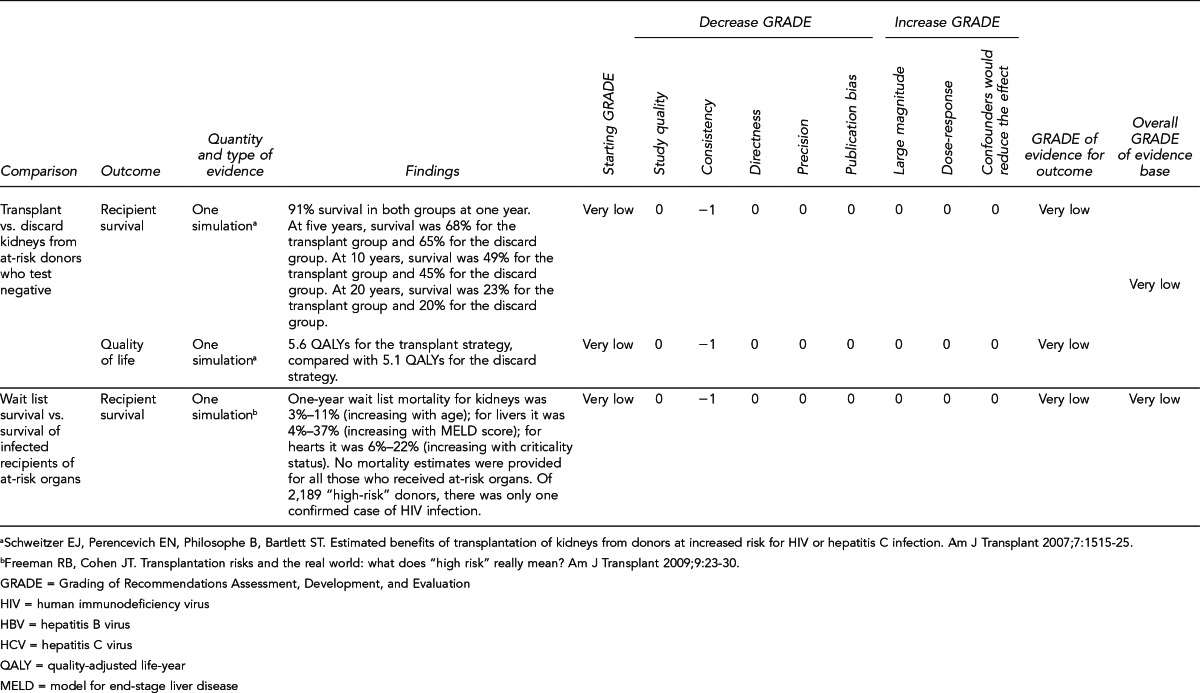
Footnotes
Ingi Lee and Craig Umscheid received funding from the Centers for Disease Control and Prevention (CDC) to support the guideline development process. Lee was employed by the University of Pennsylvania during guideline development and by Merck during guideline revision. Jonathan R. Treadwell, PhD, associate director, and Meredith Noble, MS, senior research analyst from the Evidence-Based Practice Center, ECRI Institute in Plymouth Meeting, Pennsylvania, coauthored the Evidence Report, Solid Organ Transplantation and the Probability of Transmitting HIV, HBV, or HCV: A Systematic Review to Support an Evidence-Based Guideline.
The Expert Panel and Review Committee contributed to the guideline development process and provided review and feedback on the key search questions, bibliography resulting from the literature review, and draft Evidence Report. Further input was sought from specific subject-matter experts to address selected topics in developing the guideline; in particular, the authors thank Scott Halpern, MD, PhD, MBE; Michael G. Ison, MD, MS; and Jutta Preiksaitis, MD, for writing the original drafts of the Expert Opinion summaries. The Expert Panel and Review Committee also reviewed and provided feedback on the draft guideline. The opinions of individual members of the Expert Panel or Review Committee might not be reflected in all of the recommendations, as the guideline represents the position of the U.S. Public Health Service (PHS) and is not a consensus document.
The PHS Guideline Revision Work Group performed an in-depth review of public comments submitted on the draft guideline recommendations, participated in revising the recommendations in consideration of public comment, and provided feedback on the full document.
The authors thank the many individuals and organizations that provided constructive feedback on the guideline during the public comment period.
Subject-Matter Experts
Scott Halpern, MD, PhD, MBE, Leonard Davis Institute of Health Economics; Perelman School of Medicine of the University of Pennsylvania, Philadelphia, PA.
Michael G. Ison, MD, MS, FIDSA, Northwestern University Feinberg School of Medicine, Divisions of Infectious Diseases and Organ Transplantation, Chicago, IL
Jutta Preiksaitis, MD, University of Alberta, Edmonton, Alberta, Canada
Expert Panel
Bernard M. Branson, MD, Centers for Disease Control and Prevention (CDC), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Division of HIV/AIDS Prevention, Office of the Director, Atlanta, GA
Lisa A. Grohskopf, MD, MPH, CDC, NCHHSTP, Division of HIV/AIDS Prevention, Atlanta, GA [current affiliation: CDC, National Center for Immunization and Respiratory Diseases, Influenza Division, Atlanta, GA]
Richard Hasz, BS, MFS, Gift of Life Donor Program, Philadelphia, PA
Scott Holmberg, MD, MPH, CDC, NCHHSTP, Division of Viral Hepatitis, Atlanta, GA
Michael G. Ison, MD, MS, FIDSA, Northwestern University Feinberg School of Medicine, Divisions of Infectious Diseases and Organ Transplantation, Chicago, IL
Jutta Preiksaitis, MD, University of Alberta, Edmonton, Alberta, Canada
Nicola D. Thompson, PhD, MS, CDC, NCHHSTP, Division of Viral Hepatitis, Atlanta, GA [current affiliation: CDC, National Center for Emerging Zoonotic and Infectious Diseases, Division of Healthcare Quality Promotion, Atlanta, GA]
Review Committee
Nancy D. Bridges, MD, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Transplantation Branch, Bethesda, MD
Alfred DeMaria, Jr., MD, Massachusetts Department of Public Health, Bureau of Infectious Disease, Boston, MA
Richard C. Durbin, MBA, Health Resources and Services Administration, Healthcare Systems Bureau, Rockville, MD
Jan Finn, RN, MSN, Midwest Transplant Network, Westwood, KS
Jay A. Fishman, MD, MGH Transplantation Center and Transplant Infectious Disease and Compromised Host Program, Massachusetts General Hospital and Harvard Medical School, Boston, MA
Daniel J. Lebovitz, MD, Akron Children's Hospital, Division of Critical Care, Akron, OH; MetroHealth Medical Center, Pediatric Intensive Care Unit, Cleveland, OH; Lifebanc, Cleveland, OH
Laura St. Martin, MD, MPH, Food and Drug Administration, Center for Biologics Evaluation and Research, Office of Cellular, Tissue and Gene Therapies, Division of Human Tissues, Rockville, MD
Karen L. Tritz, MSW, Centers for Medicare – Medicaid Services, Center for Clinical Standards and Quality, Survey and Certification Group, Baltimore, MD
Rainer Ziermann, PhD, Roche Molecular Systems, Pleasanton, CA [current affiliation: Cepheid, Sunnyvale, CA]
PHS Guideline Revision Work Group
James Berger, MS, MT(ASCP)SBB, Office of the Assistant Secretary for Health, Office of HIV/AIDS and Infectious Disease Policy, Division of Blood and Tissue Safety and Availability, Washington, DC
James Bowman, MD, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD
Nancy D. Bridges, MD, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Transplantation Branch, Bethesda, MD
Joanne Cono, MD, ScM, CDC, Office of Infectious Diseases, Office of the Director, Atlanta, GA
Richard C. Durbin, MBA, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD
Melissa Greenwald, MD, Food and Drug Administration, Center for Biologics Evaluation and Research, Office of Cellular, Tissue and Gene Therapies, Division of Human Tissues, Rockville, MD
Laura St. Martin, MD, MPH, Food and Drug Administration, Center for Biologics Evaluation and Research, Office of Cellular, Tissue and Gene Therapies, Division of Human Tissues, Rockville, MD
Ronald O. Valdiserri, MD, MPH, Office of the Assistant Secretary for Health, Office of HIV/AIDS and Infectious Disease Policy, Washington, DC
REFERENCES
- 1.Rogers MF, Simonds RJ, Lawton KE, Moseley RR, Jones WK. Guidelines for preventing transmission of human immunodeficiency virus through transplantation of human tissue and organs. MMWR Recomm Rep. 1994;43(RR-8):1–17. [PubMed] [Google Scholar]
- 2.Food and Drug Administration (US) Guidance for industry: eligibility determination for donors of human cells, tissues, and cellular and tissue-based products. 2007. Aug, [cited 2013 Mar 13]. Available from: URL: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances /Tissue/ucm091345.pdf. [PubMed]
