Abstract
Purpose
Hypoxia is a cause for resistance to cancer therapies. Molecularly targeted recombinant cytotoxins have shown clinical efficacy in the treatment of patients with primary brain tumors, glioblastoma multiforme, but it is not known whether hypoxia influences their antitumor effect.
Experimental Design
We have exposed glioblastoma multiforme cells, such as U-251 MG, U-373 MG, SNB-19, and A-172 MG, to either anoxia or hypoxia and then reoxygenated them while treating with an interleukin (IL)-13-based diphtheria toxin (DT)-containing cytotoxin, DT-IL13QM. We measured the levels of immunoreactive IL-13Rα2, a receptor that mediates IL-13-cytotoxin cell killing, and the levels of active form of furin, a protease that activates the bacterial toxin portion in a cytotoxin.
Results
We found that anoxia/hypoxia significantly alters the responsiveness of glioblastoma multiforme cells to DT-IL13QM. Interestingly, bringing these cells back to normoxia caused them to become even more susceptible to the cytotoxin than the cells maintained under normoxia. Anoxia/hypoxia caused a highly prominent decrease in the immunoreactive levels of both IL-13R and active forms of furin, and reoxygenation not only restored their levels but also became higher than that in normoxic glioblastoma multiforme cells.
Conclusions
Our results show that a recombinant cytotoxin directed against glioblastoma multiforme cells kills these cells much less efficiently under anoxic/hypoxic conditions. The reoxygenation brings unexpected additional benefit of making glioblastoma multiforme cells even more responsive to the killing effect of a cytotoxin.
Glioblastoma multiforme is a high-grade astrocytoma and represents the most common form of primary brain tumors. The successful treatment of patients with glioblastoma multiforme is still a major challenge, and a median survival rate is 14.5 months since diagnosis (1). In addition to the invasive nature of glioblastoma multiforme tumors, hypoxia, a unique property of solid tumors, has also been considered as an important factor affecting the efficacy of current treatments in glioblastoma multiforme (2, 3). Hypoxia is an alteration of balance between cellular proliferation and oxygen supply, resulting in significantly lower oxygen levels in focal regions than those encountered in surrounding both malignant and normal tissues (4). Evidence suggests that hypoxia influences the behavior of human tumor cells and endows hypoxic tumor cells a higher resistance to radiotherapy and certain chemotherapies and a higher mutation rate and potential for a more metastatic and malignant phenotype (2). The tumor oxygenation is negatively associated with increasing grade of human astrocytomas (5). Similarly to other solid tumors, glioblastoma multiforme tumors exhibit resistance to radiotherapy and chemotherapy largely in part due to the hypoxic tumor microenvironment (6). Several clinical trials have been done with hyperbaric oxygen or hypoxic cell radiosensitizers intending to overcome the problem of the radioresistance of hypoxic tumor cells (7-9). The results of these trials have shown benefit of proper oxygenation for glioblastoma multiforme radiotherapy.
Introduction of specific molecular targeted therapy using cytotoxins has offered new hopes for the successful treatment of glioblastoma multiforme (10). A typical recombinant cytotoxin is a single-chain fusion protein consisting of a ligand with high specificity for the overexpressed tumor-associated receptors and a potent bacterial toxin, such as the derivatives of diphtheria toxin (DT390) and Pseudomonas exotoxin A (PE38QQR; ref. 10). Cytotoxins are designed to take an advantage of the difference in receptor/target expression between normal and tumor cells, so that cytotoxins kill targeted tumor cells while sparing normal cells (11). Recombinant cytotoxins are very potent tumor cell-killing agents when compared with chemotherapeutic agents, because their IC50 values can reach the femtomolar range (10). Clinical studies using convection-enhanced delivery have employed several cytotoxins administered directly to glioblastoma multiforme tumors, such as transferrin-CRM107, interleukin (IL)-4 (38-37)-PE38KDEL, TP38, and IL-13-PE38QQR (12-16). These trials have shown clinical responses in a number of patients.
IL-13-PE38QQR, the first generation of IL-13-based cytotoxins, has been constructed in our laboratory by fusing a wild-type IL-13 to a derivative of Pseudomonas exotoxin A, PE38QQR (17). IL-13-PE38QQR was originally designed to target its physiologic receptor, IL-13/4R, which is shared with IL-4 and is found on many normal organs and glioblastoma multiforme cells (18). The study of action of IL-13-PE38QQR on glioblastoma multiforme cells (19) uncovered another IL-13 receptor, now termed IL-13Rα2, which is an IL-4-independent receptor and predominantly overexpressed on glioblastoma multiforme tumor cells (20) with very low or undetectable levels on normal brain (18). Structure-function relationship analysis has shown that two separate receptor binding areas are present in IL-13 molecule. The NH2-terminal end, especially the Glu13, is responsible for IL-13/4R binding affinity of IL-13 (21). The COOH-terminal end includes Lys105 as a key amino acid involved in the binding of IL-13 to IL-13Rα2 (21, 22). These findings have prompted development of mutated IL-13-based cytotoxins aiming at selective targeting IL-13Rα2 on glioblastoma multiforme cells and diminishing toxicity mediated by IL-13/4R, the normal tissue receptor. For example, a quadruple-mutated IL-13-based (IL-13.E13K/R66D/S69D/K105R), DT-containing (DT390) fusion protein, DT-IL13QM, has been developed that is more specifically cytotoxic to glioblastoma multiforme cells and less toxic in general (23).
