Background: The overall view of erythropoiesis remains unclear.
Results: Overexpression of α-1,6-fucosyltransferase inhibits hemoglobin production in murine and human erythroleukemia cells; down-regulation of α-1,6-fucosyltransferase promotes hemoglobin production and erythroid differentiation of human erythroleukemia cells.
Conclusion: Core fucosylation plays an important role in hemoglobin production and erythroid differentiation.
Significance: This might be the first finding that glycosylation negatively regulates erythroid differentiation.
Keywords: Erythropoeisis, Functional Genomics, Glycosyltransferases, Hemoglobin, Transcriptomics
Abstract
Erythropoiesis results from a complex combination of the expression of several transcription factor genes and cytokine signaling. However, the overall view of erythroid differentiation remains unclear. First, we screened for erythroid differentiation-related genes by comparing the expression profiles of high differentiation-inducible and low differentiation-inducible murine erythroleukemia cells. We identified that overexpression of α-1,6-fucosyltransferase (Fut8) inhibits hemoglobin production. FUT8 catalyzes the transfer of a fucose residue to N-linked oligosaccharides on glycoproteins via an α-1,6 linkage, leading to core fucosylation in mammals. Expression of Fut8 was down-regulated during chemically induced differentiation of murine erythroleukemia cells. Additionally, expression of Fut8 was positively regulated by c-Myc and c-Myb, which are known as suppressors of erythroid differentiation. Second, we found that FUT8 is the only fucosyltransferase family member that inhibits hemoglobin production. Functional analysis of FUT8 revealed that the donor substrate-binding domain and a flexible loop play essential roles in inhibition of hemoglobin production. This result clearly demonstrates that core fucosylation inhibits hemoglobin production. Third, FUT8 also inhibited hemoglobin production of human erythroleukemia K562 cells. Finally, a short hairpin RNA study showed that FUT8 down-regulation induced hemoglobin production and increase of transferrin receptor/glycophorin A-positive cells in human erythroleukemia K562 cells. Our findings define FUT8 as a novel factor for hemoglobin production and demonstrate that core fucosylation plays an important role in erythroid differentiation.
Introduction
Erythropoiesis is intricately regulated by several linage-specific factors (1). Pro-erythroblasts/colony-forming unit-erythroid cells, which arise from megakaryocytic/erythroid progenitors (MEPs),2 are stimulated by erythropoietin (Epo), differentiate into erythroblasts, and finally maturate to become erythrocytes/red blood cells (2–4). Binding of Epo to the Epo receptor (EpoR) leads to phosphorylation and activation of receptor-associated Janus-kinase 2 (JAK2) and initiation of the EpoR signaling cascade (5, 6). Epo also stimulates phosphorylation and activation of GATA-binding protein 1 (GATA-1) (7). The transcription factor GATA-1 is highly expressed in MEPs and is well known as a specific regulator of erythroid differentiation. GATA-1 induces the expression of erythroid genes such as glycophorin A, EpoR, and hemoglobin (8). In embryos of GATA-1 null mice, embryonic erythroid cells are arrested at an early pro-erythroblast-like stage of their development (9) and die thereafter by apoptosis. The transcription factor PU.1 is a hematopoietic-specific member of the ETS family that is required for the development of lymphoid and myeloid lineages (10). However, PU.1 interacts directly with GATA-1 and blocks terminal erythroid differentiation of murine erythroleukemia (MEL) cells by repression of GATA-1-mediated transcriptional activation (11). The transcription factor erythroid Kruppel-like factor (EKLF/KLF1) plays a crucial function during erythropoiesis (12). EKLF/KLF1 and KLF2 bind the c-Myc (Myc) promoter and regulate expression of the Myc gene (13). Down-regulation of Myc expression at the late stage of erythropoiesis is essential for terminal erythroid maturation (14). GATA-1 represses Myc and c-Myb (Myb) expression during erythroid differentiation (15, 16). As described above, erythropoiesis is predominantly regulated by Epo stimulation and by transcriptional control with the development-specific transcription factor GATA-1.
Friend leukemia integration 1 (FLI-1) is one of the well known regulators of erythropoiesis. FLI-1, a member of the ETS family of transcription factors that was originally identified in MEL cells, has a role in erythroleukemia induction (17). FLI-1 is also expressed in normal hematopoietic cells and suppresses erythroid differentiation (18). However, FLI-1 activates megakaryocytic differentiation (19). Both FLI-1 and EKLF/KLF1 bind to GATA-1 and are functionally antagonistic to the activation of megakaryocytic and erythrocytic gene promoters (20). Moreover, they control megakaryocytic and erythrocytic differentiation of MEPs. A recent study showed that microRNA-145 (miR-145) represses Fli-1 (21). That study also revealed the effects of miR-145 on megakaryocytic and erythroid differentiation. This finding indicates the existence of a novel differentiation regulator in addition to existing transcription factors, cytokines, and cytokine receptors. Thus, the overall view of erythroid differentiation is not yet clear.
MEL and human erythroleukemia K562 (K562) cells are widely used for the study of erythroid differentiation. MEL cells, which were isolated from Friend virus-infected mice, provide a model system for the study of erythroblast differentiation and leukemogenesis (22). The addition of chemicals, such as dimethyl sulfoxide (DMSO), hexamethylene bisacetamide (HMBA), and trichostatin A (TSA), is known to induce differentiation of MEL cells to erythroblasts that highly express hemoglobin (23–25). These chemicals act as initiators of the synthesis of β-globin and other erythroid-specific proteins (24). Expression of Myc and Myb genes are down-regulated during MEL cell differentiation, and overexpression of these genes blocks differentiation (26, 27). Furthermore, DMSO-resistant MEL cell clones were isolated and used for the study of erythroid differentiation (28, 29). K562 cells were isolated from the blood of patients with chronic myelogenous leukemia. Sodium butyrate and hemin also induce erythroid differentiation of K562 cells (30–32). In a phenotypic analysis of K562 cells, the rate of formation of transferrin receptor (CD71)/glycophorin A-positive cells was increased by hemin-mediated induction of differentiation (33). During erythroid differentiation, CD71 is highly expressed in the pre-proerythroblast and the erythroblasts that follow, whereas glycophorin A expression is delayed relative to CD71 and correlates with the transition from the pro-erythroblast to the basophilic normoblast (34). Although MEL and K562 cells are erythroleukemia cells, these cells are useful models for the study of erythroid differentiation because they can be induced to differentiate like normal erythroid cells.
We here used DNA microarrays to screen for erythroid differentiation-related genes to identify novel regulators of hemoglobin production and erythroid differentiation. We compared the expression profile of high differentiation-inducible (HD) and low differentiation-inducible (LD) MEL cells during differentiation induced by three different chemicals. We selected the consistently down-regulated genes in HD MEL cells as candidate differentiation suppressors and then overexpressed these genes in HD MEL cells to analyze for inhibition of hemoglobin production. We found that overexpression of α-1,6-fucosyltransferase (Fut8) inhibited hemoglobin production in the MEL cell. We then performed functional analysis of FUT8 to understand the mechanism of suppression of hemoglobin production and erythroid differentiation, as observed in MEL and K562 cells.
EXPERIMENTAL PROCEDURES
Cell Culture
MEL cells (T-3-Cl-2–0.fl) (35) and K562 cells were cultured in RPMI 1640 medium (Nissui Pharmaceutical) with 10% fetal calf serum (Sigma-Aldrich) at 37 °C with 5% CO2. Both cell lines were provided by RIKEN BioResource Center (Ibaraki, Japan). HD and LD MEL cells were obtained by recloning the original MEL cells. Erythroid differentiation was determined by benzidine staining. PLAT-E cells (36) and PLAT-A cells were cultured in Dulbecco's modified Eagle's media (Nissui Pharmaceutical) with 10% fetal calf serum (Sigma-Aldrich) at 37 °C with 5% CO2. PLAT-E and PLAT-A cells were kindly provided by Dr. Kitamura (University of Tokyo, Japan).
Microarray Experiments
The cDNA microarray was prepared in our laboratory using NIA mouse 15k and 7.4k cDNA clone sets (37–39). These cDNA clone sets were provided by Riken BioResource Center. Microarray preparation, RNA labeling, and hybridization procedures are described in the supplemental material.