- 3.Wolf JL, Perkins HA, Schreeder MT, Vincenti F. The transplanted kidney as a source of hepatitis B infection. Ann Intern Med. 1979;91:412–3. doi: 10.7326/0003-4819-91-3-412. [DOI] [PubMed] [Google Scholar]
- 4.Human immunodeficiency virus infection transmitted from an organ donor screened for HIV antibody—North Carolina. MMWR Morb Mortal Wkly Rep. 1987;36(20):306–8. [PubMed] [Google Scholar]
- 5.Bowen PA, 2nd, Lobel SA, Caruana RJ, Leffell MS, House MA, Rissing JP, et al. Transmission of human immunodeficiency virus (HIV) by transplantation: clinical aspects and time course analysis of viral antigenemia and antibody production. Ann Intern Med. 1988;108:46–8. doi: 10.7326/0003-4819-108-1-46. [DOI] [PubMed] [Google Scholar]
- 6.Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Bottenfield S, Conley LJ, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726–32. doi: 10.1056/NEJM199203123261102. [DOI] [PubMed] [Google Scholar]
- 7.Simonds RJ. HIV transmission by organ and tissue transplantation. AIDS. 1993;7(Suppl 2):S35–8. doi: 10.1097/00002030-199311002-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hepatitis C virus transmission from an antibody-negative organ and tissue donor—United States, 2000-2002. MMWR Morb Mortal Wkly Rep. 2003;52(13):273–6. [PubMed] [Google Scholar]
- 9.Ison MG, Llata E, Conover CS, Friedewald JJ, Gerber SI, Grigoryan A, et al. Transmission of human immunodeficiency virus and hepatitis C from an organ donor to four transplant recipients. Am J Transplant. 2011;11:1218–25. doi: 10.1111/j.1600-6143.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 10.Potential transmission of viral hepatitis through use of stored blood vessels as conduits in organ transplantation—Pennsylvania, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(6):172–4. [PubMed] [Google Scholar]
- 11.HIV transmitted from a living organ donor—New York City, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(10):297–301. [PubMed] [Google Scholar]
- 12.Notes from the field: transplant-transmitted hepatitis B virus—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(32):1087. [PubMed] [Google Scholar]
- 13.Transmission of hepatitis C virus through transplanted organs and tissue—Kentucky and Massachusetts, 2011 [published erratum appears in MMWR Morb Mortal Wkly Rep 2012;61(4):80] MMWR Morb Mortal Wkly Rep. 2011;60(50):1697–700. [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umscheid CA, Agarwal RK, Brennan PJ Healthcare Infection Control Practices Advisory Committee. Updating the guideline development methodology of the Healthcare Infection Control Practices Advisory Committee (HICPAC) Am J Infect Control. 2010;38:264–73. doi: 10.1016/j.ajic.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (US) Solid organ transplantation and the probability of transmitting HIV, HBV, or HCV: a systematic review to support an evidence-based guideline. 2010. [cited 2013 Jan 25]. Available from: URL: http://stacks.cdc.gov/view/cdc/12164/
- 20. 42 U.S.C. 273, 274.
- 21. 42 C.F.R. Part 121.
- 22. 42 U.S.C. 274(b)(2)(E)
- 23. 42 U.S.C. 273(b)(3)(C)
- 24. 42 C.F.R. Part 121.6.
- 25. 42 C.F.R. Part 121.4.
- 26.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–25. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 27.Health Resources and Services Administration (US) Organ Procurement and Transplantation Network. Policy 4: identification of transmissible diseases in organ recipients [cited 2012 Aug 15] Available from: URL: http://optn.transplant.hrsa.gov/policiesAnd Bylaws/policies.asp.
- 28.Health Resources and Services Administration (US) Organ Procurement and Transplantation Network. Policy 2: minimum procurement standards for an organ procurement organization (OPO) [cited 2012 Aug 15] Available from: URL: http://optn .transplant.hrsa.gov/policiesAndBylaws/policies.asp.
- 29.Ison MG, Hager J, Blumberg E, Burdick J, Carney K, Cutler J, et al. Donor-derived disease transmission events in the United States: data reviewed by the OPTN/UNOS Disease Transmission Advisory Committee. Am J Transplant. 2009;9:1929–35. doi: 10.1111/j.1600-6143.2009.02700.x. [DOI] [PubMed] [Google Scholar]
- 30.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 31.Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- 32.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 33.Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion. 2009;49:2454–89. doi: 10.1111/j.1537-2995.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 34.Busch MP, Glynn SA, Wright DJ, Hirschkorn D, Laycock ME, McAuley J, et al. Relative sensitivities of licensed nucleic acid amplification tests for detection of viremia in early human immunodeficiency virus and hepatitis C virus infection. Transfusion. 2005;45:1853–63. doi: 10.1111/j.1537-2995.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 35.Weber B, Berger A, Rabenau H, Doerr HW. Evaluation of a new combined antigen and antibody human immunodeficiency virus screening assay, VIDAS HIV DUO Ultra. J Clin Microbiol. 2002;40:1420–6. doi: 10.1128/JCM.40.4.1420-1426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ly TD, Ebel A, Faucher V, Fihman V, Laperche S. Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J Virol Methods. 2007;143:86–94. doi: 10.1016/j.jviromet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Perry KR, Ramskill S, Eglin RP, Barbara JA, Parry JV. Improvement in the performance of HIV screening kits. Transfus Med. 2008;18:228–40. doi: 10.1111/j.1365-3148.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- 38.Kleinman SH, Busch MP. Assessing the impact of HBV NAT on window period reduction and residual risk. J Clin Virol. 2006;36(Suppl 1):S23–9. doi: 10.1016/s1386-6532(06)80005-3. [DOI] [PubMed] [Google Scholar]
- 39.Ellingson K, Seem D, Nowicki M, Strong DM, Kuehnert MJ Organ Procurement Organization Nucleic Acid Testing Yield Project Team. Estimated risk of human immunodeficiency virus and hepatitis C virus infection among potential organ donors from 17 organ procurement organizations in the United States. Am J Transplant. 2011;11:1201–8. doi: 10.1111/j.1600-6143.2011.03518.x. [DOI] [PubMed] [Google Scholar]
- 40.Health Resources and Services Administration (US) Organ Procurement and Transplantation Network. Policy 12: living donation [cited 2013 Jan 20] Available from: URL: http://optn.transplant .hrsa.gov/policiesAndBylaws/policies.asp.
