Abstract
Context
Smoking is a possible risk factor for dementia although its impact may have been underestimated in elderly populations due to the shorter lifespan of smokers.
Objective
To examine the association between smoking history and cognitive decline in the transition from midlife to old age.
Design, Setting, and Participants
Data are from 5099 men and 2137 women in the Whitehall II study, mean age 56 years (range=44–69 years) at the first cognitive assessment (1997–1999), repeated over 2002–2004 and 2007–2009.
Main Outcome Measures
The cognitive test battery was composed of tests of memory, vocabulary, executive function (composed of one reasoning and two fluency tests), and a global cognitive score summarising performance across all five tests. Smoking status was assessed over the entire study period. Linear mixed models were used to assess the association between smoking history and 10-year cognitive decline, expressed as z-scores.
Results
In men, 10-year cognitive decline in all tests except vocabulary among never smokers ranged from a quarter to a third of the baseline standard deviation. Faster cognitive decline was observed among current smokers compared to never smokers in men [mean difference in 10-year decline in global cognition=−0.09 (95%CI:−0.15;−0.03) and executive function=−0.11 (−0.17;−0.05)]. Recent ex-smokers had greater decline in executive function (−0.08 (−0.14;−0.02)) while the decline in long-term ex-smokers was similar to that among never smokers. In analyses that additionally took drop-out and death into account, these differences were 1.2 to 1.5 times larger. In women, cognitive decline did not vary as a function of smoking status.
Conclusions
Compared to never smokers, middle-aged male smokers experienced faster cognitive decline in global cognition and executive function. In ex-smokers with at least 10-year cessation there were no adverse effects on cognitive decline.
Keywords: Adult, Aged, Cognition Disorders, etiology, physiopathology, Cohort Studies, Female, Great Britain, Humans, Male, Middle Aged, Neuropsychological Tests, Smoking, adverse effects, Time Factors
The number of dementia cases worldwide, estimated at 36 million in 2010, is on the rise and projected to double every 20 years.[1]Smoking is increasingly recognised as a risk factor for dementia in the elderly.[2–4]There is also evidence to suggest that its impact on adverse cognitive outcomes, including dementia, may have been underestimated due to selection effects as a result of greater mortality among smokers in midlife.[5, 6]The extent to which smoking increases the risk of cognitive decline remains unclear,[2]as few studies have investigated this association[2, 7–15]particularly in non-elderly populations.[7, 9, 10, 13]The fact that smokers have greater risks of respiratory and cardiovascular diseases,[16]both linked to cognitive impairment[17–19]suggests that they may also experience faster cognitive decline.
Public health messages have led many individuals to give up smoking but the extent to which this change in behaviour influences subsequent cognitive decline remains unclear.[2]We previously reported smokers compared to non-smokers to have poorer memory and greater decline in reasoning over 5 years using two waves of data.[7]The aim of the present paper is to examine the association between smoking history and decline in multiple domains of cognition using three waves of cognitive data, a total follow-up of 10 years. Smoking status was assessed over a 25-year period, starting 10 years prior to the first cognitive assessment, allowing us to investigate the impact on cognitive decline of persistent smoking, intermittent smoking, and smoking cessation. A key objective was to take into account the potential bias in the estimates of cognitive decline due to selection effects, as a result of mortality or drop-out over the follow-up. In order to this we use a method that allows joint modelling of cognitive decline, time to drop-out and time to death.[20–22]A final objective was to examine whether age modifies the association between smoking and cognitive decline.
METHODS
Study Population
The Whitehall II study is based on employees of the British Civil service.[23]At study inception (Phase 1, 1985–1988), 10,308 participants (67% men) underwent a clinical examination and completed a self-administered questionnaire. Subsequent phases of data collection have alternated between postal questionnaire alone (Phases 2 (1988–1990), 4 (1995–1996), 6 (2001) and 8 (2006)) and postal questionnaire accompanied by a clinical examination (Phases 3 (1991–1994), 5 (1997–1999), 7 (2002–2004), and 9 (2007–2009)). Cognitive testing was introduced to the study at Phase 5 (age range=44–69 years) and repeated at Phases 7 (age range=50–74 years) and 9 (age range=55–80 years). All participants provided written consent and the University College London ethics committee approved this study.
Smoking
Data on cigarette smoking were collected at Phases 1, 2, 3, 5, 7, and 9 using questions on smoking status (current, past, never), age at which the participant started smoking, average number of cigarettes per day, and ounces of tobacco smoked in hand-rolled cigarettes per week. Ex-smokers reported the age at which they had stopped smoking. The measure of smoking history at Phase 5 (to coincide with the first measure of cognition) comprised the following categories: “current smoker at Phase 5”, “recent ex-smoker” (stopped smoking between Phases 1 and 5), “long-term ex-smoker” (those who stopped before Phase 1) and “never smoker”. We also used data on the number of cigarettes smoked per day to calculate pack-years of smoking (the average number of cigarettes smoked per day/20 * number of years of smoking).
We defined smoking status over the follow-up (Phases 5, 7 and 9) as “persistent smokers” (those smoking at Phases 5 and 9), “intermittent smokers” (quitters who started smoking again), and “quitters” (stopped smoking after Phase 5). Participants corresponding to none of these categories were classified using smoking history defined at Phase 5, as described above.
Cognition
Cognitive function was assessed using a battery of five tests.
Short-term verbal memory was assessed with 20 one or two syllable words, presented at two second intervals that the participants had 2 minutes to recall in writing.
Vocabulary was assessed using the Mill Hill Vocabulary test,[24]used in its multiple-choice format, consisting of a list of 33 stimulus words ordered by increasing difficulty and six response choices.
Executive function was derived from 3 tests. One, the timed (10 minutes) Alice Heim 4-I (AH4-I) test, to assess reasoning. It is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty.[25]Two measures of verbal fluency: phonemic, assessed via “S” words, and semantic fluency using names of animals.[26]One minute was allowed for each test. The mean of the standardized z-scores of these three tests (mean=0; standard deviation (SD)=1) using the mean and standard deviation from Phase 5 was used as the executive function score.