For comparison of HD and LD MEL cell expression profiles, HD and LD MEL cells were cultured for 6, 12, 24, and 36 h with 1.0% DMSO, 3.0 mm HMBA, or 15 nm TSA, respectively. For expression analysis of HD MEL cells during differentiation, HD MEL cells were cultured for 1, 6, 12, 24, and 36 h with 1.5% DMSO, 5.0 mm HMBA, or 30 nm TSA, respectively. Total RNA from MEL cells was extracted using the RNeasy mini kit (Qiagen).
Hybridization images were scanned using a ScanArray 5000 (PerkinElmer Life Sciences). The fluorescence intensity of the cDNA microarray was quantified using QuantArray 3.0 software (PerkinElmer Life Sciences). The cDNA microarray data were analyzed using GeneSpring 6.0 (Silicon Genetics). Intensity differences between Cy3 and Cy5 were normalized by the LOWESS method. The expression ratio for each time experiment is expressed as the average of quadruplicate experiments, including two dye swaps. The complete microarray data were archived at the National Center for Biotechnology Information Gene Expression Omnibus (accession numbers GSE40754 and GSE43849) (www.ncbi.nlm.nih.gov).
Generation of Targeted Genes Overexpressing Cells
Total RNA of MEL and K562 cells was extracted using the RNeasy mini kit. cDNA was synthesized from the total RNA using SuperScript III reverse transcriptase (Invitrogen). The targeted genes were amplified by PCR using KOD Plus (Toyobo); the primers are described in the supplemental material. The PCR products were purified by isopropyl alcohol precipitation and restricted using restriction enzymes as described in the supplemental material. The DNA fragments were purified using the Wizard SV Gel and PCR Clean-Up System (Promega). The purified DNA fragments of the targeted genes were cloned into the vector pMXs-CMVp-IRES-EGFP that contains an enhancer of the cytomegalovirus promoter in the 5′-long terminal repeat sequence. This vector is a modification of the vector pMXs-IRES-EGFP (40) that was kindly provided by Dr. Kitamura. The cloned vectors were introduced into PLAT-E or PLAT-A cells using Lipofectamine 2000 (Invitrogen), and culture supernatants containing virus were collected 48 h after transfection. MEL and K562 cells were grown in the culture supernatant supplemented with 0.08–4 μg/ml Polybrene to aid infection. The infected cells were cloned using the limiting dilution method, and targeted gene overexpression was verified by measuring of the expression of enhanced green fluorescent protein using FACSCalibur (BD Biosciences).
Site-directed Mutagenesis
Site-directed mutagenesis of mouse Fut8 and human FUT8 was performed with Splicing by Overlap Extension PCR. The primer sequences are listed in the supplemental material. Additional procedures are the same as described above.
Western Blotting
FUT8 was detected in Western blotting using goat anti-FucT-VIII antibody (S-17; catalog no. sc-34629, Santa Cruz Biotechnology) and HRP-conjugated rabbit anti-goat antibody (catalog no. 61-1620, Zymed Laboratories Inc.). GAPDH was detected with mouse anti-GAPDH antibody (clone 6C5; catalog no. AM4300, Ambion) and HRP-conjugated goat anti-mouse antibody (catalog no. 62-6520, Zymed Laboratories Inc.). The experimental conditions were different between overexpression and knockdown studies. The experimental details are described in the supplemental material.
Reverse Transcription (RT-)PCR
Total RNA from MEL and K562 cells was extracted using the RNeasy mini kit. cDNA was synthesized from the total RNA using SuperScript III reverse transcriptase. PCR was performed using GoTaq Flexi DNA polymerase (Promega). The primers for RT-PCR analysis are described in the supplemental material.
Transient Transfection of FUT8 Short Hairpin RNA (shRNA)
shRNAs for FUT8 were designed by siDirect. shRNA sequences are described in the supplemental material. Forward and reverse oligonucleotides were denatured for 5 min at 95 °C and annealed for 1 h at 37 °C. The annealed, double-stranded oligonucleotide was ligated into the piGENE hU6 puro vector (iGENE Therapeutics) that had been restricted with BspMI. The plasmids containing the shRNA constructs were then restricted with EcoRI and BamHI, and the DNA containing both the shRNA and hU6 promoter sequence was introduced into the EcoRI and BamHI-digested piGENE U6 Rep vector (iGENE Therapeutics) that included EBNA1 genes and OriP sequence. The piGENE U6 Rep T7STOP vector (iGENE Therapeutics) was used as a negative control vector. These vectors were transfected into K562 cells by electroporation using Bio-Rad Gene Pulser II (Bio-Rad). The transfected cells were cultured with 2 μg/ml puromycin (Sigma-Aldrich) for 2 weeks.
Flow Cytometric Analysis
Flow cytometric analysis was performed using FACSCalibur. The cells were stained with anti-human CD235a (glycophorin A) FITC (catalog no. 11-9987; eBioscience) and anti-human CD71 (transferrin receptor) PE (catalog no. 12-0719; eBioscience).
RESULTS
Comparison of HD and LD MEL Cell Expression Profiles using cDNA Microarray
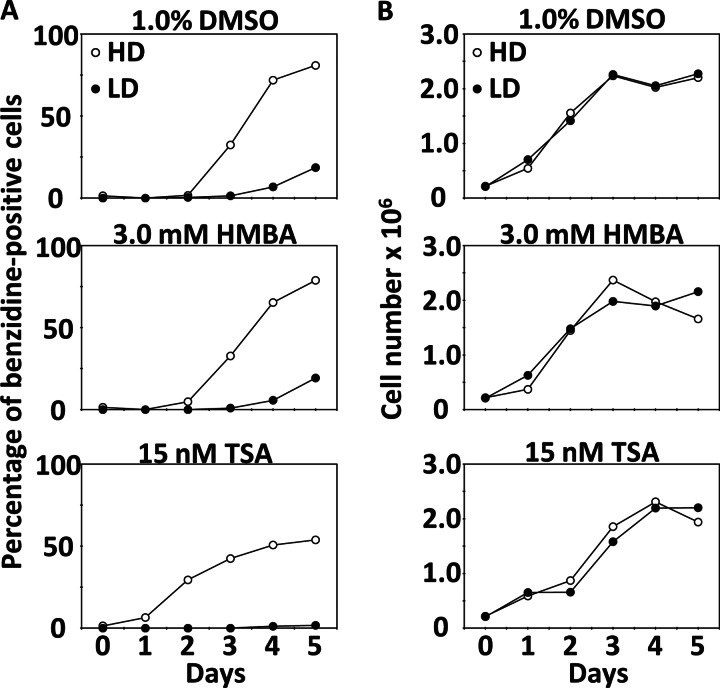
We screened for differentiation-related genes in MEL cells using cDNA microarray analysis. Prior to microarray analysis, we selected clones of HD and LD MEL cells by recloning from the original cells. HD MEL cells were more highly differentiated than LD MEL cells by treatment with 1.0% DMSO, 3.0 mm HMBA, or 15 nm TSA (Fig. 1A). The concentrations of each chemical were reduced for comparable microarray analysis, because general concentrations, 1.5% DMSO, 5.0 mm HMBA, or 30 nm TSA, were affecting the growth of LD MEL cells (data not shown). The rate of cell differentiation was determined by benzidine staining of hemoglobin. There were no differences in cell growth between HD and LD cells in chemical treatment (Fig. 1B). Next, we compared the expression profiles of HD and LD MEL cells during differentiation induced by 1.0% DMSO, 3.0 mm HMBA, or 15 nm TSA using a cDNA microarray that was prepared from NIA mouse 15k and 7.4k cDNA clone sets. We compared gene expression profiles at 6, 12, 24, and 36 h after chemical treatment. In this microarray study, we defined up-regulated and down-regulated genes of expression ratio as >1.5 and <0.66, respectively. Table 1 lists the genes from HD MEL cells that are consistently and significantly (p < 0.05) up-regulated or down-regulated at more than one time point by treatment with each of the three chemicals. These genes were considered as candidates of differentiation-related genes. We considered that not all of differentiation-related genes were continuously up-regulated or down-regulated during differentiation. In MEL cells differentiation, c-Myc mRNA decreases at 2–12 h after DMSO treatment, but it increases to pretreatment levels at 18 h (41).
FIGURE 1.
Hemoglobin-positive ratios and growth of HD and LD MEL cells after treatment with 1.0% DMSO, 3.0 mm HMBA, or 15 nm TSA. A, hemoglobin-positive ratio was measured with benzidine stain each day for 5 days after chemical treatment. B, daily cell counts/ml in culture.
TABLE 1.