- 41.Kucirka LM, Alexander C, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Viral nucleic acid testing (NAT) and OPO-level disposition of high-risk donor organs. Am J Transplant. 2009;9:620–8. doi: 10.1111/j.1600-6143.2008.02522.x. [DOI] [PubMed] [Google Scholar]
- 42.Ison MG, Stosor V. Transplantation of high-risk donor organs: a survey of US solid organ transplant center practices as reported by transplant infectious diseases physicians. Clin Transplant. 2009;23:866–73. doi: 10.1111/j.1399-0012.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 43.Humar A, Morris M, Blumberg E, Freeman R, Preiksaitis J, Kiberd B, et al. Nucleic acid testing (NAT) of organ donors: is the “best” test the right test? A consensus conference report. Am J Transplant. 2010;10:889–99. doi: 10.1111/j.1600-6143.2009.02992.x. [DOI] [PubMed] [Google Scholar]
- 44.Hidalgo G, Tejani C, Clayton R, Clements P, Distant D, Vyas S, et al. Factors limiting the rate of living-related kidney donation to children in an inner city setting. Pediatr Transplant. 2001;5:419–24. doi: 10.1034/j.1399-3046.2001.t01-2-00033.x. [DOI] [PubMed] [Google Scholar]
- 45.Renz JF, Mudge CL, Heyman MB, Tomlanovich S, Kingsford RP, Moore BJ, et al. Donor selection limits use of living-related liver transplantation. Hepatology. 1995;22(4 Pt 1):1122–6. doi: 10.1016/0270-9139(95)90618-5. [DOI] [PubMed] [Google Scholar]
- 46.Domen RE, Yen-Lieberman B, Nelson KA, Chua J, Sholtis W, Tyus H, et al. Use of an HBV-DNA hybridization assay in the evaluation of equivocal hepatitis B virus tests in solid organ donors. Prog Transplant. 2000;10:42–6. doi: 10.1177/152692480001000108. [DOI] [PubMed] [Google Scholar]
- 47.Richards PS, Nelson KA, Frazier OH, Radovancevic B, Van Buren C, Young JB. Why referred potential heart donors aren't used. Tex Heart Inst J. 1993;20:218–22. [PMC free article] [PubMed] [Google Scholar]
- 48.Shah G, Demetris AJ, Irish W, Scheffel J, Mimms L, Van Thiel DH. Frequency and severity of HCV infection following orthotopic liver transplantation. Effect of donor and recipient serology for HCV using a second generation ELISA test. J Hepatol. 1993;18:279–83. doi: 10.1016/s0168-8278(05)80270-3. [DOI] [PubMed] [Google Scholar]
- 49.Vincenti F, Lake J, Wright T, Kuo G, Weber P, Stempel C. Nontransmission of hepatitis C from cadaver kidney donors to transplant recipients. Transplantation. 1993;55:674–5. [PubMed] [Google Scholar]
- 50.Pereira BJ, Milford EL, Kirkman RL, Levey AS. Transmission of hepatitis C virus by organ transplantation. N Engl J Med. 1991;325:454–60. doi: 10.1056/NEJM199108153250702. [DOI] [PubMed] [Google Scholar]
- 51.Pereira BJ, Milford EL, Kirkman RL, Quan S, Sayre KR, Johnson PJ, et al. Prevalence of hepatitis C virus RNA in organ donors positive for hepatitis C antibody and in the recipients of their organs. N Engl J Med. 1992;327:910–5. doi: 10.1056/NEJM199209243271302. [DOI] [PubMed] [Google Scholar]
- 52.Pereira BJ, Milford EL, Kirkman RL, Quan S, Sayre KR, Johnson PJ, et al. Liver disease and HCV infection after transplantation of organs from hepatitis C antibody positive donors. Transplant Proc. 1993;25(1 Pt 2):1458–9. [PubMed] [Google Scholar]
- 53.Pereira BJ, Wright TL, Schmid CH, Bryan CF, Cheung RC, Cooper ES, et al. Screening and confirmatory testing of cadaver organ donors for hepatitis C virus infection: a U.S. National Collaborative Study. Kidney Int. 1994;46:886–92. doi: 10.1038/ki.1994.346. [DOI] [PubMed] [Google Scholar]
- 54.Roth D, Fernandez JA, Babischkin S, De Mattos A, Buck BE, Quan S, et al. Detection of hepatitis C virus infection among cadaver organ donors: evidence for low transmission of disease. Ann Intern Med. 1992;117:470–5. doi: 10.7326/0003-4819-117-6-470. [DOI] [PubMed] [Google Scholar]
- 55.Roth D, Fernandez JA, Babischkin S, De Mattos A, Buck BE, Quan S, et al. Transmission of hepatitis C virus with solid organ transplantation: incidence and clinical significance. Transplant Proc. 1993;25(1 Pt 2):1476–7. [PubMed] [Google Scholar]
- 56.Shah G, Demetris AJ, Gavaler JS, Lewis JH, Todo S, Starzl TE, et al. Incidence, prevalence, and clinical course of hepatitis C following liver transplantation. Gastroenterology. 1992;103:323–9. doi: 10.1016/0016-5085(92)91130-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou S, Dodd RY, Stramer SL, Strong DM Tissue Safety Study Group. Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751–9. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 58.Subpopulation estimates from the HIV Incidence Surveillance System—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–9. [PubMed] [Google Scholar]
- 59.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ. 2009;58(3):1–27. [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (US) Disease burden from viral hepatitis A, B, and C in the United States [cited 2009 Jun 4] Available from: URL: http://www.cdc.gov/hepatitis/PDFs /disease_burden.pdf.
- 62.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of people with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 63.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 64.HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–6. [PubMed] [Google Scholar]
- 65.Loss GE, Jr, Mason AL, Nair S, Blazek J, Farr G, Guo L, et al. Does lamivudine prophylaxis eradicate persistent HBV DNA from allografts derived from anti-HBc-positive donors? Liver Transpl. 2003;9:1258–64. doi: 10.1016/j.lts.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Prieto M, Gomez MD, Berenguer M, Cordoba J, Rayon JM, Pastor M, et al. De novo hepatitis B after liver transplantation from hepatitis B core antibody-positive donors in an area with high prevalence of anti-HBc positivity in the donor population. Liver Transpl. 2001;7:51–8. doi: 10.1053/jlts.2001.20786. [DOI] [PubMed] [Google Scholar]
- 67.Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, et al. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. Gastroenterology. 1997;113:1668–74. doi: 10.1053/gast.1997.v113.pm9352871. [DOI] [PubMed] [Google Scholar]
- 68.Yu AS, Vierling JM, Colquhoun SD, Arnaout WS, Chan CK, Khanafshar E, et al. Transmission of hepatitis B infection from hepatitis B core antibody-positive liver allografts is prevented by lamivudine therapy. Liver Transpl. 2001;7:513–7. doi: 10.1053/jlts.2001.23911. [DOI] [PubMed] [Google Scholar]
- 69.Roque-Afonso AM, Feray C, Samuel D, Simoneau D, Roche B, Emile JF, et al. Antibodies to hepatitis B surface antigen prevent viral reactivation in recipients of liver grafts from anti-HBC positive donors. Gut. 2002;50:95–9. doi: 10.1136/gut.50.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dodson SF, Bonham CA, Geller DA, Cacciarelli TV, Rakela J, Fung JJ. Prevention of de novo hepatitis B infection in recipients of hepatic allografts from anti-HBc positive donors. Transplantation. 1999;68:1058–61. doi: 10.1097/00007890-199910150-00028. [DOI] [PubMed] [Google Scholar]
- 71.Uemoto S, Inomata Y, Sannomiya A, Koshiba T, Kurokawa T, Takatsuki M, et al. Posttransplant hepatitis B infection in liver transplantation with hepatitis B core antibody-positive donors. Transplant Proc. 1998;30:134–5. doi: 10.1016/s0041-1345(97)01211-6. [DOI] [PubMed] [Google Scholar]
- 72.Uemoto S, Sugiyama K, Marusawa H, Inomata Y, Asonuma K, Egawa H, et al. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;65:494–9. doi: 10.1097/00007890-199802270-00007. [DOI] [PubMed] [Google Scholar]
- 73.Kiuchi T, Tanaka K. Living-related donor liver transplantation: status quo in Kyoto, Japan. Transplant Proc. 1998;30:687–91. doi: 10.1016/s0041-1345(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 74.Katsurada A, Marusawa H, Uemoto S, Kaburagi A, Tanaka K, Chiba T. Circulating antibody to hepatitis B core antigen does not always reflect the latent hepatitis B virus infection in the liver tissue. Hepatol Res. 2003;25:105–14. doi: 10.1016/s1386-6346(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 75.Kadian M, Hawkins L, Nespral J, Schwartz ME, Miller CM. Use of hepatitis B core antibody-positive multiorgan donors. J Transpl Coord. 1994;4:57–9. [Google Scholar]
- 76.Montalti R, Nardo B, Bertelli R, Beltempo P, Puviani L, Vivarelli M, et al. Donor pool expansion in liver transplantation. Transplant Proc. 2004;36:520–2. doi: 10.1016/j.transproceed.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 77.De Feo TM, Grossi P, Poli F, Mozzi F, Messa P, Minetti E, et al. Kidney transplantation from anti-HBc+ donors: results from a retrospective Italian study. Transplantation. 2006;81:76–80. doi: 10.1097/01.tp.0000189930.89031.1b. [DOI] [PubMed] [Google Scholar]