A global cognitive score was created using all five tests described above by first standardizing the raw scores on each test to z-scores (mean=0; SD=1) using the mean and SD at phase 5 in the entire cohort for each test. The z-scores were then averaged to yield the global cognitive score, seen to minimize problems due to measurement error.[27, 28]
Covariates at Phase 5
Socio-demographic variables included were age, sex, marital status (married/cohabiting vs others) and socioeconomic status using two measures: occupational position [high (administrative), intermediate (professional or executive), low (clerical or support)] and education [less than primary school (until age 11), lower secondary school (until age 16), higher secondary school (until age 18), university, and higher university degree].
Health behaviours: alcohol consumption, assessed via questions on the number of alcoholic drinks (“measures” of spirits, “glasses” of wine, and “pints” of beer) consumed in the last seven days, and categorized as “none or <1 unit/week” (no alcohol), “moderate drinkers” (1–14 units/week in women and 1–21 units/week in men), and “heavy drinkers” (15+ units in women and 21+ units in men); frequency of fruit and vegetable consumption, assessed using the question “How often do you eat fresh fruit or vegetable?” (responses were on an eight-point scale, ranging from ‘seldom or never’ to ‘two or more times a day’); physical activity, categorised as “active” (≥2.5 hours/week of moderate or ≥1 hour/week of vigorous physical activity), “inactive” (<1 hour/week of moderate and <1 hour/week of vigorous physical activity) or “moderately active” (if not active or inactive).[29]
Health measures included resting heart rate, serum cholesterol, systolic and diastolic blood pressure, and prevalence of coronary heart disease (CHD), stroke, and diabetes. Resting heart rate was measured via electrocardiogram with participants in the supine position and categorized as < 60, 60–80, and > 80 beats/minute.[30]Blood pressure was measured twice with the participant sitting after a 5-minute rest using the Hawksley random-zero sphygmomanometer. The average of two readings was taken to be the measured blood pressure. Fasting serum cholesterol was measured within 72 hours in serum stored at 4°C using enzymatic colorimetric methods. CHD prevalence was based on clinically verified events and included myocardial infarction and definite angina.[31]Stroke was assessed using a self-reported measure of physician diagnosis. Diabetes was defined by a fasting glucose ≥7.0 mmol/L or a 2-hour postload glucose ≥11.1 mmol/L or reported doctor diagnosed diabetes, or use of diabetes medication.[32]
Covariates over the follow-up
These included coronary heart disease and incident self-reported stroke from Phase 5 to Phase 9 and lung function from Phases 7 and 9 [33]measured using a portable flow spirometer (MicroPlus Spirometer; Micro Medical Ltd., Kent, United Kingdom) used here as forced expiratory volume in one second (FEV1).[34]
Statistical analysis
We investigated the association between smoking history and global cognition, memory, vocabulary, and executive function. In order to allow comparability across tests, all scores were converted to z-scores (mean = 0, SD = 1). Linear mixed models[35]were used to estimate the association between the smoking history and 10-year cognitive decline. These models use all available data over the follow-up, handle differences in length of follow-up, and take into account the fact that repeated measures on the same individual are correlated. We fitted both the intercept and slope as random effects, allowing individual differences both in cognitive performance at baseline and rate of cognitive decline. Interaction terms suggested sex differences in the association between smoking history and cognitive decline (p=0.03 for global cognition, p=0.54 for memory, p=0.15 for vocabulary, and p=0.02 for executive function), leading us to stratify the analyses by sex.
The linear mixed model included terms for time (individual follow-up divided by 10 to yield effects of change over 10 years), age at baseline (centered at 55 years), smoking history at baseline, education, occupational position, and marital status, and the interaction of each of the covariates with time (model 1) in order to take into account the fact that all covariates can influence the rate of cognitive decline. The interaction term between smoking history and time provides the mean difference in the 10-year decline among current smokers, long-term ex-smokers, recent ex-smokers compared to the “never smokers”. This model was subsequently expanded to include covariates and their interaction with time: first, other health behaviours and health measures at Phase 5 (model 2), then stroke and CHD as time-dependent variables (model 3) and finally the analyses presented in model 2 was repeated with lung function added as a covariate (model 4).
Using models similar to model 1, we investigated dose-response associations between smoking and cognitive decline using pack-years of smoking (at Phase 5) and the association between smoking status over the follow-up and concurrent cognitive change. A three-way interaction term between age, smoking history, and time was used to assess whether the effect of smoking on cognitive decline differed as a function of age. The results of this analysis are presented graphically, to make them easily understandable, with estimates of the regression coefficients from model 1 stratified at 55 years, the median age. In the final set of analyses, we examined the impact of missing data (due to death or dropout) on the estimates of cognitive decline using joint modelling[20, 22]that allow to take into account the correlation between cognitive decline, time to drop-out, and time to death (see eMethods).
Several sensitivity analyses were conducted. First, interactions of smoking with education (p > 0.43) and apolipoprotein allele ε4 (APOE ε4; p > 0.24) were examined; although both variables were associated with cognitive scores at baseline, they did not influence the association of smoking history with cognitive decline. Second, we repeated the analysis among participants with a Mini-Mental State Examination (MMSE) score ≥ 24 at Phases 7 and 9 to ensure that the results were not being driven by potential cases of dementia.[36]Finally, we restricted the main analysis to individuals with complete data, that is, those who had cognitive data at all three waves. All analyses were performed with SAS, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Sample description and missing data
Of the 10,308 participants at Phase 1 (1985–1988), 306 had died and 752 had dropped out from the study before the start of the cognitive data collection at Phase 5 (1997–1999). Of the 9250 remaining individuals, 7495 participated in one or more of the three cognitive function assessments over 10 years. All analyses are based on 7236 individuals who had complete data on smoking history and other covariates; this group was similar in age (55.8 vs 56.0 years, p=0.09) to those not included in the analysis but composed of more men (70.5% vs 58.7%, p<0.001) and persons from the higher occupational group (33.2% vs 20.3%, p<0.001). Of those included in the analyses, 973 (13.4%) contributed to one wave of cognitive data, 1603 (22.2%) to two waves and 4660 (64.4%) all three waves. 11.8% of those included in the analysis had less that primary school education, 35.0% had lower secondary school, 24.8% had finished school, 20.9% had a university degree, and 7.5% a postgraduate degree.