Some of the most potent bacterial toxins known, such as DT and PE, possess ADP-ribosyl transferase enzymatic activity, which inactivates elongation factor-2 and subsequently inhibits protein synthesis that leads to cell death (24). However, the toxins and their portions in cytotoxins require proteolytic activation by a calcium-dependent serine endoprotease, furin (25-28). Furin is a primary pro-protein convertase responsible for the post-translational endoproteolysis of inactive precursor proteins including most secretory proteins and membrane type I matrix metalloproteinases (29). The cleavage sites in target pro-proteins are usually paired or multiple, basic residues, such as Arg-X-Lys/Arg-Arg sequences (30). An up-regulated expression and an increased activity of furin in tumor cells have been associated with tumor invasiveness (31, 32). Furin itself is synthesized in endoplasmic reticulum as 96-kDa pro-furin and exits endoplasmic reticulum after rapid conversion to a 90-kDa active form by an intramolecular autocatalytic process (33). DT, PE, and cytotoxins based on these toxins contain a furin cleavage site that must undergo proteolysis during their entry into the host cells (25-28, 34). The peptide motif for furin cleavage is found in the 14-amino acid loop constricted by a disulfide bond between Cys186 and Cys201 in the DT toxin (34), and it is found in Cys265-Cys287 constricted loop of domain II of PE toxin (27). The expression levels, especially the conversion of pro-form to active form, of furin in targeted tumor cells are likely contributory determinants of tumoricidal efficacy of cytotoxins.
Given that glioblastoma multiforme are extensively hypooxygenated and necrotic tumors, this condition may also affect the efficacy of anti-glioblastoma multiforme recombinant cytotoxins. In the present study, we compared the cytotoxicity of IL-13-based cytotoxins in hypoxic and then reoxygenated glioblastoma multiforme cells. We also analyzed the expression of both IL-13Rα2 and furin under these conditions. Our data show that hypoxia significantly impairs the response of glioblastoma multiforme cells to the killing of the cytotoxin. Reoxygenation of hypoxic glioblastoma multiforme cells reversed this phenomenon and, surprisingly, even further increased the sensitivity of glioblastoma multiforme cells to cytotoxin. These changes in susceptibility of glioblastoma multiforme cells to an IL-13-based cytotoxin in response to hypoxia and reoxygenation were paralleled by the changes in the expression of IL-13Rα2 and in the conversion of pro-furin.
Materials and Methods
Construction, expression, and purification of DT-IL13QM fusion protein
Recombinant IL-13 quadruple mutant (IL-13QM; IL-13.E13K/R66D/S69D/K105R) was generated by site-directed mutagenesis as detailed previously (21). PCR products of IL-13QM containing HindIII/EcoRI sequences was subcloned into a TA vector using TA cloning kit (Invitrogen) and transformed to TOP-10 cells. IL-13QM-containing plasmid was amplified in TOP10 cells, digested with HindIII and EcoRI (all restriction enzymes were from Fermentas), and gel purified. Vector DNA containing DT derivates, DT389, was obtained by removing the IL-2 fragment in our previously generated DT-IL2 plasmid. The NH2-terminal end of IL-13QM was fused to the COOH-terminal of DT389 by subcloning using HindIII site and the resulted DT-IL13QM plasmid was used to transform DH5α cells. In-frame DNA sequence of DT-IL13QM was confirmed by automated sequencer before protein expression.
Recombinant DT-IL13QM fusion protein was expressed in BL21(λDE3) Escherichia coli cells and purified using fast protein liquid chromatography system (GE Healthcare) as detailed previously (22). In brief, BL21 E. coli cells were transformed with DT-IL13QM plasmid DNA and cultured in the LB medium supplemented with 100 μg/mL ampicillin in a 37 °C shaker. When A600 value of bacterial culture reaches ~ 1.5, 1 mmol/L IPTG (Inalco) was added into bacterial culture for 90 min to induce the expression of recombinant protein. The inclusion bodies were isolated, denatured in 7 mol/L guanidine HCl/dithioerythritol, refolded in arginine/oxidized glutathione solution, and dialyzed against 10 mmol/L phosphate buffer (pH 7.4). DT-IL13QM protein was then purified on an ion exchange column, Q-Sepharose using fast protein liquid chromatography system. The resulting protein is >95% purity on SDS-PAGE and is recognized by anti-IL-13 antibody (Santa Cruz Biotechnology) in Western blot analysis. Glioblastoma multiforme cells were significantly more sensitive and normal cells less sensitive to DT-IL13QM than to IL-13-PE38QQR (23), the first generation of IL-13-based cytotoxin (19), one of the most potent anti-glioblastoma multiforme agents.
Cell culture
Human glioblastoma multiforme cell lines U-251 MG, U-373 MG, SNB-19, and A-172 MG were obtained from the American Type Culture Collection. U-251 MG cells were maintained in DMEM (Invitrogen) supplemented with 1× nonessential amino acid (Invitrogen) and 10% FCS (Hyclone). U-373 MG and A-172 MG cells were cultured in DMEM containing 10% FCS. SNB-19 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 1× nonessential amino acid, 1 mmol/L sodium pyruvate (Invitrogen), and 10% FCS.
Anoxia/hypoxia treatment of glioblastoma multiforme cells
Glioblasastoma multiforme cells (1 × 106) per 100 mm2 dish or 1,000 per well in 96-well plates were incubated in an Invivo2 hypoxia workstation (Ruskinn) under anoxia (0% O2, 95% N2, and 5% CO2) or hypoxia (0.1% O2, 94.9% N2, and 5% CO2). For recovery study, glioblastoma multiforme cells, which were preincubated in hypoxia chamber for 24 h, were placed back into normoxic incubator. Cells in dishes were immediately placed in cold PBS at indicated time points to prevent hypoxia-inducible factor-1α protein from degradation. Cell lysates were prepared by incubation of cell pellets in harvesting buffer [10 mmol/L HEPES (pH 7.9), 50 mmol/L NaCl, 0.5 mol/L sucrose, 0.1 mmol/L EDTA, 0.5% Triton-X 100, 1 mmol/L DTT plus protease inhibitors (Sigma)] for 5 min on ice followed by centrifugation. The supernatants were collected and stored at −80°C. The nuclei pellets were washed once with buffer A [10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT plus protease inhibitors] and resuspended in 2× buffer C [10 mmol/L HEPES (pH 7.9), 500 mmol/L NaCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 0.1% Igepal (NP-40), 1 mmol/L DTT plus protease inhibitors] for 15 min on ice. After centrifugation, supernatants were collected as nuclear extracts and stored at −80°C until use for Western blotting analysis.