- 78.De Feo TM, Poli F, Mozzi F, Moretti MP, Scalamogna M. Risk of transmission of hepatitis B virus from anti-HBC positive cadaveric organ donors: a collaborative study. Transplant Proc. 2005;37:1238–9. doi: 10.1016/j.transproceed.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 79.Holt D, Thomas R, Van Thiel D, Brems JJ. Use of hepatitis B core antibody-positive donors in orthotopic liver transplantation. Arch Surg. 2002;137:572–5. doi: 10.1001/archsurg.137.5.572. [DOI] [PubMed] [Google Scholar]
- 80.Donataccio D, Roggen F, De Reyck C, Verbaandert C, Bodeus M, Lerut J. Use of anti-HBc positive allografts in adult liver transplantation: toward a safer way to expand the donor pool. Transpl Int. 2006;19:38–43. doi: 10.1111/j.1432-2277.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 81.Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, et al. Prevention of hepatitis B virus infection from hepatitis B core antibody-positive donor graft using hepatitis B immune globulin and lamivudine in living donor liver transplantation. Liver Int. 2005;25:1169–74. doi: 10.1111/j.1478-3231.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 82.Fabrega E, Garcia-Suarez C, Guerra A, Orive A, Casafont F, Crespo J, et al. Liver transplantation with allografts from hepatitis B core antibody-positive donors: a new approach. Liver Transpl. 2003;9:916–20. doi: 10.1053/jlts.2003.50190. [DOI] [PubMed] [Google Scholar]
- 83.Nery JR, Nery-Avila C, Reddy KR, Cirocco R, Weppler D, Levi DM, et al. Use of liver grafts from donors positive for antihepatitis B-core antibody (anti-HBc) in the era of prophylaxis with hepatitis-B immunoglobulin and lamivudine. Transplantation. 2003;75:1179–86. doi: 10.1097/01.TP.0000065283.98275.FE. [DOI] [PubMed] [Google Scholar]
- 84.Wachs ME, Amend WJ, Ascher NL, Bretan PN, Emond J, Lake JR, et al. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation. 1995;59:230–4. [PubMed] [Google Scholar]
- 85.Castells L, Vargas V, Rodriguez F, Allende H, Buti M, Sanchez-Avila JF, et al. Clinical impact and efficacy of lamivudine therapy in de novo hepatitis B infection after liver transplantation. Liver Transpl. 2002;8:892–900. doi: 10.1053/jlts.2002.35555. [DOI] [PubMed] [Google Scholar]
- 86.Loss GE, Mason AL, Blazek J, Dick D, Lipscomb J, Guo L, et al. Transplantation of livers from HBc Ab positive donors into HBc Ab negative recipients: a strategy and preliminary results. Clin Transplant. 2001;15(Suppl 6):55–8. doi: 10.1034/j.1399-0012.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 87.Madayag RM, Johnson LB, Bartlett ST, Schweitzer EJ, Constantine NT, McCarter RJ, Jr, et al. Use of renal allografts from donors positive for hepatitis B core antibody confers minimal risk for subsequent development of clinical hepatitis B virus disease. Transplantation. 1997;64:1781–6. doi: 10.1097/00007890-199712270-00027. [DOI] [PubMed] [Google Scholar]
- 88.Veroux M, Puliatti C, Gagliano M, Cappello D, Macarone M, Vizcarra D, et al. Use of hepatitis B core antibody-positive donor kidneys in hepatitis B surface antibody-positive and -negative recipients. Transplant Proc. 2005;37:2574–5. doi: 10.1016/j.transproceed.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 89.Fong TL, Bunnapradist S, Jordan SC, Cho YW. Impact of hepatitis B core antibody status on outcomes of cadaveric renal transplantation: analysis of United Network for Organ Sharing database between 1994 and 1999. Transplantation. 2002;73:85–9. doi: 10.1097/00007890-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 90.Satterthwaite R, Ozgu I, Shidban H, Aswad S, Sunga V, Zapanta R, Jr, et al. Risks of transplanting kidneys from hepatitis B surface antigen-negative, hepatitis B core antibody-positive donors. Transplantation. 1997;64:432–5. doi: 10.1097/00007890-199708150-00011. [DOI] [PubMed] [Google Scholar]
- 91.Akalin E, Ames S, Sehgal V, Murphy B, Bromberg JS. Safety of using hepatitis B virus core antibody or surface antigen-positive donors in kidney or pancreas transplantation. Clin Transplant. 2005;19:364–6. doi: 10.1111/j.1399-0012.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 92.Miedouge M, Rostaing L, Mansuy JM, Sandres-Saune K, Boudet F, Izopet J. Screening for hepatitis B virus DNA in serum of organ donors and renal transplant recipients. Eur J Clin Microbiol Infect Dis. 2003;22:246–8. doi: 10.1007/s10096-003-0889-3. [DOI] [PubMed] [Google Scholar]
- 93.Pinney SP, Cheema FH, Hammond K, Chen JM, Edwards NM, Mancini D. Acceptable recipient outcomes with the use of hearts from donors with hepatitis-B core antibodies. J Heart Lung Transplant. 2005;24:34–7. doi: 10.1016/j.healun.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 94.Ko WJ, Chou NK, Hsu RB, Chen YS, Wang SS, Chu SH, et al. Hepatitis B virus infection in heart transplant recipients in a hepatitis B endemic area. J Heart Lung Transplant. 2001;20:865–75. doi: 10.1016/s1053-2498(01)00280-7. [DOI] [PubMed] [Google Scholar]
- 95.Wang SS, Chou NK, Ko WJ, Yu HY, Chen YS, Hsu RB, et al. Heart transplantation using donors positive for hepatitis. Transplant Proc. 2004;36:2371–3. doi: 10.1016/j.transproceed.2004.08.112. [DOI] [PubMed] [Google Scholar]
- 96.Blanes M, Gomez D, Cordoba J, Almenar L, Gobernado M, Lopez-Aldeguer J, et al. Is there any risk of transmission of hepatitis B from heart donors hepatitis B core antibody positive? Transplant Proc. 2002;34:61–2. doi: 10.1016/s0041-1345(01)02666-5. [DOI] [PubMed] [Google Scholar]
- 97.Hartwig MG, Patel V, Palmer SM, Cantu E, Appel JZ, Messier RH, et al. Hepatitis B core antibody positive donors as a safe and effective therapeutic option to increase available organs for lung transplantation. Transplantation. 2005;80:320–5. doi: 10.1097/01.tp.0000165858.86067.a2. [DOI] [PubMed] [Google Scholar]
- 98.Everhart JE, Wei Y, Eng H, Charlton MR, Persing DH, Wiesner RH, et al. Recurrent and new hepatitis C virus infection after liver transplantation [published erratum appears in Hepatology 1999;30:1110] Hepatology. 1999;29:1220–6. doi: 10.1002/hep.510290412. [DOI] [PubMed] [Google Scholar]
- 99.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Koff JM, Holtzmuller KC, et al. Impact of diabetes and hepatitis after kidney transplantation on patients who are affected by hepatitis C virus. J Am Soc Nephrol. 2004;15:3166–74. doi: 10.1097/01.ASN.0000145439.48387.BF. [DOI] [PubMed] [Google Scholar]
- 100.Bucci JR, Lentine KL, Agodoa LY, Peters TG, Schnitzler MA, Abbott KC. Outcomes associated with recipient and donor hepatitis C serology status after kidney transplantation in the United States: analysis of the USRDS/UNOS database. Clin Transpl. 2004:51–61. [PubMed] [Google Scholar]
- 101.Rozental R, Bicans J, Shevelev V, Trushkov S, Amerika D. Kidney transplantation from hepatitis C virus positive donors. Transplant Proc. 2002;34:2581. doi: 10.1016/s0041-1345(02)03432-2. [DOI] [PubMed] [Google Scholar]
- 102.Tokumoto T, Tanabe K, Sonda K, Koga S, Gouya N, Yagisawa T, et al. Effect of interferon (IFN-alpha) for prevention of hepatitis C transmission from a seropositive donor to a seronegative recipient in renal transplantation. Transplant Proc. 1996;28:1503–4. [PubMed] [Google Scholar]
- 103.Wreghitt TG, Gray JJ, Allain JP, Poulain J, Garson JA, Deaville R, et al. Transmission of hepatitis C virus by organ transplantation in the United Kingdom. J Hepatol. 1994;20:768–72. doi: 10.1016/s0168-8278(05)80148-5. [DOI] [PubMed] [Google Scholar]
- 104.Mendez R, Aswad S, Bogaard T, Khetan U, Asai P, Martinez A, et al. Donor hepatitis C antibody virus testing in renal transplantation. Transplant Proc. 1993;25(1 Pt 2):1487–90. [PubMed] [Google Scholar]
- 105.Aswad S, Mendez R, Weingart RG, Mendez R. Expanding organ availability by using hepatitis C antibody positive donors. Transplant Proc. 1993;25:2270–1. [PubMed] [Google Scholar]
- 106.Tesi RJ, Waller K, Morgan CJ, Delaney S, Elkhammas EA, Henry ML, et al. Transmission of hepatitis C by kidney transplantation—the risks. Transplantation. 1994;57:826–31. doi: 10.1097/00007890-199403270-00010. [DOI] [PubMed] [Google Scholar]
- 107.Tesi RJ, Waller MK, Morgan CJ, Delaney S, Elkhammas EA, Henry ML, et al. Use of low-risk HCV-positive donors for kidney transplantation. Transplant Proc. 1993;25(1 Pt 2):1472–3. [PubMed] [Google Scholar]
- 108.Preiksaitis JK, Cockfield SM, Fenton JM, Burton NI, Chui LW. Serologic responses to hepatitis C virus in solid organ transplant recipients. Transplantation. 1997;64:1775–80. doi: 10.1097/00007890-199712270-00026. [DOI] [PubMed] [Google Scholar]