Difference in gene expression between HD and LD MEL cells after drug treatment
Microarray results were filtered at an expression ratio of >1.5 or <0.66, p < 0.05, at more than one time point, and genes consistently up-regulated or down-regulated among the three chemical treatments were selected. The expression ratios are the average of quadruplicate experiments.
| Clone name | Gene symbol | Expression ratio (HD MEL cells/LD MEL cells) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO |
HMBA |
TSA |
|||||||||||
| 6 h | 12 h | 24 h | 36 h | 6 h | 12 h | 24 h | 36 h | 6 h | 12 h | 24 h | 36 h | ||
| H3038C06 | Zfp951 | 0.48 | 0.38 | 0.39 | 0.24 | 0.42 | 0.53 | 0.42 | 0.25 | 0.62 | 0.64 | 0.48 | 0.45 |
| H3078E08 | unknown | 0.49 | 0.39 | 0.43 | 0.26 | 0.47 | 0.53 | 0.44 | 0.26 | 0.60 | 0.64 | 0.50 | 0.43 |
| H3049A07 | G430049J08Rik | 0.53 | 0.48 | 0.47 | 0.33 | 0.46 | 0.50 | 0.41 | 0.26 | 0.64 | 0.63 | 0.38 | 0.35 |
| H4070E06 | Unknown | 0.50 | 0.44 | 0.36 | 0.22 | 0.48 | 0.54 | 0.43 | 0.21 | 0.58 | 0.64 | 0.52 | 0.59 |
| H3062A11 | Clip2 | 0.59 | 0.46 | 0.51 | 0.33 | 0.45 | 0.51 | 0.47 | 0.29 | 0.76 | 0.69 | 0.46 | 0.41 |
| H4016D11 | Unknown | 0.59 | 0.55 | 0.46 | 0.34 | 0.55 | 0.61 | 0.49 | 0.27 | 0.60 | 0.59 | 0.46 | 0.49 |
| H3061H10 | Tmem123 | 0.66 | 0.62 | 0.73 | 0.48 | 0.47 | 0.61 | 0.45 | 0.27 | 0.67 | 0.61 | 0.44 | 0.36 |
| H3104H09 | Spc24 | 0.60 | 0.50 | 0.51 | 0.48 | 0.49 | 0.56 | 0.55 | 0.35 | 0.64 | 0.70 | 0.54 | 0.50 |
| H3073G11 | Rtp3 | 0.62 | 0.58 | 0.63 | 0.39 | 0.49 | 0.66 | 0.48 | 0.28 | 0.77 | 0.63 | 0.48 | 0.51 |
| H3078A01 | G430049J08Rik | 0.62 | 0.56 | 0.68 | 0.38 | 0.56 | 0.58 | 0.53 | 0.28 | 0.66 | 0.65 | 0.53 | 0.56 |
| H3046C10 | Idi1 | 0.68 | 0.56 | 0.95 | 0.61 | 0.50 | 0.57 | 0.80 | 0.61 | 0.50 | 0.49 | 0.53 | 0.56 |
| H4046D12 | Plek | 0.59 | 0.58 | 0.84 | 0.63 | 0.52 | 0.46 | 0.76 | 0.44 | 0.53 | 0.57 | 0.68 | 0.86 |
| H3037D02 | G430049J08Rik | 0.74 | 0.90 | 0.62 | 0.62 | 0.75 | 0.60 | 0.55 | 0.45 | 0.66 | 0.66 | 0.49 | 0.51 |
| H3078G11 | Drd3 | 0.76 | 0.85 | 0.94 | 0.58 | 0.63 | 0.66 | 0.59 | 0.32 | 0.75 | 0.68 | 0.49 | 0.54 |
| H4019B10 | Fut8 | 0.78 | 0.65 | 0.81 | 0.37 | 0.63 | 0.58 | 0.62 | 0.31 | 0.60 | 0.66 | 0.87 | 0.95 |
| H3078G10 | Rtp3 | 0.78 | 0.79 | 0.81 | 0.59 | 0.68 | 0.75 | 0.72 | 0.31 | 0.69 | 0.72 | 0.57 | 0.56 |
| H4073E05 | Rnpc2 | 0.72 | 0.77 | 0.89 | 0.66 | 0.46 | 0.71 | 0.85 | 0.57 | 0.72 | 0.93 | 0.55 | 0.48 |
| H3033F11 | Cst3 | 0.65 | 0.83 | 0.80 | 0.60 | 0.63 | 0.65 | 0.66 | 0.65 | 0.65 | 0.73 | 0.75 | 0.78 |
| H4040B02 | Gstt3 | 0.76 | 0.90 | 1.18 | 0.47 | 0.80 | 0.70 | 0.67 | 0.48 | 0.98 | 0.66 | 0.59 | 0.59 |
| H3138C09 | Rnf128 | 0.91 | 0.69 | 0.78 | 0.62 | 0.84 | 0.66 | 1.02 | 0.75 | 0.96 | 1.03 | 0.74 | 0.62 |
| H3032F06 | Hbb-y/Hbb-b1 | 0.61 | 0.69 | 0.79 | 0.92 | 0.57 | 0.68 | 1.14 | 1.54 | 0.62 | 0.60 | 0.69 | 0.96 |
| H4003H10 | Spn | 0.80 | 0.82 | 1.14 | 0.62 | 0.88 | 0.90 | 1.00 | 0.66 | 0.65 | 0.64 | 0.84 | 0.98 |
| H3113C01 | Hbb-y/Hbb-b1 | 0.69 | 0.59 | 0.87 | 1.07 | 0.68 | 0.75 | 1.39 | 1.59 | 0.60 | 0.68 | 0.86 | 0.96 |
| H3119H11 | Wdfy3 | 0.83 | 0.84 | 2.03 | 0.83 | 1.03 | 0.94 | 0.77 | 0.64 | 0.59 | 0.74 | 0.69 | 0.81 |
| H4020E10 | Il4ra | 0.95 | 0.89 | 0.90 | 0.60 | 1.06 | 1.00 | 1.00 | 0.54 | 0.88 | 1.17 | 1.33 | 1.66 |
| H3028F05 | Odc1 | 1.06 | 1.13 | 1.51 | 1.17 | 0.92 | 1.30 | 1.09 | 1.59 | 0.99 | 1.01 | 1.24 | 1.76 |
| H4062C02 | Mcm4 | 0.92 | 1.09 | 1.57 | 1.13 | 1.04 | 1.08 | 1.44 | 1.50 | 1.04 | 1.15 | 1.50 | 1.67 |
| H3024H12 | Hspa8 | 0.93 | 1.06 | 1.59 | 1.19 | 0.87 | 1.08 | 1.67 | 1.37 | 0.95 | 1.09 | 1.48 | 2.02 |
| H4006F09 | Hat1 | 1.12 | 0.94 | 1.68 | 1.27 | 1.08 | 1.12 | 1.69 | 1.38 | 1.01 | 1.55 | 1.30 | 1.61 |
| H3006C07 | Unknown | 1.23 | 1.24 | 1.50 | 1.16 | 1.24 | 1.65 | 1.37 | 1.57 | 0.96 | 1.06 | 1.39 | 1.80 |
| H4003E09 | Car2 | 1.05 | 1.15 | 1.63 | 1.34 | 1.01 | 1.08 | 1.82 | 1.65 | 0.87 | 0.90 | 1.79 | 2.19 |
| H3130A05 | Psmd2 | 1.26 | 1.20 | 1.67 | 1.44 | 1.19 | 1.17 | 1.85 | 1.50 | 1.27 | 1.11 | 1.48 | 2.10 |
| H3018A08 | Cct8 | 1.40 | 1.30 | 1.75 | 1.40 | 1.24 | 1.52 | 1.89 | 1.49 | 1.37 | 1.42 | 1.34 | 1.69 |
| H4034F12 | Fbxo45 | 1.33 | 1.28 | 2.14 | 1.21 | 1.22 | 1.43 | 1.65 | 1.30 | 1.30 | 1.63 | 1.39 | 2.09 |
| H3093D05 | Trfr | 1.26 | 1.42 | 1.77 | 1.51 | 1.30 | 1.28 | 1.72 | 2.06 | 1.23 | 1.17 | 1.62 | 1.68 |
| H3123F09 | Guf1 | 0.98 | 1.12 | 0.96 | 1.60 | 1.25 | 1.11 | 1.23 | 2.41 | 0.93 | 1.00 | 1.59 | 4.07 |
| H3122H09 | Hba-a1 | 0.97 | 1.19 | 1.10 | 1.69 | 1.21 | 1.07 | 1.37 | 2.85 | 1.13 | 1.01 | 1.44 | 3.50 |