Colorimetric MTS/PMS cell viability assay
Cytotoxicity of DT-IL13QM on glioblastoma multiforme cells was tested using colorimetric MTS/PMS cell viability assay (Promega) as described previously (21). Cycloheximide-treated (Calbiochem) wells served as a background control. To study the sensitivity of glioblastoma multiforme cells to IL-13-cytotoxin under anoxia or hypoxia, glioblastoma multiforme cells in 96-well plate wells were pretreated for 6 h under anoxia or hypoxia followed by incubation of cells with different concentrations of DT-IL13QM for additional 24 h in hypoxia chamber before the addition of MTS/PMS. For recovery study, glioblastoma multiforme cells were pretreated in hypoxia chamber for 24 h. Anoxic or hypoxic glioblastoma multiforme cells were placed back to normoxia. After reoxygenation for 24 h, different concentrations of DT-IL13QM were added to cells for another 48 h. In some experiments, glioblastoma multiforme cells were cultured for 30 min in the presence or absence of 100 μmol/L furin inhibitor, I Dec-RVKR-CMK (Calbiochem), followed by addition of DT-IL13QM into the wells. After 48 h incubation, 10 μL MTS/PMS mixture (1:20) was added into each well and incubated for 90 min at 37°C. The absorbance at 490 nm was recorded using a microplate reader (SpectraMax 340PC; Molecular Devices). A replicate plate was set under normoxia for comparison of sensitivity with that of anoxic/hypoxic or reoxygenated glioblastoma multiforme cells to DT-IL13QM killing. Each point in proliferation curve represents mean ± SD of duplicates.
Immunoblotting
Proteins (20 μg/well) of cell lysates or nuclear extracts was loaded onto 12% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane (Perkin-Elmer). Blots were blocked with 5% milk-PBS for 1 h at room temperature. Immunoreactive IL-13Rα2 was recognized by 2 μg/mL goat anti-human IL-13Rα2 (R&D Systems). Furin was recognized by 1 μg/mL rabbit anti-human furin polyclonal antibody (Santa Cruz Biotechnology). β-Actin was used as protein loading control and probed with 0.04 μg/mL anti-human β-actin monoclonal antibody (Sigma). The blots were incubated with primary antibodies diluted in blocking buffer overnight at 4°C. The primary antibody-protein complexes were detected by incubation for 1 h at room temperature with secondary antibody conjugated with horseradish peroxidase (Sigma) diluted 1:5,000 in blocking buffer. The detection was done using an Enhanced Chemiluminescence Plus kit (GE Healthcare).
Statistical analysis
IC50 values of DT-IL13QM on glioblastoma multiforme cells were analyzed with GraphPad Prism version 4.0 (GraphPad Software). The differences in IC50 values of DT-IL13QM in normoxic, hypoxic, and reoxygenated glioblastoma multiforme cells were analyzed using Student’s paired t test for all data points. P values < 0.05 were considered significant.
Results
Both anoxic and hypoxic glioblastoma multiforme cells are significantly less sensitive to IL-13-cytotoxin than normoxic cells
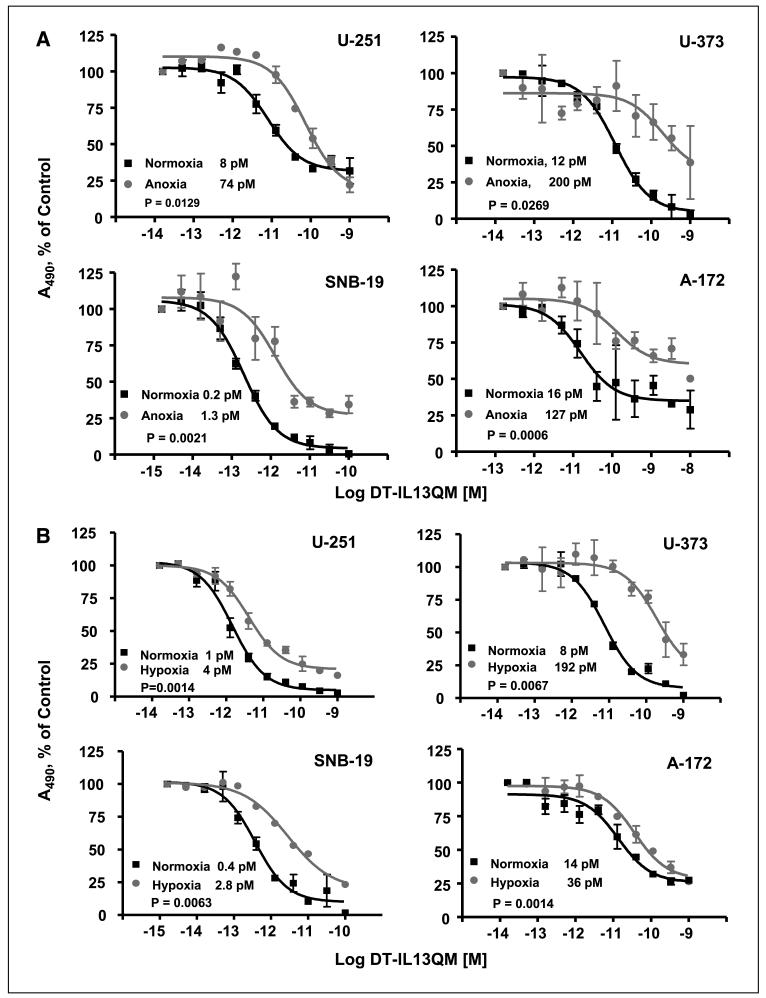
It is well known that the initial molecular response to hypoxia is an increased level of hypoxia-inducible factor 1 protein, a multisubunit protein consisting of α and β helix-loop-helix subunits (35-37). To examine whether anoxia or hypoxia affects susceptibility of glioblastoma multiforme cells to IL-13-cytotoxin killing, we employed a modified 24 h cell viability assay. We found that anoxia had a dramatic effect on the potency of DT-IL13QM in all glioblastoma multiforme cell lines studied. Anoxic glioblastoma multiforme cells were up to 16-fold less sensitive to DT-IL13QM than normoxic cells (Fig. 1A). The normoxia/anoxia ratios of IC50 values for DT-IL13QM on U-251 MG, U-373 MG, SNB-19, and A-172 MG glioblastoma multiforme cells were 8/74, 12/200, 0.2/1.3, and 16/127 pmol/L, respectively (Fig. 1A).