- 109.File E, Mehra M, Nair S, Dumas-Hicks D, Perrillo R. Allograft transmission of hepatitis C virus infection from infected donors in cardiac transplantation. Transplantation. 2003;76:1096–100. doi: 10.1097/01.TP.0000088663.76640.C9. [DOI] [PubMed] [Google Scholar]
- 110.Haji SA, Starling RC, Avery RK, Mawhorter S, Tuzcu EM, Schoenhagen P, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23:277–83. doi: 10.1016/S1053-2498(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 111.Shea KJ, Sopko NA, Ludrosky K, Hoercher K, Smedira NG, Taylor DO, et al. The effect of a donor's history of active substance on outcomes following orthotopic heart transplantation. Eur J Cardiothorac Surg. 2007;31:452–6. doi: 10.1016/j.ejcts.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 112.Ong JP, Barnes DS, Younossi ZM, Gramlich T, Yen-Lieberman B, Goormastic M, et al. Outcome of de novo hepatitis C virus infection in heart transplant recipients. Hepatology. 1999;30:1293–8. doi: 10.1002/hep.510300519. [DOI] [PubMed] [Google Scholar]
- 113.Gudmundsson GS, Malinowska K, Robinson JA, Pisani BA, Mendez JC, Foy BK, et al. Five-year follow-up of hepatitis C-naive heart transplant recipients who received hepatitis C-positive donor hearts. Transplant Proc. 2003;35:1536–8. doi: 10.1016/s0041-1345(03)00368-3. [DOI] [PubMed] [Google Scholar]
- 114.Marelli D, Bresson J, Laks H, Kubak B, Fonarow G, Tsai FC, et al. Hepatitis C-positive donors in heart transplantation. Am J Transplant. 2002;2:443–7. doi: 10.1034/j.1600-6143.2002.20508.x. [DOI] [PubMed] [Google Scholar]
- 115.McQuillan GM, Kruszon-Moran D, Kottiri BJ, Kamimoto LA, Lam L, Cowart MF, et al. Prevalence of HIV in the US household population: the National Health and Nutrition Examination Surveys, 1988 to 2002. J Acquir Immune Defic Syndr. 2006;41:651–6. doi: 10.1097/01.qai.0000194235.31078.f6. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen TQ, Gwynn RC, Kellerman SE, Begier E, Garg RK, Pfeiffer MR, et al. Population prevalence of reported and unreported HIV and related behaviors among the household adult population in New York City, 2004. AIDS. 2008;22:281–7. doi: 10.1097/QAD.0b013e3282f2ef58. [DOI] [PubMed] [Google Scholar]
- 117.Mehta SD, Hall J, Greenwald JL, Cranston K, Skolnik PR. Patient risks, outcomes, and costs of voluntary HIV testing at five testing sites within a medical center. Public Health Rep. 2008;123:608–17. doi: 10.1177/003335490812300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanchez P, Heck E, Rivera C, Sanchez A, Cavanagh HD. Risk factors for infectious disease in corneal transplant screening. Eye Contact Lens. 2006;32:124–7. doi: 10.1097/01.icl.0000178800.40166.25. [DOI] [PubMed] [Google Scholar]
- 119.Alpert PL, Shuter J, DeShaw MG, Webber MP, Klein RS. Factors associated with unrecognized HIV-1 infection in an inner-city emergency department. Ann Emerg Med. 1996;28:159–64. doi: 10.1016/s0196-0644(96)70056-2. [DOI] [PubMed] [Google Scholar]
- 120.Fischer LR, Tope DH, Conboy KS, Hedblom BD, Ronberg E, Shewmake DK, et al. Screening for hepatitis C virus in a health maintenance organization. Arch Intern Med. 2000;160:1665–73. doi: 10.1001/archinte.160.11.1665. [DOI] [PubMed] [Google Scholar]
- 121.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–8. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang LY, Kramer JR, Troisi C, Bull L, Grimes CZ, Lyerla R, et al. Relationship of cosmetic procedures and drug use to hepatitis C and hepatitis B virus infections in a low-risk population. Hepatology. 2006;44:341–51. doi: 10.1002/hep.21252. [DOI] [PubMed] [Google Scholar]
- 123.Kaur S, Rybicki L, Bacon BR, Gollan JL, Rustgi VK, Carey WD. Performance characteristics and results of a large-scale screening program for viral hepatitis and risk factors associated with exposure to viral hepatitis B and C: results of the National Hepatitis Screening Survey. Hepatology. 1996;24:979–86. doi: 10.1002/hep.510240501. [DOI] [PubMed] [Google Scholar]
- 124.Tabibian JH, Wirshing DA, Pierre JM, Guzik LH, Kisicki MD, Danovitch I, et al. Hepatitis B and C among veterans on a psychiatric ward. Dig Dis Sci. 2008;53:1693–8. doi: 10.1007/s10620-007-0045-5. [DOI] [PubMed] [Google Scholar]
- 125.Butterfield MI, Bosworth HB, Stechuchak KM, Frothingham R, Bastian LA, Meador KG, et al. Racial differences in hepatitis B and hepatitis C and associated risk behaviors in veterans with severe mental illness. J Natl Med Assoc. 2004;96:43–52. [PMC free article] [PubMed] [Google Scholar]
- 126.Butterfield CR, Shockley M, San MG, Rosa C. Routine screening for hepatitis B in an obstetric population. Obstet Gynecol. 1990;76:25–7. [PubMed] [Google Scholar]
- 127.Alter MJ, Coleman PJ, Alexander WJ, Kramer E, Miller JK, Mandel E, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201–5. [PubMed] [Google Scholar]
- 128.Murphy EL, Bryzman SM, Glynn SA, Ameti DI, Thomson RA, Williams AE, et al. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS) Hepatology. 2000;31:756–62. doi: 10.1002/hep.510310329. [DOI] [PubMed] [Google Scholar]
- 129.Hand WL, Vasquez Y. Risk factors for hepatitis C on the Texas-Mexico border. Am J Gastroenterol. 2005;100:2180–5. doi: 10.1111/j.1572-0241.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 130.Orton SL, Stramer SL, Dodd RY, Alter MJ. Risk factors for HCV infection among blood donors confirmed to be positive for the presence of HCV RNA and not reactive for the presence of anti-HCV. Transfusion. 2004;44:275–81. doi: 10.1111/j.1537-2995.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 131.Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–6. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen MT, Herrine SK, Laine CA, Ruth K, Weinberg DS. Description of a new hepatitis C risk assessment tool. Arch Intern Med. 2005;165:2013–8. doi: 10.1001/archinte.165.17.2013. [DOI] [PubMed] [Google Scholar]
- 133.Gasink LB, Blumberg EA, Localio AR, Desai SS, Israni AK, Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843–50. doi: 10.1001/jama.296.15.1843. [DOI] [PubMed] [Google Scholar]
- 134.Zetola NM, Kaplan B, Dowling T, Jensen T, Louie B, Shahkarami M, et al. Prevalence and correlates of unknown HIV infection among patients seeking care in a public hospital emergency department. Public Health Rep. 2008;123(Suppl 3):41–50. doi: 10.1177/00333549081230S306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turner SB, Kunches LM, Gordon KF, Travers PH, Mueller NE. Occupational exposure to human immunodeficiency virus (HIV) and hepatitis B virus (HBV) among embalmers: a pilot seroprevalence study. Am J Public Health. 1989;79:1425–6. doi: 10.2105/ajph.79.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034–40. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 137.Hann HW, Hann RS, Maddrey WC. Hepatitis B virus infection in 6,130 unvaccinated Korean-Americans surveyed between 1988 and 1990. Am J Gastroenterol. 2007;102:767–72. doi: 10.1111/j.1572-0241.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 138.Lee H, Levin MJ, Kim F, Warner A, Park W. Hepatitis B infection among Korean Americans in Colorado: evidence of the need for serologic testing and vaccination. Hepatitis Monthly. 2008;8:91–6. [Google Scholar]
- 139.Luban NL, Colvin CA, Mohan P, Alter HJ. The epidemiology of transfusion-associated hepatitis C in a children's hospital. Transfusion. 2007;47:615–20. doi: 10.1111/j.1537-2995.2007.01162.x. [DOI] [PubMed] [Google Scholar]
- 140.Murphy EL, Bryzman S, Williams AE, Co-Chien H, Schreiber GB, Ownby HE, et al. Demographic determinants of hepatitis C virus seroprevalence among blood donors. JAMA. 1996;275:995–1000. [PubMed] [Google Scholar]
- 141.McGinn T, O'Connor-Moore N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. Arch Intern Med. 2008;168:2009–13. doi: 10.1001/archinte.168.18.2009. [DOI] [PubMed] [Google Scholar]
- 142.Barbe F, Klein M, Badonnel Y. Early detection of antibodies to human immunodeficiency virus 1 by a third-generation enzyme immunoassay. A comparative study with the results of second-generation immunoassays and western blot. Ann Biol Clin (Paris) 1994;52:341–5. [PubMed] [Google Scholar]
- 143.Owen SM, Yang C, Spira T, Ou CY, Pau CP, Parekh BS, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol. 2008;46:1588–95. doi: 10.1128/JCM.02196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Food and Drug Administration (US) Vaccines, blood – biologics. Genetic systems HIV-1/HIV-2 plus O EIA [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs /BloodDonorScreening/InfectiousDisease/ucm091151.htm.