| H3126A10 | Hba-a1 | 1.03 | 1.13 | 1.06 | 1.59 | 1.11 | 1.10 | 1.36 | 2.93 | 1.21 | 1.09 | 1.51 | 3.55 |
| H3026B04 | Ppat | 1.84 | 1.49 | 1.81 | 1.65 | 1.37 | 1.47 | 1.78 | 1.76 | 1.21 | 1.65 | 1.22 | 1.63 |
| H3126G09 | Hba-a1 | 1.00 | 1.08 | 1.05 | 2.13 | 1.18 | 0.95 | 1.35 | 3.39 | 1.05 | 1.06 | 1.34 | 3.65 |
| H3125H07 | Hba-a1 | 0.97 | 1.08 | 1.11 | 1.99 | 1.23 | 1.15 | 1.55 | 3.45 | 0.90 | 1.10 | 1.62 | 3.78 |
| H3121B01 | Hba-a1 | 1.04 | 1.23 | 1.31 | 2.05 | 1.43 | 1.11 | 1.63 | 3.81 | 1.06 | 1.08 | 1.68 | 3.97 |
| H3124F12 | unknown | 0.99 | 0.99 | 1.25 | 1.64 | 1.26 | 1.07 | 1.42 | 4.07 | 1.29 | 1.17 | 1.79 | 4.58 |
| H3122H11 | Hba-a1 | 1.02 | 1.20 | 1.29 | 2.03 | 1.25 | 1.30 | 1.40 | 4.25 | 1.17 | 1.03 | 1.85 | 4.27 |
| H3045A12 | Hba-a1 | 1.03 | 1.16 | 1.59 | 2.09 | 1.43 | 1.16 | 1.81 | 4.45 | 1.19 | 1.31 | 2.30 | 5.50 |
| H3123A01 | Hba-a1 | 1.06 | 1.40 | 1.90 | 2.73 | 1.25 | 1.32 | 2.27 | 5.18 | 1.35 | 1.01 | 1.64 | 4.09 |
| H3083F09 | Phtf1,Rsbn1 | 1.10 | 1.09 | 1.58 | 1.87 | 1.47 | 1.16 | 2.16 | 4.91 | 1.20 | 1.47 | 2.47 | 5.70 |
| H3123E05 | Hba-a1 | 1.34 | 1.30 | 1.90 | 2.72 | 1.51 | 1.49 | 2.33 | 5.70 | 1.42 | 1.22 | 2.02 | 4.85 |
Fut8 Overexpression Inhibits Hemoglobin Production in MEL Cells
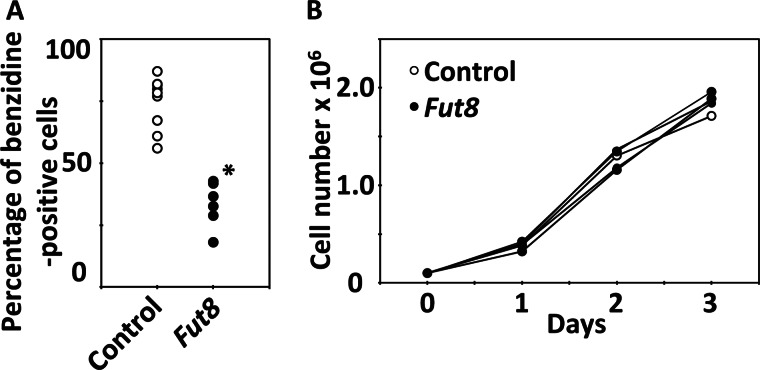
We investigated an association between the down-regulated genes and hemoglobin production in an overexpression study. Idi1, Plek, Fut8, Rnpc2, Cst3, Gstt3, and Spn were selected as candidate differentiation suppressor genes. Because these genes were strongly down-regulated in HD MEL cells during chemically induced differentiation, annotations on these cDNA clones were available at that time. Each gene was introduced into HD MEL cells using the retroviral vector pMXs-CMVp-IRES-EGFP, thereby constructing MEL cell lines in which the candidate gene was overexpressed. After cell cloning, we measured the hemoglobin-positive ratio of the overexpressing cells 4 days after 1.5% DMSO treatment. We observed that the rate of appearance of benzidine-positive cells in clones overexpressing Fut8 was significantly (p < 0.01) less than that in control cell clones that contained the empty vector (Fig. 2A). There were no differences in cell growth between clones overexpressing Fut8 and control cell clones (Fig. 2B). These data indicate that the low hemoglobin-positive rate of MEL cells overexpressing Fut8 was caused by Fut8-mediated differential suppression of hemoglobin production and not by differential suppression of growth. Overexpression of the other genes did not show statistically significant differential suppression of hemoglobin production in MEL cells (data not shown).
FIGURE 2.
Hemoglobin-positive ratios and growth of MEL cells overexpressing Fut8. A, hemoglobin-positive ratios of MEL cells containing the empty vector (control), and Fut8 overexpression 4 days after 1.5% DMSO treatment. Individual circles mean different cell clone; there were nine control cell clones, and six Fut8-overexpressing clones were analyzed. The asterisk indicates statistically significant difference (p < 0.01). B, daily cell counts/ml in culture without DMSO. One control clone and five Fut8-overexpressing clones were analyzed.
Fut8 Expression Is Down-regulated during MEL Cell Differentiation
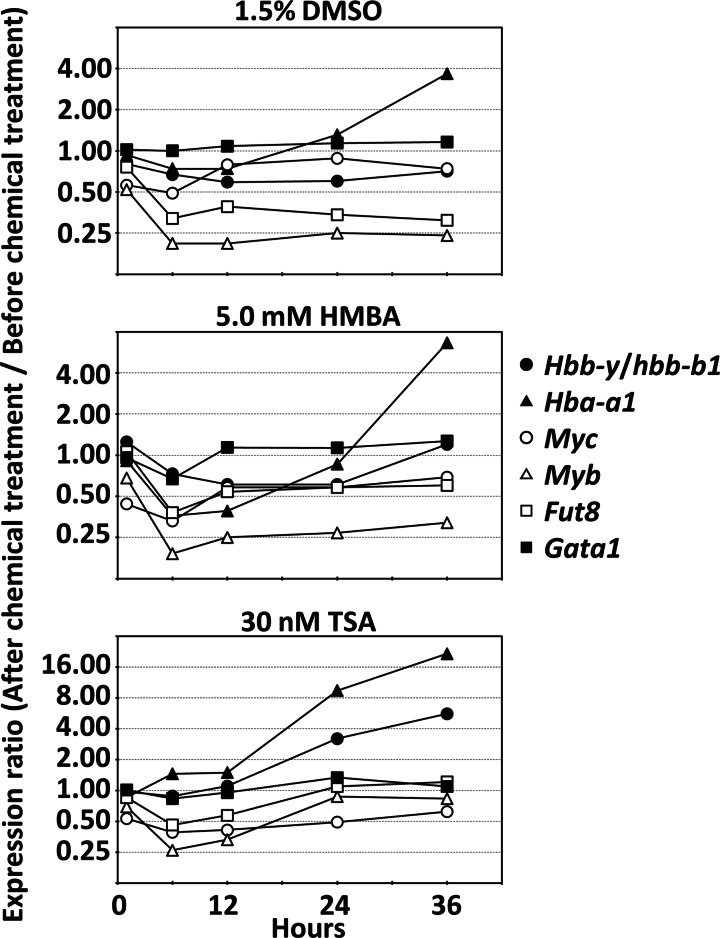
We analyzed the expression profile of Fut8 and other erythroid differentiation-related genes during chemically induced differentiation of HD MEL cells by another microarray study. We compared gene expression before and after chemical treatment. Fig. 3 shows expression profiles of representative cDNA clones of erythroid differentiation-related genes and Fut8. Fut8 expression was rapidly down-regulated after 1.5% DMSO, 5.0 mm HMBA, or 30 nm TSA treatments, the same as Myc and Myb that were known as suppressors of erythroid differentiation. However, hemoglobin genes (Hba-a1 and Hbb-y/Hbb-b1) were up-regulated during MEL cell differentiation. Especially, up-regulation of hemoglobin genes with TSA treatment was faster than DMSO or HMBA treatment. In the same conditions, Fut8 decreased at 6–12 h, but it increased to before TSA treatment levels at 24 h. These data indicate that temporary down-regulation of Fut8 at an early time point is important for hemoglobin expression, and continuous down-regulation of Fut8 is not necessary for hemoglobin expression. Significant expression change of Gata-1 was not detected. The expression profile indicates that Fut8 expression is well correlated to Myb expression among the three chemical treatments.
