Abstract
CD4+CD25highCD127low/− forkhead box p3 (Foxp3)+ regulatory T cells (Treg cells) possess functional plasticity. Here we describe a higher frequency of T helper type 1 (TH1)-like, interferon-γ (IFN-γ)-secreting Foxp3+ T cells in untreated subjects with relapsing remitting multiple sclerosis (RRMS) as compared to healthy control individuals. In subjects treated with IFN-β, the frequency of IFN-γ+Foxp3+ T cells is similar to that in healthy control subjects. In vitro, human Treg cells from healthy subjects acquire a TH1-like phenotype when cultured in the presence of interleukin-12 (IL-12). TH1-like Treg cells show reduced suppressive activity in vitro, which can partially be reversed by IFN-γ–specific antibodies or by removal of IL-12.
Multiple sclerosis is a genetically mediated chronic inflammatory disease of the central nervous system (CNS). Activated CD4+ inflammatory cells in the circulation of affected individuals infiltrate into the CNS and damage both myelin and axons1,2. A general loss of immune regulation is commonly seen in human autoimmune diseases3–6.
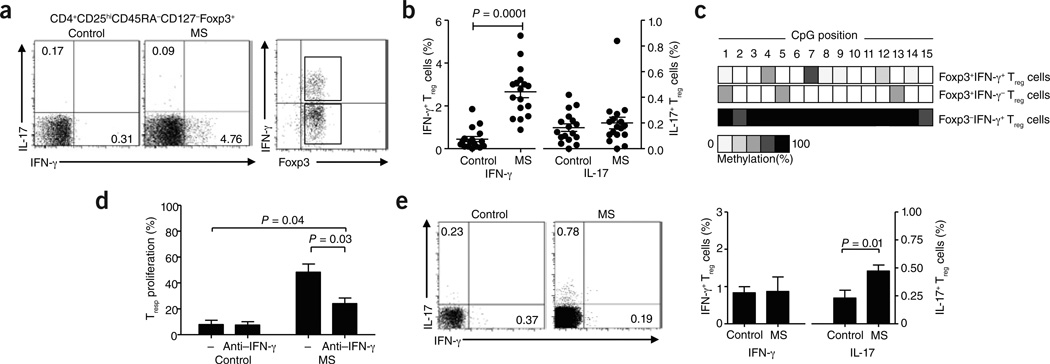
In light of recent findings suggesting that Foxp3+ Treg cells show functional and phenotypic plasticity and are capable of secreting proinflammatory cytokines7–9, we were interested in analyzing ex vivo secretion of cytokines by Treg cells from individuals with relapsing/remitting multiple sclerosis (RRMS) that had not been receiving any immunomodulatory treatment as compared to age-matched healthy controls (Supplementary Methods and Supplementary Table 1). We stimulated FACS-sorted CD4+CD45RA−CD25highCD127low/− Treg cells (Supplementary Fig. 1a) for 4 h with phorbol 12-myristate 13-acetate (PMA) and ionomycin. The percentage of Treg cells producing IFN-γ was significantly higher in untreated individuals with RRMS as compared to healthy control individuals (Fig. 1a,b). The frequency of Treg cells producing IL-17 did not differ between individuals with RRMS and control subjects. Analysis of the Foxp3+ Treg cell–specific demethylated region (TSDR) in sorted IFN-γ+Foxp3+ and IFN-γ−Foxp3+ multiple sclerosis Treg cells revealed that IFN-γ–producing Foxp3+ Treg cells possessed a similar pattern of demethylation in the TSDR region to that of IFN-γ−Foxp3+ Treg cells (Fig. 1c) and healthy control Foxp3+ Treg cells (data not shown).
Figure 1.