Fig. 1.
Cytotoxicity of IL-13-based cytotoxins in anoxic, hypoxic, or normoxic glioblastoma multiforme cells. Glioblastoma multiforme cells were maintained under anoxia or hypoxia condition for 6 h and various concentrations of DT-IL13QM were then added for additional 24 h in hypoxia chamber. All assays were done in duplicates. A, cytotoxicity of DT-IL13QM in anoxic and normoxic U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells. B, cytotoxicity of DT-IL13QM in hypoxic and normoxic U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells.
In solid tumors, anoxia is the most extreme expression of lack of oxygen that can even lead to necrosis. Next, we tested how the cytotoxin cell killing is influenced by hypoxia. U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells were thus treated with DT-IL13QM under hypoxia (Fig. 1B). In all the tested glioblastoma multiforme cells, similarly to anoxic conditions, hypoxia significantly altered the potency of DT-IL13QM, reflected by a 4-fold (U-251 cells) to 24-fold (U-373 cells) decrease in the IC50 values (Fig. 1B).
Reoxygenation of anoxic/hypoxic glioblastoma multiforme cells restores and even further increases the sensitivity of glioblastoma multiforme cells to DT-IL13QM
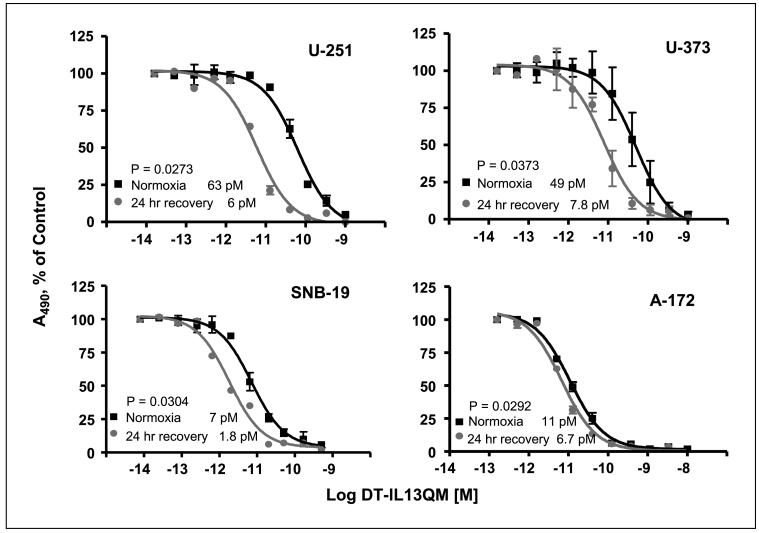
Both anoxia and hypoxia significantly decreased a response of glioblastoma multiforme cells to IL-13-cytotoxin killing (Fig. 1). We next tested whether reoxygenation may reverse this effect. U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells were cultured for 24 h either under anoxia/hypoxia conditions. The cells were then reoxygenated for 24 h in normoxic incubator before the addition of DT-IL13QM. We have found that reoxygenation for 24 h not only restored the susceptibility to the cytotoxin fully but also statistically significantly increased the sensitivity of previously anoxic U-251, U-373, or SNB-19 glioblastoma multiforme cells to the killing by DT-IL13QM when compared with cells maintained in normoxia (Fig. 2). The reoxygenated anoxic glioblastoma multiforme cells were 2- to 10-fold more sensitive to DT-IL13QM killing than normoxic glioblastoma multiforme cells. The IC50 ratios of normoxic/recovered anoxic U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells were 63/6, 49/7.8, 7/1.8, and 11/6.7 pmol/L, respectively.
Fig. 2.
Cytotoxicity of DT-IL13QM in reoxygenated, previously anoxic glioblastoma multiforme cells. Glioblastoma multiforme cells were maintained under anoxia for 24 h. Anoxic glioblastoma multiforme cells were then placed back to normoxic CO2 incubator for another 24 h. Reoxygenated glioblastoma multiforme cells were then incubated with various concentrations of DT-IL13QM for 48 h. All assays were done in duplicates.
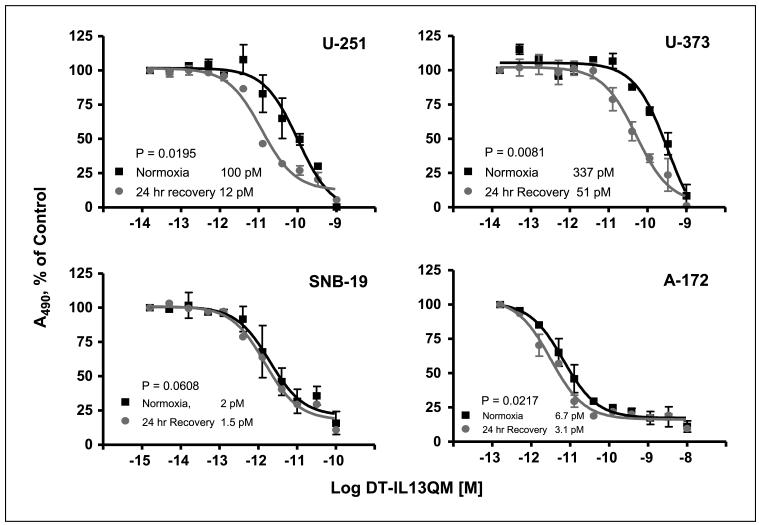
An increase in susceptibility to DT-IL13QM in previously anoxic and subsequently reoxygenated glioblastoma multiforme cells was unexpected and we continued these experiments with cells kept first under hypoxic conditions. Reoxygenation of previously hypoxic U-251, U-373, and A-172 cells for 24 h significantly improved DT-IL13QM killing when compared with normoxic cells (Fig. 3). The normoxia/hypoxia ratio of IC50 values for DT-IL13QM in 24 h reoxygenated U-251 and U-373 cells were 100/12 and 337/51 pmol/L, respectively. In some of these assays, the IC50 values were higher than usual as a result of prolonged duration. In hypoxic SNB-19 cells, reoxygenation caused a restoration of the cytotoxin potency without further improvement in killing efficiency (Fig. 3). Interestingly, SNB-19 cells were by far the most responsive to DT-IL13QM among other studied cells to start with (Figs. 1-3).