- 145.Food and Drug Administration (US) Vaccines, blood – biologics. ABBOTT HIVAB HIV-1/HIV-2 (rDNA) EIA [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs /BloodDonorScreening/InfectiousDisease/ucm091149.htm.
- 146.Vargo J, Smith K, Knott C, Wang S, Fang C, McDonough S, et al. Clinical specificity and sensitivity of a blood screening assay for detection of HIV-1 and HCV RNA. Transfusion. 2002;42:876–85. doi: 10.1046/j.1537-2995.2002.00130.x. [DOI] [PubMed] [Google Scholar]
- 147.Jackson JB, Smith K, Knott C, Korpela A, Simmons A, Piwowar-Manning E, et al. Sensitivity of the Procleix HIV-1/HCV assay for detection of human immunodeficiency virus type 1 and hepatitis C virus RNA in a high-risk population. J Clin Microbiol. 2002;40:2387–91. doi: 10.1128/JCM.40.7.2387-2391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Candotti D, Richetin A, Cant B, Temple J, Sims C, Reeves I, et al. Evaluation of a transcription-mediated amplification-based HCV and HIV-1 RNA duplex assay for screening individual blood donations: a comparison with a minipool testing system. Transfusion. 2003;43:215–25. doi: 10.1046/j.1537-2995.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 149.Kolk DP, Dockter J, Linnen J, Ho-Sing-Loy M, Gillotte-Taylor K, McDonough SH, et al. Significant closure of the human immunodeficiency virus type 1 and hepatitis C virus preseroconversion detection windows with a transcription-mediated-amplification-driven assay. J Clin Microbiol. 2002;40:1761–6. doi: 10.1128/JCM.40.5.1761-1766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang Y, Wisbeski MH, Mendoza M, Dorf S, Xu D, Nguyen M, et al. Performance characteristics of the AmpliScreen HIV-1 test, an assay designed for screening plasma mini-pools. Biologicals. 1999;27:315–23. doi: 10.1006/biol.1999.0226. [DOI] [PubMed] [Google Scholar]
- 151.Food and Drug Administration (US) Vaccines, blood – biologics. Procleix HIV-1/HCV assay [cited 2009 Sep 17] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts /ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening /InfectiousDisease/ucm092022.htm.
- 152.Food and Drug Administration (US) Vaccines, blood – biologics. COBAS AmpliScreen HIV-1 Test [cited 2009 Sep 17] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs /BloodDonorScreening/InfectiousDisease/ucm093493.htm.
- 153.Bamaga MS, Bokhari FF, Aboud AM, Al-Malki M, Alenzi FQ. Nucleic acid amplification technology screening for hepatitis C virus and human immunodeficiency virus for blood donations. Saudi Med J. 2006;27:781–7. [PubMed] [Google Scholar]
- 154.Aghokeng AF, Ewane L, Awazi B, Nanfack A, Delaporte E, Peeters M, et al. Evaluation of four simple/rapid assays and two fourth-generation ELISAs for the identification of HIV infection on a serum panel representing the HIV-1 group M genetic diversity in Cameroon. J Acquir Immune Defic Syndr. 2004;37:1632–40. doi: 10.1097/00126334-200412150-00018. [DOI] [PubMed] [Google Scholar]
- 155.Bourlet T, Pretis C, Pillet S, Lesenechal M, Piche J, Pozzetto B. Comparative evaluation of the VIDAS HIV DUO Ultra assay for combined detection of HIV-1 antigen and antibodies to HIV. J Virol Methods. 2005;127:165–7. doi: 10.1016/j.jviromet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 156.Kwon JA, Yoon SY, Lee CK, Lim CS, Lee KN, Sung HJ, et al. Performance evaluation of three automated human immunodeficiency virus antigen-antibody combination immunoassays. J Virol Methods. 2006;133:20–6. doi: 10.1016/j.jviromet.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 157.Ly TD, Servant-Delmas A, Bagot S, Gonzalo S, Ferey MP, Ebel A, et al. Sensitivities of four new commercial hepatitis B virus surface antigen (HBsAg) assays in detection of HBsAg mutant forms. J Clin Microbiol. 2006;44:2321–6. doi: 10.1128/JCM.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ly TD, Laperche S, Brennan C, Vallari A, Ebel A, Hunt J, et al. Evaluation of the sensitivity and specificity of six HIV combined p24 antigen and antibody assays. J Virol Methods. 2004;122:185–94. doi: 10.1016/j.jviromet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 159.Sickinger E, Stieler M, Kaufman B, Kapprell HP, West D, Sandridge A, et al. Multicenter evaluation of a new, automated enzyme-linked immunoassay for detection of human immunodeficiency virus-specific antibodies and antigen. J Clin Microbiol. 2004;42:21–9. doi: 10.1128/JCM.42.1.21-29.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weber B, Gürtler L, Thorstensson R, Michl U, Mühlbacher A, Burgisser P, et al. Multicenter evaluation of a new automated fourth-generation human immunodeficiency virus screening assay with a sensitive antigen detection module and high specificity. J Clin Microbiol. 2002;40:1938–46. doi: 10.1128/JCM.40.6.1938-1946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saville RD, Constantine NT, Cleghorn FR, Jack N, Bartholomew C, Edwards J, et al. Fourth-generation enzyme-linked immunosorbent assay for the simultaneous detection of human immunodeficiency virus antigen and antibody. J Clin Microbiol. 2001;39:2518–24. doi: 10.1128/JCM.39.7.2518-2524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seyoum E, Wolday D, Mekonen T, Girma M, Meselle T, Källander C, et al. Alternative approach to blood screening using the ExaVir reverse transcriptase activity assay. Curr HIV Res. 2005;3:371–6. doi: 10.2174/157016205774370438. [DOI] [PubMed] [Google Scholar]
- 163.Delieu E, Donovan LG, Perry KR, Parry JV. Murex HIV Ag/Ab combination EIA (product code: GE41/GE42) London: Medical Devices Agency, Microbiological Diagnostics Assessment Service; 2001. MDA Evaluation Report No. 01123. [Google Scholar]
- 164.Diepersloot RJ, van Zantvliet-van OY, Gleaves CA. Comparison of a chemiluminescent immunoassay with two microparticle enzyme immunoassays for detection of hepatitis B virus surface antigen. Clin Diagn Lab Immunol. 2000;7:865–6. doi: 10.1128/cdli.7.6.865-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huzly D, Schenk T, Jilg W, Neumann-Haefelin D. Comparison of nine commercially available assays for quantification of antibody response to hepatitis B virus surface antigen. J Clin Microbiol. 2008;46:1298–306. doi: 10.1128/JCM.02430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Food and Drug Administration (US) Vaccines, blood – biologics. Genetic Systems HBsAg EIA 3.0 [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/ucm085808.htm.
- 167.Food and Drug Administration (US) Medical devices. AxSYM HBsAg Assay—P050049 [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures /DeviceApprovalsandClearances/Recently-ApprovedDevices /ucm078174.htm.
- 168.Food and Drug Administration (US) Medical devices. ADVIA Centaur HBsAg ReadyPack Reagents, ADVIA Centaur HBsAg Confirmatory ReadyPack Reagents, and ADVIA Centaur HBsAg Quality Control Material—P030049 [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/MedicalDevices/Productsand MedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm078604.htm.
- 169.Food and Drug Administration (US) Vaccines, blood – biologics. COBAS AmpliScreen HBV Test [cited 2009 Sep 17] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs /BloodDonorScreening/InfectiousDisease/ucm077872.htm.
- 170.Ortho Clinical Diagnostics. Donor screening. Genetic Systems HBsAg EIA 3.0. 2006 [cited 2009 Sep 17] Available from: URL: http://www.orthoclinical.com/en-us/ProductInformation/Trans fusionMedicine/DonorScreening/Pages/ScreeningAssays.aspx.