FIGURE 3.
Expression profile of Fut8 and other erythroid differentiation-related genes during HD MEL cells differentiation with 1.5% DMSO, 5.0 mm HMBA, or 30 nm TSA treatment. The expression ratios are the average of quadruplicate experiments.
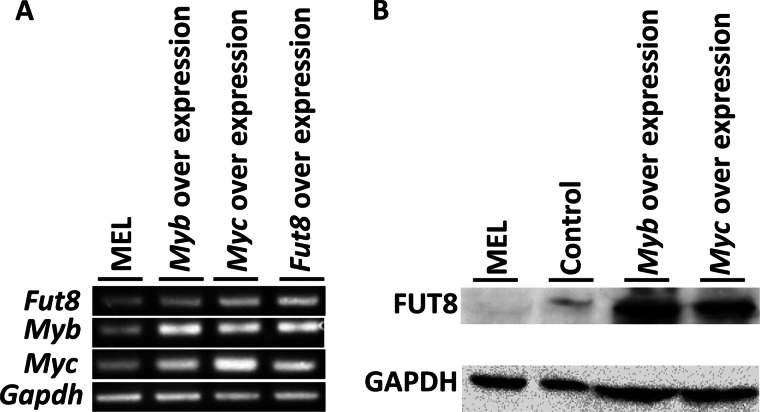
Overexpression of Myc and Myb Increase Fut8 Expression
We investigated whether Myc and Myb were effect on Fut8 expression. We constructed Myc- and Myb-overexpressing MEL cell clones and measured Fut8 expression by RT-PCR and Western blotting. As a result, Fut8 was more highly expressed in Myc- and Myb-overexpressing MEL cells than in normal MEL cells and empty vector introduced cells (Fig. 4, A and B). This result indicates that Fut8 expression was positively regulated by Myc and Myb.
FIGURE 4.
Effect of Myb and Myc for Fut8 expression. A, RT-PCR analysis of Fut8, Myb, and Myc overexpression in MEL cells. B, Western blot analysis of FUT8 in Myb- and Myc-overexpressing MEL cells.
Fut8 Is the Only Suppressor of Hemoglobin Production among the Fut Gene Family
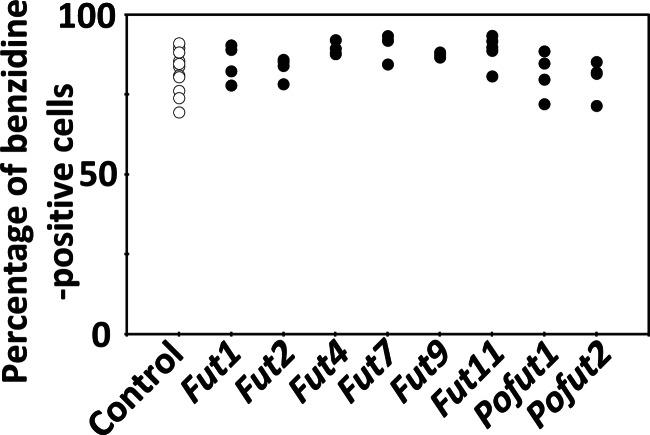
We also performed overexpression studies of other Fut family genes to determine that suppression of hemoglobin production was caused not by general function of fucosyltransferase but by the unique function of FUT8. We cloned the Fut1, Fut2, Fut4, Fut7, Fut9, Fut11, Pofut1, and Pofut2 genes into the same vector, and these genes were overexpressed in HD MEL cells as described for Fut8 above. These overexpression clones were treated with 1.5% DMSO for 4 days, and the rates of appearance of benzidine-positive cells were determined. However, these clones did not show statistically significant differential suppression of hemoglobin production compared with control cell clones that contained the empty vector (Fig. 5). These results indicate that Fut8 is the only fucosyltransferase that is capable of inhibition of hemoglobin production in MEL cell.
FIGURE 5.
Hemoglobin-positive ratios of MEL cells overexpressing Fut family genes. Hemoglobin-positive ratio of MEL cells containing the empty vector (control) or overexpressing Fut family genes 4 days after 1.5% DMSO treatment. Individual circles denote different cell clones. The following were analyzed: 12 control cell clones; four clones overexpressing Fut1; four clones overexpressing Fut2; four clones overexpressing Fut4; four clones overexpressing Fut7; three clones overexpressing Fut9; five clones overexpressing Fut11; four clones overexpressing Pofut1, and four clones overexpressing Pofut2.
FUT8-mediated Core Fucosylation Is Essential for Hemoglobin Production
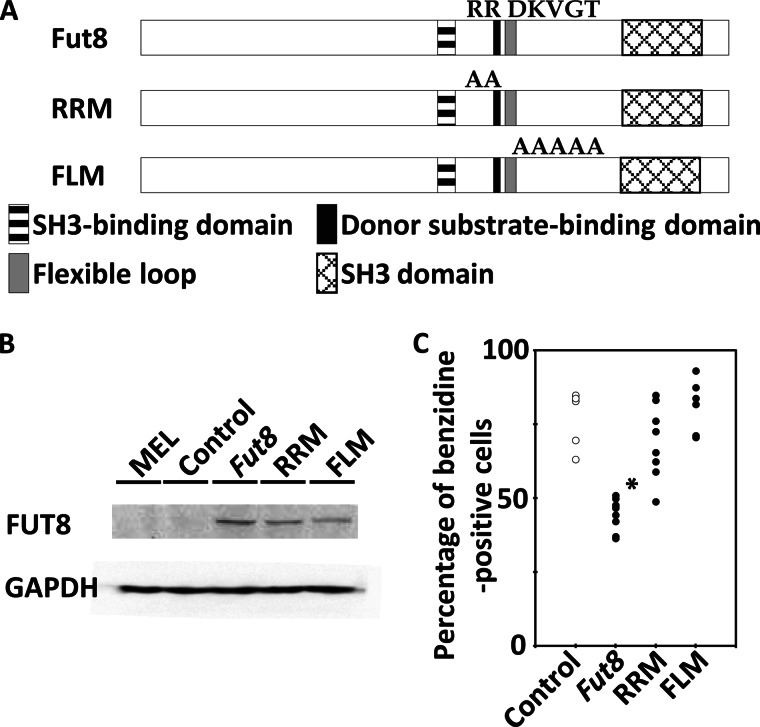
We next investigated whether the FUT8-mediated differential suppression of hemoglobin production could be cancelled by mutation of FUT8 functional domains. The structure of FUT8 has an SH3 domain, an SH3-binding domain, a donor substrate-binding domain, and a flexible loop. Arg-365 and Arg-366 are essential for substrate binding (42), and the flexible loop, which includes Asp-368 and Lys-369, is also essential for enzyme activity (43). On the basis of these findings, we constructed FUT8 mutants of the donor substrate-binding domain (the R365A/R366A double mutant (RRM)) and the flexible loop (the D368A/K369A/V370A/G371A/T372A quintuple mutant (FLM)) and introduced them into MEL cells (Fig. 6A). Both FUT8 mutant proteins were overexpressed in MEL cells (Fig. 6B). We observed that introduction of the RRM or FLM mutations into FUT8 cancelled the FUT8-mediated differential inhibition of hemoglobin production in MEL cells (Fig. 6C). These results strongly indicate that FUT8-mediated core fucosylation is associated with hemoglobin production in MEL cells.
FIGURE 6.
Hemoglobin-positive ratios of MEL cells with overexpression of Fut8 mutant. A, constructs of FUT8 mutant. RRM means R365A/R366A mutant of FUT8. FLM means flexible loop (D368A/K369A/V370A/G371A/T372A) mutant of FUT8. B, Western blot analysis of overexpression of FUT8 and FUT8 mutant. C, hemoglobin-positive ratio of MEL cells with the empty vector (control), overexpression of Fut8 and Fut8 mutants 4 days after 1.5% DMSO treatment. Individual circles indicate different cell clone. Four control cell clones, nine clones overexpressing Fut8, eight clones overexpressing RRM, and six clones overexpressing FLM were analyzed. The asterisk indicates statistically significant difference (p < 0.01).