Treg cells from individuals with RRMS secrete IFN-γ ex vivo. (a) The frequency of FACS-sorted IFN-γ+ and IL-17+ Treg cells in healthy control individuals (left) and untreated individuals with RRMS (middle, n = 17) gated on Foxp3+ Treg cells. Right, purity analysis of the sorted IFN-γ+Foxp3+ and IFN-γ Foxp3+ populations from subjects with RRMS used for methylation analysis in c. (b) Percentage of IFN-γ+Foxp3+ and IL-17+Foxp3+ Treg cells (n = 17) as a proportion of total Foxp3+ Treg cells. (c) Representative example of methylation analysis of the TSDR region of the FOXP3 locus in sorted IFN-γ+Foxp3+ and IFN-γ− Foxp3+ Treg cells from subjects with RRMS. An analysis of IFN-γ+Foxp3− memory T cells from subjects with RRMS is shown as a control. (d) Proliferation of responder T (Tresp) cells cultured with ex vivo FACS-sorted Treg cells from healthy control subjects and untreated subjects with multiple sclerosis (MS; Treg cell:Tresp cell ratio of 1:2) in the presence or absence of an IFN-γ–specific antibody (n = 4). (e) The frequency of IFN-γ+ and IL-17+ Treg cells in healthy control subjects (left) or IFN-β–treated patients with RRMS (right) as assessed by intracellular cytokine staining and FACS analysis. The bar diagram (right) shows the percentage of IFN-γ+Foxp3+ and IL-17+Foxp3+ cells as a proportion of total Foxp3+ Treg cells in healthy controls or IFN-β–treated patients with RRMS (n = 12). Approval for studies was obtained from the Brigham and Women’s Hospital Institutional Review Board, and informed consent was obtained from all donors.
After a 4-h stimulation with PMA and ionomycin, Treg cells isolated ex vivo from untreated subjects with RRMS showed a TH1-like phenotype, including secretion of IFN-γ, upregulation of mRNA expression of the TH1-associated transcription factor TBET (encoded by TBX21) and downregulation of RORC (encoding RAR-related orphan receptor C) and TGFB1 (encoding transforming growth factor β1) mRNA (Supplementary Fig. 2 and Supplementary Table 1). CXCR3, but not CCR5 or IL10, was upregulated in Treg cells from subjects with RRMS as compared to healthy controls (Supplementary Fig. 2). Treg cells from subjects with RRMS downregulated CTLA4 (encoding cytotoxic T lymphocyte–associated protein 4) (271.8 ± 86.9 (arbitary units) in controls compared to 43.91 ± 11.7 in RRMS Treg cells).
To ascertain whether IFN-γ secretion by RRMS Treg cells reduces their suppressive activity, we cultured Treg cells and responder T cells ex vivo in the presence of an IFN-γ–specific antibody. The suppressive activity of multiple sclerosis Treg cells was significantly increased upon IFN-γ blockade, whereas healthy control Treg cells were not affected (Fig. 1d).
IFN-β has been shown to affect the IL-12–IFN-γ axis in people with multiple sclerosis10, among its other immunomodulatory effects. To examine the possible in vivo role of this axis in the generation of IFNγ+ Foxp3+ T cells in people with RRMS, we performed a cross-sectional investigation, isolating Treg cells from patients with RRMS treated for at least 1 year with IFN-β and comparing them to Treg cells from healthy control subjects. Intracellular staining revealed that the frequency of IFN-γ+Foxp3+ T cells in IFN-β–treated subjects with RRMS was similar to that in age- and sex-matched healthy control subjects (Fig. 1e). This was accompanied by an increase in the frequency of IL-17+Foxp3+ T cells in IFN-β–treated patients with RRMS compared to healthy controls (Fig. 1e). Although these alterations in cytokine release could be due to a direct effect of IFN-β on IL-17 secretion by Treg cells, IFNβ–mediated decreases in the amount of IL-12 could also induce a change in the cytokine milieu, driving increased IL-17 production.