Table 1 shows characteristics of study participants as a function of smoking history. 10-year cognitive decline in men aged 55 (results not shown) was estimated at −0.34 of the baseline standard deviation (95% confidence interval (CI): −0.35, −0.32) for global cognition, −0.28 (−0.31, −0.25) for memory, and −0.39 (−0.41, −0.37) for executive function. There was a small improvement in vocabulary scores (0.02 (0.00, 0.03)). The corresponding figures for women were −0.30 (−0.33, −0.28) for global cognition, −0.25 (−0.30, −0.20) for memory, −0.37 (−0.40, −0.34) for executive function, and 0.05 (0.02, 0.07) for vocabulary. Older individuals experienced faster decline; for example men (women) aged 65 compared to 55 years at baseline declined −0.10 (−0.11) of the baseline standard deviation more in global cognition, −0.06 (−0.10) in memory, −0.10 (−0.10) in executive function, and −0.06 (−0.04) in vocabulary.
Table 1.
Characteristic of the population as a function of smoking history at Phase 5 (1997–1999).
| Phase 5 characteristics | Sex | Current smoker | Recent ex-smoker | Long-term ex-smoker | Never smoker | p* |
|---|---|---|---|---|---|---|
| N (N, %) | M | 468 (9.2) | 408 (8.0) | 1825 (35.8) | 2398 (47.0) | <0.001 |
| F | 262 (12.3) | 191 (8.9) | 507 (23.7) | 1177 (55.1) | ||
|
| ||||||
| Age (M, SD) | M | 54.5 (5.6) | 55.7 (6.1) | 56.2 (5.9) | 55.3 (6.1) | <0.001 |
| F | 56.5 (5.9) | 56.8 (5.9) | 56.5 (6.1) | 56.0 (6.0) | 0.15 | |
|
| ||||||
| Married/cohabiting (N, %) | M | 331 (70.7) | 327 (80.2) | 1579 (86.5) | 2008 (83.7) | 0.001 |
| F | 145 (55.3) | 117 (61.3) | 315 (62.1) | 704 (59.8) | 0.32 | |
|
| ||||||
| High occupational position (N, %) | M | 155 (33.1) | 182 (44.6) | 924 (50.6) | 1367 (57.0) | <0.001 |
| F | 39 (14.9) | 32 (16.8) | 123 (24.3) | 230 (19.5) | 0.009 | |
|
| ||||||
| University degree or higher (N, %) | M | 93 (19.9) | 125 (30.6) | 494 (27.1) | 918 (38.3) | <0.001 |
| F | 22 (8.4) | 29 (15.2) | 110 (21.7) | 263 (22.3) | <0.001 | |
|
| ||||||
| Heavy alcohol consumption† (N, %) | M | 181 (38.7) | 146 (35.8) | 608 (33.3) | 516 (21.5) | <0.001 |
| F | 61 (23.3) | 37 (19.4) | 119 (23.5) | 147 (12.5) | <0.001 | |
|
| ||||||
| Physically active‡ (N, %) | M | 230 (49.2) | 242 (59.3) | 1126 (61.7) | 1434 (59.8) | <0.001 |
| F | 75 (28.6) | 83 (43.5) | 219 (43.2) | 418 (35.5) | <0.001 | |
|
| ||||||
| Daily consumption of fruits & vegetables (N, %) | M | 244 (52.1) | 282 (69.1) | 1329 (72.8) | 1747 (72.9) | <0.001 |
| F | 114 (56.5) | 3154 (80.6) | 398 (78.5) | 944 (80.2) | <0.001 | |
|
| ||||||
| Heart rate>80 beats/min (N, %) | M | 72 (15.4) | 68 (16.7) | 238 (13.0) | 313 (13.1) | 0.05 |
| F | 25 (9.5) | 33 (17.3) | 85 (16.8) | 193 (16.4) | 0.03 | |
|
| ||||||
| SBP (mmHg) (M, SD) | M | 123.1 (15.0) | 125.6 (17.0) | 125.0 (15.9) | 123.1 (15.9) | <0.001 |
| F | 119.8 (17.2) | 121.8 (17.6) | 122.6 (17.2) | 122.8 (17.5) | 0.09 | |
|
| ||||||
| DBP (mmHg) (M, SD) | M | 77.4 (9.9) | 79.4 (11.4) | 79.0 (10.1) | 78.5 (10.6) | 0.01 |
| F | 73.4 (9.6) | 74.5 (10.5) | 75.7 (9.9) | 75.7 (10.2) | 0.005 | |
|
| ||||||
| Cholesterol (mmol/l) (M, SD) | M | 6.0 (1.1) | 6.1 (1.2) | 6.0 (1.0) | 5.8 (1.0) | <0.001 |
| F | 6.1 (1.0) | 6.0 (1.0) | 6.1 (1.1) | 6.0 (1.1) | 0.40 | |
|
| ||||||
| Prevalence of CHD (N, %) | M | 24 (6.4) | 101(6.3) | 28 (7.8) | 24 (6.4) | 0.15 |
| F | 12 (4.6) | 17 (8.9) | 19 (3.8) | 70 (6.0) | 0.04 | |
|
| ||||||
| Prevalence of Diabetes (N, %) | M | 35 (7.5) | 37 (9.1) | 106 (5.8) | 123 (5.1) | 0.007 |
| F | 6 (2.3) | 16 (8.4) | 37 (7.3) | 87 (7.4) | 0.02 | |
|
| ||||||
| Prevalence of Stroke (N, %) | M | 5 (1.1) | 7 (1.7) | 16 (0.9) | 28 (1.2) | 0.50 |
| F | 2 (0.8) | 2 (1.1) | 8 (1.6) | 4 (0.3) | 0.06 | |
p for heterogeneity
Heavy alcohol consumption was defined as 15+ units/week in women and 21+ units/week in men.