FUT8 Overexpression Inhibits Hemoglobin Production in K562 Cells
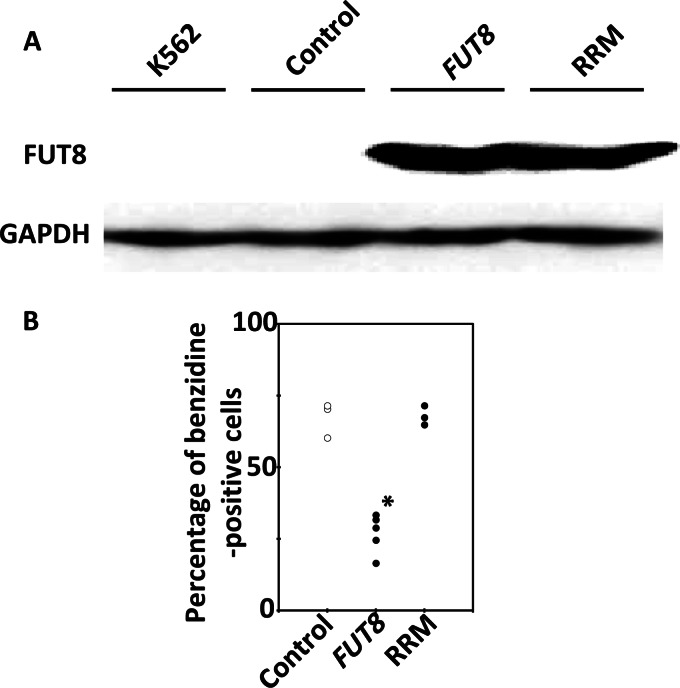
We next investigated whether the results with MEL cells were consistent between species. We investigated whether FUT8 functions as an inhibitor of hemoglobin production in humans using K562 cells. We constructed overexpression clones containing wild-type FUT8 or its donor substrate-binding domain mutant RRM (Fig. 7A). Differentiation was induced in these clones by treatment with 100 μm hemin, and the rate of appearance of benzidine-positive cells was determined 4 days after treatment. We observed that FUT8 overexpression also inhibited hemoglobin production during hemin-inducible differentiation of K562 cells (Fig. 7B). The RRM mutant did not inhibit hemoglobin production. This result indicates that FUT8 is also involved in the inhibition of hemoglobin production of human cells.
FIGURE 7.
Hemoglobin-positive ratios of K562 cells overexpressing FUT8 and FUT8 mutants. A, Western blot analysis of FUT8 and FUT8 mutant overexpression. B, hemoglobin-positive ratios of K562 cells containing the empty vector (control), overexpressing FUT8, and overexpressing FUT8 mutant 4 days after 100 μm hemin treatment. Individual circles denote different cell clones. Three control cell clones, five clones overexpressing FUT8, and three clones overexpressing RRM were analyzed. The asterisk indicates statistically significant difference (p < 0.01).
shRNA-mediated FUT8 Knockdown Promotes Hemoglobin Production and Differentiation of K562 Cells
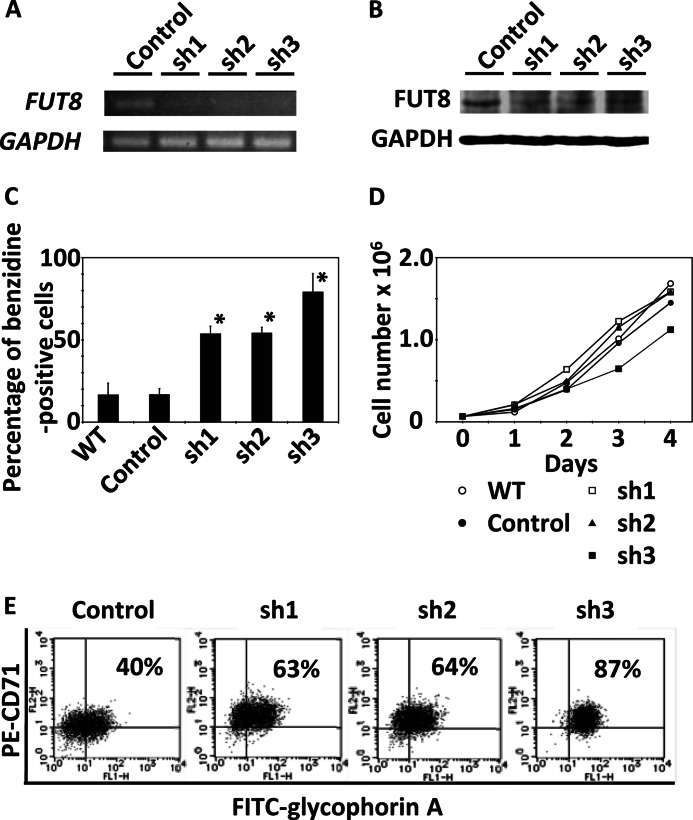
We examined the effect of FUT8 knockdown using shRNA constructs in K562 cells. Three different FUT8 shRNA sequences were designed and transiently introduced into K562 cells. The cells containing each shRNA construct were selected with puromycin for 2 weeks after transfection, and then the decreases in FUT8 mRNA and protein expression were confirmed by RT-PCR and Western blotting, respectively (Fig. 8A and B). Using benzidine staining, we observed that the hemoglobin-positive rates of the cells into which shRNA constructs had been introduced were higher than cells into which a negative control vector had been introduced (Fig. 8C). The introduction of shRNA constructs did not affect cell growth, except for SH3, which may be related to the high differentiation ratio of cells into which the SH3 construct had been introduced (Fig. 8D). Furthermore, we performed flow cytometric analysis of K562 cells using glycophorin A and CD71 as markers of erythroblast differentiation. Flow cytometric analysis showed that the rate of appearance of glycophorin A- and CD71-positive cells was increased in cells into which shRNA constructs had been introduced (Fig. 8E). The increase in the rate of coexpression of these markers indicates that FUT8 regulates not only hemoglobin production but also the differentiation of pre-erythroblasts to basophilic normoblasts and polychromatic erythroblasts.
FIGURE 8.
shRNA-mediated FUT8 knockdown in K562 cells. Negative control vector (control) or FUT8 shRNA constructs (sh1, sh2, and sh3) were transfected into K562 cells by electroporation. The cells containing each construct were selected with puromycin for 2 weeks after transfection, and then these cells and nontransfected K562 cells (WT) were analyzed. A, RT-PCR analysis of FUT8 expression. B, Western blot analysis of FUT8 expression. C, hemoglobin-positive ratios of K562 cells. The mean values and S.D. bars for triplicate experiments are shown. The asterisk indicates statistically significant difference (p < 0.01). D, daily cell counts/ml in culture. E, flow cytometric analysis of CD71 and glycophorin A.
DISCUSSION
DNA microarray is a powerful tool to understand biological phenomena regulated by multiple genes, such as cell differentiation, because it can comprehensively analyze gene expression at one time point. However, it also detects the noise of gene expression, such as responses to the stresses of chemical addition and cell proliferation that occur during chemical induction of the differentiation of cell lines. In this study, we compensated for the shortcomings of our microarray experiments by comparing the expression profiles of HD and LD MEL cells. The only difference between these cells was their ability to differentiate in response to chemicals that induce cell differentiation. We also effectively selected for differentiation-related genes by focusing on the genes consistently regulated among the three chemical inducers.
Next, we evaluated the direct relationship between the targeted genes and differentiation by gain-of-function, which was performed using an overexpression study. We observed that Fut8 overexpression inhibited hemoglobin production during MEL cell differentiation; the same effect was observed in human K562 cells.
Fut8 is a member of the fucosyltransferase gene family. Fucosyltransferases are involved in various biological and pathological processes in eukaryotic organisms, including tissue development, angiogenesis, fertilization, cell adhesion, inflammation, and tumor metastasis (44). There are two main types of fucosyltransferases in the mouse. One of the main types of fucosyltransferases catalyzes fucosylation of N-linked glycans at asparagine residues. FUT1 and FUT2 transfer fucose to galactose in an α-1,2 linkage. FUT3 and FUT5 transfer fucose to N-acetylglucosamine in an α-1,3 or α-1,4 linkage. FUT4, FUT6, FUT7, FUT9, FUT10, and FUT11 transfer fucose to N-acetylglucosamine in an α-1,3 linkage. Finally, FUT8 transfers fucose to N-acetylglucosamine in an α-1,6 linkage. Another main type of fucosyltransferase in mice catalyzes fucosylation of O-linked glycans at serine or threonine residues. There are two O-fucosyltransferases, POFUT1 and POFUT2. In this study, FUT8 was the only fucosyltransferase that showed inhibition of hemoglobin production. This indicates that core fucosylation plays a key role in hemoglobin production and cell differentiation because FUT8 is the only fucosyltransferase that adds fucose to the core asparagine-linked glycans.