Fig. 3.
Cytotoxicity of DT-IL13QM in reoxygenated, previously hypoxic glioblastoma multiforme cells. Glioblastoma multiforme cells were maintained under hypoxia for 24 h followed by incubation under normoxia for 24 h before addition of various concentrations of DT-IL13QM for another 48 h. All assays were done in duplicates.
Inhibition of furin protease activity diminishes cytotoxicity of DT-IL13QM on glioblastoma multiforme cells
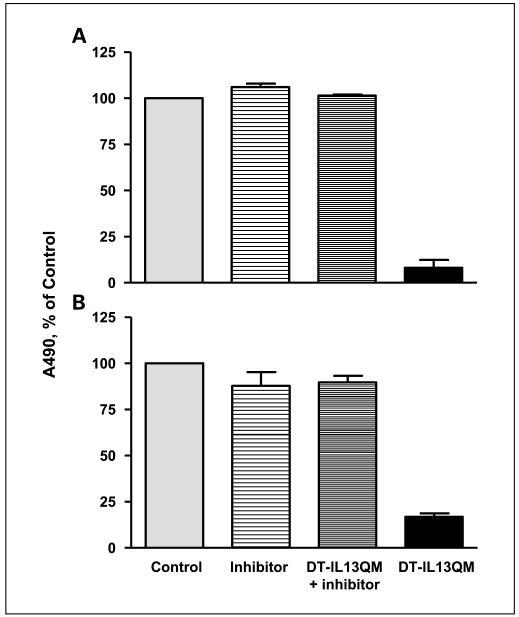
An overexpression of IL-13Rα2 in glioblastoma multiforme cells determines the susceptibility of glioblastoma multiforme cells to IL-13-cytotoxin (19, 38, 39) and furin protease is responsible for the intracellular process of cytotoxin to exert cytotoxicity (25-28, 34). We now tested directly whether furin protease activity plays a role in the killing potency of IL-13-based cytotoxin on glioblastoma multiforme cells in the presence of an overexpressed IL-13Rα2. As shown in Fig. 4A, >90% of U-251 cells were killed by DT-IL13QM alone. However, the potent cytotoxic effect of DT-IL13QM on U-251 cells was completely blocked by 100 μmol/L furin inhibitor, I Dec-RVKR-CMK (Fig. 4A). Furin inhibitor alone did not change cell viability when compared with control. A similar result was observed in another glioblastoma multiforme cell line, SNB-19 cells. DT-IL13QM alone killed SNB-19 cells potently and the inhibition of furin protease activity prevented this cell killing (Fig. 4B). These experiments further documented an essential role of furin in bacterial toxins’ processing and subsequent targeted cytotoxin potency.
Fig. 4.
Inhibition of furin activity blocks the cytotoxicity of DT-IL13QM on glioblastoma multiforme cells. U-251 (A) and SNB-19 (B) cells were preincubated with or without 100 μmol/L furin inhibitor, I Dec-RVKR-CMK, for 30 min, and 3 and 1.5 pmol/L DT-IL13QM were added into pretreated U-251and SNB-19 cells for 48 h, respectively. Assay was done in duplicates.
IL-13Rα2 expression in glioblastoma multiforme cells is dependent on oxygenation status
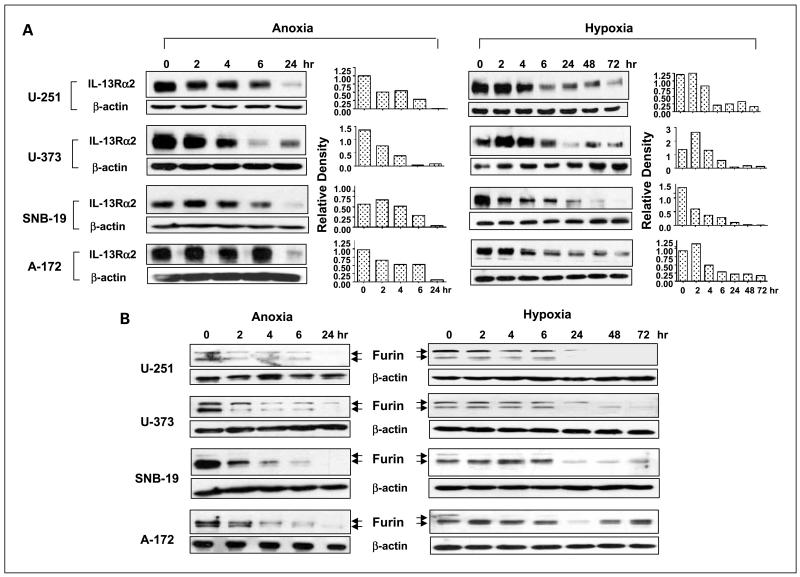
We reasoned that the regulation of IL-13Rα2 expression in glioblastoma multiforme cells by anoxia or hypoxia may be responsible, at least in part, for relative insensitivity of anoxic or hypoxic glioblastoma multiforme cells to DT-IL13QM. We have thus begun analyzing the levels of immunoreactive IL-13Rα2 in U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells kept under anoxia or hypoxia conditions. In response to anoxia or hypoxia, IL-13Rα2 expression in glioblastoma multiforme cells gradually decreased starting even before 6 h of anoxia/hypoxia with dramatically decreased levels found after 24 h (anoxia) or 72 h (hypoxia) in all four glioblastoma multiforme cell lines studied (Fig. 5A).