Corresponds to more than 2.5h/week of moderate physical activity or more 1h/week of vigorous physical activity.
Abbrevations: M: Mean, SD: Standard deviation, CHD Coronary heart disease, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure
Cross-sectional and longitudinal association as a function of smoking history
Mean raw baseline cognitive scores and 10-year cognitive change for all 5 cognitive tests are presented in eTable 1. The cross-sectional associations between smoking history and cognitive function at Phase 5, estimated from the mixed models (model 1) suggested that long-term ex-smokers had better cognitive scores than never smokers on all tests except memory, in both men and women (eTable 2). Table 2 shows the estimates of subsequent cognitive change over 10 years derived from the same models. In men, compared to never smokers, current smokers had a greater 10-year decline in global cognition (mean difference in decline (95%CI)=−0.09 (−0.15; −0.03)) and executive function (−0.11 (−0.17; −0.05)). This effect size was similar to the effect of 10 years of age on cognitive decline. Among recent ex-smokers, decline in executive function (−0.08 (−0.14, −0.02)) was faster than among never smokers. Smoking history was not associated with cognitive change in women.
Table 2.
Association of smoking history at Phase 5 (1997–99) and cognitive change over the subsequent 10 years.†
| N | Cognitive change over 10 years
|
||||
|---|---|---|---|---|---|
| Global cognition | Memory | Vocabulary | Executive function | ||
|
| |||||
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | ||
| MEN (N=5099) | |||||
| Current smokers | 468 | −0.09 (−0.15; −0.03)* | −0.05 (−0.16; 0.06) | −0.04 (−0.09; 0.01) | −0.11 (−0.17; −0.05)* |
| Recent ex-smokers | 408 | −0.04 (−0.09; 0.02) | 0.04 (−0.07; 0.15) | 0.00 (−0.05; 0.05) | −0.08 (−0.14; −0.02)* |
| Long-term ex-smokers | 1825 | 0.00 (−0.03; 0.03) | −0.05 (−0.11; 0.02) | 0.00 (−0.03; 0.02) | 0.02 (−0.02; 0.05) |
| Never smokers | 2398 | Ref | Ref | Ref | Ref |
| Estimates in never smokers | 2398 | −0.32 (−0.35; −0.29) | −0.24 (−0.29; −0.18) | 0.02 (0.00; 0.05) | −0.37 (−0.41; −0.34) |
| WOMEN (N=2137) | |||||
| Current smokers | 262 | 0.03 (−0.05; 0.12) | 0.04 (−0.14; 0.21) | 0.02 (−0.06; 0.10) | 0.03 (−0.06; 0.12) |
| Recent ex-smokers | 191 | 0.01 (−0.08; 0.10) | −0.03 (−0.22; 0.16) | 0.00 (−0.08; 0.09) | 0.04 (−0.07; 0.14) |
| Long-term ex-smokers | 507 | −0.01 (−0.07; 0.05) | −0.01 (−0.14; 0.11) | −0.02 (−0.07; 0.04) | 0.00 (−0.07; 0.07) |
| Never smokers | 1177 | Ref | Ref | Ref | Ref |
| Estimates in never smokers | 1177 | −0.28 (−0.33; −0.23) | −0.24 (−0.34; −0.14) | 0.04 (0.00; 0.09) | −0.35 (−0.40; −0.30) |
p<0.05
Estimates from a mixed model adjusted for educational level (ordinal variable, 5 levels), occupational position (categorical variable, 3 levels), marital status, age at baseline. A negative value for cognitive change corresponds to a higher decline compared to that in the never smokers.
In men, the associations between smoking history and decline in global cognition and executive function were not attenuated after adjustment for other health behaviours and health measures (eTable 3). Entering CHD and stroke events as time-dependent covariates did not change these results (eTable 4). In men with data on lung function (N=4100), adjustment for the mean FEV1 over the follow-up (Phases 7 and 9) also did not reduce the association (results not shown).
Analysis using pack-years of smoking in men showed that for every ten pack-years there was greater decline in global cognition (mean 10-year cognitive decline (95%CI)=−0.009 (−0.017; −0.001)) and executive function (−0.010 (−0.019; −0.001)). No association with pack-years of smoking was observed in women.
Smoking status over the follow-up and concurrent cognitive change
In men, compared to never smokers, persistent smokers over the follow-up were more likely to show faster decline in global cognition (−0.12 (−0.19; −0.04)), memory (−0.15 (−0.29; −0.01)), and executive function (−0.11 (−0.20; −0.03)), Table 3. Intermittent smokers also had greater decline in global cognition (−0.10 (−0.20; 0.00)). The 168 men who stopped smoking after Phase 5 did not show greater cognitive decline than the never smokers but their decline was not statistically different from that in persistent smokers (p=0.21 for global cognition, p=0.71 for memory, p=0.43 for vocabulary, and p=0.36 for executive function). In women there was no evidence of an association.