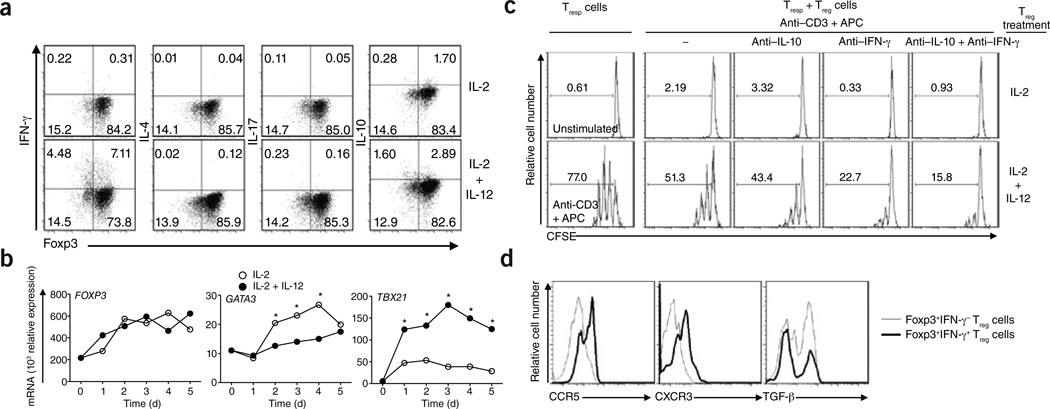
Individuals with autoimmune disease have elevated IL-12 expression, and Treg cells isolated from such individuals show reduced suppressive activity3,11,12. To elucidate the mechanism by which Treg cells produce IFN-γ, we hypothesized that IL-12, a cytokine associated with TH1 responses, would induce a TH1-like phenotype in Treg cells, similar to the phenotype we observed ex vivo in Treg cells from subjects with RRMS. We cultured Treg cells from healthy control subjects in the presence or absence of IL-12. After 4 d, a significant (P = 0.009) percentage of IL-12–stimulated Treg cells secreted IFN-γ (Fig. 2a and Supplementary Fig. 1b). This increase was even more pronounced when we stimulated Treg cells from subjects with RRMS with IL-12 (Supplementary Fig. 1c), suggesting a higher intrinsic responsiveness to IL-12 in these individuals.
Figure 2.
Characterization of IL-12–driven, IFN-γ+Foxp3+ Treg cells in vitro from healthy controls. (a) Intracellular staining for IFN-γ, IL-4, IL-17 and IL-10 of untreated (upper row) and IL-12-stimulated (bottom row) human Treg cells from healthy controls at day 4. (b) mRNA expression of FOXP3 (left), GATA3 (middle) and TBX21 (right) in Treg cells stimulated in the presence or absence of IL-12 for 5 d (data are a representative example of three experiments performed with similar results; *P < 0.05). (c) Proliferation of Tresp cells cocultured for 3 d with Treg cells (Treg cell:Tresp cell ratio of 1:2) previously treated with IL-2 (top) or IL-2 + IL-12 (bottom), as assessed by carboxyfluorescein succinimidyl ester (CFSE) dilution. Histograms depict unstimulated Tresp cells alone (top left), Tresp cells stimulated with antibody to CD3 (anti-CD3) and antigen-presenting cells without Treg cells (bottom left) and Tresp cell and Treg cell cocultures without blocking antibodies (second column), in the presence of an IL-10–specific blocking antibody (anti–IL-10; third column), IFN-γ–specific blocking antibody (anti–IFN-γ; fourth column) or both antibodies (fifth column). (d) Representative example of staining for TH1-associated chemokines(CCR5 and CXCR3) and TGF-β on IFN-γ+Foxp3+ and IFN-γ− Foxp3+ Treg cells (data are representative of three experiments).
The capacity of Treg cells from healthy subjects to secrete IFN-γ was not accompanied by loss of Foxp3 expression (Supplementary Figs. 1d and 3) and was dependent on the dose of IL-12 (Supplementary Fig. 4). IL-12 also induced a modest increase in IL-10, but not in IL-17 or IL-4, production (Fig. 2a and Supplementary Fig. 5). Other members of the IL-12 family of cytokines, including IL-23 and IL-27, did not induce either IFN-γ or IL-10 production (Supplementary Fig. 6). We confirmed at a single-cell level that IL-12 could induce IFN-γ and IL-10 production in Treg cell clones (Supplementary Figs. 7 and 8).
As expected given the induction of IFN-γ secretion by IL-12, and similar to what we observed in ex vivo Treg cells from subjects with RRMS IL-12–stimulated Treg cells expressed significantly more TBX21 mRNA and protein and less GATA3 mRNA as compared to Treg cells not treated with IL-12 (Fig. 2b and Supplementary Fig. 9). There was no change in the expression of FOXP3, RORC, IRF1 (encoding interferon regulatory factor 1) and MAF, a transcription factor related to IL-10 production (Fig. 2b and Supplementary Fig. 9). We obtained similar results with single-cell–derived Treg cell clones stimulated for 10 d in the presence of IL-12 (Supplementary Fig. 10).