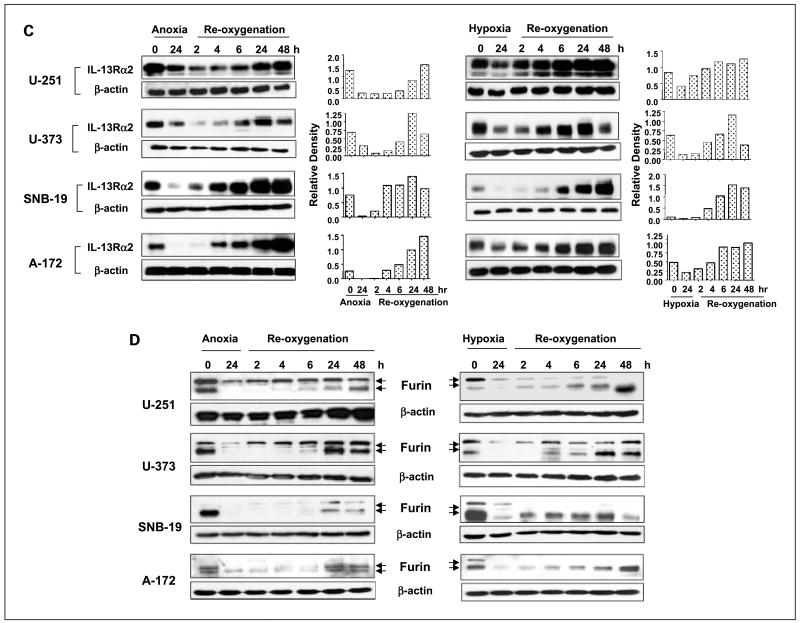
Fig. 5.
Changes of IL-13Rα2 and active furin protein levels in glioblastoma multiforme cells during anoxia/hypoxia and consequential reoxygenation treatments. Glioblastoma multiforme cells were incubated either for up to 24 h under anoxia or up to 72 h under hypoxia. For reoxygenation studies, glioblastoma multiforme cells were maintained under anoxia or hypoxia condition for 24 h followed by incubation under normoxia for 2 to 48 h. Cell lysates were prepared at indicated time points for Western blot analyses of IL-13Rα2 or furin, respectively. Densitometry was done using Scion Image software. A, IL-13Rα2 expression in anoxic and hypoxic glioblastoma multiforme cells. B, furin expression and conversion in anoxic and hypoxic glioblastoma multiforme cells. C, IL-13Rα2 expression in reoxygenated anoxic and reoxygenated hypoxic glioblastoma multiforme cells. D, furin expression and conversion in reoxygenated anoxic and reoxygenated hypoxic glioblastoma multiforme cells. Left, anoxic glioblastoma multiforme cells; right, hypoxic glioblastoma multiforme cells.
Levels of mature furin change dramatically under anoxia/hypoxia in glioblastoma multiforme cells
Inhibition of furin activity using a specific inhibitor blocked the glioblastoma multiforme cell killing by IL-13-cytotoxin (Fig. 4). Hence, we analyzed the effect of anoxia or hypoxia on the levels of mature form of furin in U-251, U-373, SNB-19, and A-172 glioblastoma multiforme cells. Immunoreactive activated furin (90 kDa) protein levels in glioblastoma multiforme cells started to decrease even after 2 h of anoxia until almost undetectable levels at and past 24 h, although the levels of 96-kDa pro-furin also changed and became close to the detection limit in some cell lines after 24 h of anoxia (Fig. 5B). Similar patterns of furin protein expression and pro-furin conversion in glioblastoma multiforme cells was observed in response to hypoxia stress (Fig. 5B).
Reoxygenation causes a rebound or even a further increase in protein levels of IL-13Rα2 and active furin in glioblastoma multiforme cells subjected to anoxia or hypoxia
We next investigated the effect of reoxygenation of anoxic glioblastoma multiforme cells on IL-13Rα2 expression and the conversion of pro-furin to mature furin, in view of previous results showing restoration or even better killing activity of DT-IL13QM in glioblastoma multiforme cells (Figs. 2 and 3). The U-251, U-373, SNB-19, or A-172 cells were kept under anoxia or hypoxia for 24 h. Cells were then reoxygenated in 5% CO2 incubator under normoxia from 2 to 48 h. IL-13Rα2 expression in 24 h anoxic cells notably decreased compared with that in normoxic cells (Fig. 5C). However, IL-13Rα2 expression rebounded in anoxic glioblastoma multiforme cells to at least basal, or even higher, levels after 6 to 48 h of reoxygenation (Fig. 5C).
The protein levels of activated furin in reoxygenated anoxic glioblastoma multiforme cells exhibited a similar pattern to that by IL-13Rα2. Activated form of furin in anoxic glioblastoma multiforme cells (90 kDa) started to rebound after 4 h of reoxygenation and steadily increased until 48 h of reoxygenation. Reoxygenation of hypoxic cells resulted again in a similar pattern of changes in activated furin levels with tendency to recover furin to at least basal if not higher levels (Fig. 5D).
Discussion
We have found that anoxia/hypoxia altered prominently the responsiveness of glioblastoma multiforme cells to the killing by IL-13-based cytotoxins. This alteration was associated with a significant decrease in the levels of IL-13Rα2, targeted by the cytotoxin plasma membrane receptor, and of active furin, a protease that activates the toxin portion of a cytotoxin. Interestingly, the cells that were subjected to anoxia/hypoxia first and then brought back to normoxic conditions became better responders to IL-13-cytotoxin than the cells maintained continuously in normoxia. These reoxygenated glioblastoma multiforme cells that were killed more efficiently using a recombinant cytotoxin had their levels of both IL-13Rα2 and activated furin also elevated when compared with normoxic cells. We have thus shown for the first time that anoxia/hypoxia negatively affects the potency of recombinant cytotoxins in killing glioblastoma multiforme cells. Furthermore, reoxygenation offers even better efficacy of the cytotoxins.