To evaluate whether FUT8-mediated core fucosylation directly affects hemoglobin production, we performed mutagenesis studies of the donor substrate-binding domain and the flexible loop that are essential for core fucosylation activity. We observed that FUT8-mediated inhibition of hemoglobin production was cancelled by these FUT8 mutations in MEL cells. Moreover, the same results were observed in K562 cells. This is the first report that the regulation of core fucosylation is essential for hemoglobin production and erythroid differentiation. In the development of other tissues, it has previously been reported that FUT8-mediated core fucosylation of signal-transducing receptors positively regulates the function of the receptors. However, there have been no reports of FUT8-mediated negative regulation of differentiation. Fut8 knock-out mice lack core fucosylation in both their epidermal growth factor receptors and platelet-derived growth factor receptors, and they experience early death during postnatal development (∼70% Fut8 null mice) or growth retardation (45). The lack of core fucosylation of transforming growth factor β1 (TGF-β) receptors led to abnormal lung development and an emphysema-like phenotype in Fut8 null mice (46). In B cell development, the loss of core fucosylation in both α4β1 integrin and vascular cell adhesion molecule 1 led to a decrease in binding between pre-B cells and stromal cells, which impaired the generation of pre-B cells in Fut8 null mice (47). There were no reports about Fut8 null mice of defective of erythroid phenotypes, because it seem that down-regulation of Fut8 promotes erythroid differentiation. These reports predict that signal-transducing receptors other than EpoR may play a role in erythroid differentiation and that core fucosylation of these receptors may play a key part in this role. We think the TGF-β receptor is one of the candidate targets of FUT8. This receptor was identified as the target of core fucosylation (46), and TGF-β induces hemoglobin production in K562 and other human erythroleukemia cells (48, 49).
FUT8 knockdown studies using shRNA in K562 cells indicated that the decrease in FUT8 expression was important for hemoglobin production and differentiation. In the microarray experiments, we also observed that Fut8 expression was decreased during HD MEL cell differentiation. Our results indicate that Fut8 is regulated downstream of Myc and Myb. Both transcription factors were already known as regulators of erythroid differentiation (13–16, 26, 27). The upstream sequence of human FUT8 has binding sites for MYB and GATA-1 (50). In a study exploring the regulation of erythroid gene expression by GATA-1, Fut8 expression was rapidly down-regulated after GATA-1 induction (51). This result also indicates that Fut8 expression is down-regulated downstream of GATA-1.
In contrast, the enzyme activity of FUT8 was increased during megakaryocytic differentiation (52). This fact is the opposite to our findings that a decrease in FUT8 progressed differentiation of pre-erythroblasts to basophilic normoblasts and polychromatic erythroblasts. FUT8 may be involved in the decision whether MEPs differentiate into erythrocytes or megakaryocytes, a role similar to FLI-1, which suppresses erythroid differentiation but activates megakaryocytic differentiation.
In conclusion, expression profile analysis of the differential differentiation ability of MEL cells using DNA microarrays identified FUT8 as a suppressor of differentiation and demonstrated that FUT8-mediated core fucosylation regulates hemoglobin production and erythroid differentiation. In a previous erythropoiesis study, several transcription factors and cytokines, including GATA-1 and erythropoietin, were shown to regulate hemoglobin production and erythroid differentiation. In addition, our findings clearly demonstrate that modification of proteins by glycosylation is important for hemoglobin production and erythroid differentiation. We are currently exploring the target proteins of FUT8-mediated core fucosylation. Further study of FUT8-mediated core fucosylation during erythroid differentiation will help to clarify the additional details of the mechanism of differentiation.
This work was supported by Bio Matrix Research Inc.

This article contains supplemental material.
- MEP
- megakaryocytic/erythroid progenitor
- FUT8
- α-1,6-fucosyltransferase
- MEL
- murine erythroleukemia
- Epo
- erythropoietin
- EpoR
- erythropoietin receptor
- HMBA
- hexamethylene bisacetamide
- TSA
- trichostatin A
- HD
- high differentiation-inducible
- LD
- low differentiation-inducible
- RRM
- R365A/R366A double mutant
- FLM
- D368A/K369A/V370A/G371A/T372A quintuple mutant
- TGF-β
- transforming growth factor β1
- SH
- Src homology.
REFERENCES
- 1. Orkin S. H., Zon L. I. (2008) Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu H., Liu X., Jaenisch R., Lodish H. F. (1995) Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 83, 59–67 [DOI] [PubMed] [Google Scholar]
- 3. Lin C. S., Lim S. K., D'Agati V., Costantini F. (1996) Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 10, 154–164 [DOI] [PubMed] [Google Scholar]
- 4. Kieran M. W., Perkins A. C., Orkin S. H., Zon L. I. (1996) Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc. Natl. Acad. Sci. U.S.A. 93, 9126–9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. (1993) JAK2 associates with the erythropoietin receptor and is tyrosine-phosphorylated and activated following stimulation with erythropoietin. Cell 74, 227–236 [DOI] [PubMed] [Google Scholar]
- 6. Ghaffari S., Kitidis C., Fleming M. D., Neubauer H., Pfeffer K., Lodish H. F. (2001) Erythropoiesis in the absence of Janus-kinase 2: BCR-ABL induces red cell formation in JAK2(−/−) hematopoietic progenitors. Blood 98, 2948–2957 [DOI] [PubMed] [Google Scholar]
- 7. Zhao W., Kitidis C., Fleming M. D., Lodish H. F., Ghaffari S. (2006) Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood 107, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss M. J., Orkin S. H. (1995) Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl. Acad. Sci. U.S.A. 92, 9623–9627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujiwara Y., Browne C. P., Cunniff K., Goff S. C., Orkin S. H. (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. U.S.A. 93, 12355–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scott E. W., Simon M. C., Anastasi J., Singh H. (1994) Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265, 1573–1577 [DOI] [PubMed] [Google Scholar]
- 11. Rekhtman N., Radparvar F., Evans T., Skoultchi A. I. (1999) Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev. 13, 1398–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siatecka M., Bieker J. J. (2011) The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood 118, 2044–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pang C. J., Lemsaddek W., Alhashem Y. N., Bondzi C., Redmond L. C., Ah-Son N., Dumur C. I., Archer K. J., Haar J. L., Lloyd J. A., Trudel M. (2012) Kruppel-like factor 1 (KLF1), KLF2, and Myc control a regulatory network essential for embryonic erythropoiesis. Mol. Cell. Biol. 32, 2628–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jayapal S. R., Lee K. L., Ji P., Kaldis P., Lim B., Lodish H. F. (2010) Down-regulation of Myc is essential for terminal erythroid maturation. J. Biol. Chem. 285, 40252–40265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rylski M., Welch J. J., Chen Y. Y., Letting D. L., Diehl J. A., Chodosh L. A., Blobel G. A., Weiss M. J. (2003) GATA-1-mediated proliferation arrest during erythroid maturation. Mol. Cell. Biol. 23, 5031–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartnek P., Králová J., Blendinger G., Dvorák M., Zenke M. (2003) GATA-1 and c-myb crosstalk during red blood cell differentiation through GATA-1 binding sites in the c-myb promoter. Oncogene 22, 1927–1935 [DOI] [PubMed] [Google Scholar]