- 171.Food and Drug Administration (US) Medical devices. ADVIA Centaur HBc Total ReadyPack Reagents, ADVIA Centaur HBc Total Quality Control Materials—P040004 [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/MedicalDevices /ProductsandMedicalProcedures/DeviceApprovalsandClearances /Recently-ApprovedDevices/ucm078871.htm.
- 172.Food and Drug Administration (US) Medical devices. Bayer ADVIA Centaur HCV Assay—P030056 [cited 2009 Sep 29] Available from: URL: http://www.fda.gov/MedicalDevices/ProductsandMedical Procedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm079236.htm.
- 173.Kleinman SH, Strong DM, Tegtmeier GG, Holland PV, Gorlin JB, Cousins C, et al. Hepatitis B virus (HBV) DNA screening of blood donations in minipools with the COBAS AmpliScreen HBV test. Transfusion. 2005;45:1247–57. doi: 10.1111/j.1537-2995.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 174.Romano L, Velati C, Baruffi L, Fomiatti L, Colucci G, Zanetti AR, et al. Multicenter evaluation of a semiautomated, standardized assay for detection of hepatitis B virus DNA in blood donations. J Clin Microbiol. 2005;43:2991–3. doi: 10.1128/JCM.43.6.2991-2993.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anderson SC, Hathaway T, Kuramoto IK, Holland PV, Gilcher R, Koch T, et al. Comparison of two second-generation anti-hepatitis C virus ELISA on 21431 US blood donor samples. J Viral Hepat. 1995;2:55–61. doi: 10.1111/j.1365-2893.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 176.Laycock KA, Essary LR, Delaney S, Kuhns MC, Pepose JS. A critical evaluation of hepatitis C testing of cadaveric corneal donors. Cornea. 1997;16:146–50. [PubMed] [Google Scholar]
- 177.Leon P, Lopez JA, Domingo C, Echevarria JM. Evaluation of laboratory assays for screening antibody to hepatitis C virus. Transfusion. 1993;33:268–70. doi: 10.1046/j.1537-2995.1993.33393174455.x. [DOI] [PubMed] [Google Scholar]
- 178.Vrielink H, Zaaijer HL, Reesink HW, Lelie PN, van der Poel CL. Comparison of two anti-hepatitis C virus enzyme-linked immunosorbent assays. Transfusion. 1995;35:601–4. doi: 10.1046/j.1537-2995.1995.35795357885.x. [DOI] [PubMed] [Google Scholar]
- 179.Vrielink H, Zaaijer HL, Reesink HW, van der Poel CL, Cuypers HT, Lelie PN. Sensitivity and specificity of three third-generation anti-hepatitis C virus ELISAs. Vox Sang. 1995;69:14–7. doi: 10.1111/j.1423-0410.1995.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 180.Kita M, Deguchi M, Kagita M, Yoshioka N, Kobayashi E, Watanabe M, et al. Clinical utility and characteristics of nine anti-HCV antibody screening reagents used in Japan. Clin Lab. 2009;55:9–22. [PubMed] [Google Scholar]
- 181.Denoyel G, van Helden J, Bauer R, Preisel-Simmons B. Performance of a new hepatitis C assay on the Bayer ADVIA Centaur Immunoassay System. Clin Lab. 2004;50:75–82. [PubMed] [Google Scholar]
- 182.Galel SA, Strong DM, Tegtmeier GE, Holland PV, Kuramoto IK, Kemper M, et al. Comparative yield of HCV RNA testing in blood donors screened by 2.0 versus 3.0 antibody assays. Transfusion. 2002;42:1507–13. doi: 10.1046/j.1537-2995.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 183.Barrera JM, Francis B, Ercilla G, Nelles M, Achord D, Darner J, et al. Improved detection of anti-HCV in post-transfusion hepatitis by a third-generation ELISA. Vox Sang. 1995;68:15–8. doi: 10.1111/j.1423-0410.1995.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 184.Food and Drug Administration (US) Vaccines, blood – biologics. COBAS AmpliScreen HCV Test [cited 2009 Sep 17] Available from: URL: http://www.fda.gov/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs /BloodDonorScreening/InfectiousDisease/ucm173197.htm.
- 185.Katsoulidou AS, Moschidis ZM, Gialeraki RE, Paraskevis DN, Sypsa VA, Lazanas MC, et al. Clinical evaluation of an HIV-1 and HCV assay and demonstration of significant reduction of the HCV detection window before seroconversion. Transfusion. 2004;44:59–66. doi: 10.1111/j.0041-1132.2003.00591.x. [DOI] [PubMed] [Google Scholar]
- 186.Laperche S, Elghouzzi MH, Morel P, Asso-Bonnet M, Le Marrec N, Girault A, et al. Is an assay for simultaneous detection of hepatitis C virus core antigen and antibody a valuable alternative to nucleic acid testing? Transfusion. 2005;45:1965–72. doi: 10.1111/j.1537-2995.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 187.Laperche S, Le Marrec N, Girault A, Bouchardeau F, Servant-Delmas A, Maniez-Montreuil M, et al. Simultaneous detection of hepatitis C virus (HCV) core antigen and anti-HCV antibodies improves the early detection of HCV infection. J Clin Microbiol. 2005;43:3877–83. doi: 10.1128/JCM.43.8.3877-3883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dean L, Perry K, Nichols T. Evaluation of MONOLISA HCV Ag-Ab ULTRA. London: Health Protection Agency, Centre for Infections; 2006. [Google Scholar]
- 189.Zucker K, Cirocco R, Roth D, Olson L, Burke GW, Nery J, et al. Depletion of hepatitis C virus from procured kidneys using pulsatile perfusion preservation. Transplantation. 1994;57:832–40. doi: 10.1097/00007890-199403270-00011. [DOI] [PubMed] [Google Scholar]
- 190.Roth D, Zucker K, Cirocco R, Burke G, Olson L, Esquenazi V, et al. Transmission of hepatitis C virus by kidney transplantation: impact of perfusion techniques and course of viremia post transplant. Pediatr Nephrol. 1995;(9 Suppl):S29–34. doi: 10.1007/BF00867680. [DOI] [PubMed] [Google Scholar]
- 191.Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Peters TG, Schnitzler MA. The impact of transplantation with deceased donor hepatitis C-positive kidneys on survival in wait-listed long-term dialysis patients. Am J Transplant. 2004;4:2032–7. doi: 10.1046/j.1600-6143.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 192.Lai MK, Huang CC, Chu SH, Chuang CK, Chen CS, Chen HW. The outcome of kidney transplantation from HBsAg-positive donors. Asian J Surg. 1994;17:340–3. [Google Scholar]
- 193.Lai MK, Chang KS, Chueh SC, Huang CC, Chen SC, Chu SH. Kidney transplantation from hepatitis B surface antigen (HBsAg)-positive donors: changes of relative HBV genomic copy number after transplantation. Transplant Proc. 1996;28:1518–9. [PubMed] [Google Scholar]
- 194.Chen JB, Hsieh H, Hsu KT, Chen CH. Impact of donor hepatitis B antigenemia on renal allograft in hepatitis B recipients. Transplant Proc. 1996;28:1490–2. [PubMed] [Google Scholar]
- 195.Saab S, Chang AJ, Comulada S, Geevarghese SK, Anselmo RD, Durazo F, et al. Outcomes of hepatitis C- and hepatitis B core antibody-positive grafts in orthotopic liver transplantation. Liver Transpl. 2003;9:1053–61. doi: 10.1053/jlts.2003.50208. [DOI] [PubMed] [Google Scholar]
- 196.Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, Peters TG, et al. Donor hepatitis C seropositivity: clinical correlates and effect on early graft and patient survival in adult cadaveric kidney transplantation. J Am Soc Nephrol. 2002;13:2974–82. doi: 10.1097/01.asn.0000034944.90425.75. [DOI] [PubMed] [Google Scholar]
- 197.Abbott KC, Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, et al. Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14:2908–18. doi: 10.1097/01.asn.0000090743.43034.72. [DOI] [PubMed] [Google Scholar]
- 198.Velidedeoglu E, Desai NM, Campos L, Olthoff KM, Shaked A, Nunes F, et al. Effect of donor hepatitis C on liver graft survival. Transplant Proc. 2001;33:3795–6. doi: 10.1016/s0041-1345(01)02606-9. [DOI] [PubMed] [Google Scholar]
- 199.Velidedeoglu E, Desai NM, Campos L, Olthoff KM, Shaked A, Nunes F, et al. The outcome of liver grafts procured from hepatitis C-positive donors. Transplantation. 2002;73:582–7. doi: 10.1097/00007890-200202270-00018. [DOI] [PubMed] [Google Scholar]