Recombinant cytotoxins have been already tested in the clinic showing considerable promise (10) and human IL-13-PE38QQR, the first generation of IL-13-based cytotoxins, showed a highly significantly better progression-free survival in patients with recurrent glioblastoma multiforme when compared with standard of care (16). Our current study clearly indicates that anoxia/hypoxia, which is so characteristic of glioblastoma multiforme tumors, has hampered the efficacy of the cytotoxins in glioblastoma multiforme cells. However, when cells were reoxygenated, the cytotoxin became even more potent. For example, reoxygenation reversed the diminished sensitivity of hypoxic glioblastoma multiforme cells to IL-13-cytotoxin resulting in a 100-fold lower IC50 values for DT-IL13QM in reoxygenated glioblastoma multiforme cells than that in hypoxic glioblastoma multiforme cells. This strongly implies that an effort should be made to diminish possible tumor anoxia/hypoxia in glioblastoma multiforme patients before cytotoxin treatment initiation. One of the possible means to overcome the hypoxia-associated resistance of glioblastoma multiforme therapies is to prenormalize oxygen status with hyperbaric oxygen before therapy. It has been shown that hyperbaric oxygen therapy can efficiently improves oxygen supply to hypoxic cells (7, 8). The results of clinical trials with combination of hyperbaric oxygen therapy and radiotherapy in glioblastoma multiforme patients encourage its application to other therapies such as targeted therapy with recombinant cytotoxins. Being that the hyperbaric oxygen is not widely available clinically, combination of antiangiogenic therapy with cytotoxins therapy provides another opportunity to overcome hypoxia-associated resistance to drugs. Recent attempts at antiangiogenic therapy (e.g., with AZD2171, a pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor) have shown “normalization” of hypoxia and favored radiotherapy of glioblastoma multiforme patients (40, 41).
Hypoxia regulates expression of a panel of genes during the adaptation period to stress conditions with subsequent functional changes (35). Overexpressions of genes and proteins of vascular endothelial growth factor and chemokine (C-X-C motif) receptor 4 in glioblastoma multiforme cells have been associated with a well-developed neovascularization and invasive nature of glioblastoma multiforme tumors (42, 43). The regulation of expression of tumor marker proteins and enzymes by hypoxia that are crucial to recombinant cytotoxins therapy of glioblastoma multiforme has not been illustrated previously. The cytotoxic effect of a cytotoxin parallels the number of receptor molecules in targeted cells (19, 38). For example, turning off IL-13Rα2 gene in IL-13-PE38QQR-sensitive glioblastoma multiforme cells resulted in the resistance to IL-13-PE38QQR killing (39). In the present study, we found that the changes in IL-13Rα2 protein levels correlated with DT-IL13QM cytotoxicity on glioblastoma multiforme cells in response to anoxia/hypoxia stress and subsequent reoxygenation. IL-13Rα2 protein levels dramatically decreased in glioblastoma multiforme cells after 24 h anoxia and steadily rebounded to even higher than the background levels during the reoxygenation. A similar pattern in IL-13Rα2 protein levels change was seen in hypoxic and reoxygenated glioblastoma multiforme cells. It is thus likely that the changes in expression levels of a target molecule under anoxic and/or hypoxic tumor cells are in part responsible for the changes of their sensitivity to cytotoxin killing. Currently, some models of glioblastoma multiforme reflect hypoxia and necrosis, especially when large tumors are developed in rodents (44).
In addition to the levels of targeted receptor molecule on tumor cells that are important to the cytotoxin efficacy, the intracellular processing of cytotoxin by furin is another significant determinant of cytotoxin potency. Furin is encoded by fur gene, which is overexpressed in glioblastoma multiforme cells to start with (31), similarly to IL-13Rα2. fur gene decodes the full-length pro-furin followed by a intracellular processing to convert pro-form to mature form of furin through intramolecular autocatalytic cleavage in endoplasmic reticulum (33). Only the mature form of furin can release an active domain of the toxin into the cytosol by cleavage at the furin recognition sequence RXK/RR that is located in the translocation domain of DT and PE toxins (25-28, 34). Therefore, not only the fur gene transcription but also the conversion of pro-furin to active furin could be factors determining the sensitivity of targeted tumor cells to cytotoxin therapy. We observed that the inhibition of furin activity using a specific inhibitor significantly blocked the cytotoxicity of IL-13-cytotoxin in glioblastoma multiforme cells. The protein levels of active furin gradually declined to almost undetectable levels when cells were exposed to anoxia/hypoxia. The levels of furin, however, rebounded to even higher than the background levels after reoxygenation. These changes were closely associated with the cytotoxic potency of DT-IL13QM in glioblastoma multiforme cells and thus indicate on an indispensable role of furin in the potency of cytotoxins in glioblastoma multiforme cells.
In summary, prenormalization of tumor hypoxia status should be attempted to further improve the results of targeted therapy of glioblastoma multiforme using IL-13-based cytotoxins.
Translational Relevance.
Hypoxia is very frequent in glioblastoma multiforme and, in its severe form, leads to necrosis, a hallmark of this brain tumor. There are few effective modalities of treatment of glioblastoma multiforme, and one of them is recombinant cytotoxins. We have found that hypoxia has a detrimental effect on the efficacy of recombinant IL-13 mutant-based cytotoxin on glioblastoma multiforme cells. However, improving oxygenation status not only brings the effectiveness of the cytotoxin back to the basal levels but also makes the killing by the cytotoxinmore effective. Therefore, the clinical maneuvers that either normalize tumor circulation, and subsequently oxygenation, or apply direct hyperbaric approach should be considered when recombinant cytotoxins are used in patients with glioblastoma multiforme.
Acknowledgments
Grant support: National Cancer Institute grant R01CA118261.