Table 3.
| N | Cognitive change over 10 years
|
||||
|---|---|---|---|---|---|
| Global cognition | Memory | Vocabulary | Executive function | ||
|
| |||||
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | ||
| MEN (N=4800) | |||||
| Persistent smokers | 240 | −0.12 (−0.19; −0.04)* | −0.15 (−0.29; −0.01)* | −0.04 (−0.10; 0.03) | −0.11 (−0.20; −0.03)* |
| Intermittent smokers | 106 | −0.10 (−0.20; 0.00)* | −0.16 (−0.36; 0.04) | 0.00 (−0.08; 0.09) | −0.10 (−0.21; 0.01) |
| Quitters after Phase 5 | 168 | −0.04 (−0.13; 0.03) | 0.08 (−0.08; 0.24) | 0.08 (−0.09; 0.25) | −0.08 (−0.17; 0.01) |
| Never smokers | 2242 | Ref | Ref | Ref | Ref |
| Estimates in never smokers | 2242 | −0.32 (−0.35; −0.29) | −0.24 (−0.30; −0.18) | 0.02 (−0.01; 0.04) | −0.38 (−0.41; −0.35) |
|
| |||||
| WOMEN (N=1993) | |||||
| Persistent smokers | 128 | 0.01 (−0.11; 0.12) | 0.09 (−0.15; 0.33) | 0.03 (−0.08; 0.14) | −0.03 (−0.15; 0.09) |
| Intermittent smokers | 16 | −0.36 (−0.69; −0.04)* | −0.53 (−1.21; 0.15) | −0.23 (−0.54; 0.09) | −0.20 (−0.56; 0.17) |
| Quitters after Phase 5 | 100 | 0.05 (−0.07; 0.16) | −0.08 (−0.32; 0.16) | 0.00 (−0.11; 0.10) | 0.08 (−0.04; 0.21) |
| Never smokers | 1104 | Ref | Ref | Ref | Ref |
| Estimates in never smokers | 1104 | −0.29 (−0.34; −0.24) | −0.26 (−0.36; −0.15) | 0.04 (0.00; 0.09) | −0.36 (−0.41; −0.30) |
p<0.05
Smoking status at Phase 9 defined as: persistent smokers (smokers at both Phases 5 and 9), intermittent smokers (ex-smokers at Phase 5 and current smokers at Phase 9), quitters (current smokers at Phase 5 and ex-smokers at Phase 9). If participants drop-out at Phase 7, smoking status at Phase 7 was used in the analysis using a similar definition. Participants without information on smoking status at Phases 7 or 9 were excluded from this analysis (N=299 men and N=144 women). Results among ex-smokers at both Phases 5 and 9 are not shown.
Estimates from a mixed model adjusted for educational level (ordinal variable, 5 levels), occupational position (categorical variable, 3 levels), marital status, age at baseline.
Smoking history at Phase 5 and cognitive decline as a function of age in men
The interaction term between age (continuous variable) at baseline, smoking, and time suggested differences in the effect of smoking for global cognition (p=0.08) and executive function (p=0.04) as a function of age. These findings are summarized in Figure 1 which shows the analyses reported in Table 2 (differences in cognitive decline between the smoking history categories with the never smokers as the reference group) but stratified by median age (55 years). There was some evidence that the impact of smoking on cognitive decline was weaker in the older group.
Figure 1. Association between smoking history at Phase 5 and cognitive change over the subsequent 10 years in men as a function of age group (reference group: never smokers)*.
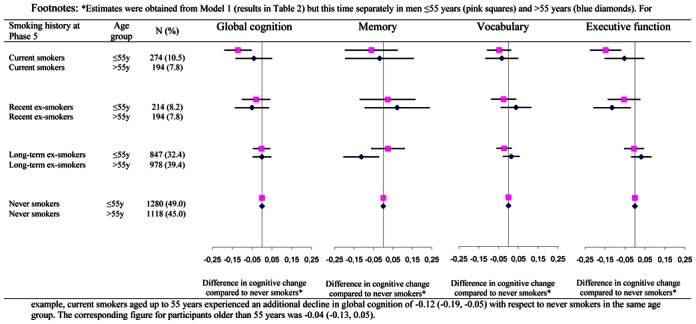
*Estimates were obtained from Model 1 (results in Table 2) but this time separately in men ≤55 years (pink squares) and >55 years (blue diamonds). For example, current smokers aged up to 55 years experienced an additional decline in global cognition of −0.12 (−0.19, −0.05) with respect to never smokers in the same age group. The corresponding figure for participants older than 55 years was −0.04 (−0.13, 0.05).
Joint models
These analyses assessed the effect of drop-out, due to death or non-participation during the follow-up, on the association between smoking history and cognitive decline (see eResults for more details). Joint model estimates of cognitive change were around 10% higher than those using mixed models alone, with larger differences seen in current smokers than in never smokers (Figure 2). The relative differences between the estimates from the mixed model and the joint models were more evident in the oldest group (> 55 years), with estimates being 100% stronger in the joint models in this age group compared to 17% stronger in the youngest group.
Figure 2.
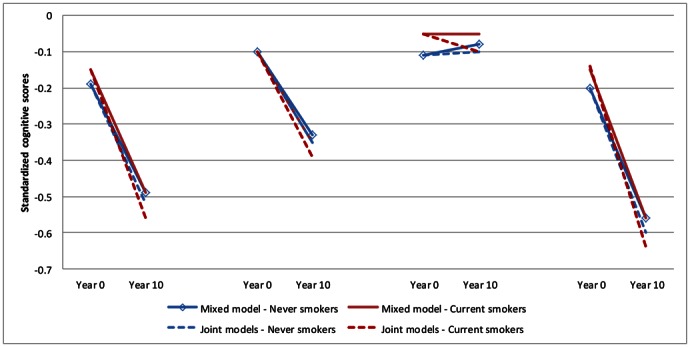
Mixed and joint models showing standardized cognitive scores at baseline and 10 years cognitive decline in current and never smokers at Phase 5 (1997–99).
Sensitivity analysis
Analyses restricted to those with a MMSE score ≥24 (N=7165) or those with complete data at all three waves of cognitive data yielded results similar to that in the main analysis (not shown).
COMMENT
Our analysis of data using six assessments of smoking status over 25 years and three cognitive assessments over ten years presents four key findings. One, in men, smoking was associated with faster cognitive decline; analyses using pack-years of smoking suggested a dose-response relation. Two, men who continued smoking over the follow-up experienced greater decline in all cognitive tests. Three, men who quit smoking in the 10 years preceding the first cognitive measure were still at risk of greater cognitive decline, particularly in executive function. However, long-term ex-smokers did not show faster cognitive decline. Finally, our results show that the association between smoking and cognition, particularly at older ages, is likely to be underestimated due to higher risk of death and dropping-out among smokers.