- 17. Ben-David Y., Giddens E. B., Bernstein A. (1990) Identification and mapping of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc. Natl. Acad. Sci. U.S.A. 87, 1332–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Athanasiou M., Mavrothalassitis G., Sun-Hoffman L., Blair D. G. (2000) FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia 14, 439–445 [DOI] [PubMed] [Google Scholar]
- 19. Athanasiou M., Clausen P. A., Mavrothalassitis G. J., Zhang X. K., Watson D. K., Blair D. G. (1996) Increased expression of the ETS-related transcription factor FLI-1/ERGB correlates with and can induce the megakaryocytic phenotype. Cell Growth & Differ. 7, 1525–1534 [PubMed] [Google Scholar]
- 20. Starck J., Cohet N., Gonnet C., Sarrazin S., Doubeikovskaia Z., Doubeikovski A., Verger A., Duterque-Coquillaud M., Morle F. (2003) Functional cross-antagonism between transcription factors FLI-1 and EKLF. Mol. Cell. Biol. 23, 1390–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar M. S., Narla A., Nonami A., Mullally A., Dimitrova N., Ball B., McAuley J. R., Poveromo L., Kutok J. L., Galili N., Raza A., Attar E., Gilliland D. G., Jacks T., Ebert B. L. (2011) Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q− syndrome. Blood 118, 4666–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ney P. A., D'Andrea A. D. (2000) Friend erythroleukemia revisited. Blood 96, 3675–3680 [PubMed] [Google Scholar]
- 23. Friend C., Scher W., Holland J. G., Sato T. (1971) Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc. Natl. Acad. Sci. U.S.A. 68, 378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marks P. A., Sheffery M., Ramsay R., Ikeda K., Rifkind R. A. (1987) Induction of transformed cells to terminal differentiation. Ann. N.Y. Acad. Sci. 511, 246–255 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida M., Nomura S., Beppu T. (1987) Effects of trichostatins on differentiation of murine erythroleukemia cells. Cancer Res. 47, 3688–3691 [PubMed] [Google Scholar]
- 26. Kaneko-Ishino T., Kume T. U., Sasaki H., Obinata. M., Oishi M. (1988) Effect of c-myc gene expression on early inducible reactions required for erythroid differentiation in vitro. Mol. Cell. Biol. 8, 5545–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Todokoro K., Watson R. J., Higo H., Amanuma H., Kuramochi S., Yanagisawa H., Ikawa Y. (1988) Down-regulation of c-myb gene expression is a prerequisite for erythropoietin-induced erythroid differentiation. Proc. Natl. Acad. Sci. U.S.A. 85, 8900–8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujita H., Yamamoto M., Yamagami T., Hayashi N., Sassa S. (1991) Erythroleukemia differentiation. Distinctive responses of the erythroid-specific and the nonspecific δ-aminolevulinate synthase mRNA. J. Biol. Chem. 266, 17494–17502 [PubMed] [Google Scholar]
- 29. Fukuda Y., Fujita H., Garbaczewski L., Sassa S. (1994) Regulation of β-globin mRNA accumulation by heme in dimethyl sulfoxide (DMSO)-sensitive and DMSO-resistant murine erythroleukemia cells. Blood 83, 1662–1667 [PubMed] [Google Scholar]
- 30. Lozzio C. B., Lozzio B. B. (1975) Human chronic myelogenous leukemia cell line with positive Philadelphia chromosome. Blood 45, 321–334 [PubMed] [Google Scholar]
- 31. Andersson L. C., Jokinen M., Gahmberg C. G. (1979) Induction of erythroid differentiation in the human leukaemia cell line K562. Nature 278, 364–365 [DOI] [PubMed] [Google Scholar]
- 32. Rutherford T., Clegg J. B., Higgs D. R., Jones R. W., Thompson J., Weatherall D. J. (1981) Embryonic erythroid differentiation in the human leukemic cell line K562. Proc. Natl. Acad. Sci. U.S.A. 78, 348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Pietro R., di Giacomo V, Caravatta L., Sancilio S., Rana R. A., Cataldi A. (2007) Cyclic nucleotide response element binding (CREB) protein activation is involved in K562 erythroleukemia cells differentiation. J. Cell. Biochem. 100, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 34. Wojda U., Noel P., Miller J. L. (2002) Fetal and adult hemoglobin production during adult erythropoiesis: coordinate expression correlates with cell proliferation. Blood 99, 3005–3013 [PubMed] [Google Scholar]
- 35. Ikawa Y., Aida M., Inoue Y. (1976) Isolation and characterization of high and low differentiation-inducible Friend leukemia lines. Gann 67, 767–770 [PubMed] [Google Scholar]
- 36. Morita S., Kojima T., Kitamura T. (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka T. S., Jaradat S. A., Lim M. K., Kargul G. J., Wang X., Grahovac M. J., Pantano S., Sano Y., Piao Y., Nagaraja R., Doi H., Wood W. H., 3rd, Becker K. G., Ko M. S. (2000) Genome-wide expression profiling of mid-gestation placenta and embryo using a 15,000 mouse developmental cDNA microarray. Proc. Natl. Acad. Sci. U.S.A. 97, 9127–9132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kargul G. J., Dudekula D. B., Qian Y., Lim M. K., Jaradat S. A., Tanaka T. S., Carter M. G., Ko M. S. (2001) Verification and initial annotation of the NIA mouse 15K cDNA clone set. Nat. Genet. 28, 17–18 [DOI] [PubMed] [Google Scholar]
- 39. VanBuren V., Piao Y., Dudekula D. B., Qian Y., Carter M. G., Martin P. R., Stagg C. A., Bassey U. C., Aiba K., Hamatani T., Kargul G. J., Luo A. G., Kelso J., Hide W., Ko M. S. (2002) Assembly, verification, and initial annotation of the NIA mouse 7.4K cDNA clone set. Genome Res. 12, 1999–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nosaka T, Kawashima T., Misawa K., Ikuta K., Mui A. L., Kitamura T. (1999) STAT5 as a molecular regulator of proliferation, differentiation, and apoptosis in hematopoietic cells. EMBO J. 18, 4754–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lachman H. M., Skoultchi A. I. (1984) Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature 310, 592–594 [DOI] [PubMed] [Google Scholar]
- 42. Takahashi T., Ikeda Y., Tateishi A., Yamaguchi Y., Ishikawa M., Taniguchi N. (2000) A sequence motif involved in the donor substrate binding by α1,6-fucosyltransferase: the role of the conserved arginine residues. Glycobiology 10, 503–510 [DOI] [PubMed] [Google Scholar]
- 43. Ihara H., Ikeda Y., Toma S., Wang X., Suzuki T., Gu J., Miyoshi E., Tsukihara T., Honke K., Matsumoto A., Nakagawa A., Taniguchi N. (2007) Crystal structure of mammalian α1,6-fucosyltransferase, FUT8. Glycobiology 17, 455–466 [DOI] [PubMed] [Google Scholar]
- 44. Ma B., Simala-Grant J. L., Taylor D. E. (2006) Fucosylation in prokaryotes and eukaryotes. Glycobiology 16, 158–184 [DOI] [PubMed] [Google Scholar]
- 45. Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. (2006) Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 281, 2572–2577 [DOI] [PubMed] [Google Scholar]
- 46. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li W., Ishihara K., Yokota T., Nakagawa T., Koyama N., Jin J., Mizuno-Horikawa Y., Wang X., Miyoshi E., Taniguchi N., Kondo A. (2008) Reduced α4β1 integrin/VCAM-1 interactions lead to impaired pre-B cell repopulation in α1,6-fucosyltransferase deficient mice. Glycobiology 18, 114–124 [DOI] [PubMed] [Google Scholar]
- 48. Burger P. E., Dowdle E. B., Lukey P. T., Wilson E. L. (1994) Basic fibroblast growth factor antagonizes transforming growth factor β-mediated erythroid differentiation in K562 cells. Blood 83, 1808–1812 [PubMed] [Google Scholar]
- 49. Kaneko K., Furuyama K., Aburatani H., Shibahara S. (2009) Hypoxia induces erythroid-specific 5-aminolevulinate synthase expression in human erythroid cells through transforming growth factor-β signaling. FEBS J. 276, 1370–1382 [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi Y., Ikeda Y., Takahashi T., Ihara H., Tanaka T., Sasho C., Uozumi N., Yanagidani S., Inoue S., Fujii J., Taniguchi N. (2000) Genomic structure and promoter analysis of the human α1,6-fucosyltransferase gene (FUT8). Glycobiology 10, 637–643 [DOI] [PubMed] [Google Scholar]
- 51. Welch J. J., Watts J. A., Vakoc C. R., Yao Y., Wang H., Hardison R. C., Blobel G. A., Chodosh L. A., Weiss M. J. (2004) Global regulation of erythroid gene expression by transcription factor GATA-1. Blood 104, 3136–3147 [DOI] [PubMed] [Google Scholar]
- 52. Bany-Łaszewicz U., Kamińska J., Klimczak-Jajor E., Kocielak J. (2004) The activity of α1,6-fucosyltransferase during human megakaryocytic differentiation. Cell. Mol. Biol. Lett. 9, 145–152 [PubMed] [Google Scholar]