To examine whether IL-12–stimulated Foxp3+T-bet+ Treg cells were functionally suppressive, we cocultured CD4+CD25low/− responder T cells with Treg cells that had been prestimulated for 4 d with IL-2 alone or with IL-2 and IL-12, along with antibody to CD3 and irradiated T cell–depleted peripheral blood mononuclear cells. After 3 d, Treg cells cultured with IL-2 and IL-12 were significantly less effective at inhibiting responder T cell proliferation as compared to IL-2–treated Treg cells (Fig. 2c and Supplementary Fig. 9c). Blocking IFN-γ increased the suppressive activity of Treg cells cultured with IL-2 and IL-12 but not that of control cells treated with only IL-2. These data suggest that other mechanisms may also account for the defect in suppression. We obtained the same results in antigen-presenting cell–free coculture assays (Supplementary Fig. 11). We also observed diminished suppressive activity of IL-12–treated Treg cell clones (Supplementary Fig. 12). Although our data point to a general defect in the suppressive activity of TH1-like, Foxp3+ T cells, it is unclear whether these cells are able to specifically suppress TH1 responses, as has been shown in a mouse model of inflammation13.
Next we examined the reversible nature of the TH1-like Treg cell phenotype. We collected Treg cells cultured with IL-12 for 4 d and split them into two populations; one was washed to eliminate IL-12, whereas the other was cultured with IL-12 for 3 d longer. The frequency of IFN-γ+Foxp3+ T cells decreased when we cultured the cells without IL-12 for the last 72 h, and these Treg cells reacquired suppressive activity (Supplementary Fig. 13). Viability staining of the populations excluded the possibility of selective apoptosis induced by the removal IL-12 (data not shown), suggesting that IL-12 did not induce permanent changes in the amount of IFN-γ expressed or the frequency of Treg cells expressing IFN-γ.
IL-12–stimulated Treg cells showed a TH1 chemokine receptor profile14,15 characterized by expression of both CXCR3 and CCR5 and decreased TGFB1 mRNA and protein expression (Fig. 2d and Supplementary Fig. 14). In contrast to Treg cells from subjects with RRMS, Treg cells upregulated CTLA4 mRNA and protein expression in response to IL-12 (Supplementary Fig. 15).
These data provide a general mechanism by which proinflammatory cytokines such as IL-12 can rapidly alter the phenotype and function of Treg cells, decreasing their suppressive activity. Our results suggest one possible mechanism to account for the diminished suppressive activity of Treg cells from individuals with multiple sclerosis3–6, although prospective studies on the frequency of IFN-γ+Foxp3+ T cells in populations at risk for developing multiple sclerosis and in patients before and after treatment with IFN-β are needed to confirm whether these TH1-like Treg cells are associated with disease pathogenesis. Taken together, our results underscore the plasticity of Treg cells in a proinflammatory environment.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank K.C. O’Connor and members of the Hafler lab for valuable discussions and comments and D. Kozoriz for technical assistance. This work was supported by the US National Institutes of Health grants U19AI070352, R01NS024247, P01AI03971 and P01NS038037 (D.A.H.). D.A.H. is a Jacob Javits Scholar of the National Institute of Neurological Disorders and Stroke. C.M.B.-A. is supported by a Dana Scholars Grant from the Dana Foundation.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
M.D.-V. designed and performed the experiments, analyzed data and wrote the manuscript; C.M.B.-A. and D.A.H. supervised the study and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.McFarland HF, Martin R. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Hafler DA, et al. N. Engl. J Med. 1985;312:1405–1411. doi: 10.1056/NEJM198505303122201. [DOI] [PubMed] [Google Scholar]
- 3.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Forero I, et al. Eur. J. Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- 5.Kumar M, et al. J. Neuroimmunol. 2006;180:178–184. doi: 10.1016/j.jneuroim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Astier AL, Meiffren G, Freeman S, Hafler DA. J. Clin. Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beriou G, et al. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voo KS, et al. Proc. Natl. Acad. Sci. USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, et al. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrnes AA, McArthur JC, Karp CL. Ann. Neurol. 2002;51:165–174. doi: 10.1002/ana.10084. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch A, Suarez-Pinzon WL, Sorensen O. J. Autoimmun. 1996;9:645–651. doi: 10.1006/jaut.1996.0084. [DOI] [PubMed] [Google Scholar]
- 12.Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Proc. Natl. Acad. Sci. USA. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch MA, et al. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beima KM, et al. J. Biol. Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 15.Loetscher P, et al. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.