Our previous paper based on data from the first two waves of cognitive assessment showed smoking in midlife to be associated with poor memory and 5-year decline in reasoning abilities.[7]We also showed long-term ex-smokers to have better memory and verbal fluency scores than never smokers. In the present paper, the third wave of cognitive data allowed us: 1) to estimate the association between smoking history and 10-year cognitive decline; 2) to cover an age window from 45 to 80 years; 3) to use mixed models with multiple repeated measures rather than analysis of change using two waves of data. The third measurement reduces potential biases related to practice effects and regression to the mean, which are particularly encountered in studies with only two measurements.[37–39]Thus, the present analyses provide more robust estimates of the impact of smoking on cognitive decline.
Comparison with other studies
At least four previous studies[7, 9, 10, 13]have examined the association between smoking and cognitive decline with cognition first assessed in midlife. In the 1946 Birth Cohort,[13]smoking was associated with a greater decline in memory but not visual search. In the Doetinchem Cohort study,[10]smokers had faster decline in memory, cognitive flexibility, and global cognition, but not processing speed. Finally, the ARIC-MRI study, the only other study with more than 2 waves of cognitive data in a non-elderly population,[9]did not find smoking to influence cognitive decline. One possible explanation for the lack of association in the ARIC-MRI study is that the study population was composed mainly of women, 62% of total population. Our results show no association between smoking and cognitive decline in women, but the underlying reasons remain unclear. Some studies have reported sex differences in this association,[13, 40]while others report no differences.[10, 15]One explanation for the sex difference we observed might be the greater quantity of tobacco smoked by men.[40]Indeed, the mean pack-years of smoking (36 vs. 31, p=0.05) as well as the number of cigarettes smoked (19 vs. 16, p=0.007) was higher in men than women. It is also possible that smoking clusters with other risk factors differently in men and women. Alcohol consumption greater than the recommended quantities were seen in 38.7% of male and 23.3% female smokers; mean alcohol consumption in smokers was considerably higher in men than in women, 23.0 vs 9.6 units/week, p<0.0001. Future research needs to explore possible reasons for these differences.
Few observational studies have distinguished long-term ex-smokers from recent ex-smokers. In the 1946 British Birth Cohort study,[13]long-term ex-smokers had better memory and slower decline in memory compared with never smokers but in the Honolulu-Asia Aging study,[41]long-term ex-smokers did not have a lower risk of cognitive impairment than never smokers, and recent ex-smokers had the same increased risk of impairment as current smokers. In the Doetinchem Cohort study,[10]no difference was found between recent ex-smokers, long-term ex-smokers and never smokers, although the point estimates of decline increased steadily from never smokers to long-term ex-smokers, then to recent ex-smokers and to smokers. Our results show that long-term ex-smokers did not have greater cognitive decline than never smokers while male recent ex-smokers had on average greater decline in executive function than never smokers. These results suggest that residual effects of smoking on cognition might wear off approximately a decade after smoking cessation. A recent non-randomized trial[42]of smoking cessation on 95 non-smokers and 228 smokers aged 68 to 88 years found recent quitters (defined as a minimum of 18 smoking free months over the 24-month period of follow-up) not to have greater cognitive decline than never smokers. The discrepancy with our results might be explained by factors such as the older and smaller study population in the trial as well as the use of a cognitive test battery (the Wechsler Logical memory test and Alzheimer’s Disease Assessment Scale) designed to assess changes in memory and symptoms of Alzheimer’s disease.
Mechanisms
In the present study the adverse impact of smoking was greater on executive function than memory or vocabulary. Executive function, an umbrella term for various complex cognitive processes involved in achieving a particular goal,[43]has been shown to be particularly affected in vascular dementia.[44]We assessed executive function using measures of reasoning and verbal fluency, as these tasks require the combination of different cognitive abilities such as memory, attention, and speed of information processing.[25, 26]Smoking is an important risk factor for vascular diseases[45]and could influence executive function via vascular pathways. Nevertheless the inclusion of heart rate (a marker of cardiovascular fitness),[46]cardiovascular diseases and cardiovascular risk factors such as blood pressure and cholesterol in the analysis did not attenuate the association with smoking. Although the mechanisms by which smoking affects cognitive decline remain unclear, it has been shown to be associated with periventricular and subcortical white matter lesion progression, themselves associated with greater cognitive decline,[47]independently of other cardiovascular risk factors.
Another mechanism that could underlie the association between smoking and cognitive decline is lung function.[8]Smoking is a risk factor for lung injuries[16]that can increase risk of cognitive impairment and dementia.[17, 18]However, the association between smoking and cognitive decline in our study was not explained by lung function, measured by FEV1. As this measure was introduced only 5 years after the first cognitive assessment, further research is required to examine this potential mechanism in greater detail.
Influence of drop-out
In longitudinal studies, drop-out is common and death is also a cause of sample attrition, particularly in older populations. Drop-out is a potential source of bias if it is non-random, in that it is associated with either the exposure and/or the outcome under investigation, independently of observed data. Individuals who drop-out are more likely to have health problems and experience greater cognitive decline.[48, 49]Smoking history in our data was associated with both mortality and drop-out during the follow-up, suggesting that cognitive decline may be underestimated among smokers. Our results from the joint models of cognitive decline and drop-out are consistent with this possibility; the estimated differences in cognitive change between current smokers and never smokers were 1.2 to 1.5 times larger than those from the mixed models. Furthermore, in older men mixed models suggested weaker association between smoking and cognitive decline compared to the younger group. These estimates increased by up to 100% when information on drop-outs was included in the joint models, thus reducing the apparent difference between the younger and older men in the association between smoking and cognitive decline. These results illustrate the selection biases encountered in studies investigating the association between smoking, a strong risk factor for mortality, and cognitive ageing in the elderly.[5, 6]Indeed, such studies have led to speculation as to whether smoking is a risk factor for dementia or whether nicotine has a protective effect on the brain.[5]This confusion stems from the fact that smokers susceptible of dying or developing dementia may already have done so by the age of inclusion in ageing studies, and thus the group of elderly participants free of dementia at baseline in ageing studies are depleted of susceptible smokers.[5]Our results on cognitive decline in a non-elderly population might therefore better capture the potential impact of smoking on cognitive function. Further research on elderly populations, possibly even reanalysis of published data, using joint models is needed to understand the impact of smoking on cognitive decline.