- 200.Morales JM, Campistol JM, Castellano G, Andres A, Colina F, Fuertes A, et al. Transplantation of kidneys from donors with hepatitis C antibody into recipients with pre-transplantation anti-HCV. Kidney Int. 1995;47:236–40. doi: 10.1038/ki.1995.29. [DOI] [PubMed] [Google Scholar]
- 201.Woodside KJ, Ishihara K, Theisen JE, Early MG, Covert LG, Hunter GC, et al. Use of kidneys from hepatitis C seropositive donors shortens waitlist time but does not alter one-yr outcome. Clin Transplant. 2003;17:433–7. doi: 10.1034/j.1399-0012.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 202.Ali MK, Light JA, Barhyte DY, Sasaki TM, Currier CB, Jr, Grandas O, et al. Donor hepatitis C virus status does not adversely affect short-term outcomes in HCV+ recipients in renal transplantation. Transplantation. 1998;66:1694–7. doi: 10.1097/00007890-199812270-00021. [DOI] [PubMed] [Google Scholar]
- 203.Kasprzyk T, Kwiatkowski A, Wszola M, Ostrowski K, Danielewicz R, Domagala P, et al. Long-term results of kidney transplantation from HCV-positive donors. Transplant Proc. 2007;39:2701–3. doi: 10.1016/j.transproceed.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 204.Salizzoni M, Lupo F, Zamboni F, Franchello A, Lavezzo B, Cerutti E, et al. Outcome of patients transplanted with liver from hepatitis C positive donors. Transplant Proc. 2001;33:1507–8. doi: 10.1016/s0041-1345(00)02821-9. [DOI] [PubMed] [Google Scholar]
- 205.Testa G, Goldstein RM, Netto G, Abbasoglu O, Brooks BK, Levy MF, et al. Long-term outcome of patients transplanted with livers from hepatitis C-positive donors. Transplantation. 1998;65:925–9. doi: 10.1097/00007890-199804150-00011. [DOI] [PubMed] [Google Scholar]
- 206.Marroquin CE, Marino G, Kuo PC, Plotkin JS, Rustgi VK, Lu AD, et al. Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers. Liver Transpl. 2001;7:762–8. doi: 10.1053/jlts.2001.27088. [DOI] [PubMed] [Google Scholar]
- 207.Vargas HE, Laskus T, Wang LF, Lee R, Radkowski M, Dodson F, et al. Outcome of liver transplantation in hepatitis C virus-infected patients who received hepatitis C virus-infected grafts. Gastroenterology. 1999;117:149–53. doi: 10.1016/s0016-5085(99)70561-5. [DOI] [PubMed] [Google Scholar]
- 208.Mandal AK, Kraus ES, Samaniego M, Rai R, Humphreys SL, Ratner LE, et al. Shorter waiting times for hepatitis C virus seropositive recipients of cadaveric renal allografts from hepatitis C virus seropositive donors. Clin Transplant. 2000;14(4 Pt 2):391–6. doi: 10.1034/j.1399-0012.2000.14040602.x. [DOI] [PubMed] [Google Scholar]
- 209.Schweitzer EJ, Perencevich EN, Philosophe B, Bartlett ST. Estimated benefits of transplantation of kidneys from donors at increased risk for HIV or hepatitis C infection. Am J Transplant. 2007;7:1515–25. doi: 10.1111/j.1600-6143.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- 210.Freeman RB, Cohen JT. Transplantation risks and the real world: what does “high risk” really mean? Am J Transplant. 2009;9:23–30. doi: 10.1111/j.1600-6143.2008.02476.x. [DOI] [PubMed] [Google Scholar]
- 211.Organ Procurement and Transplantation Network. 2007 annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients [cited 2013 Apr 6] Available from: URL: http://www.ustransplant.org/annual_reports /current/ar_archives.htm.
- 212.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–14. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 213.Fishman JA, Greenwald MA, Kuehnert MJ. Enhancing transplant safety: a new era in the microbiologic evaluation of organ donors? Am J Transplant. 2007;7:2652–4. doi: 10.1111/j.1600-6143.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 214.Kahneman D, Tversky A, editors. Choices, values and frames. New York: Cambridge University Press; 2000. [Google Scholar]
- 215.Halpern SD, Shaked A, Hasz RD, Caplan AL. Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med. 2008;358:2832–7. doi: 10.1056/NEJMsb0800674. [DOI] [PubMed] [Google Scholar]
- 216.Kucirka LM, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Formal policies and special informed consent are associated with higher provider utilization of CDC high-risk donor organs. Am J Transplant. 2009;9:629–35. doi: 10.1111/j.1600-6143.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 217.Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52. [PubMed] [Google Scholar]
- 218.Panlilio AL, Cardo DM, Grohskopf L, Heneine W, Ross CS. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1–17. [PubMed] [Google Scholar]
- 219.Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54(RR-2):1–20. [PubMed] [Google Scholar]
- 220.Occupational Safety and Health Administration (US) Bloodborne pathogens. Post-exposure follow-up (29 C.F.R. 1910.1030) [cited 2010 Sep 2] Available from: URL: http://www.osha.gov/SLTC/etools/hospital/hazards/bbp/bbp.html#PostExposureFollow-up.
- 221.Ison MG. The epidemiology and prevention of donor-derived infections. Adv Chronic Kidney Dis. 2009;16:234–41. doi: 10.1053/j.ackd.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 222.Tugwell BD, Patel PR, Williams IT, Hedberg K, Chai F, Nainan OV, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143:648–54. doi: 10.7326/0003-4819-143-9-200511010-00008. [DOI] [PubMed] [Google Scholar]
- 223.Ison MG, Friedewald JJ. Transmission of human immunodeficiency virus and hepatitis C virus through liver transplantation. Liver Transpl. 2009;15:561. doi: 10.1002/lt.21756. [DOI] [PubMed] [Google Scholar]
- 224.Busch MP, Wilber JC, Johnson P, Tobler L, Evans CS. Impact of specimen handling and storage on detection of hepatitis C virus RNA. Transfusion. 1992;32:420–5. doi: 10.1046/j.1537-2995.1992.32592327714.x. [DOI] [PubMed] [Google Scholar]
- 225.Halfon P, Khiri H, Gerolami V, Bourliere M, Feryn JM, Reynier P, et al. Impact of various handling and storage conditions on quantitative detection of hepatitis C virus RNA. J Hepatol. 1996;25:307–11. doi: 10.1016/s0168-8278(96)80116-4. [DOI] [PubMed] [Google Scholar]
- 226.Gessoni G, Barin P, Valverde S, Giacomini A, Di Natale C, Orlandini E, et al. Biological qualification of blood units: considerations about the effects of sample's handling and storage on stability of nucleic acids. Transfus Apher Sci. 2004;30:197–203. doi: 10.1016/j.transci.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 227.Strong DM, Nelson K, Pierce M, Stramer SL. Preventing disease transmission by deceased tissue donors by testing blood for viral nucleic acid. Cell Tissue Bank. 2005;6:255–62. doi: 10.1007/s10561-005-2834-4. [DOI] [PubMed] [Google Scholar]
- 228.Eastlund T. Hemodilution due to blood loss and transfusion and reliability of cadaver tissue donor infectious disease testing. Cell Tissue Bank. 2000;1:121–7. doi: 10.1023/A:1010120115451. [DOI] [PubMed] [Google Scholar]
- 229.Rose C, Mohr J, Gross M, Lee S. Hemodilution—an overview of current Canadian practices. Cell Tissue Bank. 2001;2:41–4. doi: 10.1023/A:1011509515563. [DOI] [PubMed] [Google Scholar]
- 230.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–93. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 231.Comanor L, Holland P. Hepatitis B virus blood screening: unfinished agendas [published erratum appears in Vox Sang 2007;92:278] Vox Sang. 2006;91:1–12. doi: 10.1111/j.1423-0410.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 232.Preiksaitis JK, Sandhu J, Strautman M. The risk of transfusion-acquired CMV infection in seronegative solid-organ transplant recipients receiving non-WBC-reduced blood components not screened for CMV antibody (1984 to 1996): experience at a single Canadian center. Transfusion. 2002;42:396–402. doi: 10.1046/j.1525-1438.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 233.Dow BC, Yates P, Galea G, Munro H, Buchanan I, Ferguson K. Hepatitis B vaccinees may be mistaken for confirmed hepatitis B surface antigen-positive blood donors. Vox Sang. 2002;82:15–7. doi: 10.1046/j.0042-9007.2001.00125.x. [DOI] [PubMed] [Google Scholar]