Footnotes
Disclosure of Potential Conflicts of Interest
W. Debinski has an ownership interest in and has served as a consultant for Targepeutics.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM. The hypoxic cell: a target for selective cancer therapy—Eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1999;59:5863–70. [PubMed] [Google Scholar]
- 3.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–8. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrall DE, Rosner GL, Azuma C, McEntee MC, Raleigh JA. Hypoxia marker labeling in tumor biopsies: quantification of labeling variation and criteria for biopsy sectioning. Radiother Oncol. 1997;44:171–6. doi: 10.1016/s0167-8140(97)01931-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–84. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 6.Irie N, Matsuo T, Nagata I. Protocol of radiotherapy for glioblastoma according to the expression of HIF-1. BrainTumor Pathol. 2004;21:1–6. doi: 10.1007/BF02482169. [DOI] [PubMed] [Google Scholar]
- 7.Kohshi K, Yamamoto H, Nakahara A, Katoh T, Takagi M. Fractionated stereotactic radiotherapy using gamma unit after hyperbaric oxygenation on recurrent high-grade gliomas. JNeurooncol. 2007;82:297–303. doi: 10.1007/s11060-006-9283-1. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa K, Yoshii Y, Inoue O, et al. Phase II trial of radiotherapy after hyperbaric oxygenation with chemotherapy for high-grade gliomas. BrJ Cancer. 2006;95:862–8. doi: 10.1038/sj.bjc.6603342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JE, Khuntia D, Robins HI, Mehta MP. Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol. 2007;5:894–902. 907–15. [PubMed] [Google Scholar]
- 10.Debinski W. Local treatment of brain tumors with targeted chimera cytotoxic proteins. Cancer Invest. 2002;20:801–9. doi: 10.1081/cnv-120003545. [DOI] [PubMed] [Google Scholar]
- 11.Debinski W. Molecular targeting of brain tumors with recombinant cytotoxins. Res Adv Cancer. 2007;7:155–65. [Google Scholar]
- 12.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–14. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami M, Kawakami K, Puri RK. Interleukin-4-Pseudomonas exotoxin chimeric fusion protein for malignant glioma therapy. J Neurooncol. 2003;65:15–25. doi: 10.1023/a:1026294416718. [DOI] [PubMed] [Google Scholar]
- 14.Sampson JH, Akabani G, Archer GE, et al. Progress report of a phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-α and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 15.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 16.Kunwar S, Westphal M, Medhorn M, et al. Results from PRECISE: a randomized phase 3 study in patients with first recurrent GBM comparing cintredekin besudotox administered via convection-enhanced delivery with Gliadel wafers. Neuro-oncol. 2007;9:531. [Google Scholar]
- 17.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–80. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 18.Debinski W, Gibo DM. Molecular expression analysis of a restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–8. [PubMed] [Google Scholar]
- 20.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271:22428–33. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JP, Debinski W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J Biol Chem. 1999;274:29944–50. doi: 10.1074/jbc.274.42.29944. [DOI] [PubMed] [Google Scholar]
- 22.Madhankumar AB, Mintz A, Debinski W. Alanine-scanning mutagenesis of α-helix D segment of interleukin-13 reveals new functionally important residues of the cytokine. J Biol Chem. 2002;277:43194–205. doi: 10.1074/jbc.M205047200. [DOI] [PubMed] [Google Scholar]
- 23.Liu TF, Cai J, Gibo D, Debinski W. Variant IL13-directed diphtheria toxin fusion recombinant cytotoxin for the treatment of malignant gliomas. Neuro-oncol. 2006;8:429. [Google Scholar]
- 24.Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci U S A. 1975;72:2284–8. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moehring JM, Inocencio NM, Robertson BJ, Moehring TJ. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem. 1993;268:2590–4. [PubMed] [Google Scholar]
- 26.Inocencio NM, Moehring JM, Moehring TJ. Furin activates Pseudomonas exotoxin A by specific cleavage in vivo and in vitro. J Biol Chem. 1994;269:31831–5. [PubMed] [Google Scholar]
- 27.Chiron MF, Fryling CM, FitzGerald D. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J Biol Chem. 1997;272:31707–11. doi: 10.1074/jbc.272.50.31707. [DOI] [PubMed] [Google Scholar]
- 28.Ogata M, Chaudhary VK, Pastan I, FitzGerald DJ. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990;265:20678–85. [PubMed] [Google Scholar]
- 29.Tellier E, Négre-Salvayre A, Bocquet B, et al. Role for furin in tumor necrosis factor α-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Mol Cell Biol. 2007;27:2997–3007. doi: 10.1128/MCB.01485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosaka M, Nagahama M, Kim WS, et al. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991;266:12127–30. [PubMed] [Google Scholar]
- 31.Mercapide J, Lopez De Cicco R, Bassi DE, Castresana JS, Thomas G, Klein-Szanto AJ. Inhibition of furin-mediated processing results in suppression of astrocytoma cell growth and invasiveness. Clin Cancer Res. 2002;8:1740–6. [PubMed] [Google Scholar]
- 32.Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci U S A. 2001;98:10326–31. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leduc R, Molloy SS, Thorne BA, Thomas G. Activation of human furin precursor processing endoprotease occurs by an intramolecular autoproteolytic cleavage. J Biol Chem. 1992;267:14304–8. [PubMed] [Google Scholar]
- 34.Hu HY, Huynh PD, Murphy JR, vanderSpek JC. The effects of helix breaking mutations in the diphtheria toxin transmembrane domain helix layers of the fusion toxin DAB389IL-2. Protein Eng. 1998;11:811–7. doi: 10.1093/protein/11.9.811. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 36.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its a subunit. J Biol Chem. 1996;271:32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 37.Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1α: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci U S A. 1997;94:5667–72. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu TF, Cohen KA, Ramage JG, Willingham MC, Thorburn AM, Frankel AE. A diphtheria toxin-epidermal growth factor fusion protein is cytotoxic to human glioblastoma multiforme cells. Cancer Res. 2003;63:1834–7. [PubMed] [Google Scholar]
- 39.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Rα2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–99. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1 in brain tumors: association with angiogenesis, invasion and progression. Cancer. 2000;88:2606–18. [PubMed] [Google Scholar]
- 43.Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 44.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–48. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]