Limitations
Our study has limitations. First, although the sample covered a wide socioeconomic range, with annual full-time salaries ranging from £4,995 to £150,000, data are from white-collar civil servants and cannot be assumed to be representative of the general population, particularly the unemployed or blue-collar workers. Second, smoking was self-reported and is likely to have been under-reported. Third, we could not ascertain dementia cases and the extent to which this impacts our results is unclear but our findings regarding stronger relations before age 55, when dementia is exceptional, suggest that dementia might not influence the results. The fourth limitation relates to the cognitive tests being dependent on writing speed. Finally, it must be noted that the method we used to model jointly the longitudinal cognitive change, the time to drop-out, and the time to death[20]is not yet widely used and makes assumptions that cannot be tested using observed data such as the jointly multivariate Gaussian random effects.[50]Other methods to take into account missing data may not produce the same estimate of cognitive decline.[51]The extent to which estimates of cognitive decline vary as a function of the method used to correct for drop-out remains unclear. Nevertheless, the differences seen between the estimates from mixed models and the joint models can be reliably used to conclude that non-response leads to underestimation of the impact of smoking on cognitive decline.
Implications
Much research on uncovering risk factors for dementia or adverse cognitive ageing profiles has been carried out in elderly populations. It is increasingly recognized that age-related cognitive pathologies such as dementia result from long term processes, perhaps beginning as long as 20 to 30 years before the clinical diagnosis of dementia.[1, 52]Our study illustrates the importance of examining risk factors for cognitive decline much earlier in the lifecourse. However, cognitive tests and age-specific norms for detecting ‘abnormal’ cognitive decline do not yet exist. Thus, it is difficult to quantify the clinical significance of our findings. We observe that the effect size associated with smoking is similar to that associated with 10 years of age. The extent to which the steeper cognitive trajectories observed in smokers will lead to dementia later in life cannot yet be addressed using our data and is an important research question.
Conclusion
Our results show that, compared to never smokers, middle-aged male smokers are likely to experience faster 10-year cognitive decline in global cognition and executive function. Intermittent smokers and recent ex-smokers also exhibited greater cognitive decline although no residual adverse effect of smoking on cognitive decline was observable in the group of men who stopped smoking 10 years prior to cognitive testing. Public health messages on smoking should continue to target smokers at all ages.
Acknowledgments
Funding/Support: ASM is supported by a “European Young Investigator Award” from the European Science Foundation and the National Institute on Aging, NIH (R01AG013196; R01AG034454). MK is supported by the Academy of Finland, the BUPA Foundation, the National Institutes of Health (R01HL036310; R01AG034454). MJS is supported by the British Heart Foundation. JH is supported by the National Institute on Aging, NIH (R01AG013196). The Whitehall II study is also supported by the British Medical Research Council (G0902037). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
Footnotes
Data access and responsibility: Dr Sabia had full access to all of the data in the study and performed the statistical analysis. She takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors had full access to all the data in the study.
Disclosures: None.
Reference List
- 1.Alzheimer’s Disease International. World Alzheimer Report. 2009. Ref Type: Online Source. [Google Scholar]
- 2.Anstey KJ, von SC, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007 Aug 15;166(4):367–78. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 3.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusanen M, Kivipelto M, Quesenberry CP, Jr, Zhou J, Whitmer RA. Heavy Smoking in Midlife and Long-term Risk of Alzheimer Disease and Vascular Dementia. Arch Intern Med. 2010 Oct 25; doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- 5.Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008 May;19(3):448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 6.Elbaz A, Alperovitch A. Bias in association studies resulting from gene-environment interactions and competing risks. Am J Epidemiol. 2002 Feb 1;155(3):265–72. doi: 10.1093/aje/155.3.265. [DOI] [PubMed] [Google Scholar]
- 7.Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008 Jun 9;168(11):1165–73. doi: 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins N, Sachs-Ericsson N, Preacher KJ, Sheffield KM, Markides K. Smoking increases risk for cognitive decline among community-dwelling older Mexican Americans. Am J Geriatr Psychiatry. 2009 Nov;17(11):934–42. doi: 10.1097/JGP.0b013e3181b0f8df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009 May;5(3):207–14. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Nooyens AC, van Gelder BM, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health. 2008 Dec;98(12):2244–50. doi: 10.2105/AJPH.2007.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A, Launer LJ. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004 Mar 23;62(6):920–4. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- 12.Reitz C, Luchsinger J, Tang MX, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005 Sep 27;65(6):870–5. doi: 10.1212/01.wnl.0000176057.22827.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003 Jun;93(6):994–8. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters R, Beckett N, Geneva M, Tzekova M, Lu FH, Poulter R, Gainsborough N, Williams B, de Vernejoul MC, Fletcher A, Bulpitt C. Sociodemographic and lifestyle risk factors for incident dementia and cognitive decline in the HYVET. Age Ageing. 2009 Sep;38(5):521–7. doi: 10.1093/ageing/afp094. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009 Jun 9;72(23):2029–35. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The 2004 United States Surgeon General’s Report: The Health Consequences of Smoking. N S W Public Health Bull. 2004 May;15(5–6):107. [PubMed] [Google Scholar]
- 17.Pathan SS, Gottesman RF, Mosley TH, Knopman DS, Sharrett AR, Alonso A. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011 Jan 18;18(6):888–98. doi: 10.1111/j.1468-1331.2010.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh-Manoux A, Dugravot A, Kauffmann F, Elbaz A, Ankri J, Nabi H, Kivimaki M, Sabia S. Association of lung function with physical, mental and cognitive function in early old age. Age (Dordr ) 2010 Sep 29; doi: 10.1007/s11357-010-9189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, Wolf PA. Dementia after stroke: the Framingham Study. Stroke. 2004 Jun;35(6):1264–8. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 20.Diggle P, Henderson R, Philipson P. Random-effects models for joint analysis of repeated-measurement and time-to-event outcomes. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal Data Analysis. Chapman & Hall/CRC; 2009. pp. 349–66. [Google Scholar]
- 21.Guo X, Carlin BP. Separate and Joint Modeling of Longitudinal and Event Time Data Using Standard Computer Packages. The American Statistician. 2004 Feb 1;58(1):16–24. [Google Scholar]
- 22.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000 Dec;1(4):465–80. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 23.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005 Apr;34(2):251–6. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 24.Raven JC. Guide to using the Mill Hill vocabulary test with progressive matrices. London, UK: HK Lewis; 1965. [Google Scholar]
- 25.Heim AW. AH 4 group test of general Intelligence. Windsor, UK: NFER-Nelson Publishing Company Ltd; 1970. [Google Scholar]
- 26.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologica. 1967;5:135–40. [Google Scholar]
- 27.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010 Sep 21;75(12):1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arvanitakis Z, Grodstein F, Bienias JL, Schneider JA, Wilson RS, Kelly JF, Evans DA, Bennett DA. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008 Jun 3;70(23):2219–25. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- 29.Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010 Mar 24;303(12):1159–66. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Levy S, McNamara RL, Prystowsky EN, Wann LS, Wyse DG, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC, Klein WW, Alonso-Garcia A, Blomstrom-Lundqvist C, De BG, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001 Oct;38(4):1231–66. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 31.Ferrie JE, Langenberg C, Shipley MJ, Marmot MG. Birth weight, components of height and coronary heart disease: evidence from the Whitehall II study. Int J Epidemiol. 2006 Dec;35(6):1532–42. doi: 10.1093/ije/dyl184. [DOI] [PubMed] [Google Scholar]
- 32.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003 Jan 1;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 33.Sabia S, Shipley M, Elbaz A, Marmot M, Kivimaki M, Kauffmann F, Singh-Manoux A. Why does lung function predict mortality? Results from the Whitehall II Cohort Study. Am J Epidemiol. 2010 Dec 15;172(12):1415–23. doi: 10.1093/aje/kwq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes D, Jr, Kraman SS. The physiologic basis of spirometry. Respir Care. 2009 Dec;54(12):1717–26. [PubMed] [Google Scholar]
- 35.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982 Dec;38(4):963–74. [PubMed] [Google Scholar]
- 36.Anstey KJ, Burns RA, Birrell CL, Steel D, Kiely KM, Luszcz MA. Estimates of probable dementia prevalence from population-based surveys compared with dementia prevalence estimates based on meta-analyses. BMC Neurol. 2010;10:62. doi: 10.1186/1471-2377-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke PS. Analysing change based on two measures taken under different conditions. Stat Med. 2005 Nov 30;24(22):3401–15. doi: 10.1002/sim.2198. [DOI] [PubMed] [Google Scholar]
- 38.Dugravot A, Guéguen A, Kivimaki M, Vahtera J, Shipley M, Marmot M, Singh-Manoux A. Socioeconomic position and cognitive decline using data from 2 waves: What is the role of the wave 1 cognitive measure? J Epidemiol Comm Health. 2009;63(8):675–80. doi: 10.1136/jech.2008.081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005 Aug 1;162(3):267–78. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 40.Stewart MC, Deary IJ, Fowkes FG, Price JF. Relationship between lifetime smoking, smoking status at older age and human cognitive function. Neuroepidemiology. 2006;26(2):83–92. doi: 10.1159/000090253. [DOI] [PubMed] [Google Scholar]
- 41.Galanis DJ, Petrovitch H, Launer LJ, Harris TB, Foley DJ, White LR. Smoking history in middle age and subsequent cognitive performance in elderly Japanese-American men. The Honolulu-Asia Aging Study. Am J Epidemiol. 1997 Mar 15;145(6):507–15. doi: 10.1093/oxfordjournals.aje.a009138. [DOI] [PubMed] [Google Scholar]
- 42.Almeida OP, Garrido GJ, Alfonso H, Hulse G, Lautenschlager NT, Hankey GJ, Flicker L. 24-Month effect of smoking cessation on cognitive function and brain structure in later life. Neuroimage. 2011 Jan 31; doi: 10.1016/j.neuroimage.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 43.Elliott R. Executive functions and their disorders. Br Med Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004 Nov 15;226(1–2):3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 45.WHO. Cardiovascular diseases. 2007. Feb, Available at: URL: http://www.who.int/mediacentre/factsheets/fs317/en/print.html.
- 46.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002 Mar 14;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 47.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008 Oct;39(10):2712–9. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 48.Tyas SL, Tate RB, Wooldrage K, Manfreda J, Strain LA. Estimating the incidence of dementia: the impact of adjusting for subject attrition using health care utilization data. Annals of Epidemiology. 2006 Jun;16(6):477–84. doi: 10.1016/j.annepidem.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008 May;19(3):440–7. doi: 10.1097/EDE.0b013e31816a1d31. [DOI] [PubMed] [Google Scholar]
- 50.Diggle PJ, Sousa I, Chetwynd AG. Joint modelling of repeated measurements and time-to-event outcomes: the fourth Armitage lecture. Stat Med. 2008 Jul 20;27(16):2981–98. doi: 10.1002/sim.3131. [DOI] [PubMed] [Google Scholar]
- 51.Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal Data with Follow-up Truncated by Death: Match the Analysis Method to Research Aims. Stat Sci. 2009;24(2):211. doi: 10.1214/09-STS293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Launer LJ. The epidemiologic study of dementia: a life-long quest? Neurobiol Aging. 2005 Mar;26(3):335–40. doi: 10.1016/j.neurobiolaging.2004.03.016. [DOI] [PubMed] [Google Scholar]


